Anti-Thrombogenicity Study of a Covalently-Attached Monolayer on Stent-Grade Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Mechanical and Electrochemical Polishing of Steel Samples
2.3. Surface Cleaning of Steel Samples
2.4. Surface Silanization of Steel Samples
2.5. Surface Characterization
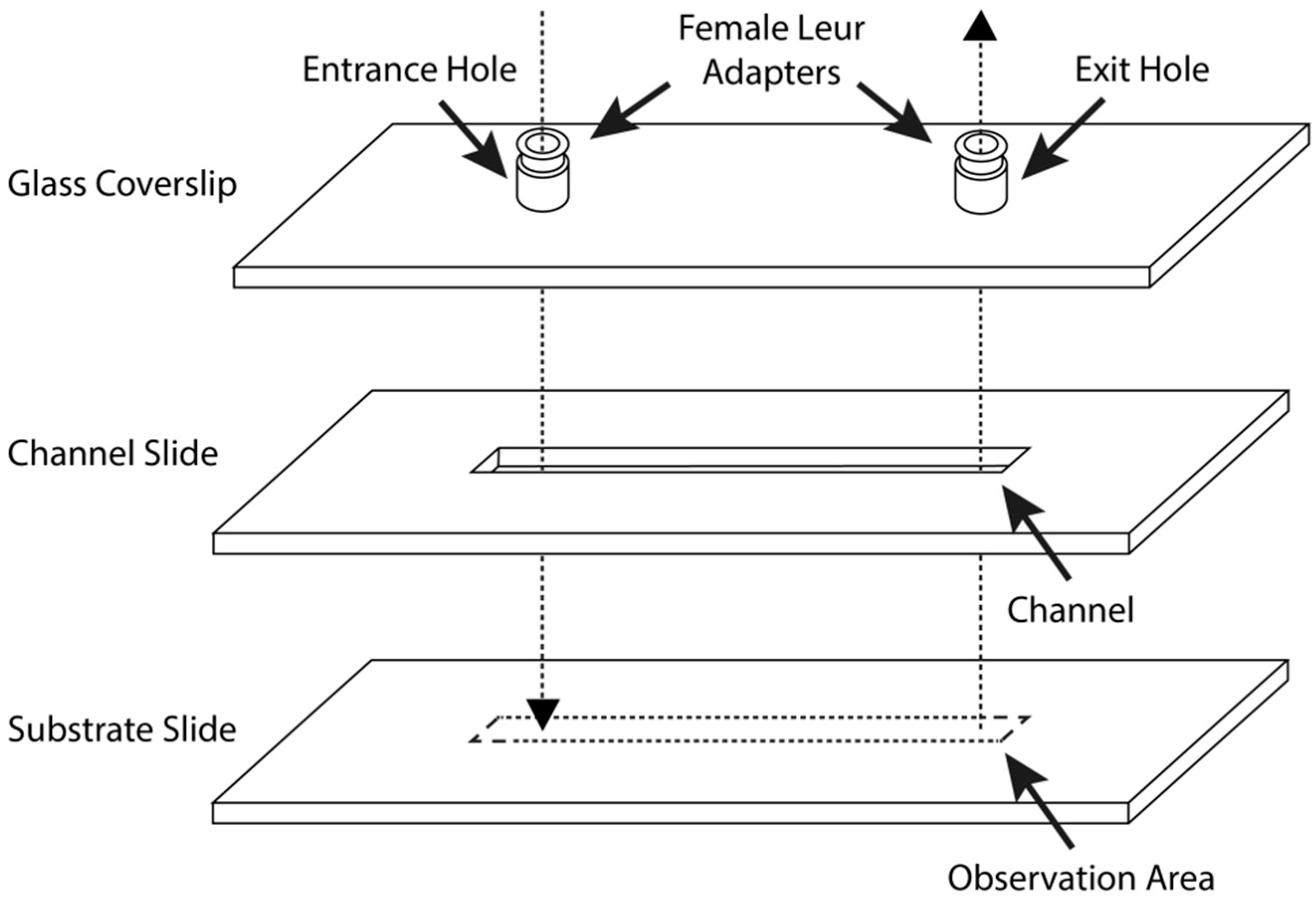
2.6. Parallel-Plate Flow Chamber Preparation and Assembly
2.7. Blood Flow Shear Rate and Regulation
2.8. Fluorescent Labelling of Platelets
2.9. Equipment for Blood Perfusion Experiments
3. Results and Discussion
3.1. Surface Analysis
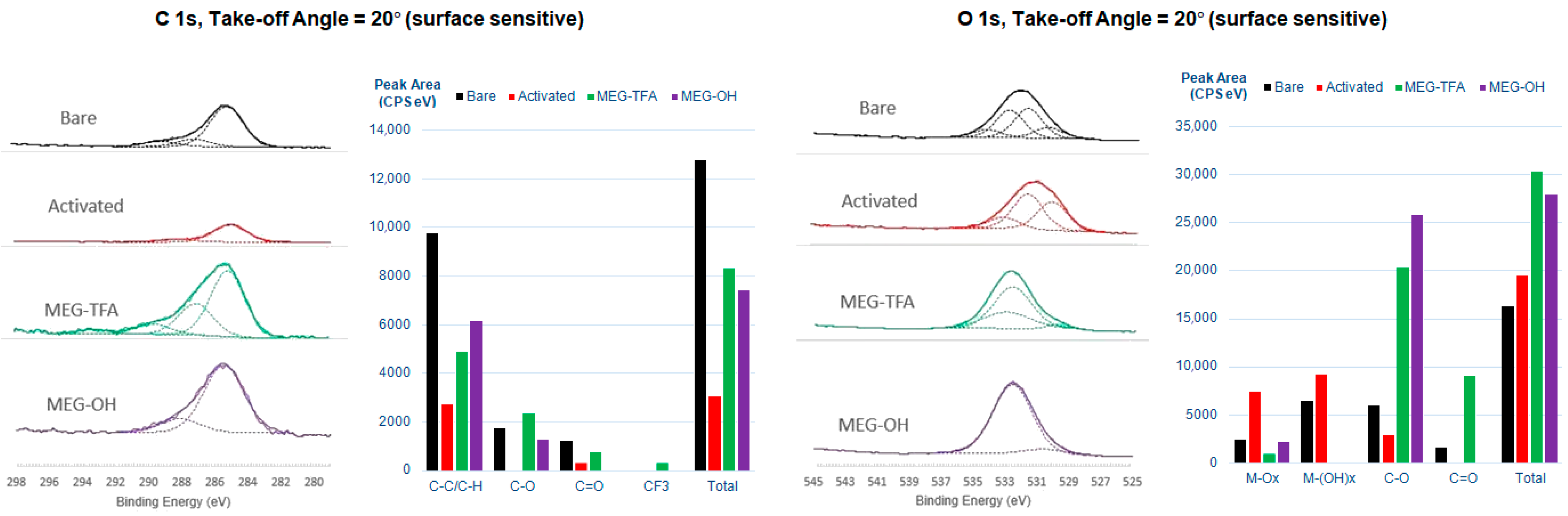
3.1.1. X-ray Photoelectron Spectroscopy
3.1.2. Contact Angle Goniometry
3.2. Thrombogenicity Study of the Blood–Surface Interaction
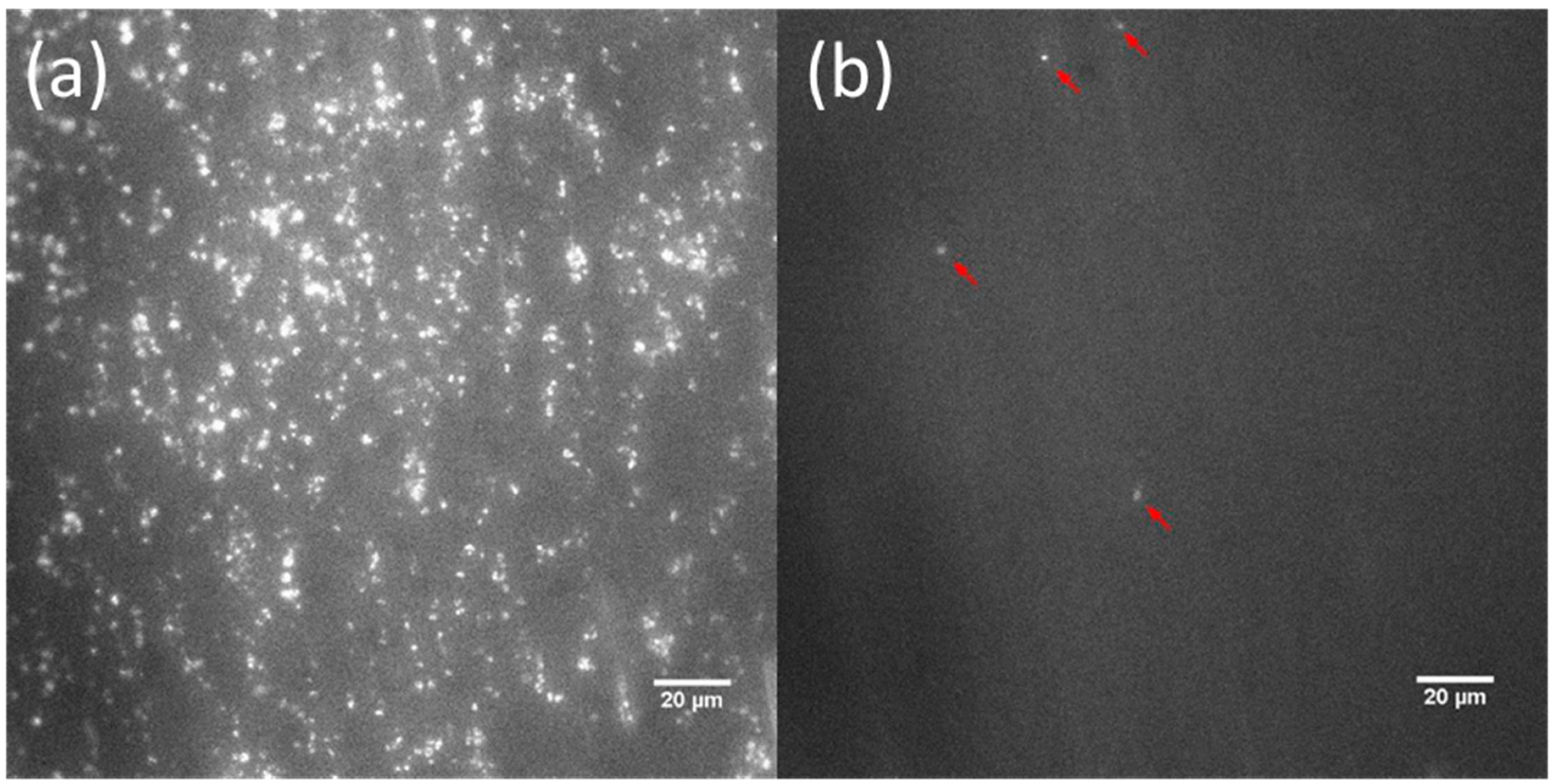
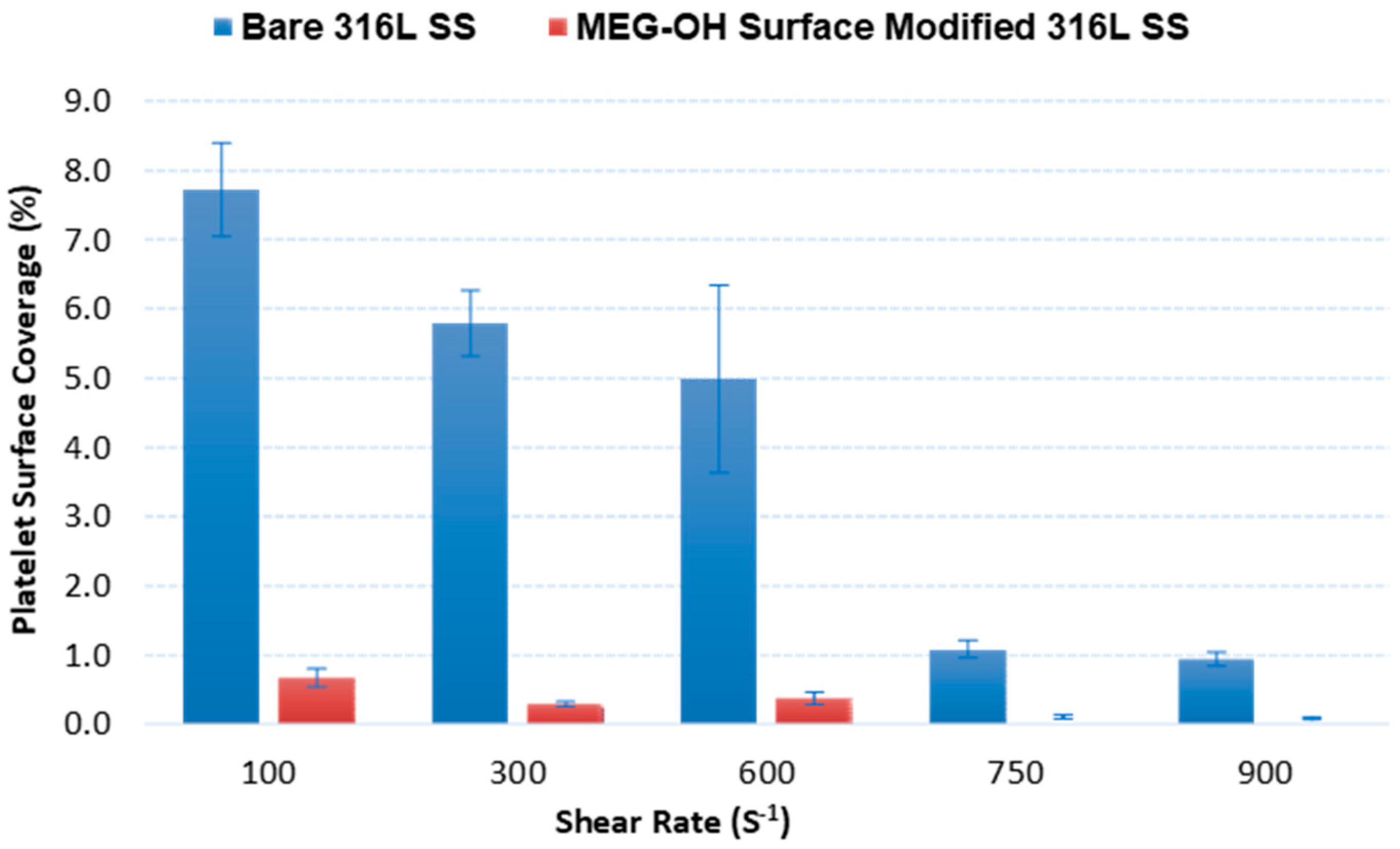
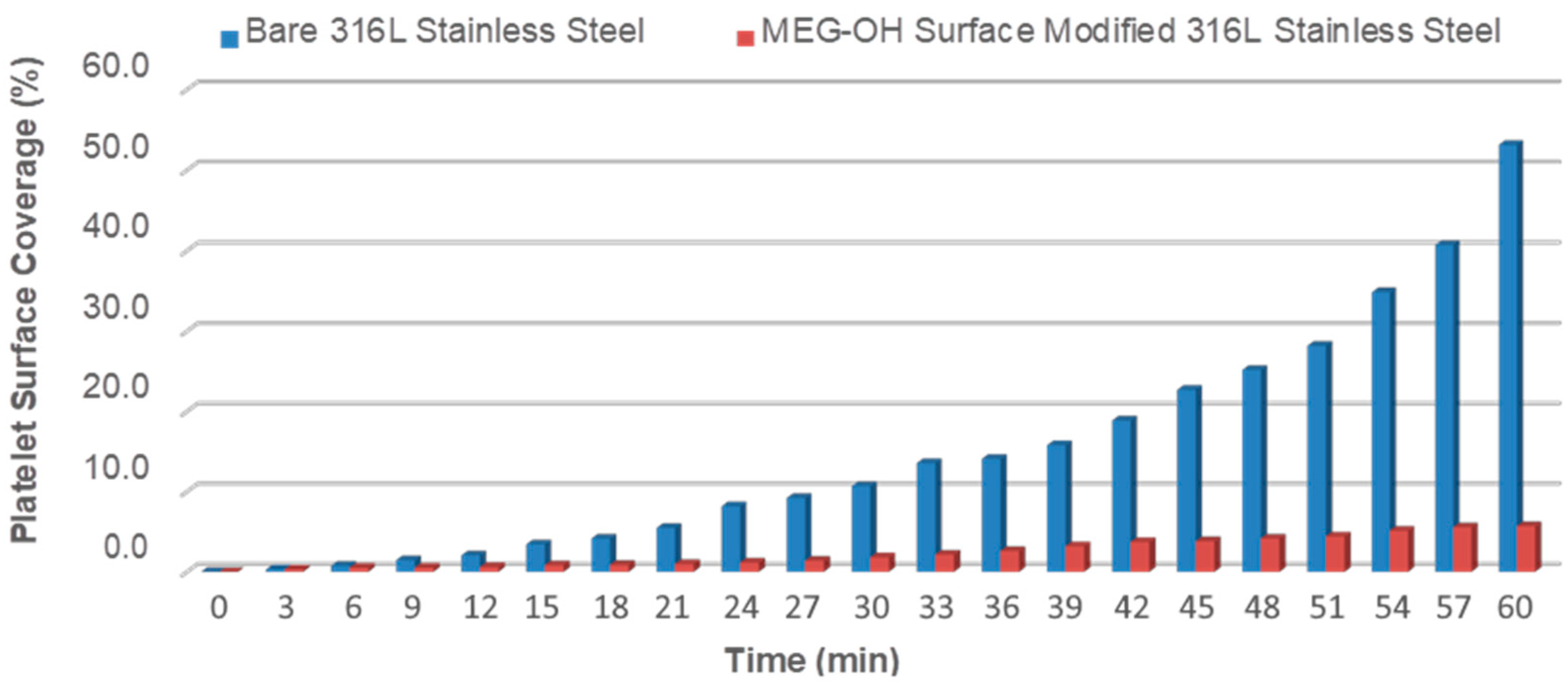
3.2.1. End-Stage Measurement of Surface Platelet Adhesion
3.2.2. Real-Time Measurement of Surface Platelet Adhesion
3.3. Basis of Reduction of Platelet Adhesion by MEG-OH Surface Modification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeewandara, T.M.; Wise, S.G.; Ng, M.K.C. Biocompatibility of Coronary Stents. Materials 2014, 7, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Nazneen, F.; Herzog, G.; Arrigan, D.W.M.; Caplice, N.; Benvenuto, P.; Galvin, P.; Thompson, M. Surface chemical and physical modification in stent technology for the treatment of coronary artery disease. J. Biomed. Mater. Res. B 2012, 100, 1989–2014. [Google Scholar] [CrossRef]
- Farb, A.; Sangiorgi, G.; Carter, A.J.; Walley, V.M.; Edwards, W.D.; Schwartz, R.S.; Virmani, R. Pathology of acute and chronic coronary stenting in humans. Circulation 1999, 99, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Zheng, Y.; Diao, Z.; Qiu, J.; Wang, G. Review: Research progress and future prospects for promoting endothelialization on endovascular stents and preventing restenosis. J. Med. Biol. Eng. 2011, 31, 307–316. [Google Scholar] [CrossRef]
