Influence of Non-Thermal Atmospheric Pressure Plasma Treatment on Retentive Strength between Zirconia Crown and Titanium Implant Abutment
Abstract
1. Introduction
2. Materials and Methods
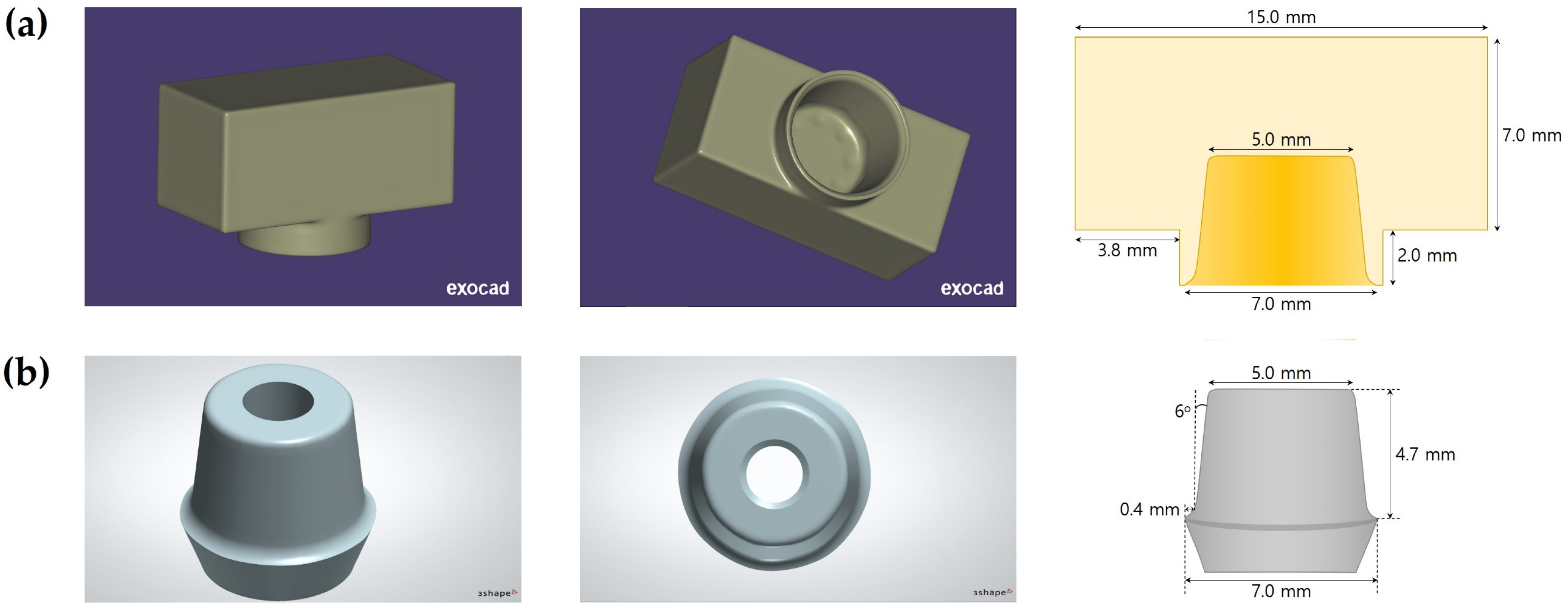
2.1. Preparation of Zirconia Specimens and Titanium Implant Abutments
2.2. Non-Thermal Atmospheric Pressure Plasma (NTP) Treatment
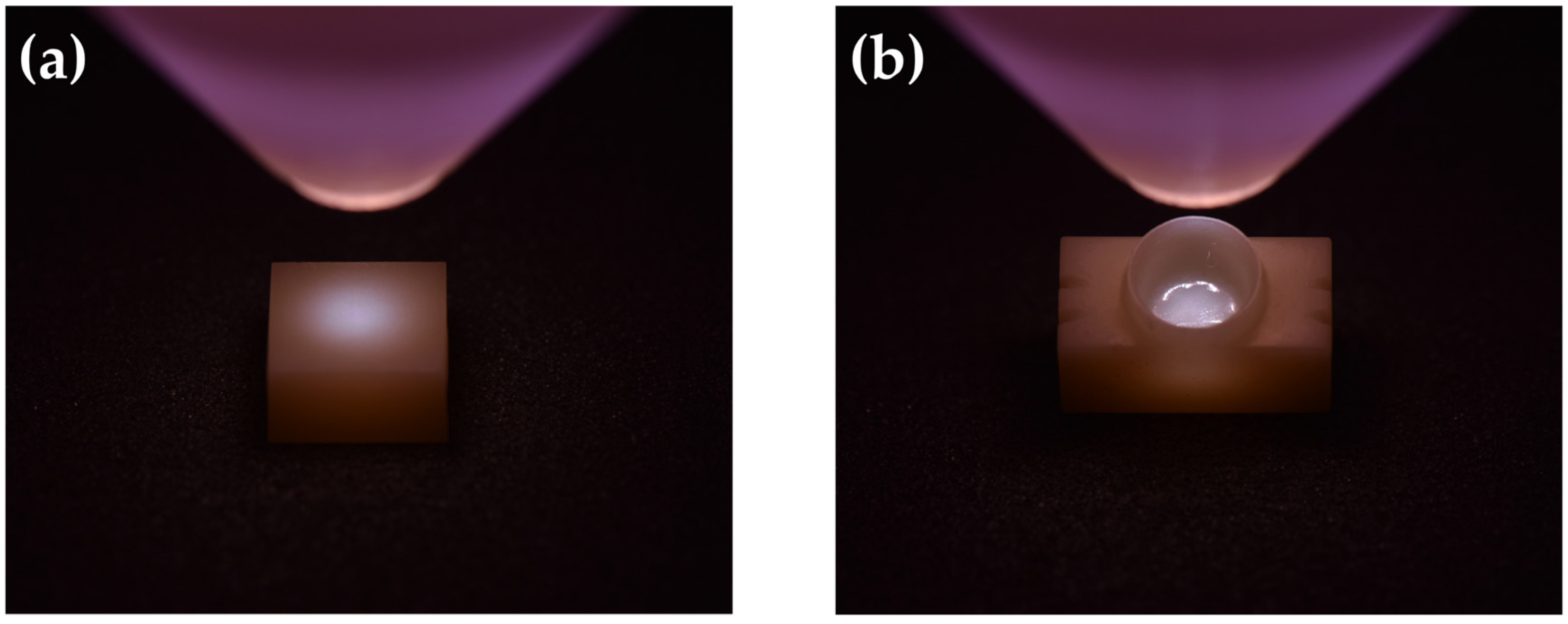
