Size Control of Synthesized Silver Nanoparticles by Simultaneous Chemical Reduction and Laser Fragmentation in Origanum majorana Extract: Antibacterial Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
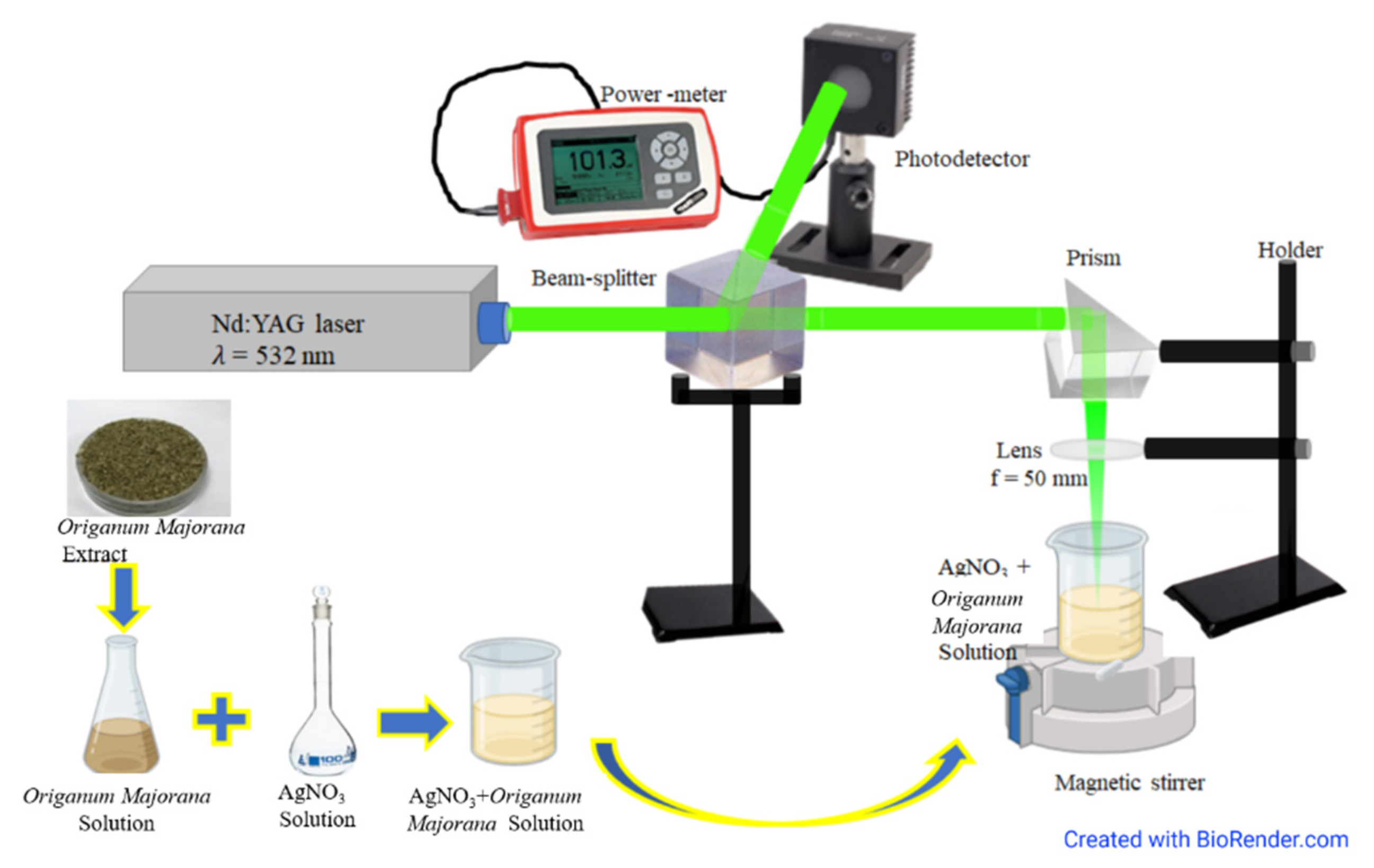
2.2. Experimental Set-Up
2.3. Characterization
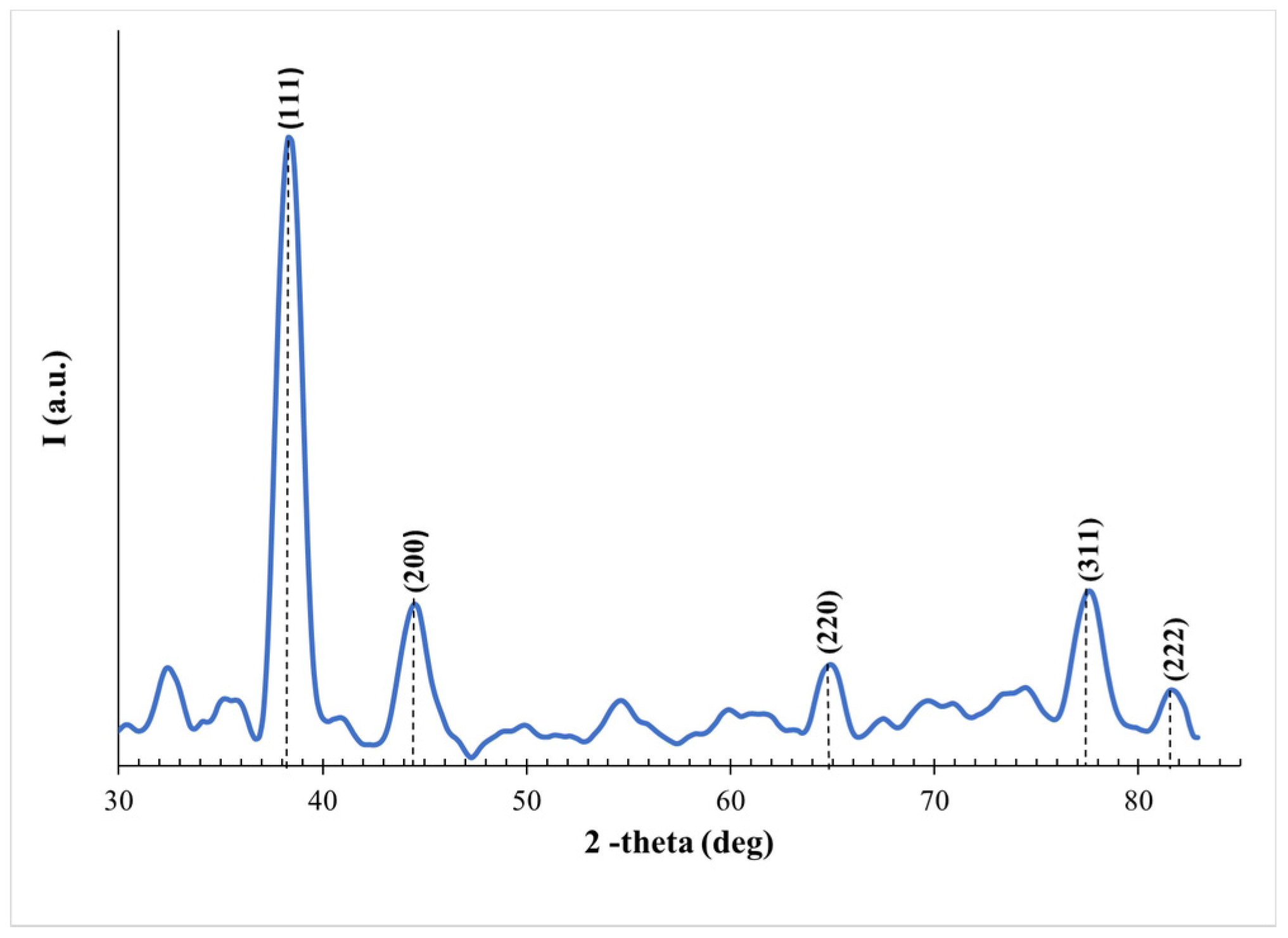
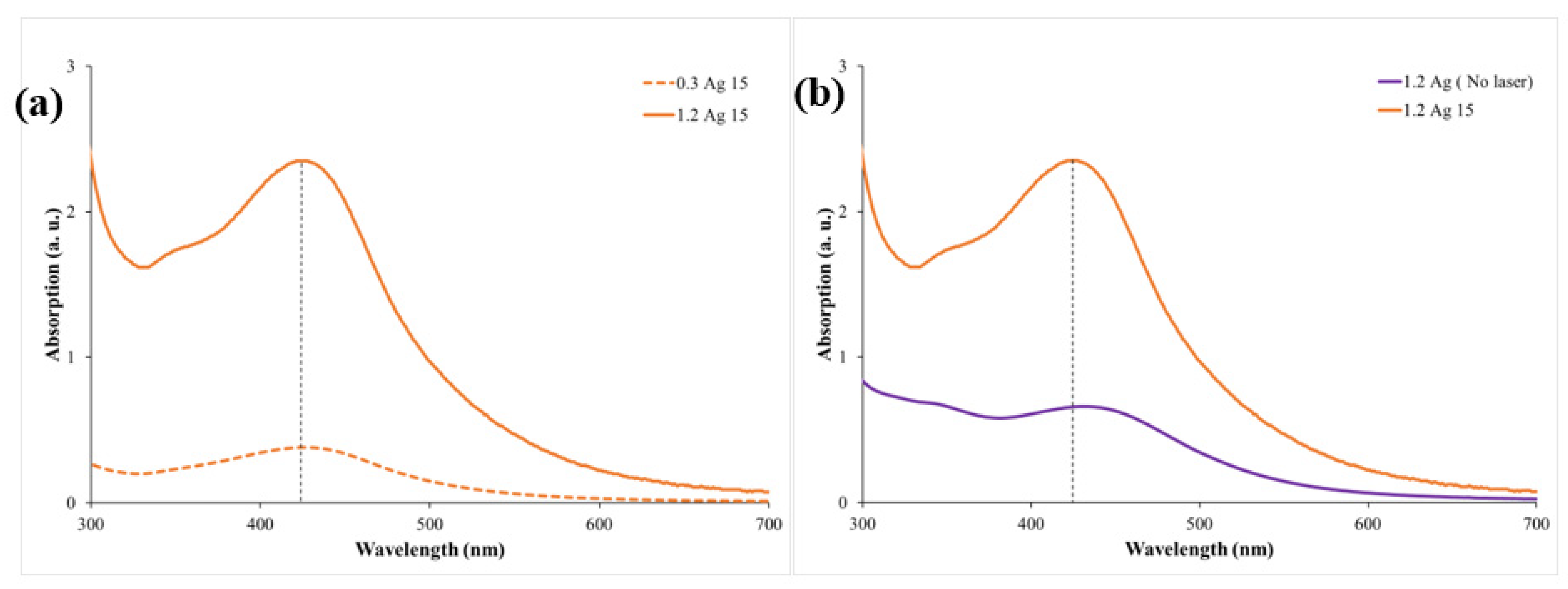
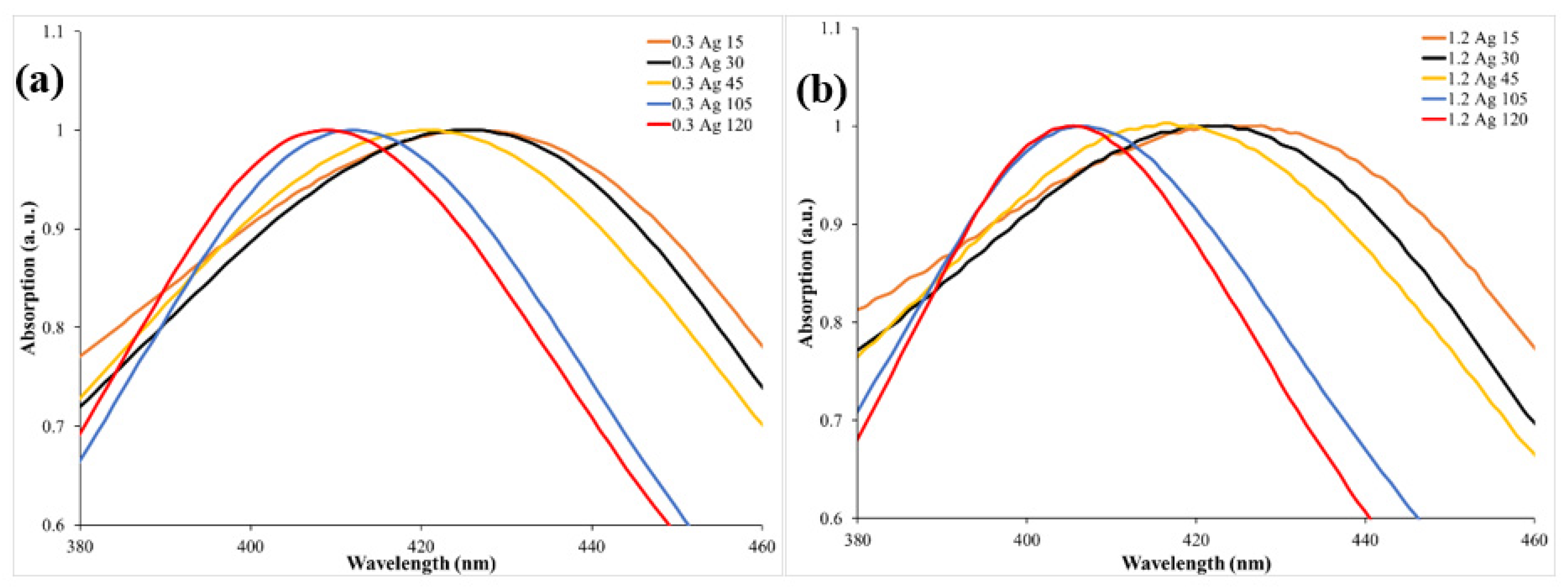
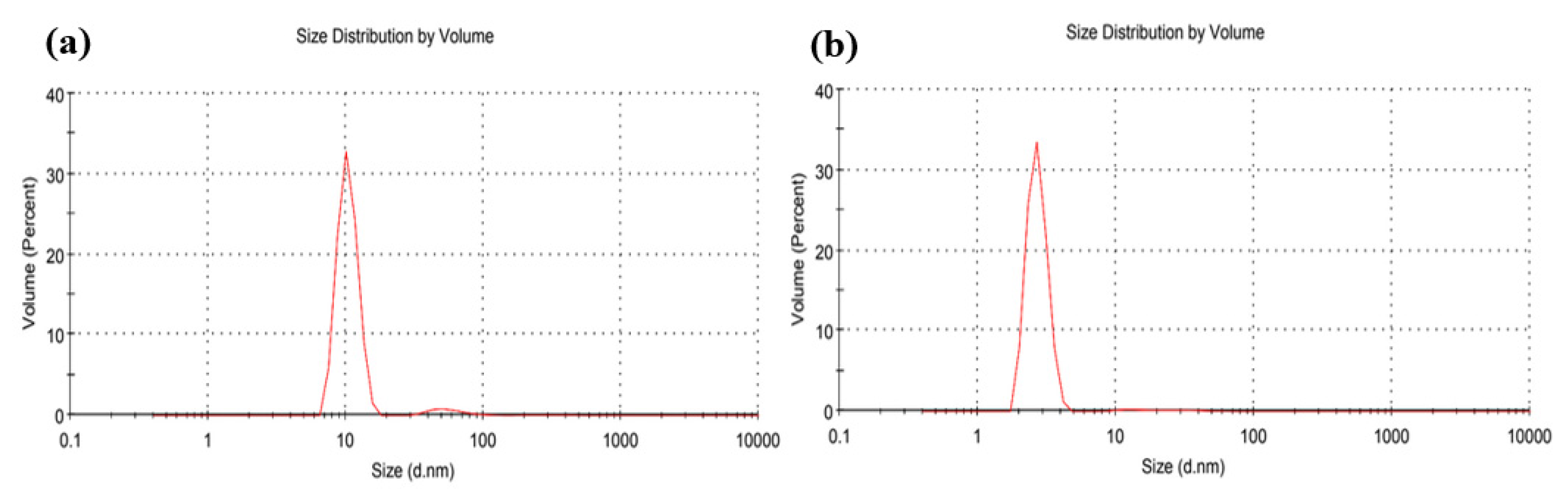
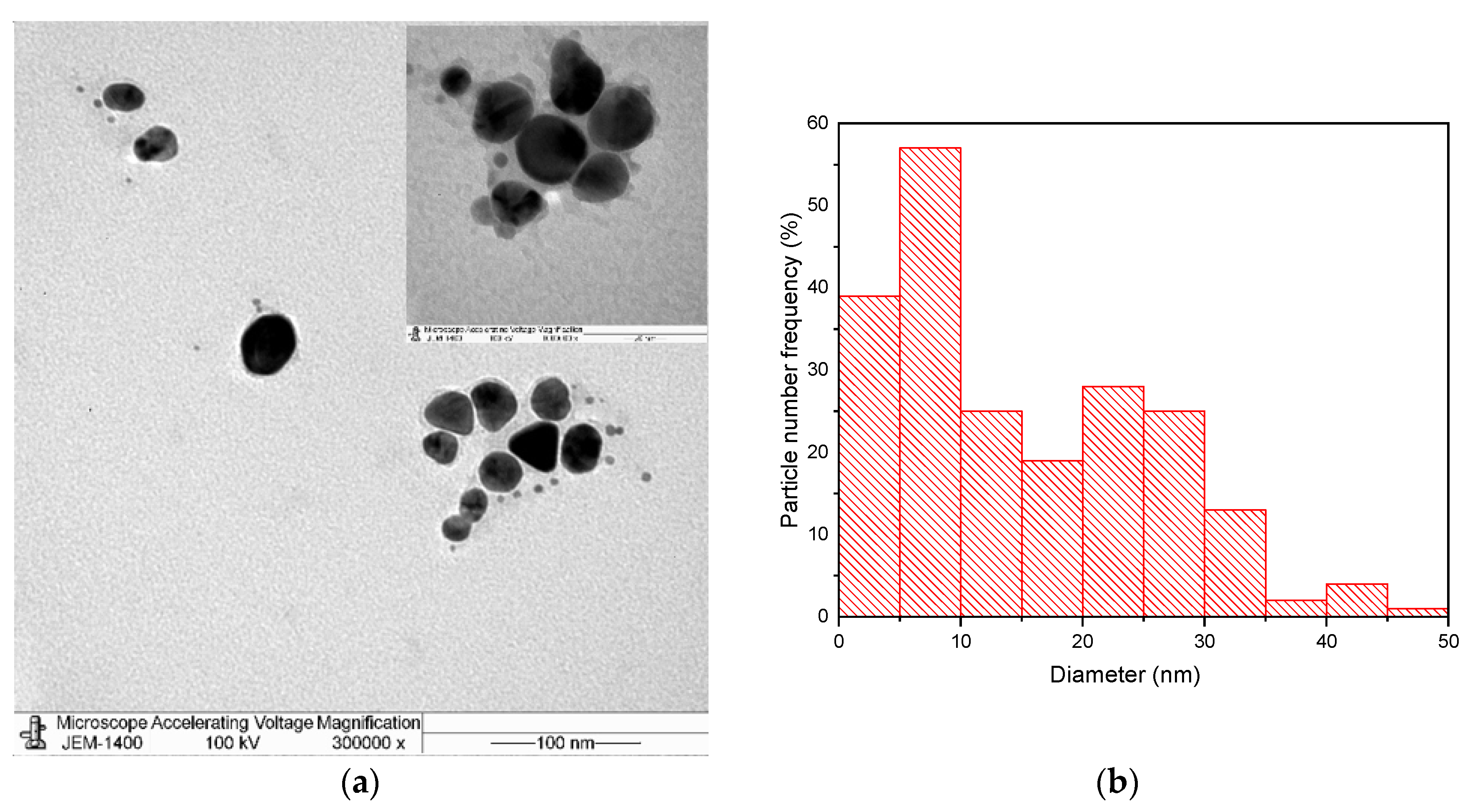
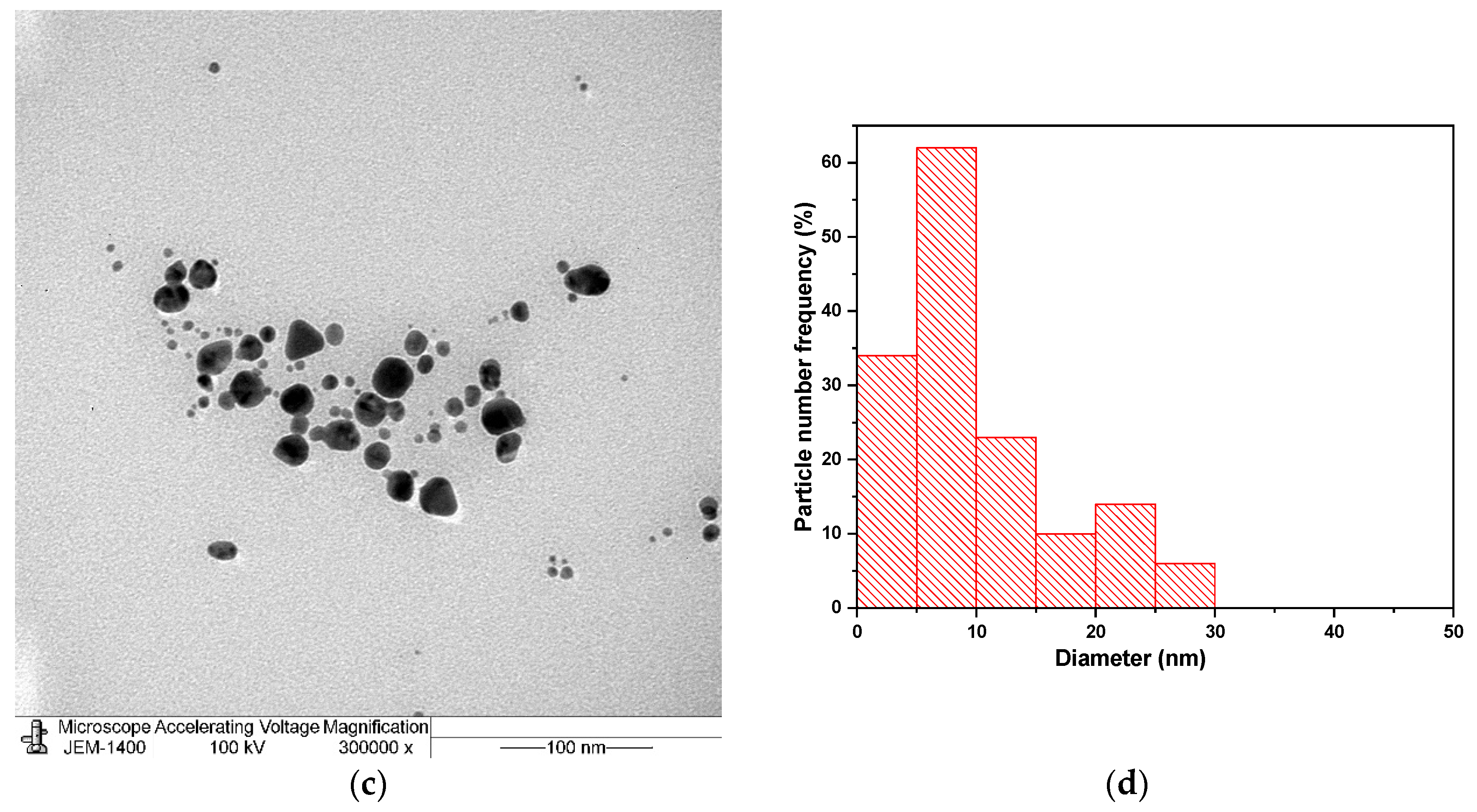
3. Results and Discussion
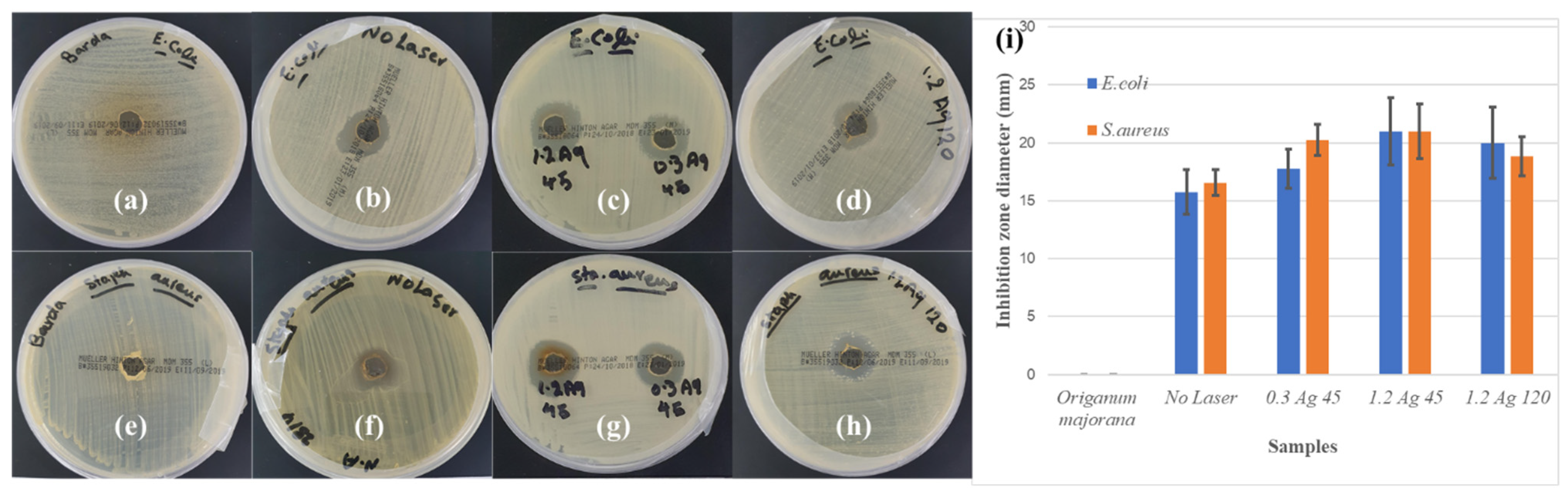
Ag NP Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef]
- Rafique, M.; Rafique, M.S.; Kalsoom, U.; Afzal, A.; Butt, S.H.; Usman, A. Laser Ablation Synthesis of Silver Nanoparticles in Water and Dependence on Laser Nature. Opt. Quantum Electron. 2019, 51, 179. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by Laser Ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Zhang, D.S.; Göekce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Altuwirqi, R.M.; Albakri, A.S.; Al-Jawhari, H.; Ganash, E.A. Green Synthesis of Copper Oxide Nanoparticles by Pulsed Laser Ablation in Spinach Leaves Extract. Optik 2020, 219, 165280. [Google Scholar] [CrossRef]
