Adsorption of Hexavalent Chromium by Sodium Alginate Fiber Biochar Loaded with Lanthanum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
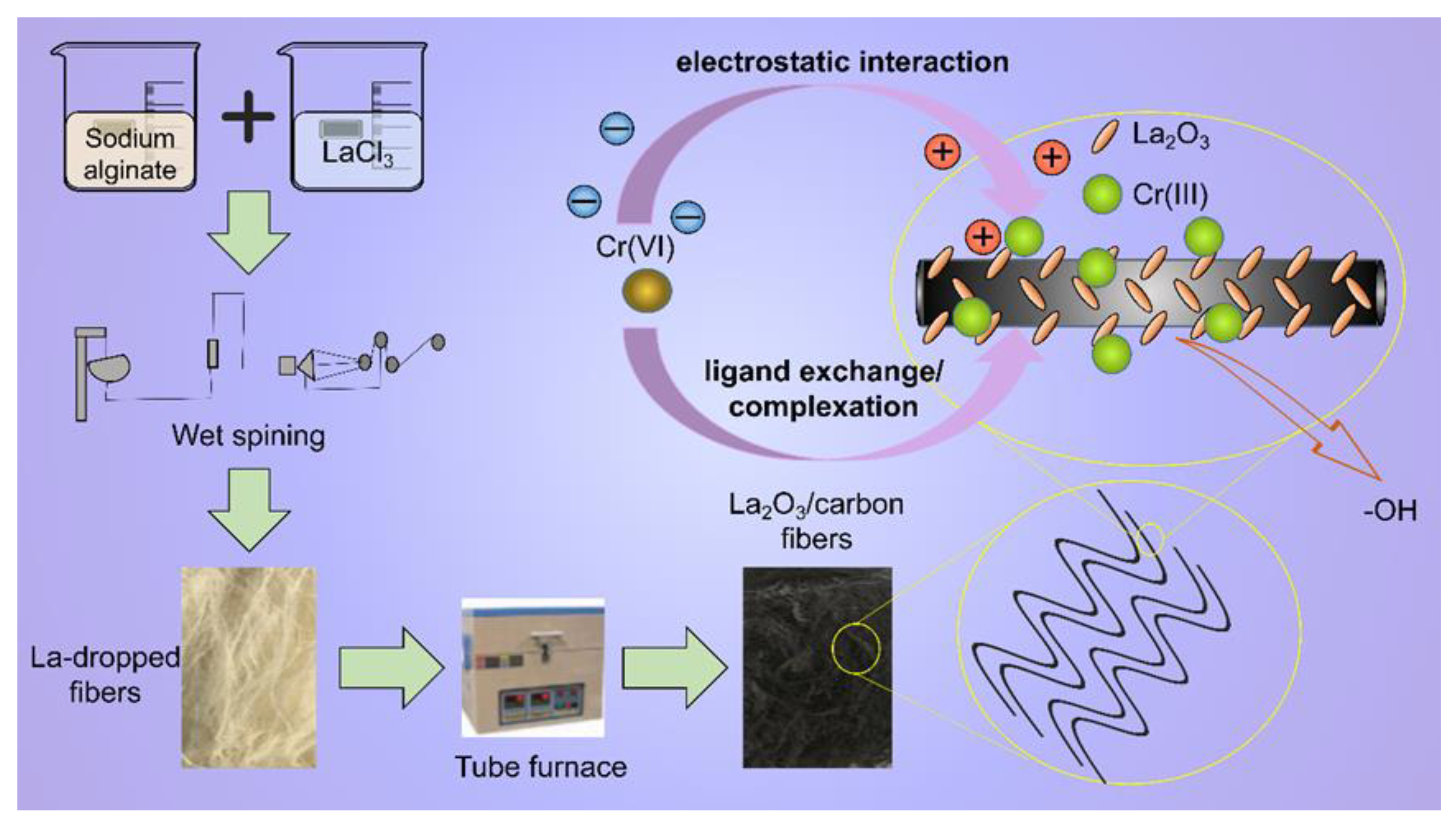
2.2. Preparation of the Adsorbent
2.3. Characterization
2.4. Adsorption and Desorption Experiments
3. Results and Discussion
3.1. Preliminary Experiments
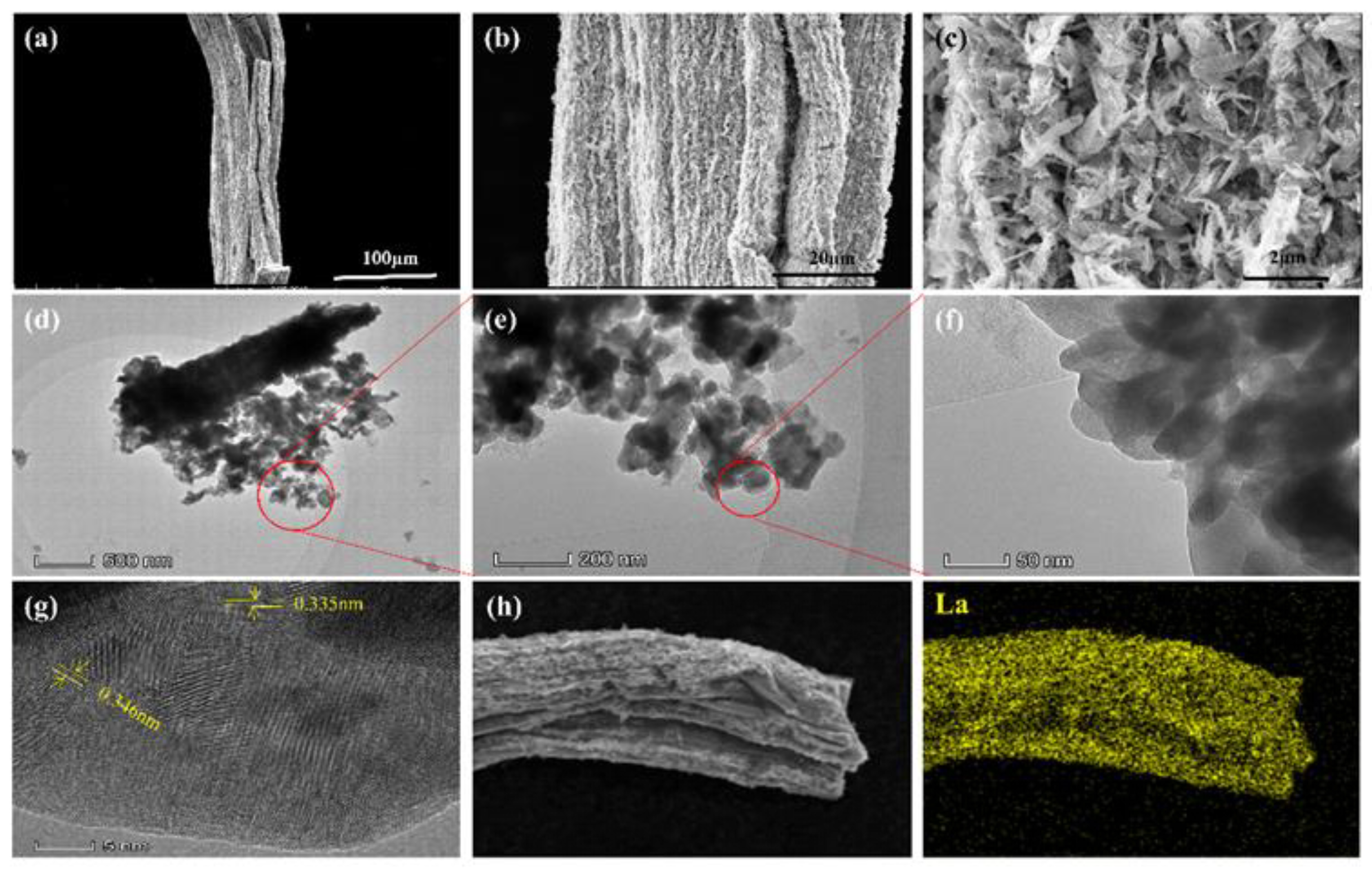
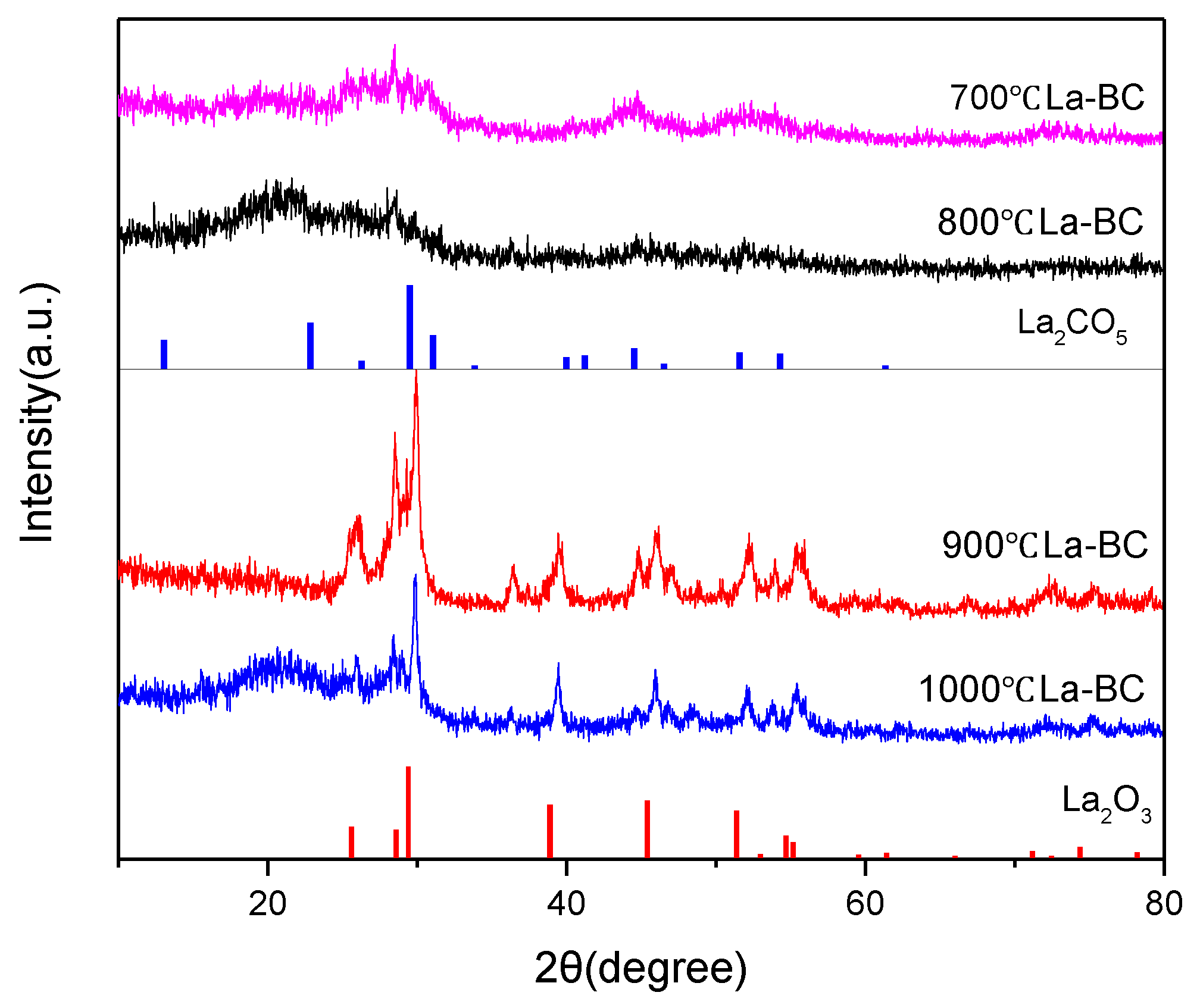
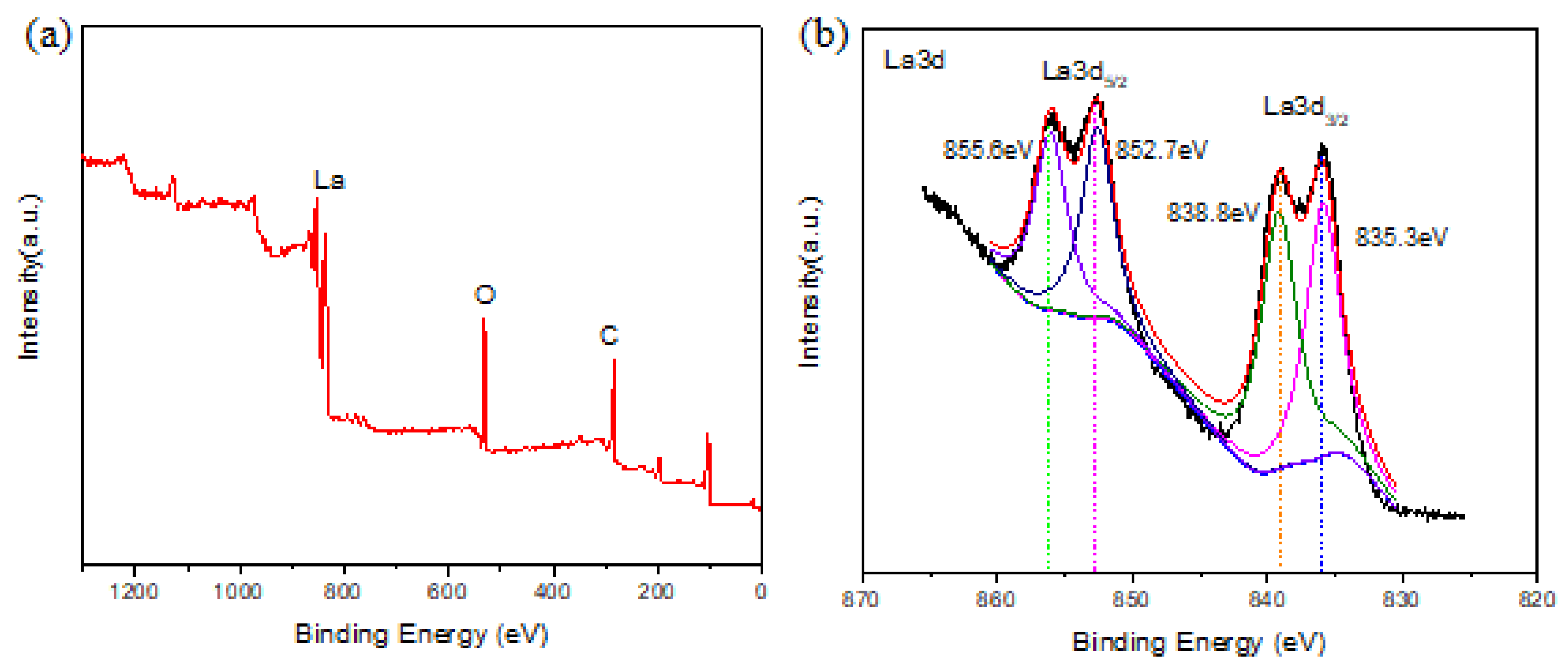
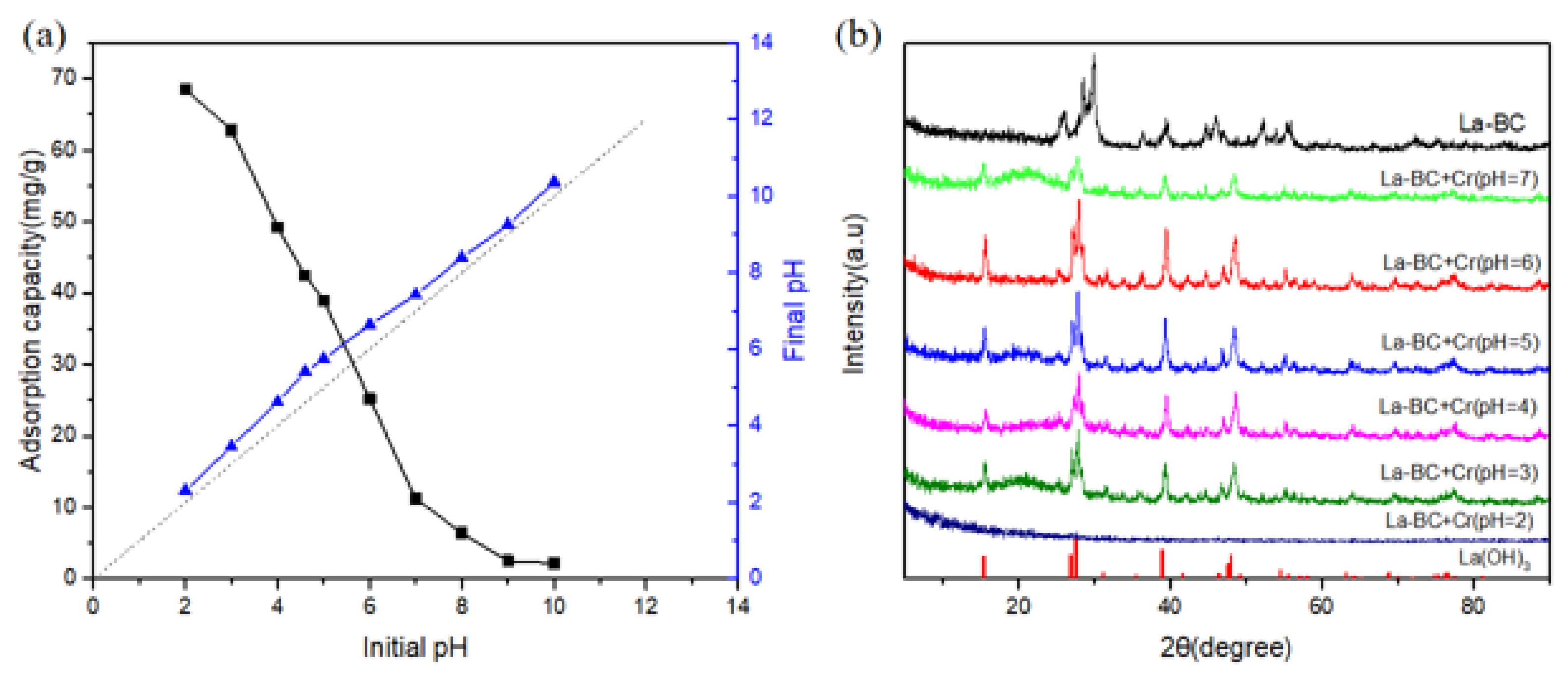
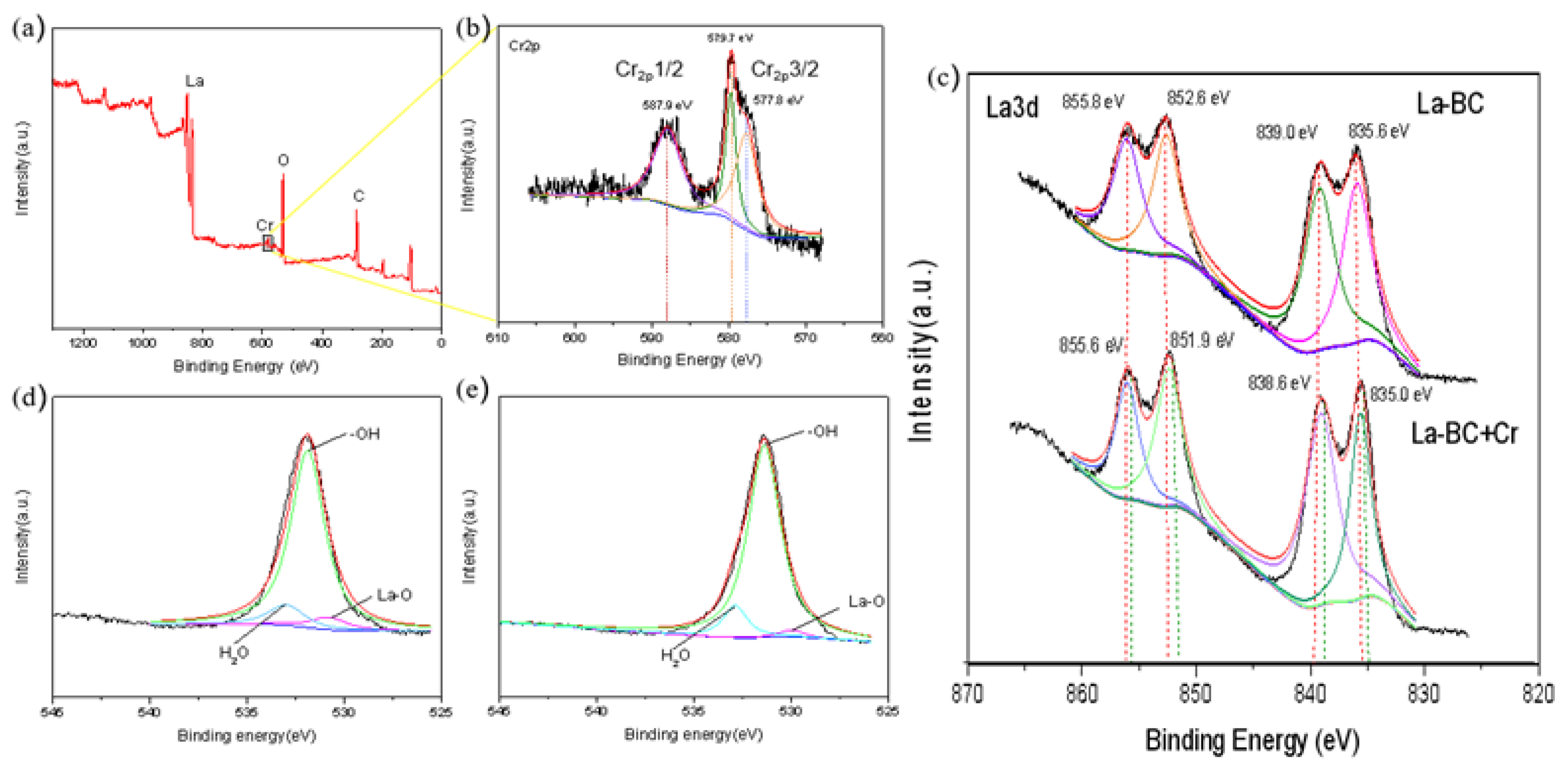
3.2. Characterization of the La-BC
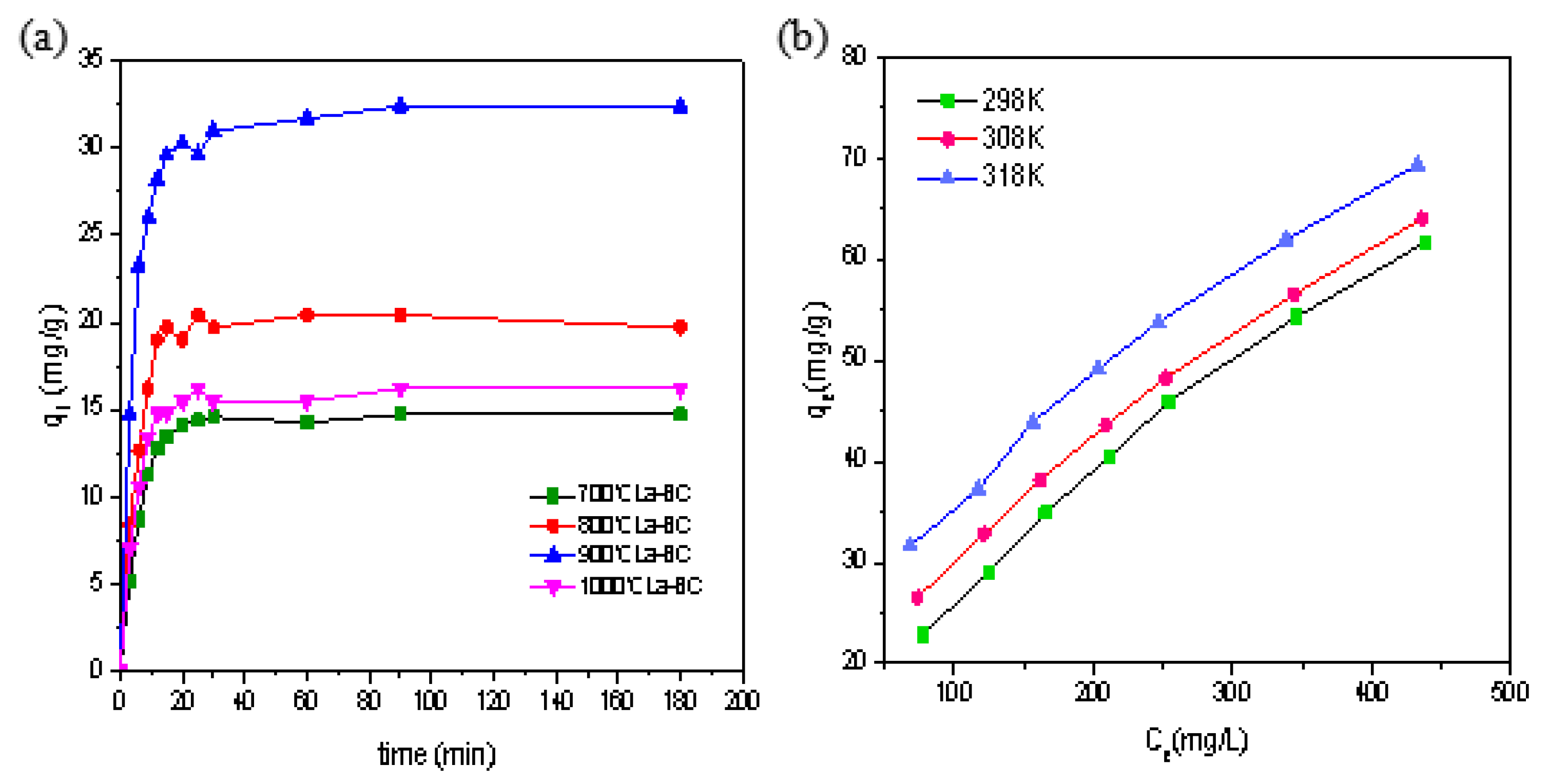
3.3. Adsorption Isotherm
3.4. Adsorption Kinetics
3.5. Adsorption Thermodynamics
3.6. Effect of the Solution pH on the Adsorption Experiment
3.7. Effect of Coexisting Acid Ions on the Adsorption Experiments
3.8. Cycle Experiments
3.9. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, Z.; Zhang, Q.; Gao, B.; Li, M.; Liu, C.; Qiu, Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 2019, 217, 85–94. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Shi, S.; Yang, J.; Liang, S.; Li, M.; Gan, Q.; Xiao, K.; Hu, J. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci. Total Environ. 2018, 628–629, 499–508. [Google Scholar] [CrossRef]