- Wilcox, J.N.; Okamoto, E.I.; Nakahara, K.I.; Vinten-Johansen, J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: A role for circulating myofibroblast precursors? Ann. N. Y. Acad. Sci. 2001, 947, 68–92. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Sheikh, S.; Blaszykowski, C.; Rodriguez-Emmenegger, C.; Pereira, A.S. Biological-Fluid Surface Interactions in Detection and Medical Devices; Royal Society of Chemistry Publishing: Cambridge, UK, 2016. [Google Scholar]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, J.E.; Costa, M.A.; Abizaid, A.C.; Rensing, B.J.; Abizaid, A.S.; Tanajura, L.F.; Kozuma, K.; Van Langenhove, G.; Sousa, A.G.M.T.; Falotico, R.; et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents. Circulation 2001, 104, 2007–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffel, J.; Latini, R.A.; Akhmedov, A.; Zimmermann, D.; Zimmerling, P.; Luscher, T.; Tanner, F.C. Rapamycin, but not FK-506, Increases endothelial tissue factor expression: Implications for drug-eluting stent design. Circulation 2005, 112, 2002–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matter, C.M.; Rozenberg, I.; Jaschko, A.; Greutert, H.; Kruz, D.J.; Wnendt, S.; Kuttler, B.; Joch, H.; Grunenfelder, J.; Zund, G.; et al. Effects of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2006, 48, 286–292. [Google Scholar] [CrossRef]
- Parry, T.J.; Brosius, R.; Thyagarajan, R.; Carter, D.; Argentieri, D.; Falotico, R.; Siekierka, J. Drug-Eluting stents: Sirolimus and Paclitaxel differentially affects cultured cells and injured arteries. Eur. J. Pharmacol. 