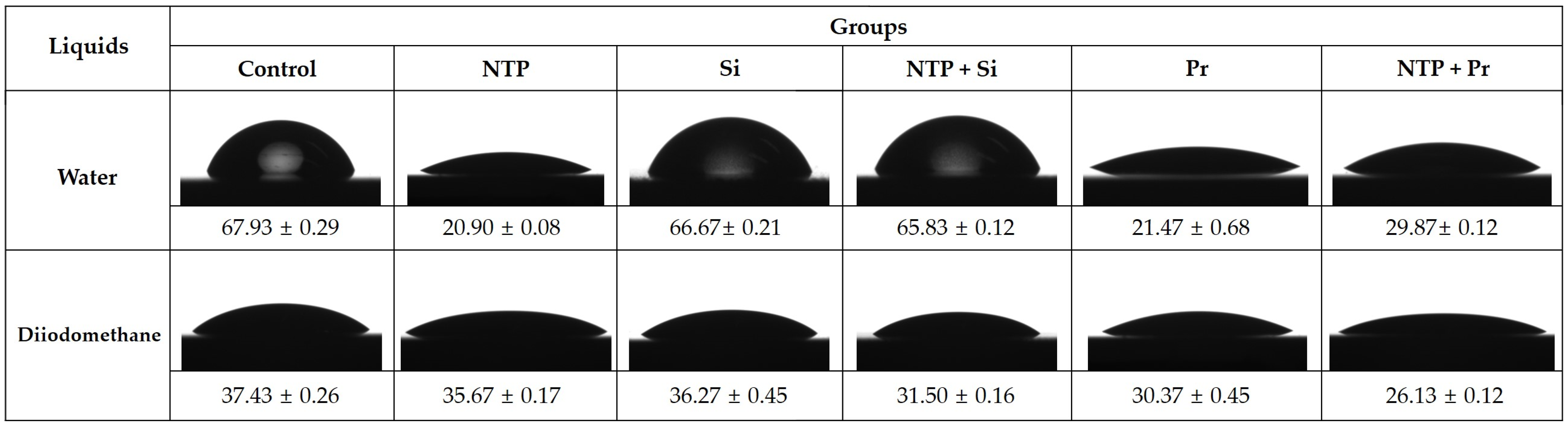
2.3. Contact Angle Measurement and Surface Free Energy (SFE) Analysis
2.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
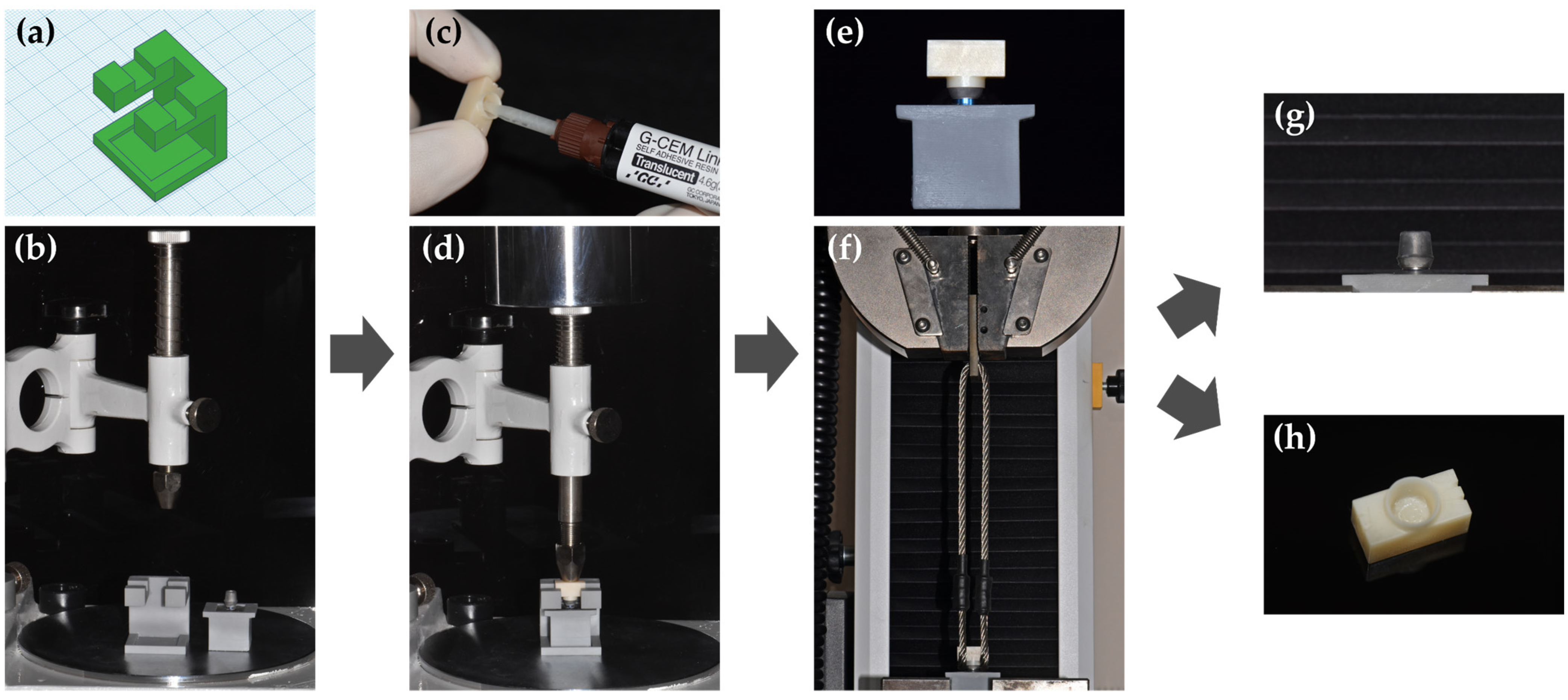
2.5. Retentive Strength (RS) Test before and after Thermocycling
2.6. Failure Mode Analysis
2.7. Energy-Dispersive X-ray Spectroscopy (EDS) Analysis
2.8. Statistical Analysis