- Kieber, R.J.; Helz, G.R. Indirect photoreduction of aqueous chromium(VI). Environ. Sci. Technol. 1992, 26, 307–312. [Google Scholar] [CrossRef]
- Fu, H.; Zhou, Z.; Zheng, S.; Xu, Z.; Alvarez, P.J.J.; Yin, D.; Qu, X.; Zhu, D. Dissolved Mineral Ash Generated by Vegetation Fire Is Photoactive under the Solar Spectrum. Environ. Sci. Technol. 2018, 52, 10453–10461. [Google Scholar] [CrossRef] [PubMed]
- Hausladen, D.M.; Alexander-Ozinskas, A.; McClain, C.N.; Fendorf, S. Hexavalent Chromium Sources and Distribution in California Groundwater. Environ. Sci. Technol. 2018, 52, 8242–8251. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Sun, Y.; Zhang, J.; Hao, Z. Simultaneous redox conversion and sequestration of chromate(VI) and arsenite(III) by iron(III)-alginate based photocatalysis. Appl. Catal. B Environ. 2019, 259, 118046. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Sun, D.; Tan, X. Entrapment of nanoscale zero-valent iron in chitosan beads for hexavalent chromium removal from wastewater. J. Hazard. Mater. 2010, 184, 724–730. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Lu, A.; Zhong, S.; Chen, J.; Shi, J.; Tang, J.; Lu, X. Removal of Cr(VI) and Cr(III) from Aqueous Solutions and Industrial Wastewaters by Natural Clinopyrrhotite. Environ. Sci. Technol. 2006, 40, 3064–3069. [Google Scholar] [CrossRef]