2005, 524, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.; Schweiss, R.B.; Streller, U.; Werner, C. In Vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials 2005, 26, 6547–6557. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, M.; Anderson, J.M.; Bosworth, C.A.; Andukuri, A.; Minor, W.P.; Lancaster, J.R.J.; Anderson, P.G.; Brott, B.C.; Jun, H.W. A nitric oxide releasing, self-assembled peptide amphiphile matrix that mimics native endothelium for coating implant able cardiovascular devices. Biomaterials 2010, 31, 1502–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andukuri, A.; Kushwaha, M.; Tambralli, A.; Anderson, J.M.; Dean, D.R.; Berry, J.L.; Sohn, Y.D.; Yoon, Y.-S.; Brott, B.C.; Jun, H.-W. A hybrid biomimetic nanomatrix composed of electrospun polycaprolactone and bioactive peptide amphiphiles for cardiovascular implants. Acta Biomater. 2011, 7, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagkiozaki, V.C.; Logothetidis, S.D.; Kassavetis, S.N.; Giannoglou, G.D. Nanomedicine for the reduction of the thrombogenicity of stent coatings. Int. J. Nanomed. 2010, 5, 239. [Google Scholar]
- Lewis, A.L.; Tolhurst, L.A.; Stratford, P.W. Analysis of a phosphorylcholine-based polymer coating on a stent pre- and post-implantation. Biomaterials 2002, 23, 1697–1706. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Gong, F.; Jiang, S.; Yu, H.; An, Y. Anti-CD133 Antibody immobilized on the surface of stents enhances endothelialization. J. Biomed. Res. Int. 2014, 2014, 902782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Wang, Q.; Zhou, J.; Zhu, M.; Qiao, T.; Liu, C.; Mao, C.; Zhou, M. Effect of a novel stent on re-endothelialization, platelet adhesion, and neointimal formation. J. Atheroscler. Thromb. 2016, 23, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Grove, E.C.L.; Kristensen, S.D. Stent thrombosis: Definitions, mechanisms and prevention. ESC Counc. Cardiol. Pract. 2007, 32. [Google Scholar]