- Norsyuhada, W.; Shukri, W.M.; Bakhtiar, H.; Islam, S.; Bidin, N. Synthesis and Characterization of Gold-Silver Nanoparticles in Deionized Water by Pulsed Laser Ablation (PLAL) Technique at Different Laser Parameter. Int. J. Nanosci. 2019, 18, 9. [Google Scholar] [CrossRef] [Green Version]
- Ganash, E.A.; Al-Jabarti, G.A.; Altuwirqi, R.M. The Synthesis of Carbon-Based Nanomaterials by Pulsed Laser Ablation in Water. Mater. Res. Express 2019, 7, 015002. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, V.V.; Zhilnikova, M.I.; Barmina, E.V.; Shafeev, G.A.; Kobtsev, V.D.; Kostritsa, S.A.; Pridvorova, S.M. Laser Fragmentation of Aluminum Nanoparticles in Liquid Isopropanol. Chem. Phys. Lett. 2021, 763, 138211. [Google Scholar] [CrossRef]
- Schaumberg, C.A.; Wollgarten, M.; Rademann, K. Fragmentation Mechanism of the Generation of Colloidal Copper(I) Iodide Nanoparticles by Pulsed Laser Irradiation in Liquids. Phys. Chem. Chem. Phys. 2015, 17, 17934–17938. [Google Scholar] [CrossRef]
- Charipar, K.; Kim, H.; Piqué, A.; Charipar, N. ZnO Nanoparticle/Graphene Hybrid Photodetectors via Laser Fragmentation in Liquid. Nanomaterials 2020, 10, 1648. [Google Scholar] [CrossRef]
- Hajiesmaeilbaigi, F.; Mohammadalipour, A.; Sabbaghzadeh, J.; Hoseinkhani, S.; Fallah, H.R. Preparation of Silver Nanoparticles by Laser Ablation and Fragmentation in Pure Water. Laser Phys. Lett. 2006, 3, 252–256. [Google Scholar] [CrossRef]