- Ai, Z.; Cheng, Y.; Zhang, L.; Qiu, J. Efficient Removal of Cr(VI) from Aqueous Solution with Fe@Fe2O3Core−Shell Nanowires. Environ. Sci. Technol. 2008, 42, 6955–6960. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, A.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. Hexavalent chromium adsorption on virgin, biochar, and chemically modified carbons prepared from Phanera vahlii fruit biomass: Equilibrium, kinetics, and thermodynamics approach. Environ. Sci. Pollut. Res. 2019, 26, 32137–32150. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Li, W.; Lan, Y.; Li, Y. Efficient removal of As(III) from aqueous solution by S-doped copper-lanthanum bimetallic oxides: Simultaneous oxidation and adsorption. Chem. Eng. J. 2020, 384, 123274. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Khan, T.A. Gelatin-polyvinyl alcohol/lanthanum oxide composite: A novel adsorbent for sequestration of arsenic species from aqueous environment. J. Water Process Eng. 2020, 34, 101071. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, D.; Wang, Q.; He, T.; Chu, Y.; Li, R. Adsorption of Chromate from Aqueous Solution by Polyethylenimine Modified Multi-Walled Carbon Nanotubes. J. Nanosci. Nanotechnol. 2018, 18, 4006–4013. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Wang, L. Enhanced hexavalent chromium (Cr(VI)) removal from aqueous solution by Fe–Mn oxide-modified cattail biochar: Adsorption characteristics and mechanism. Chem. Ecol. 2019, 36, 138–154. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhanga, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Gupta, V.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, Z.; He, C.; Lyu, W.; Yan, W.; Yang, L. Enhanced antimonate (Sb(V)) removal from aqueous solution by La-doped magnetic biochars. Chem. Eng. J. 2018, 354, 623–632. [Google Scholar] [CrossRef]
- Koilraj, P.; Sasaki, K. Selective removal of phosphate using La-porous carbon composites from aqueous solutions: Batch and column studies. Chem. Eng. J. 2017, 317, 1059–1068. [Google Scholar] [CrossRef]
- Yang, B.; Feng, Y.; Yu, Y.; He, S.; Liu, H.; Xue, L.; Yang, L. Lanthanum ferrite nanoparticles modification onto biochar: Derivation from four different methods and high performance for phosphate adsorption. Environ. Sci. Pollut. Res. 2019, 26, 2010–22020. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Bender, J.; Xu, S. Removal of Arsenate and Chromate by Lanthanum-modified Granular Ceramic Material: The Critical Role of Coating Temperature. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Catalano, J.G.; Park, C.; Fenter, P.; Zhang, Z. Simultaneous inner- and outer-sphere arsenate adsorption on corundum and hematite. Geochim. Cosmochim. Acta 2008, 72, 1986–2004. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, Z.; Khan, Z.H.; Liu, X.; Qiu, W. Enhanced As(III) removal from aqueous solution by Fe-Mn-La-impregnated biochar composites. Sci. Total Environ. 2019, 686, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, L.; Huo, J.B.; Gupta, K.; Fu, M.-L. Simultaneous removal of butylparaben and arsenite by MOF-derived porous carbon coated lanthanum oxide: Combination of persulfate activation and adsorption. Chem. Eng. J. 2020, 391, 123552. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Zhang, Z.; Kuang, Y.; Yang, R.; Wu, D. Interactions of phosphate and dissolved organic carbon with lanthanum modified bentonite: Implications for the inactivation of phosphorus in lakes. Water Res. 2020, 181, 115941. [Google Scholar] [CrossRef]
- Shan, S.; Wang, W.; Liu, D.; Zhao, Z.; Shi, W.; Cui, F. Remarkable phosphate removal and recovery from wastewater by magnetically recyclable La2O2CO3/gamma-Fe2O3 nanocomposites. J. Hazard. Mater. 2020, 397, 122597. [Google Scholar] [CrossRef]
