Preliminary In Vitro Study of Fluoride Release from Selected Ormocer Materials
Abstract
:1. Introduction
2. Materials and Methods
- Admira (A), (Voco, Cuxhaven, Germany) has a 78% content of inorganic fillers (by weight) which are a mixture of ceramic glass with an average particle size of 0.7 μm and of pyrogenic silica with the size of about 0.04 μm. The particles are prepared chemically, or silanized, to obtain a good connection between the matrix and the filler. The matrix is an inorganic osmolar copolymer and dimethacrylate mix.
- Admira Flow (AF), (Voco, Cuxhaven, Germany) is a light-curing, liquid osmic material for low-viscosity restorations.
- Admira Seal (AS), (Voco, Cuxhaven, Germany) is a material consisting mainly of borosilicate glass (16%).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaczmarek, U.; Kosior, P. Fluoride release from selected compomers—An in vitro study. Polish J. Environ. Stud. 2007, 16, 460–465. [Google Scholar]
- Kosior, P.; Dobrzyński, M.; Korczyński, M.; Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Piesiak-Pańczyszyn, D.; Fita, K.; Janeczek, M. Long-term release of fluoride from fissure sealants—In vitro study. J. Trace Elem. Med. Biol. 2017, 41, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kosior, P.; Kaczmarek, U. Effect in vitro of environmental parameters on the release of fluoride ions from some materials used in dentistry. Ann. Acad. Med. Stetin. 2004, 50 (Suppl. 1), 65–68. [Google Scholar] [PubMed]
- Kosior, P.; Kaczmarek, U. Release of fluoride ions into saliva from some dental materials. Ann. Acad. Med. Stetin. 2004, 50 (Suppl. 1), 62–64. [Google Scholar] [PubMed]
- Hamilton, I.R. Biochemical Effects of Fluoride on Oral Bacteria. J. Dent. Res. 1990, 69, 660–667. [Google Scholar] [CrossRef]
- Scholz, K.J.; Federlin, M.; Hiller, K.A.; Ebensberger, H.; Ferstl, G.; Buchalla, W. EDX-analysis of fluoride precipitation on human enamel. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Simmer, J.P.; Hardy, N.C.; Chinoy, A.F.; Bartlett, J.D.; Hu, J.C.-C. How fluoride protects dental enamel from demineralization. J. Int. Soc. Prev. Community Dent. 2020, 10, 134. [Google Scholar] [CrossRef]
- Kobierska-Brzoza, J.; Dobrzyński, M.; Fita, K.; Bader-Orłowska, D.; Szymonowicz, M. Currently Recommended Restorative Materials in Modern Conservative Dentistry. Polym. Med. 2015, 45, 37–43. [Google Scholar]