- Alghoraibi, I.; Zein, R. Silver Nanoparticles: Advances in Research and Applications is Approaching. In Silver Nanoparticles: Advances in Research and Applications; Edwards, B., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017. [Google Scholar]
- Dankovich, T.A.; Gray, D.G. Bactericidal Paper Impregnated with Silver Nanoparticles for Point-of-Use Water Treatment. Environ. Sci. Technol. 2011, 45, 1992–1998. [Google Scholar] [CrossRef]
- Moustafa, M.T. Removal of Pathogenic Bacteria from Wastewater Using Silver Nanoparticles Synthesized by Two Fungal Species. Water Sci. 2017, 31, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a New Generation of Nanoproduct in Biomedical Applications. Trends. Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Pandey, J.K.; Swarnkar, R.K.; Soumya, K.K.; Dwivedi, P.; Singh, M.K.; Sundaram, S.; Gopal, R. Silver Nanoparticles Synthesized by Pulsed Laser Ablation: As a Potent Antibacterial Agent for Human Enteropathogenic Gram-Positive and Gram-Negative Bacterial Strains. Appl. Biochem. Biotechnol. 2014, 174, 1021–1031. [Google Scholar] [CrossRef]
- Gao, M.J.; Sun, L.; Wang, Z.Q.; Zhao, Y.B. Controlled Synthesis of Ag Nanoparticles with Different Morphologies and their Antibacterial Properties. Mater. Sci. Eng. C 2013, 33, 397–404. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Zhang, Y.; Zhang, Z.; Yang, X.; Ali, M.A.; Fox, E.M.; Gobius, K.S.; Man, C. Silver Nanoparticles: A Novel Antibacterial Agent for Control of Cronobacter Sakazakii. J. Dairy Sci. 2018, 101, 10775–10791. [Google Scholar] [CrossRef] [Green Version]
- Ganash, A.A. Electrochemical Properties and Mechanistic Study of the Green Synthesis of Silver Nanoparticles Using Bardaqush Extract Solution. Mater. Res. Express 2019, 6, 065024. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green Synthesis and Characterization of Silver Nanoparticles using Banana Peel Extract and their Antimicrobial Activity against Representative Microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.Y.; Lu, J.R.; Xu, H.Z.; Patel, A.; Chen, Z.S.; Chen, G.F. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.Y.; Gao, Z.W.; Yang, K.F.; Zhang, W.Q.; Xu, L.W. Nanosilver as a New Generation of Silver Catalysts in Organic Transformations for Efficient Synthesis of Fine Chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, Morphological Control, and Properties of Silver Nanoparticles in Potential Applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Tasharrofi, N.; Saber, M.M. Green Synthesis of Silver Nanoparticles Using Teucrium Polium Leaf Extract and Assessment of their Antitumor Effects Against MNK45 Human Gastric Cancer Cell Line. J. Mol. Struct. 2020, 1208, 127889. [Google Scholar] [CrossRef]