3. Results
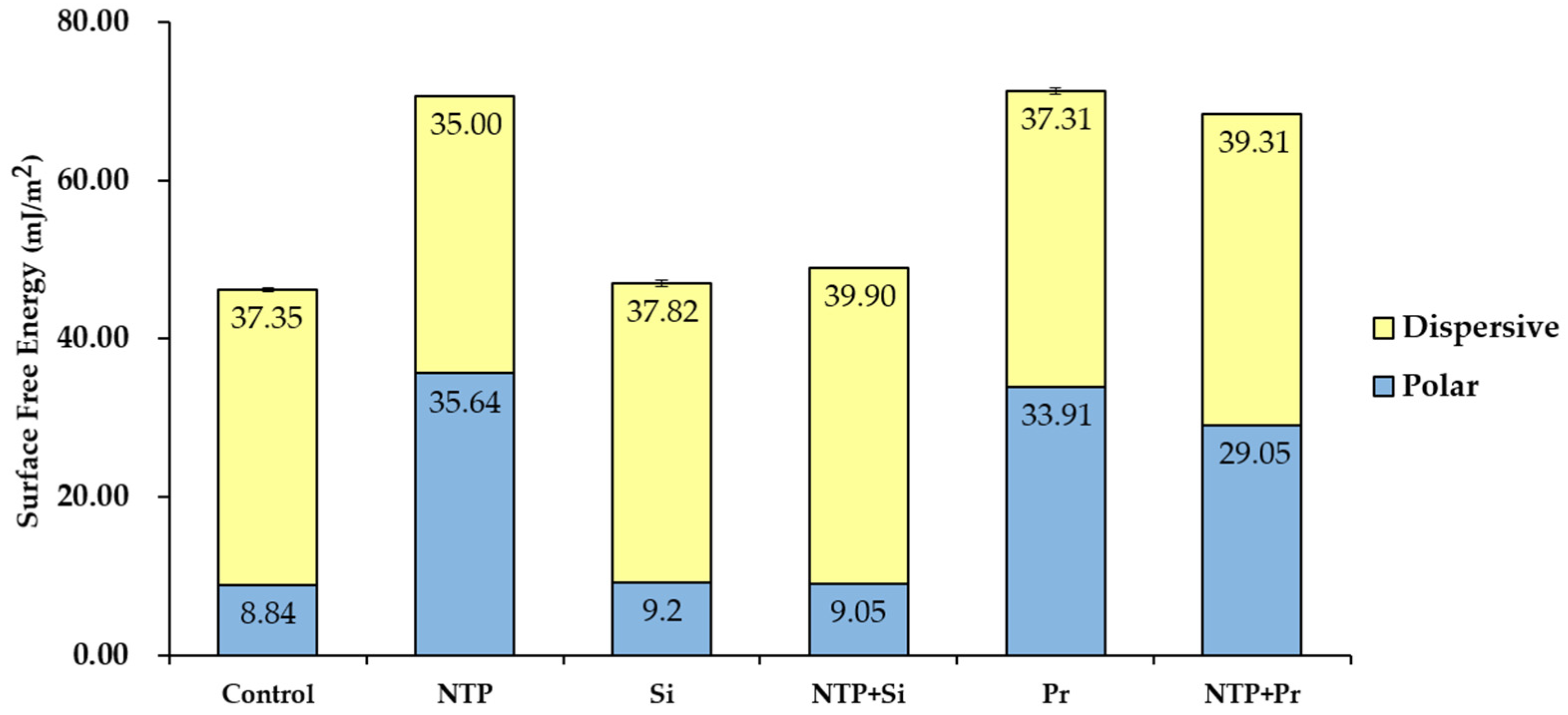
3.1. Contact Angle Measurement and Surface Free Energy (SFE) Analysis
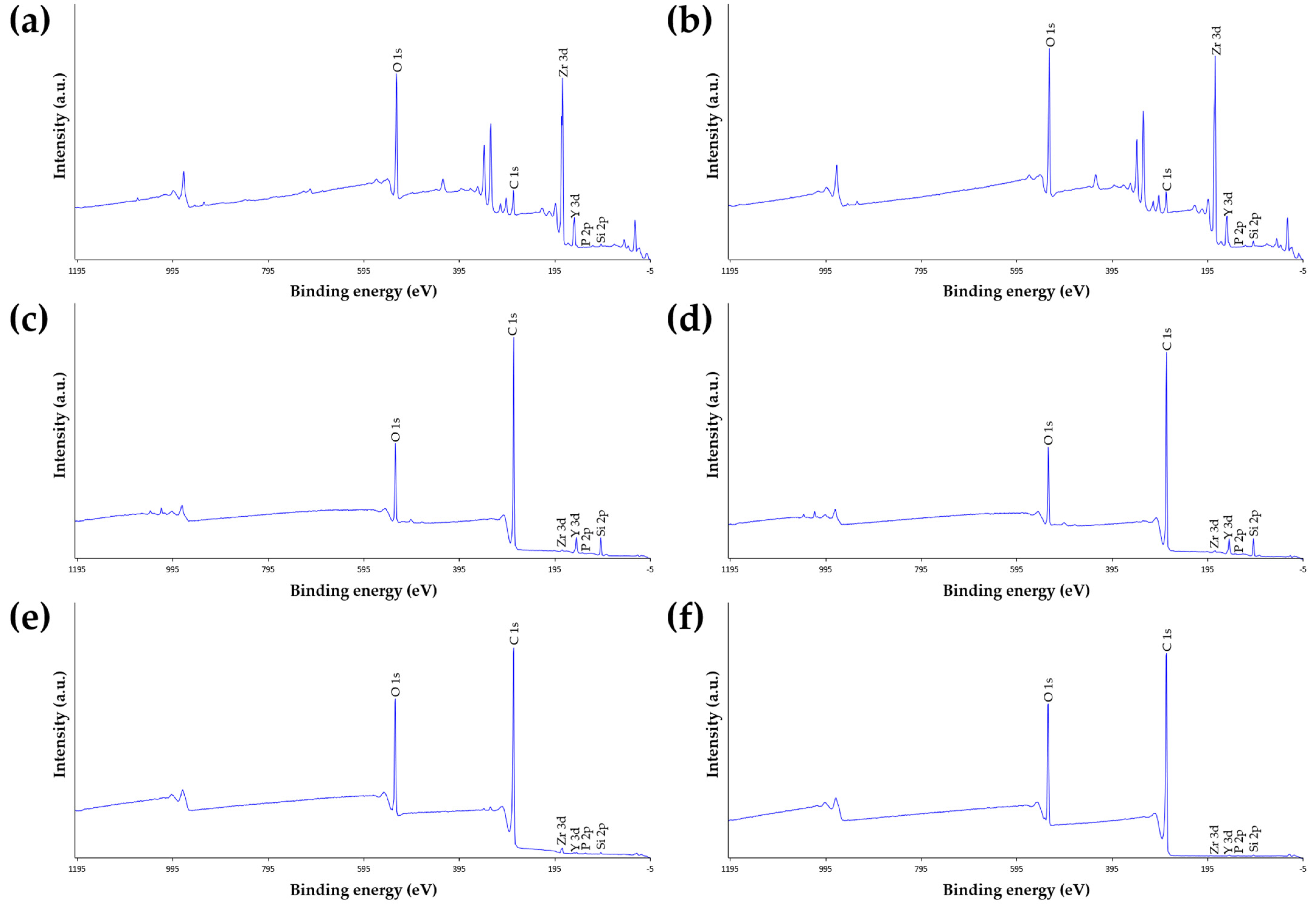
3.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
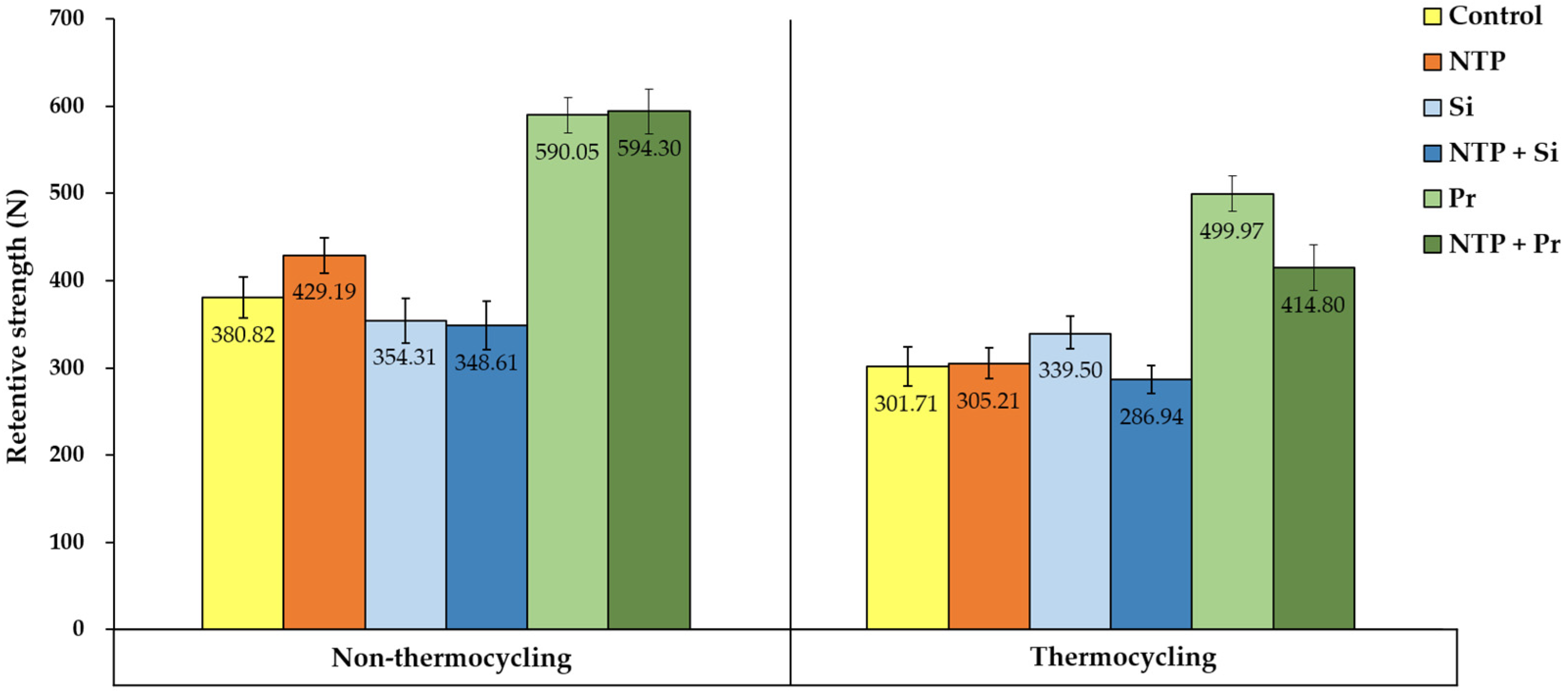
3.3. Retentive Strength (RS) Test
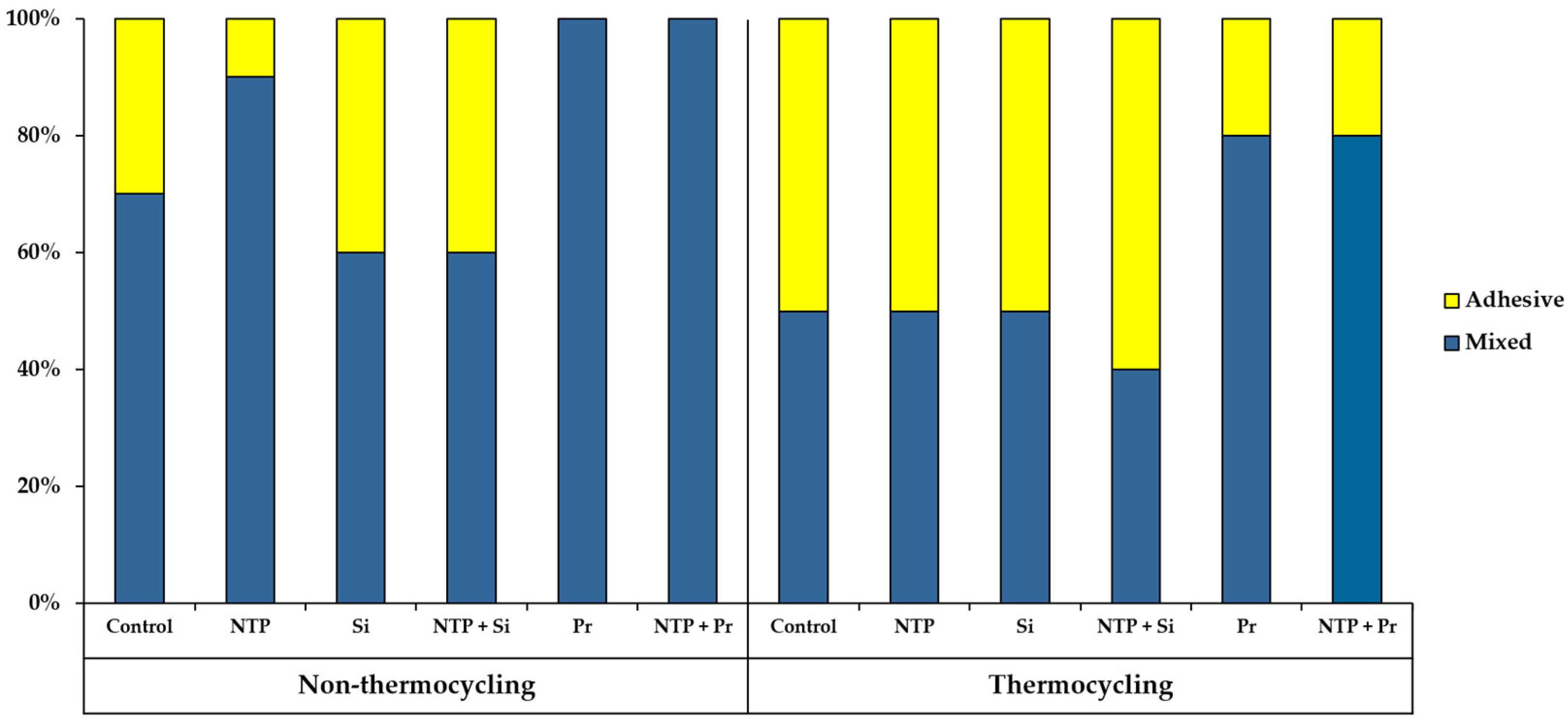
3.4. Failure Mode Analysis
3.5. Energy-Dispersive X-ray Spectroscopy (EDS) Analysis
4. Discussion
5. Conclusions
- NTP single treatment increases SFE of zirconia.
- NTP single treatment increases the initial RS between zirconia crown and titanium implant abutment but has little effect on the RS after thermocycling.
- Regardless of thermocycling, NTP pre-treatment does not show a positive effect on the RS when applied with Silane or Z-Prime Plus.