- Zhong, Z.; Lu, X.; Yan, R.; Lin, S.; Wu, X.; Huang, M.; Liu, Z.; Zhang, F.; Zhang, B.; Zhu, H.; et al. Phosphate sequestration by magnetic La-impregnated bentonite granules: A combined experimental and DFT study. Sci. Total Environ. 2020, 738, 139636. [Google Scholar] [CrossRef]
- Preethi, J.; Vigneshwaran, S.; Meenakshi, S. Performance of chitosan engraved iron and lanthanum mixed oxyhydroxide for the detoxification of hexavalent chromium. Int. J. Biol. Macromol. 2019, 130, 491–498. [Google Scholar] [CrossRef]
- Xing, Y.; Cheng, J.; Wu, J.; Zhang, M.; Li, X.-A.; Pan, W. Direct electrospinned La2O3 nanowires decorated with metal particles: Novel 1 D adsorbents for rapid removal of dyes in wastewater. J. Mater. Sci. Technol. 2020, 45, 84–91. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium(VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J. Hazard. Mater. 2009, 163, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Li, A. Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal. J. Environ. Manag. 2018, 206, 989–998. [Google Scholar] [CrossRef]
- You, N.; Wang, X.F.; Li, J.Y.; Fan, H.T.; Shen, H.; Zhang, Q. Synergistic removal of arsanilic acid using adsorption and magnetic separation technique based on Fe3O4@ graphene nanocomposite. J. Ind. Eng. Chem. 2019, 70, 346–354. [Google Scholar] [CrossRef]