- Tsuji, T.; Thang, D.H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of Silver Nanoparticles by Laser Ablation in Polyvinylpyrrolidone Solutions. Appl. Surf. Sci. 2008, 254, 5224–5230. [Google Scholar] [CrossRef]
- Hamad, A.H. Nanosecond Laser Generation of Silver Nanoparticles in Ice Water. Chem. Phys. Lett. 2020, 755, 137782. [Google Scholar] [CrossRef]
- Moura, C.G.; Pereira, R.S.F.; Andritschky, M.; Lopes, A.L.B.; Grilo, J.P.D.F.; Nascimento, R.M.D.; Silva, F.S. Effects of Laser Fluence and Liquid Media on Preparation of Small Ag Nanoparticles by Laser Ablation in Liquid. Opt. Laser Technol. 2017, 97, 20–28. [Google Scholar] [CrossRef]
- Qayyum, H.; Ali, R.; Rehman, Z.U.; Ullah, S.; Shafique, B.; Dogar, A.H.; Shah, A.; Qayyum, A. Synthesis of Silver and Gold Nanoparticles by Pulsed Laser Ablation for Nanoparticle Enhanced Laser-Induced Breakdown Spectroscopy. J. Laser Appl. 2019, 31, 022014. [Google Scholar] [CrossRef]
- Qayyum, H.; Ahmed, W.; Hussain, S.; Khan, G.A.; Rehman, Z.U.; Ullah, S.; Rahman, T.U.; Dogar, A.H. Laser Synthesis of Surfactant-Free Silver Nanoparticles for Toxic Dyes Degradation and SERS Applications. Opt. Laser Technol. 2020, 129, 106313. [Google Scholar] [CrossRef]