- Salmerón-Valdés, E.; Scougall-Vilchis, R.; Alanis-Tavira, J.; Morales-Luckie, R. Comparative study of fluoride released and recharged from conventional pit and fissure sealants versus surface prereacted glass ionomer technology. J. Conserv. Dent. 2016, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Cagetti, M.G.; Carta, G.; Cocco, F.; Sale, S.; Congiu, G.; Mura, A.; Strohmenger, L.; Lingström, P.; Campus, G.; Bossù, M.; et al. Effect of Fluoridated Sealants on Adjacent Tooth Surfaces. J. Dent. Res. 2014, 93, 59S–65S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaga, M.; Kakuda, S.; Ida, Y.; Toshima, H.; Hashimoto, M.; Endo, K.; Sano, H. Inhibition of enamel demineralization by buffering effect of S-PRG filler-containing dental sealant. Eur. J. Oral Sci. 2014, 122, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Townsend, J.; Wang, Y.; Lee, E.C.; Evans, K.; Hender, E.; Hagan, J.L.; Xu, X. Formulation and characterization of antibacterial fluoride-releasing sealants. Pediatr. Dent. 2013, 35, 8E–13E. [Google Scholar]
- Shimazu, K.; Ogata, K.; Karibe, H. Evaluation of the ion-releasing and recharging abilities of a resin-based fissure sealant containing S-PRG filler. Dent. Mater. J. 2011, 30, 923–927. [Google Scholar] [CrossRef] [Green Version]
- Bayrak, S.; Tunc, E.S.; Aksoy, A.; Ertas, E.; Guvenc, D.; Ozer, S. Fluoride Release and Recharge from Different Materials Used as Fissure Sealants. Eur. J. Dent. 2010, 04, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Kavaloglu Cildir, S.; Sandalli, N. Compressive Strength, Surface Roughness, Fluoride Release and Recharge of Four New Fluoride-releasing Fissure Sealants. Dent. Mater. J. 2007, 26, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Cuijpers, V.; Fan, M.; Frencken, J.E. Marginal leakage of two newer glass-ionomer-based sealant materials assessed using micro-CT. J. Dent. 2010, 38, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Czarnecka, B. Materials for the Direct Restoration of Teeth; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081004913. [Google Scholar]
- Rezk-Lega, F.; Ögaard, B.; Rölla, G. Availability of fluoride from glass-ionomer luting cements in human saliva. Eur. J. Oral Sci. 1991, 99, 60–63. [Google Scholar] [CrossRef]