- Regardless of NTP pre-treatment and thermocycling, Z-Prime Plus shows higher RS than Silane.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alao, A.R.; Stoll, R.; Song, X.F.; Miyazaki, T.; Hotta, Y.; Shibata, Y.; Yin, L. Surface quality of yttria-stabilized tetragonal zirconia polycrystal in CAD/CAM milling, sintering, polishing and sandblasting processes. J. Mech. Behav. Biomed. Mater. 2017, 65, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas Fernandes Junior, V.; Barbosa Dantas, D.C.; Bresciani, E.; Rocha Lima Huhtala, M.F. Evaluation of the bond strength and characteristics of zirconia after different surface treatments. J. Prosthet. Dent. 2018, 120, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Keul, C.; Liebermann, A.; Roos, M.; Uhrenbacher, J.; Stawarczyk, B.; Ing, D. The effect of ceramic primer on shear bond strength of resin composite cement to zirconia: A function of water storage and thermal cycling. J. Am. Dent. Assoc. 2013, 144, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Edelhoff, D.; Schweiger, J.; Prandtner, O.; Stimmelmayr, M.; Güth, J.F. Metal-free implant-supported single-tooth restorations. Part I: Abutments and cemented crowns. Quintessence Int. 2019, 50, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implants Res. 2018, 29, 199–214. [Google Scholar] [CrossRef]
- Zahoui, A.; Bergamo, E.T.; Marun, M.M.; Silva, K.P.; Coelho, P.G.; Bonfante, E.A. Cementation Protocol for Bonding Zirconia Crowns to Titanium Base CAD/CAM Abutments. Int. J. Prosthodont. 2020, 33, 527–535. [Google Scholar] [CrossRef]
- Valente, F.; Mavriqi, L.; Traini, T. Effects of 10-MDP Based Primer on Shear Bond Strength between Zirconia and New Experimental Resin Cement. Materials 2020, 13, 235. [Google Scholar] [CrossRef]
- Vechiato Filho, A.J.; dos Santos, D.M.; Goiato, M.C.; de Medeiros, R.A.; Moreno, A.; Bonatto Lda, R.; Rangel, E.C. Surface characterization of lithium disilicate ceramic after nonthermal plasma treatment. J. Prosthet. Dent. 2014, 112, 1156–1163. [Google Scholar] [CrossRef]
- Piascik, J.R.; Swift, E.J.; Braswell, K.; Stoner, B.R. Surface fluorination of zirconia: Adhesive bond strength comparison to commercial primers. Dent. Mater. 2012, 28, 604–608. [Google Scholar] [CrossRef]
- Liu, T.; Hong, L.; Hottel, T.; Dong, X.; Yu, Q.; Chen, M. Non-thermal plasma enhanced bonding of resin cement to zirconia ceramic. Clin. Plasma Med. 2016, 4, 50–55. [Google Scholar] [CrossRef]
- Ahn, J.S.; Yi, Y.A.; Lee, Y.; Seo, D.G. Shear Bond Strength of MDP-Containing Self-Adhesive Resin Cement and Y-TZP Ceramics: Effect of Phosphate Monomer-Containing Primers. Biomed. Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.M.; Kern, M. Long-term Resin Bond Strength to Zirconia Ceramic. J. Adhes. Dent. 2000, 2, 139–147. [Google Scholar] [PubMed]
- Mattiello, R.D.L.; Coelho, T.M.K.; Insaurralde, E.; Coelho, A.A.K.; Terra, G.P.; Kasuya, A.V.B.; Favarão, I.N.; Gonçalves, L.d.S.; Fonseca, R.B. A Review of Surface Treatment Methods to Improve the Adhesive Cementation of Zirconia-Based Ceramics. Biomaterials 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Melo, R.M.; Souza, R.O.; Dursun, E.; Monteiro, E.B.; Valandro, L.F.; Bottino, M.A. Surface Treatments of Zirconia to Enhance Bonding Durability. Oper. Dent. 2015, 40, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Vonderheide, M.; Conejo, J. The Effect of Resin Bonding on Long-Term Success of High-Strength Ceramics. J. Dent. Res. 2018, 97, 132–139. [Google Scholar] [CrossRef]
- Kern, M. Bonding to oxide ceramics-laboratory testing versus clinical outcome. Dent. Mater. 2015, 31, 8–14. [Google Scholar] [CrossRef]
- Kern, M.; Wegner, S.M. Bonding to zirconia ceramic: Adhesion methods and their durability. Dent. Mater. 1998, 14, 64–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R.; Rekow, E.D.; Thompson, V.P. Effect of sandblasting on the long-term performance of dental ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 381–386. [Google Scholar] [CrossRef]
- Pilo, R.; Dimitriadi, M.; Palaghia, A.; Eliades, G. Effect of tribochemical treatments and silane reactivity on resin bonding to zirconia. Dent. Mater. 2018, 34, 306–316. [Google Scholar] [CrossRef]