- Labied, R.; Benturki, O.; Hamitouche, A.E.; Donnot, A. Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujubacores): Kinetic, equilibrium, and thermodynamic study. Adsorpt. Sci. Technol. 2018, 36, 1066–1099. [Google Scholar] [CrossRef]
- Koilraj, P.; Sasaki, K. Eco-Friendly Alkali-Free Arginine-Assisted Hydrothermal Synthesis of Different Layered Double Hydroxides and Their Chromate Adsorption/Reduction Efficiency. ChemistrySelect 2017, 2, 10459–10469. [Google Scholar] [CrossRef]
- Yan, E.; Cao, M.; Ren, X.; Jiang, J.; An, Q.; Zhang, Z.; Gao, J.; Yang, X.; Zhang, D. Synthesis of Fe3O4 nanoparticles functionalized polyvinyl alcohol/chitosan magnetic composite hydrogel as an efficient adsorbent for chromium (VI) removal. J. Phys. Chem. Solids 2018, 121, 102–109. [Google Scholar] [CrossRef]
- Bagheri, M.; Younesi, H.; Hajati, S.; Borghei, S.M. Application of chitosan-citric acid nanoparticles for removal of chromium (VI). Int. J. Biol. Macromol. 2015, 80, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, B.; Zhang, L.; Huang, R. Adsorptive removal of Cr(VI) from aqueous solutions by cross-linked chitosan/bentonite composite. Korean J. Chem. Eng. 2015, 32, 1314–1322. [Google Scholar] [CrossRef]



| Adsorbent | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| MgAl-LDH | 84.2 | [42] |
| MWCNTs-70 | 17.2 | [15] |