- El Mallakh, B.F.; Sarkar, N.K. Fluoride release from glass-ionomer cements in de-ionized water and artificial saliva. Dent. Mater. 1990, 6, 118–122. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials-Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Vahid-Dastjerdi, E.; Borzabadi-Farahani, A.; Pourmofidi-Neistanak, H.; Amini, N. An in-vitro assessment of weekly cumulative fluoride release from three glass ionomer cements used for orthodontic banding. Prog. Orthod. 2012, 13, 49–56. [Google Scholar] [CrossRef]
- Williams, J.A.; Billington, R.W.; Pearson, G.J. The influence of sample dimensions on fluoride ion release from a glass ionomer restorative cement. Biomaterials 1999, 20, 1327–1337. [Google Scholar] [CrossRef]
- Harhash, A.Y.; ElSayad, I.I.; Zaghloul, A.G.S. A comparative in vitro study on fluoride release and water sorption of different flowable esthetic restorative materials. Eur. J. Dent. 2017, 11, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed EL-Sharkawy, F.; Mohamed Zaghloul, N.; Mohsen Ell-kappaney, A. Effect of Water Absorption on Color Stability of Different Resin Based Restorative Materials in Vitro Study. Int. J. Compos. Mater. 2012, 2, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Al-Hiyasat, A.S.; Darmani, H.; Milhem, M.M. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin. Oral Investig. 2005, 9, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, N.M.; Craig, R.G.; Filisko, F.E. The effects of moisture on the dielectric relaxation of urethane dimethacrylate polymer and composites. J. Oral Rehabil. 2001, 28, 376. [Google Scholar] [CrossRef]
- Itota, T.; Carrick, T.E.; Rusby, S.; Al-Naimi, O.T.; Yoshiyama, M.; McCabe, J.F. Determination of fluoride ions released from resin-based dental materials using ion-selective electrode and ion chromatograph. J. Dent. 2004, 32, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Janda, R.; Roulet, J.-F.; Latta, M.; Rüttermann, S. Water sorption and solubility of contemporary resin-based filling materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82B, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.C.; Monteiro, G.Q.d.M.; Montes, M.A.J.R. Polymerization Shrinkage and Flexural Modulus of Flowable Dental Composites. Mater. Res. 2010, 13, 380–384. [Google Scholar] [CrossRef]
- Gunwal, M.K.; Shenoi, P.R.; Paranjape, T.; Dhote, S.; Tongya, R.; Kumar, M.; Rastogi, S. Evaluation of fracture resistance and mode of failure of premolars restored with nanohybrid composite, ORMOCER and ceramic inlays. J. Oral Biol. Craniofacial Res. 2018, 8, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.; Valiathan, A. Dental ceramics and ormocer technology—Navigating the future! Trends Biomater. Artif. Organs 2006, 20, 40–43. [Google Scholar]