- Chen, L.; Suh, B.I. Bonding of Resin Materials to All-Ceramics: A Review. Curr. Res. Dent. 2012, 3, 7–17. [Google Scholar] [CrossRef]
- Nagaoka, N.; Yoshihara, K.; Feitosa, V.P.; Tamada, Y.; Irie, M.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Chemical interaction mechanism of 10-MDP with zirconia. Sci. Rep. 2017, 7, 45563. [Google Scholar] [CrossRef]
- Thompson, J.Y.; Stoner, B.R.; Piascik, J.R.; Smith, R. Adhesion/cementation to zirconia and other non-silicate ceramics: Where are we now? Dent. Mater. 2011, 27, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, B.; Xie, H.; Chen, Y.; Chen, Y.; Chen, C. Durability of Resin Bonding to Zirconia Using Products Containing 10-Methacryloyloxydecyl Dihydrogen Phosphate. J. Adhes. Dent. 2018, 20, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Mirmohamadi, H.; Matinlinna, J.P.; Kukk, E.; Ounsi, H.F.; Salameh, Z. Innovations in bonding to zirconia-based materials. Part II: Focusing on chemical interactions. Dent. Mater. 2009, 25, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chae, S.; Lee, Y.; Han, G.J.; Cho, B.H. Comparison of shear test methods for evaluating the bond strength of resin cement to zirconia ceramic. Acta. Odontol. Scand. 2014, 72, 745–752. [Google Scholar] [CrossRef]
- Scaminaci Russo, D.; Cinelli, F.; Sarti, C.; Giachetti, L. Adhesion to Zirconia: A Systematic Review of Current Conditioning Methods and Bonding Materials. Dent. J. 2019, 7, 74. [Google Scholar] [CrossRef]
- Tzanakakis, E.G.; Tzoutzas, I.G.; Koidis, P.T. Is there a potential for durable adhesion to zirconia restorations? A systematic review. J. Prosthet. Dent. 2016, 115, 9–19. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Sky Driver, M.; Caruso, A.N.; Yu, Q.; Wang, Y. Surface modification of several dental substrates by non-thermal, atmospheric plasma brush. Dent. Mater. 2013, 29, 871–880. [Google Scholar] [CrossRef]
- Da Silva, B.T.F.; Trevelin, L.T.; Teixeira, F.D.S.; Salvadori, M.C.; Cesar, P.F.; Bona Matos, A. Non-thermal plasma increase bond strength of zirconia to a resin cement. Braz. Dent. Sci. 2018, 21, 210–219. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hsieh, J.P.; Chen, Y.C.; Kang, L.L.; Hwang, C.S.; Chuang, S.F. Promoting porcelain-zirconia bonding using different atmospheric pressure gas plasmas. Dent. Mater. 2018, 34, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Vechiato-Filho, A.J.; Matos, A.O.; Landers, R.; Goiato, M.C.; Rangel, E.C.; De Souza, G.M.; Barao, V.A.R.; Dos Santos, D.M. Surface analysis and shear bond strength of zirconia on resin cements after non-thermal plasma treatment and/or primer application for metallic alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 284–292. [Google Scholar] [CrossRef]
- Park, C.; Yoo, S.H.; Park, S.W.; Yun, K.D.; Ji, M.K.; Shin, J.H.; Lim, H.P. The effect of plasma on shear bond strength between resin cement and colored zirconia. J. Adv. Prosthodont. 2017, 9, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Pott, P.C.; Syvari, T.S.; Stiesch, M.; Eisenburger, M. Influence of nonthermal argon plasma on the shear bond strength between zirconia and different adhesives and luting composites after artificial aging. J. Adv. Prosthodont. 2018, 10, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Ahn, J.J.; Bae, E.B.; Kim, G.C.; Jeong, C.M.; Huh, J.B.; Lee, S.H. Influence of Non-Thermal Atmospheric Pressure Plasma Treatment on Shear Bond Strength between Y-TZP and Self-Adhesive Resin Cement. Materials 2019, 12, 3321. [Google Scholar] [CrossRef]
- Ahn, J.J.; Kim, D.S.; Bae, E.B.; Kim, G.C.; Jeong, C.M.; Huh, J.B.; Lee, S.H. Effect of Non-Thermal Atmospheric Pressure Plasma (NTP) and Zirconia Primer Treatment on Shear Bond Strength between Y-TZP and Resin Cement. Materials 2020, 13, 3934. [Google Scholar] [CrossRef]
- Elias, A.B.; Simao, R.A.; Prado, M.; Cesar, P.F.; Botelho Dos Santos, G.; Moreira da Silva, E. Effect of different times of nonthermal argon plasma treatment on the microtensile bond strength of self-adhesive resin cement to yttria-stabilized tetragonal zirconia polycrystal ceramic. J. Prosthet. Dent. 2019, 121, 485–491. [Google Scholar] [CrossRef]