- Ong, A.T.; McFadden, E.P.; Regar, E.; de Jaegere, P.P.; van Domburg, R.T.; Serruys, P.W. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J. Am. Coll. Cardiol. 2005, 45, 2088–2092. [Google Scholar] [CrossRef] [Green Version]
- Sharkawi, T.; Cornhill, F.; Lafont, A.; Sabaria, P.; Vert, M. Intravascular bioresorbable polymeric stents: A potential alternative to current drug eluting metal stents. J. Pharm. Sci. 2007, 96, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Yang, M.C.; Tsou, H.M.; Liu, T.Y. Hemocompatibility and anti-fouling behavior of multilayer biopolymers immobilized on gold-thiolized drug-eluting cardiovascular stents. Colloids Surf. B Biointerfaces 2019, 173, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, P.; Wang, J.; Tu, Q.; Bai, L.; Xiong, K.; Qiu, H.; Zhao, X.; Maitz, M.F.; Wang, H.; et al. Endothelium-Mimicking multifunctional coating modified cardiovascular stents via a stepwise metal-catechol-(amine) surface engineering strategy. Research 2020, 2020, 9203906. [Google Scholar] [CrossRef] [Green Version]
- Link, A.; Cattaneo, G.; Brynda, E.; Riedel, T.; Kucerova, J.; Schlensak, C.; Wendel, H.P.; Krajewski, S.; Michel, T. Hemocompatibility testing of blood-contacting implants in a flow loop model mimicking human blood flow. JoVE (J. Vis. Exp.) 2020, 157, e60610. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, L.; Bei, H.P.; Li, X.; Du, Z.; Maitz, M.F.; Huang, N.; Tu, Q.; Zhao, X.; Yang, Z. Self-Protonating, plasma polymerized, superimposed multi-layered biomolecule nanoreservoir as blood-contacting surfaces. Chem. Eng. J. 2021, 410, 128313. [Google Scholar] [CrossRef]