- Olczak-Kowalczyk, D.; Borysewicz-Lewicka, M.; Adamowicz-Klepalska, B.; Jackowska, T.; Kaczmarek, U.; Kaczmarek, U. Consensus statement of Polish experts on individual caries prevention with fluoride in children and adolescents. Nowa Stomatol. 2016, 21, 47–73. [Google Scholar] [CrossRef]
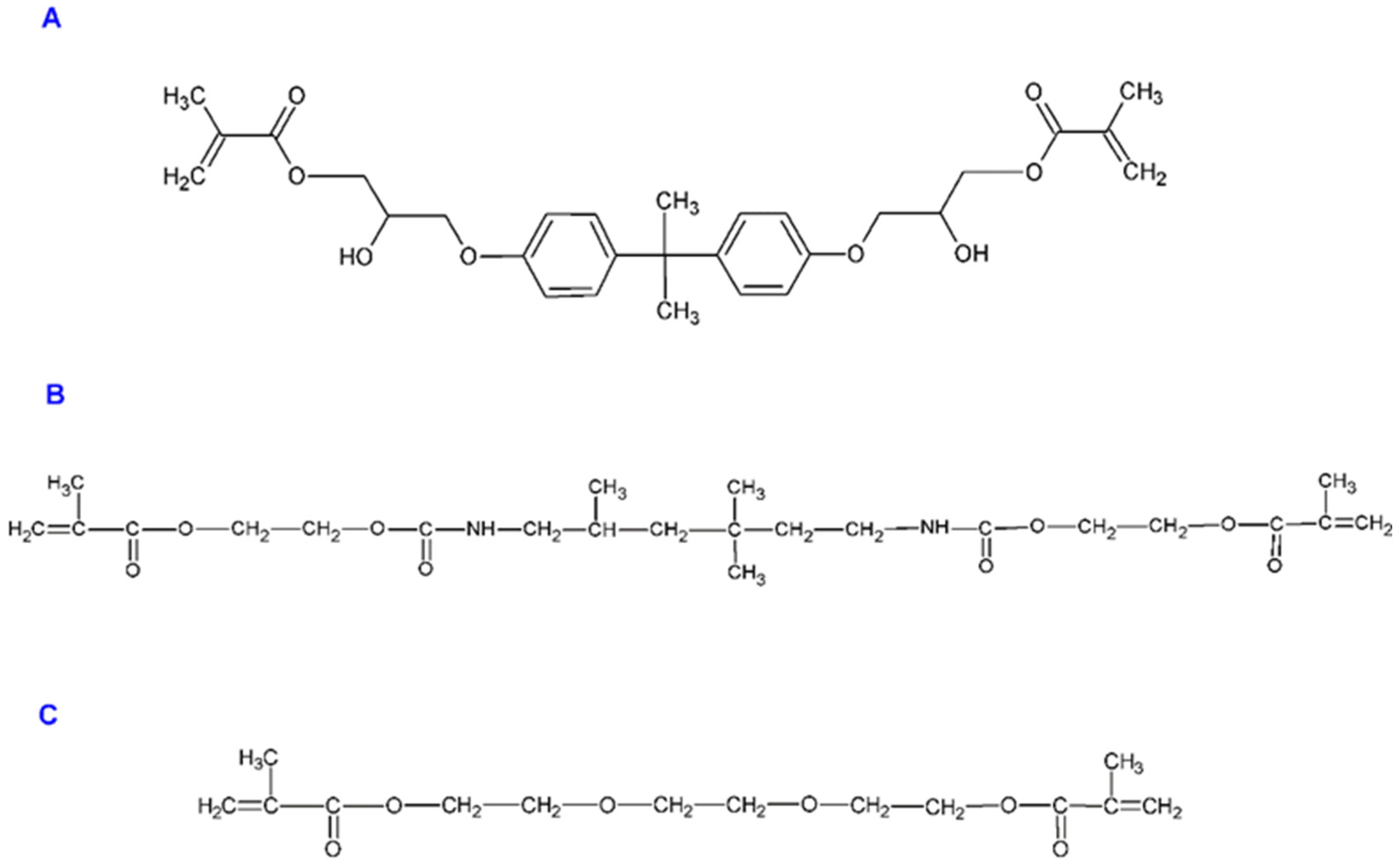




| Material | Manufacturer | Composition |
|---|---|---|
| Admira (A) | Voco, Germany | ormocers (10–25%), Bis-GMA (5–10%), urethane di-methacrylate (5–10%), sodium fluoride |
| Admira Flow (AF) | Voco, Germany | ormocers (10–25%), 1,6-hexanediyl bismoacrylate (10–25%), Bis-GMA (5–10%), urethane di-methacrylate (5–10%), triethylene glycol di-methacrylate (≤2.5%), sodium fluoride |
| Admira Seal (AS) | Voco, Germany | borosilicate glass (16%), di-methacrylates, silicate fillers, ormocers and additives (with sodium fluoride) |
| Time (Hours) | A (n = 5) (μg/mm2) Mean ± SD | AF (n = 5) (μg/mm2) Mean ± SD | AS (n = 5) (μg/mm2) Mean ± SD |
|---|---|---|---|
| 1 | 0.20 ± 0.03 | 0.57 ± 0.06 | 0.19 ± 0.02 |
| 3 | 0.14 ± 0.05 | 0.34 ± 0.02 | 0.29 ± 0.02 |
| 24 | 0.23 ± 0.04 | 0.20 ± 0.02 | 0.13 ± 0.02 |
| 48 | 0.23 ± 0.03 | 0.13 ± 0.02 | 0.10 ± 0.03 |
| 72 | 0.11 ± 0.03 | 0.20 ± 0.03 | 0.12 ± 0.03 |
| 168 | 0.18 ± 0.03 | 0.23 ± 0.02 | 0.10 ± 0.03 |
| 336 | 0.37 ± 0.03 | 0.23 ± 0.04 | 0.28 ± 0.02 |
| 504 | 0.09 ± 0.02 | 0.15 ± 0.02 | 0.09 ± 0.02 |
| 672 | 0.31 ± 0.03 | 0.48 ± 0.01 | 1.13 ± 0.08 |
| 840 | 0.36 ± 0.03 | 0.46 ± 0.02 | 0.36 ± 0.03 |
| 1008 | 0.11 ± 0.06 | 0.43 ± 0.02 | 0.35 ± 0.02 |
| 1176 | 0.43 ± 0.02 | 0.65 ± 0.05 | 0.46 ± 0.02 |
| 1344 | 0.43 ± 0.02 | 0.66 ± 0.04 | 0.50 ± 0.02 |
| 1512 | 0.34 ± 0.02 | 0.50 ± 0.03 | 0.40 ± 0.02 |
| 1680 | 0.63 ± 0.02 | 0.61 ± 0.02 | 0.62 ± 0.03 |
| 1848 | 0.65 ± 0.02 | 0.64 ± 0.02 | 0.59 ± 0.02 |
| 2016 | 0.51 ± 0.03 | 0.55 ± 0.03 | 0.46 ± 0.03 |
| 2184 | 0.52 ± 0.02 | 0.50 ± 0.02 | 0.49 ± 0.02 |
| 2352 | 0.59 ± 0.02 | 0.53 ± 0.02 | 0.54 ± 0.02 |
| Mean ± SD | 0.34 ± 0.18 | 0.42 ± 0.18 | 0.38 ± 0.25 |
| Cumulative release of F-ions | 6.73 ± 1.10 | 8.08 ± 1.30 | 7.36 ± 0.30 |
| p-value (ANOVA for dependent samples) | <0.0001 * | <0.0001 * | <0.0001 * |
| Time (Hours) | A (n = 5) (μg/mm2) Mean ± SD | AF (n = 5) (μg/mm2) Mean ± SD | AS (n = 5) (μg/mm2) Mean ± SD |