- Valverde, G.B.; Coelho, P.G.; Janal, M.N.; Lorenzoni, F.C.; Carvalho, R.M.; Thompson, V.P.; Weltemann, K.D.; Silva, N.R. Surface characterisation and bonding of Y-TZP following non-thermal plasma treatment. J. Dent. 2013, 41, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Min, B.K.; Son, J.S.; Kwon, T.Y. Influence of Different Post-Plasma Treatment Storage Conditions on the Shear Bond Strength of Veneering Porcelain to Zirconia. Materials 2016, 9, 43. [Google Scholar] [CrossRef]
- Ito, Y.; Okawa, T.; Fujii, T.; Tanaka, M. Influence of plasma treatment on surface properties of zirconia. J. Osaka Dent. Univ. 2016, 50, 79–84. [Google Scholar] [CrossRef]
- Park, C.; Park, S.W.; Yun, K.D.; Ji, M.K.; Kim, S.; Yang, Y.P.; Lim, H.P. Effect of Plasma Treatment and Its Post Process Duration on Shear Bonding Strength and Antibacterial Effect of Dental Zirconia. Materials 2018, 11, 2233. [Google Scholar] [CrossRef]
- Ito, Y.; Okawa, T.; Fukumoto, T.; Tsurumi, A.; Tatsuta, M.; Fujii, T.; Tanaka, J.; Tanaka, M. Influence of atmospheric pressure low-temperature plasma treatment on the shear bond strength between zirconia and resin cement. J. Prosthodont. Res. 2016, 60, 289–293. [Google Scholar] [CrossRef]
- Tabari, K.; Hosseinpour, S.; Mohammad-Rahimi, H. The Impact of Plasma Treatment of Cercon(R) Zirconia Ceramics on Adhesion to Resin Composite Cements and Surface Properties. J. Lasers Med. Sci. 2017, 8, S56–S61. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C.; Gengembre, L.; Payen, E. Treatment of polymer films with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7066–7075. [Google Scholar] [CrossRef]
- Yaman, N.; Özdoǧan, E.; Seventekin, N. Atmospheric plasma treatment of polypropylene fabric for improved dyeability with insoluble textile dyestuff. Fibers Polym. 2011, 12, 35–41. [Google Scholar] [CrossRef]
- Sun, S.; Sun, J.; Yao, L.; Qiu, Y. Wettability and sizing property improvement of raw cotton yarns treated with He/O2 atmospheric pressure plasma jet. Appl. Surf. Sci. 2011, 257, 2377–2382. [Google Scholar] [CrossRef]
- Lee, E.S.; Huh, Y.H.; Park, C.J.; Cho, L.R. Effect of silica-containing glass-ceramic liner treatment on zirconia coping retention. J. Prosthet. Dent. 2018, 120, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, L.; Ulmer, P.; Lehmann, F.; Wille, S.; Polonskyi, O.; Johannes, M.; Kobel, S.; Trottenberg, T.; Bornholdt, S.; Haase, F.; et al. Effect of surface modifications on the bond strength of zirconia ceramic with resin cement resin. Dent. Mater. 2016, 32, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Lumkemann, N.; Eichberger, M.; Stawarczyk, B. Different surface modifications combined with universal adhesives: The impact on the bonding properties of zirconia to composite resin cement. Clin. Oral Investig. 2019, 23, 3941–3950. [Google Scholar] [CrossRef]
- Labriaga, W.; Song, S.Y.; Park, J.H.; Ryu, J.J.; Lee, J.Y.; Shin, S.W. Effect of non-thermal plasma on the shear bond strength of resin cements to Polyetherketoneketone (PEKK). J. Adv. Prosthodont. 2018, 10, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E.; Lindén, L.Å.; Rabek, J.F. The role of oxygen in camphorquinone-initiated photopolymerization. Macromol. Chem. Phys. 1998, 199, 441–449. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Stangel, I.; Ellis, T.H.; Zhu, X.X.; Gauthier, M.A.; Stangel, I.; Ellis, T.H.; Zhu, X.X. Oxygen inhibition in dental resins. J. Dent. Res. 2005, 84, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.Y.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef]
- Tabatabaei, M.H.; Chiniforush, N.; Namdar, S.F. Effects of different ceramic primers and surface treatments on the shear bond strength of restorative composite resin to zirconium. Laser Ther. 2018, 27, 111–117. [Google Scholar] [CrossRef]
- Derand, T.; Molin, M.; Kvam, K. Bond strength of composite luting cement to zirconia ceramic surfaces. Dent. Mater. 2005, 21, 1158–1162. [Google Scholar] [CrossRef]
- Piascik, J.R.; Swift, E.J.; Thompson, J.Y.; Grego, S.; Stoner, B.R. Surface modification for enhanced silanation of zirconia ceramics. Dent. Mater. 2009, 25, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; De Munck, J.; Maruo, Y.; Nishigawa, G.; Minagi, S.; Osaka, A.; et al. Adhesive interfacial interaction affected by different carbon-chain monomers. Dent. Mater. 2013, 29, 888–897. [Google Scholar] [CrossRef]