- Qiu, H.; Qi, P.; Liu, J.; Yang, Y.; Tan, X.; Xiao, Y.; Maitz, M.F.; Huang, N.; Yang, Z. Biomimetic engineering endothelium-like coating on cardiovascular stent through heparin and nitric oxide-generating compound synergistic modification strategy. Biomaterials 2019, 207, 10–22. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, M.; Gao, W.; Fang, D.; Liu, Z.; Wu, G.; Wan, M.; Mao, C.; Shen, J. Coronary Stents Decorated by Heparin/NONOate Nanoparticles for Anticoagulant and Endothelialized Effects. Langmuir 2020, 36, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Kelton, J.G.; Warkentin, T.E. Heparin-Induced thrombocytopenia: A historical perspective. Blood J. Am. Soc. Hematol. 2008, 112, 2607–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Tu, Q.; Gao, P.; Li, X.; Maitz, M.F.; Xiong, K.; Huang, N.; Yang, Z. Phenolic-Amine chemistry mediated synergistic modification with polyphenols and thrombin inhibitor for combating the thrombosis and inflammation of cardiovascular stents. Biomaterials 2021, 269, 120626. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyńska, M.; Bil-Lula, I.; Krzywonos-Zawadzka, A.; Arkowski, J.; Łukaszewicz, M.; Hreniak, D.; Stręk, W.; Sawicki, G.; Woźniak, M.; Drab, M.; et al. Biocompatible carbon-based coating as potential endovascular material for stent surface. BioMed Res. Int. 2018, 2018, 2758347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, S.; Xi, Y.; Du, R.; Ren, Y.; Xu, Z.; Tan, Y.; Wang, Y.; Yin, T.; Wang, G. Inhibition of in-stent restenosis after graphene oxide double-layer drug coating with good biocompatibility. Regen. Biomater. 2019, 6, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.C.; Tsou, H.M.; Hsiao, Y.S.; Cheng, Y.W.; Liu, C.C.; Huang, L.Y.; Peng, X.Y.; Liu, T.Y.; Yung, M.C.; Hsu, C.C. Electrochemical Polymerization of PEDOT–Graphene Oxide–Heparin Composite Coating for Anti-Fouling and Anti-Clotting of Cardiovascular Stents. Polymers 2019, 11, 1520. [Google Scholar] [CrossRef] [Green Version]