|---|---|---|---|
| 1 | 0.65 ± 0.02 | 0.43 ± 0.03 | 0.48 ± 0.08 |
| 3 | 0.44 ± 0.02 | 0.26 ± 0.03 | 0.37 ± 0.13 |
| 24 | 0.49 ± 0.03 | 0.35 ± 0.05 | 0.49 ± 0.07 |
| 48 | 0.43 ± 0.04 | 0.32 ± 0.01 | 0.44 ± 0.13 |
| 72 | 0.46 ± 0.06 | 0.38 ± 0.05 | 0.37 ± 0.32 |
| 168 | 0.98 ± 0.03 | 0.65 ± 0.03 | 1.00 ± 0.18 |
| 336 | 0.44 ± 0.01 | 0.31 ± 0.04 | 0.29 ± 0.02 |
| 504 | 0.94 ± 0.14 | 0.75 ± 0.06 | 0.93 ± 0.11 |
| 672 | 0.48 ± 0.08 | 0.82 ± 0.03 | 1.43 ± 0.11 |
| 840 | 0.42 ± 0.02 | 0.36 ± 0.08 | 0.68 ± 0.15 |
| 1008 | 1.90 ± 0.15 | 1.59 ± 0.03 | 1.51 ± 0.1 |
| 1176 | 1.25 ± 0.04 | 1.3 ± 0.28 | 2.38 ± 1.04 |
| 1344 | 1.27 ± 0.09 | 1.52 ± 0.45 | 1.92 ± 0.23 |
| 1512 | 1.36 ± 0.14 | 1.66 ± 0.5 | 1.97 ± 0.2 |
| 1680 | 1.48 ± 0.09 | 1.52 ± 0.13 | 1.89 ± 0.17 |
| 1848 | 1.42 ± 0.07 | 1.32 ± 0.24 | 1.87 ± 0.39 |
| 2016 | 1.18 ± 0.07 | 1.03 ± 0.06 | 1.67 ± 0.52 |
| 2184 | 2.26 ± 0.19 | 1.53 ± 0.11 | 2.34 ± 0.32 |
| 2352 | 5.41 ± 2.87 | 0.69 ± 0.05 | 1.92 ± 0.03 |
| Mean ± SD | 1.22 ± 1.15 | 0.88 ± 0.52 | 1.26 ± 0.73 |
| Cumulative release of F-ions | 23.26 ± 4.16 | 16.79 ± 2.26 | 23.95 ± 4.30 |
| p-value (ANOVA for dependent samples) | <0.0001 * | <0.0001 * | <0.0001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosior, P.; Dobrzynski, M.; Zakrzewska, A.; Grosman, L.; Korczynski, M.; Blicharski, T.; Gutbier, M.; Watras, A.; Wiglusz, R.J. Preliminary In Vitro Study of Fluoride Release from Selected Ormocer Materials. Materials 2021, 14, 2244. https://doi.org/10.3390/ma14092244
Kosior P, Dobrzynski M, Zakrzewska A, Grosman L, Korczynski M, Blicharski T, Gutbier M, Watras A, Wiglusz RJ. Preliminary In Vitro Study of Fluoride Release from Selected Ormocer Materials. Materials. 2021; 14(9):2244. https://doi.org/10.3390/ma14092244
Chicago/Turabian StyleKosior, Piotr, Maciej Dobrzynski, Aneta Zakrzewska, Lukasz Grosman, Mariusz Korczynski, Tomasz Blicharski, Martina Gutbier, Adam Watras, and Rafal J. Wiglusz. 2021. "Preliminary In Vitro Study of Fluoride Release from Selected Ormocer Materials" Materials 14, no. 9: 2244. https://doi.org/10.3390/ma14092244
APA StyleKosior, P., Dobrzynski, M., Zakrzewska, A., Grosman, L., Korczynski, M., Blicharski, T., Gutbier, M., Watras, A., & Wiglusz, R. J. (2021). Preliminary In Vitro Study of Fluoride Release from Selected Ormocer Materials. Materials, 14(9), 2244. https://doi.org/10.3390/ma14092244