- Hass, V.; Abuna, G.; Pinheiro Feitosa, V.; Martini, E.C.; Sinhoreti, M.A.; Furtado Carvalho, R.; Coelho Bandeca, M.; Sauro, S.; Loguercio, A.D. Self-Etching Enamel Bonding Using Acidic Functional Monomers with Different-length Carbon Chains and Hydrophilicity. J. Adhes. Dent. 2017, 19, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Beutel, B.G.; Danna, N.R.; Gangolli, R.; Granato, R.; Manne, L.; Tovar, N.; Coelho, P.G. Evaluation of bone response to synthetic bone grafting material treated with argon-based atmospheric pressure plasma. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Balkenhol, M.; Nothdurft, F.P.; Hannig, M.; Schindler, A.; Lehmann, A.; Arnold, T.; Knauber, A.; Rupf, S. Bonding to zirconia ceramic: The effect of cold plasma treatment and 4-META. Clin. Plasma Med. 2017, 5, 8–13. [Google Scholar] [CrossRef]
- Swartz, J.M.; Davis, R.D.; Overton, J.D. Tensile bond strength of resin-modified glass-ionomer cement to microabraded and silica-coated or tin-plated high noble ceramic alloy. J. Prosthodont. 2000, 9, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Pujari, M.; Prithviraj, D.R.; Khare, S. Retentiveness of various luting agents used with implant-supported prosthesis: An in vitro study. J. Oral Implantol. 2014, 40, 649–654. [Google Scholar] [CrossRef] [PubMed]


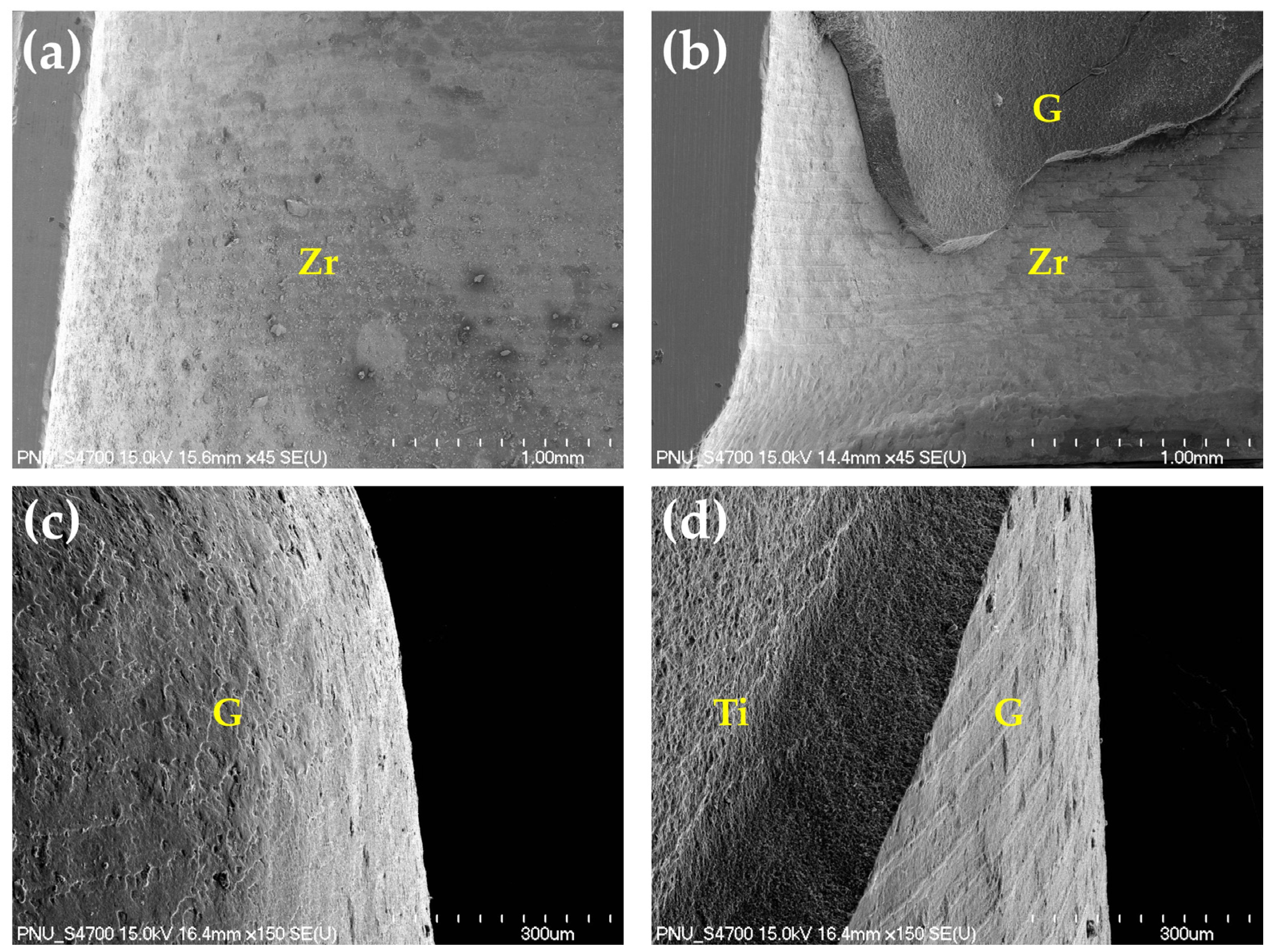
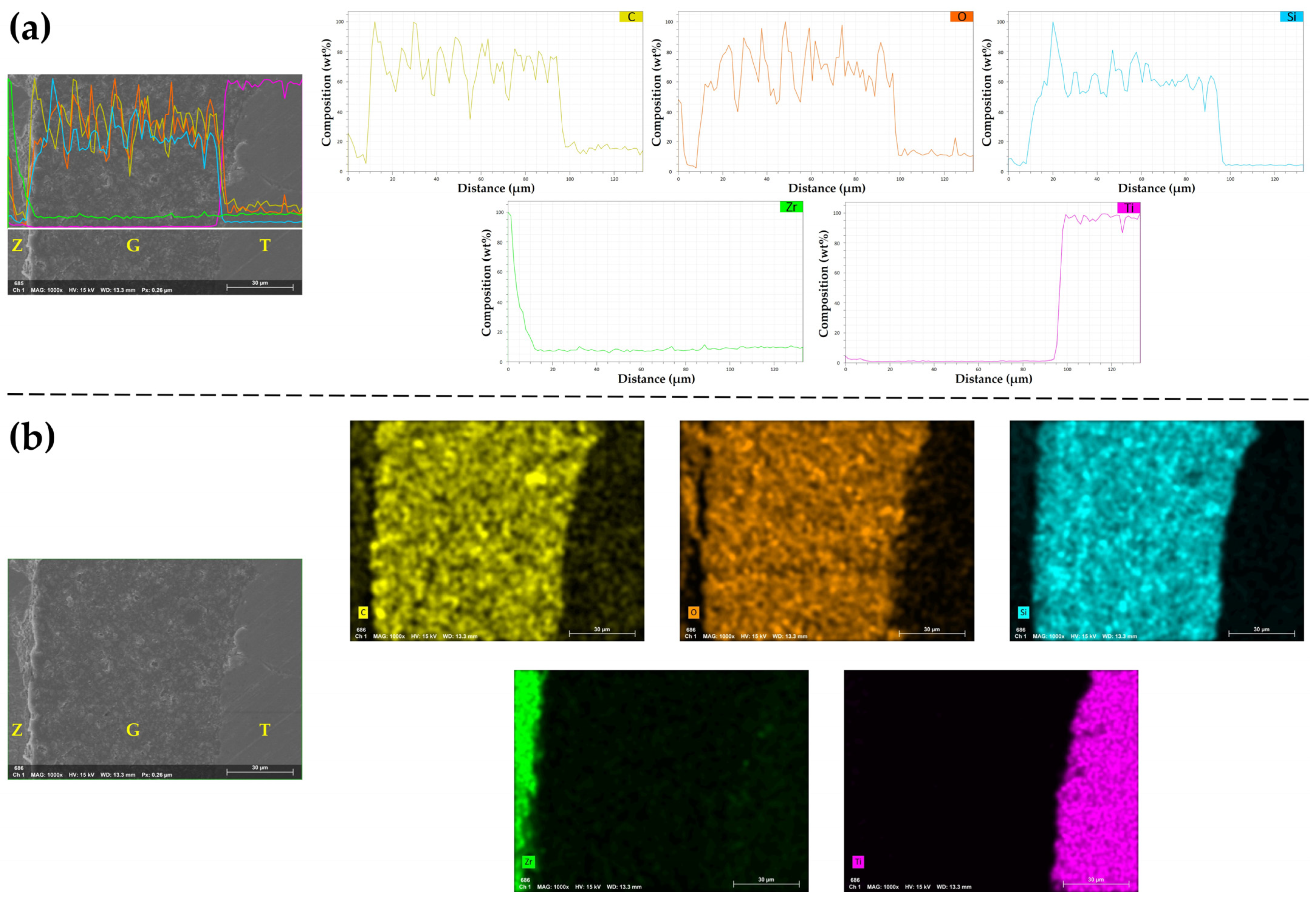
| Groups | Distribution of Zirconia Specimens (n) | |||
|---|---|---|---|---|
| Cube-Shaped Blocks | Crowns | |||
| Surface Free Energy (SFE) Analysis | X-ray Photoelectron Spectroscopy (XPS) Analysis | Retentive Strength (RS) Test | ||
| Non-Thermocycling | Thermocycling | |||
| Control | 3 | 1 | 10 | 10 |
| NTP | 3 | 1 | 10 | 10 |
| Si | 3 | 1 | 10 | 10 |
| NTP + Si | 3 | 1 | 10 | 10 |
| Pr | 3 | 1 | 10 | 10 |
| NTP + Pr | 3 | 1 | 10 | 10 |
| Material | Manufacturer | Type | Composition |
|---|---|---|---|
| G-CEM LinkAce | GC Corporation, Tokyo, Japan | Self-adhesive resin cement | Paste A: fluoroalumino silicate glass, urethane dimethacrylate (UDMA), dimethacrylate, pigment, silicon dioxide, initiator, inhibitor |
| Paste B: urethane dimethacrylate (UDMA), dimethacrylate, phosphoric acid ester monomer, initiator, stabilizer | |||
| Silane | Ultradent Products Inc., South Jordan, UT, USA | Silane agent | 3-Methacryloxypropyltrimethoxysilane (MPS), isopropyl alcohol |
| Z-Prime Plus | Bisco Inc., Schaumberg, IL, USA | MDP primer | Ethanol, bisphenol A-glycidyl methacrylate (Bis-GMA), 2-hydroxyethyl methacrylate, 10-methacryloyloxydecyl dihydrogen phosphate (MDP) |
| Elements | Groups | |||||
|---|---|---|---|---|---|---|
| Control | NTP | Si | NTP + Si | Pr | NTP + Pr | |
| C | 21.10 | 14.56 | 71.14 | 79.33 | 81.19 | 82.72 |
| O | 49.90 | 55.96 | 16.97 | 12.02 | 17.61 | 16.77 |
| Zr | 20.25 | 20.76 | 0.17 | 0.15 | 0.37 | 0.00 |
| Si | 1.42 | 2.45 | 9.92 | 7.00 | 0.52 | 0.33 |
| P | 0.02 | 0.02 | 0.12 | 0.21 | 0.23 | 0.10 |
| Y | 7.25 | 6.24 | 1.39 | 1.07 | 0.07 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Ahn, J.-J.; Kim, G.-C.; Jeong, C.-M.; Huh, J.-B.; Lee, S.-H. Influence of Non-Thermal Atmospheric Pressure Plasma Treatment on Retentive Strength between Zirconia Crown and Titanium Implant Abutment. Materials 2021, 14, 2352. https://doi.org/10.3390/ma14092352
Kim D-S, Ahn J-J, Kim G-C, Jeong C-M, Huh J-B, Lee S-H. Influence of Non-Thermal Atmospheric Pressure Plasma Treatment on Retentive Strength between Zirconia Crown and Titanium Implant Abutment. Materials. 2021; 14(9):2352. https://doi.org/10.3390/ma14092352
Chicago/Turabian StyleKim, Dae-Sung, Jong-Ju Ahn, Gyoo-Cheon Kim, Chang-Mo Jeong, Jung-Bo Huh, and So-Hyoun Lee. 2021. "Influence of Non-Thermal Atmospheric Pressure Plasma Treatment on Retentive Strength between Zirconia Crown and Titanium Implant Abutment" Materials 14, no. 9: 2352. https://doi.org/10.3390/ma14092352
APA StyleKim, D.-S., Ahn, J.-J., Kim, G.-C., Jeong, C.-M., Huh, J.-B., & Lee, S.-H. (2021). Influence of Non-Thermal Atmospheric Pressure Plasma Treatment on Retentive Strength between Zirconia Crown and Titanium Implant Abutment. Materials, 14(9), 2352. https://doi.org/10.3390/ma14092352