| Chitosan engraved iron and lanthanum Mixed oxyhydroxide | 106.04 | [32] |
| Fe3O4 nanoparticles functionalized polyvinyl alcohol/chitosan magnetic composite hydrogel | 24.69 | [43] |
| Chitosan-citric acid nanoparticles | 106.15 | [44] |
| Cross-linked chitosan bentonite composite | 89.13 | [45] |
| Fe–Mn oxide-modified biochar | 59.8 | [16] |
| Biochar supported nZVI composite | 31.53 | [1] |
| La2O3-dropped biochar | 104.9 | This article |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Guo, P.; Sun, Y.; Cui, Y. Adsorption of Hexavalent Chromium by Sodium Alginate Fiber Biochar Loaded with Lanthanum. Materials 2021, 14, 2224. https://doi.org/10.3390/ma14092224
Sun X, Guo P, Sun Y, Cui Y. Adsorption of Hexavalent Chromium by Sodium Alginate Fiber Biochar Loaded with Lanthanum. Materials. 2021; 14(9):2224. https://doi.org/10.3390/ma14092224
Chicago/Turabian StyleSun, Xinzhe, Peng Guo, Yuanyuan Sun, and Yuqian Cui. 2021. "Adsorption of Hexavalent Chromium by Sodium Alginate Fiber Biochar Loaded with Lanthanum" Materials 14, no. 9: 2224. https://doi.org/10.3390/ma14092224
APA StyleSun, X., Guo, P., Sun, Y., & Cui, Y. (2021). Adsorption of Hexavalent Chromium by Sodium Alginate Fiber Biochar Loaded with Lanthanum. Materials, 14(9), 2224. https://doi.org/10.3390/ma14092224