- Obiweluozor, F.O.; Maharjan, B.; Emechebe, A.G.; Park, C.H.; Kim, C.S. Mussel-Inspired elastic interpenetrated network hydrogel as an alternative for anti-thrombotic stent coating membrane. Chem. Eng. J. 2018, 347, 932–943. [Google Scholar] [CrossRef]
- Parada, G.; Yu, Y.; Riley, W.; Lojovich, S.; Tshikudi, D.; Ling, Q.; Zhang, Y.; Wang, J.; Ling, L.; Yang, Y.; et al. Ultrathin and Robust Hydrogel Coatings on Cardiovascular Medical Devices to Mitigate Thromboembolic and Infectious Complications. Adv. Healthc. Mater. 2020, 9, 2001116. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A. Metals. In Endoluminal Stenting; Sigwart, U., Ed.; WB Saunders: London, UK, 1996; pp. 28–33. [Google Scholar]
- Fischman, D.; Leon, M.; Baim, D. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, K.; Blaszykowski, C.; Sheikh, S.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of thrombogenesis from whole human blood on plastic polymer by ultrathin monoethylene glycol silane adlayer. Langmuir 2014, 30, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, K.; Jankowski, A.; Sheikh, S.; Blaszykowski, C.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of surface-induced thrombogenesis on poly (vinyl chloride). J. Mat. Chem. B 2015, 3, 8623–8628. [Google Scholar] [CrossRef]
- Fedorov, K.; Sheikh, S.; Romaschin, A.; Thompson, M. Enhanced long-term antithrombogenicity instigated by a covalently-attached surface modifier on biomedical polymers. Res. Prog. Mater. Spec. Issue Appl. Dev. Biomater. Med. 2020, 2. [Google Scholar] [CrossRef]
- Pawlowska, N.M.; Fritzsche, H.; Vezvaie, M.; Blaszykowski, C.; Sheikh, S.; Thompson, M. Probing the hydration of ultra-thin antifouling silane adlayers using neutron reflectometry. Langmuir 2014, 30, 1199. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Blaszykowski, C.; Thompson, D.; Thompson, M. On the hydration of subnanometric antifouling organosilane adlayers: A molecular dynamics simulation. J. Coll. Interface Sci. 2015, 437, 197–204. [Google Scholar] [CrossRef]