- Nikov, R.G.; Nedyalkov, N.; Atanasov, P.A.; Karashanova, D.B. Characterization of Colloidal Silver Nanostructures Produced by Pulsed Laser Ablation in Different Liquids. In Proceedings of the 19th International Conference and School on Quantum Electronics: Laser Physics and Applications, Sozopol, Bulgaria, 26–30 September 2016; Dreischuh, T., Gateva, S., Daskalova, A., Serafetinide, A., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2017; Volume 10226. [Google Scholar]
- Valverde-Alva, M.A.; García-Fernández, T.; Villagrán-Muniz, M.; Sánchez-Aké, C.; Castañeda-Guzmán, R.; Esparza-Alegría, E.; Sánchez-Valdés, C.F.; Llamazares, J.L.S.; Herrera, C.E.M. Synthesis of Silver Nanoparticles by Laser Ablation in Ethanol: A Pulsed Photoacoustic Study. Appl. Surf. Sci. 2015, 355, 341–349. [Google Scholar] [CrossRef]
- Solati, E.; Mashayekh, M.; Dorranian, D. Effects of Laser Pulse Wavelength and Laser Fluence on the Characteristics of Silver Nanoparticle Generated by Laser Ablation. Appl. Phys. A. 2013, 112, 689–694. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Al-Azawi, M.A.; Bidin, N.; Ali, A.K.; Hassoon, K.I.; Abdullah, M. Effect of Liquid Layer Thickness on the Ablation Efficiency and the Size-Control of Silver Colloids Prepared by Pulsed Laser Ablation. Mod. Appl. Sci. 2015, 9, 20. [Google Scholar] [CrossRef]
- Mehrabi, M.; Parvin, P.; Reyhani, A.; Mortazavi, S.Z. Hybrid Laser Ablation and Chemical Reduction to Synthesize Ni/Pd Nanoparticles Decorated Multi-Wall Carbon Nanotubes for Effective Enhancement of Hydrogen Storage. Int. J. Hydrog. Energy 2018, 43, 12211–12221. [Google Scholar] [CrossRef]
- Menazea, A.A. Femtosecond Laser Ablation-Assisted Synthesis of Silver Nanoparticles in Organic and Inorganic Liquids Medium and their Antibacterial Efficiency. Radiat. Phys. Chem. 2020, 168, 108616. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Menazea, A.A. Polyvinyl Alcohol/Silver Nanoparticles Film Prepared via Pulsed Laser Ablation: An Eco-friendly Nano-Catalyst for 4-Nitrophenol Degradation. J. Mol. Struct. 2020, 1212, 128125. [Google Scholar] [CrossRef]
- Etacheri, V.; Georgekutty, R.; Michael, K.S.; Suresh, C.P. Single Step Morphology-Controlled Synthesis of Silver Nanoparticles. MRS Online Proc. Libr. 2010, 1217, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, S.J. Silver Nanoparticles: Properties and Applications. 2021. Available online: https://www.sigmaaldrich.com/technical-documents/articles/materials-science/nanomaterials/silver-nanoparticles.html (accessed on 11 April 2021).
- Kaasalainen, M.; Aseyev, V.; Von Haartman, E.; Karaman, D.S.; Makila, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size, Stability, and Porosity of Mesoporous Nanoparticles Characterized with Light Scattering. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of Silver Nanoparticles with Different Shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef] [Green Version]