- Sheikh, S.; Sheng, J.C.-C.; Blaszykowski, C.; Thompson, M. New oligoethylene glycol linkers for the surface modification of an ultra-high frequency acoustic wave biosensor. Chem. Sci. 2010, 1, 271–275. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T. XPS study of Aisi 316L stainless steel surfaces after mechanical and electrochemical polishing and chelating/electro-chelating treatments. Adv. Mat. Sci. 2014, 14, 31. [Google Scholar]
- Lane, W.O.; Jantzen, A.E.; Carlon, T.A.; Jamiolkowski, R.M.; Grenet, J.E.; Ley, M.M.; Haseltine, J.M.; Galinat, L.J.; Lin, F.-H.; Allen, J.D.; et al. Parallel-Plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. J. Vis. Expts. 2012, 59, e3349. [Google Scholar] [CrossRef] [Green Version]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers—The Scienta ESCA300 Database; Wiley Intersci.: Chichester, UK, 1992; Appendices 3.1 and 3.2. [Google Scholar]
- Functional assays. In Platelets and Megakaryocytes; Gibbins, J.M.; Mahaut-Smith, M.P. (Eds.) Humana Press: Totowa, NJ, USA, 2012; Volume 1. [Google Scholar]
- De La Franier, B.; Asker, D.; van den Berg, D.; Hatton, B.; Thompson, M. Reduction of microbial adhesion on polyurethane by a sub-nanometer covalently-attached surface modifier. Colloids Surf. B Biointerfaces 2021, 200, 111579. [Google Scholar] [CrossRef] [PubMed]
- Sakariassen, K.S.; Orning, L.; Turitto, V.T. The impact of blood shear rate on arterial thrombus formation. Futur. Sci. OA 2015, 1, FSO30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhairamadgi, N.S.; Pujari, S.P.; Trovela, F.G.; Debrassi, A.; Khamis, A.A.; Alonso, J.M.; Al Zahrani, A.A.; Wennekes, T.; Al-Turaif, H.A.; van Rijn, C.; et al. Hydrolytic and thermal stability of organic monolayers on Various inorganic substrates. Langmuir 2014, 30, 5829–5839. [Google Scholar] [CrossRef]