| Item | 0.3 mL | 1.2 mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 0.3Ag15 | 0.3Ag30 | 0.3Ag45 | 0.3Ag105 | 0.3Ag120 | 1.2Ag15 | 1.2Ag30 | 1.2Ag45 | 1.2Ag105 | 1.2Ag120 |
| Irradiation time (min) | 15 | 30 | 45 | 105 | 120 | 15 | 30 | 45 | 105 | 120 |
| Peak position (nm) | 425 | 424 | 420 | 412 | 409 | 421 | 419 | 414 | 406 | 405 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganash, E.A.; Altuwirqi, R.M. Size Control of Synthesized Silver Nanoparticles by Simultaneous Chemical Reduction and Laser Fragmentation in Origanum majorana Extract: Antibacterial Application. Materials 2021, 14, 2326. https://doi.org/10.3390/ma14092326
Ganash EA, Altuwirqi RM. Size Control of Synthesized Silver Nanoparticles by Simultaneous Chemical Reduction and Laser Fragmentation in Origanum majorana Extract: Antibacterial Application. Materials. 2021; 14(9):2326. https://doi.org/10.3390/ma14092326
Chicago/Turabian StyleGanash, Entesar Ali, and Reem Mohammad Altuwirqi. 2021. "Size Control of Synthesized Silver Nanoparticles by Simultaneous Chemical Reduction and Laser Fragmentation in Origanum majorana Extract: Antibacterial Application" Materials 14, no. 9: 2326. https://doi.org/10.3390/ma14092326