| Shear Rate (s−1) | Blood Flow Rate (μL min−1) | Minimum Blood Volume Required (mL) | |
|---|---|---|---|
| 20 min | 60 min | ||
| 100 | 10.3 | 0.21 | 0.62 |
| 300 | 31 | 0.62 | 1.86 |
| 600 | 61.3 | 1.24 | 3.72 |
| 750 | 77.4 | 1.55 | 4.65 |
| 900 | 92.9 | 1.86 | 5.58 |
| Sample | TOA | C (1s) | O (1s) | Si (1s) | F (1s) | Cr (2p) | Fe (2p) | Ni (2p) | Mo (2p) |
|---|---|---|---|---|---|---|---|---|---|
| Bare | 20° | 64.1 | 28.7 | 6.4 | 0 | 0.8 | 0 | 0 | 0 |
| 90° | 37.8 | 42.6 | 12.5 | 0 | 3.5 | 3.5 | 0 | 0 | |
| Activated | 20° | 11.6 | 58.6 | 0 | 0 | 15.1 | 14.8 | 0 | 0 |
| 90° | 9.7 | 59.3 | 0 | 0 | 10.1 | 8.2 | 6.4 | 6.4 | |
| MEG-TFA | 20° | 33.2 | 44.5 | 17.2 | 4.3 | 0.8 | 0 | 0 | 0 |
| 90° | 27.3 | 45.3 | 13.3 | 12.6 | 1.6 | 0 | 0 | 0 | |
| MEG-OH | 20° | 35.7 | 47.9 | 15.2 | 0 | 1.2 | 0 | 0 | 0 |
| 90° | 29.6 | 53.1 | 14.2 | 0 | 1.9 | 0.9 | 0 | 0.3 |
| Shear Rate (s−1) | 100 | 300 | 600 | 750 | 900 |
|---|---|---|---|---|---|
| Student’s t-value | 52.7 | 23.0 | 20.9 | 33.5 | 26.1 |
| at 99% confidence | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; De La Franier, B.; Thompson, M. Anti-Thrombogenicity Study of a Covalently-Attached Monolayer on Stent-Grade Stainless Steel. Materials 2021, 14, 2342. https://doi.org/10.3390/ma14092342
Yang T, De La Franier B, Thompson M. Anti-Thrombogenicity Study of a Covalently-Attached Monolayer on Stent-Grade Stainless Steel. Materials. 2021; 14(9):2342. https://doi.org/10.3390/ma14092342
Chicago/Turabian StyleYang, Tairan, Brian De La Franier, and Michael Thompson. 2021. "Anti-Thrombogenicity Study of a Covalently-Attached Monolayer on Stent-Grade Stainless Steel" Materials 14, no. 9: 2342. https://doi.org/10.3390/ma14092342






