Dynamic Response of Polyindole Coated Zinc Ferrite Particle Suspension under an Electric Field
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Synthesis
2.1.1. Synthesis of ZnFe2O4
2.1.2. Fabrication of ZnFe2O4/PIn
2.1.3. Fabrication of ER Fluid
2.2. Characterization
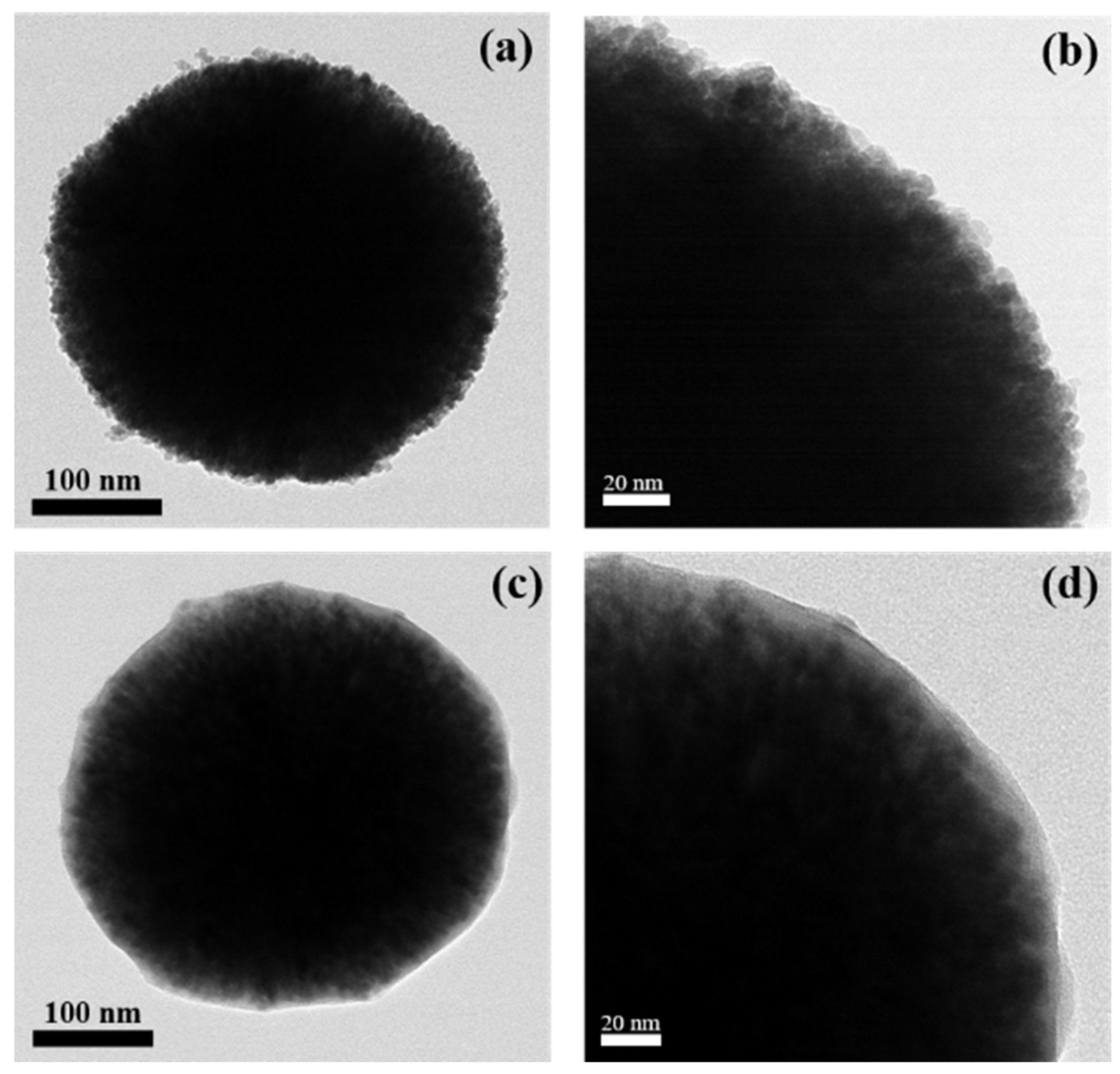
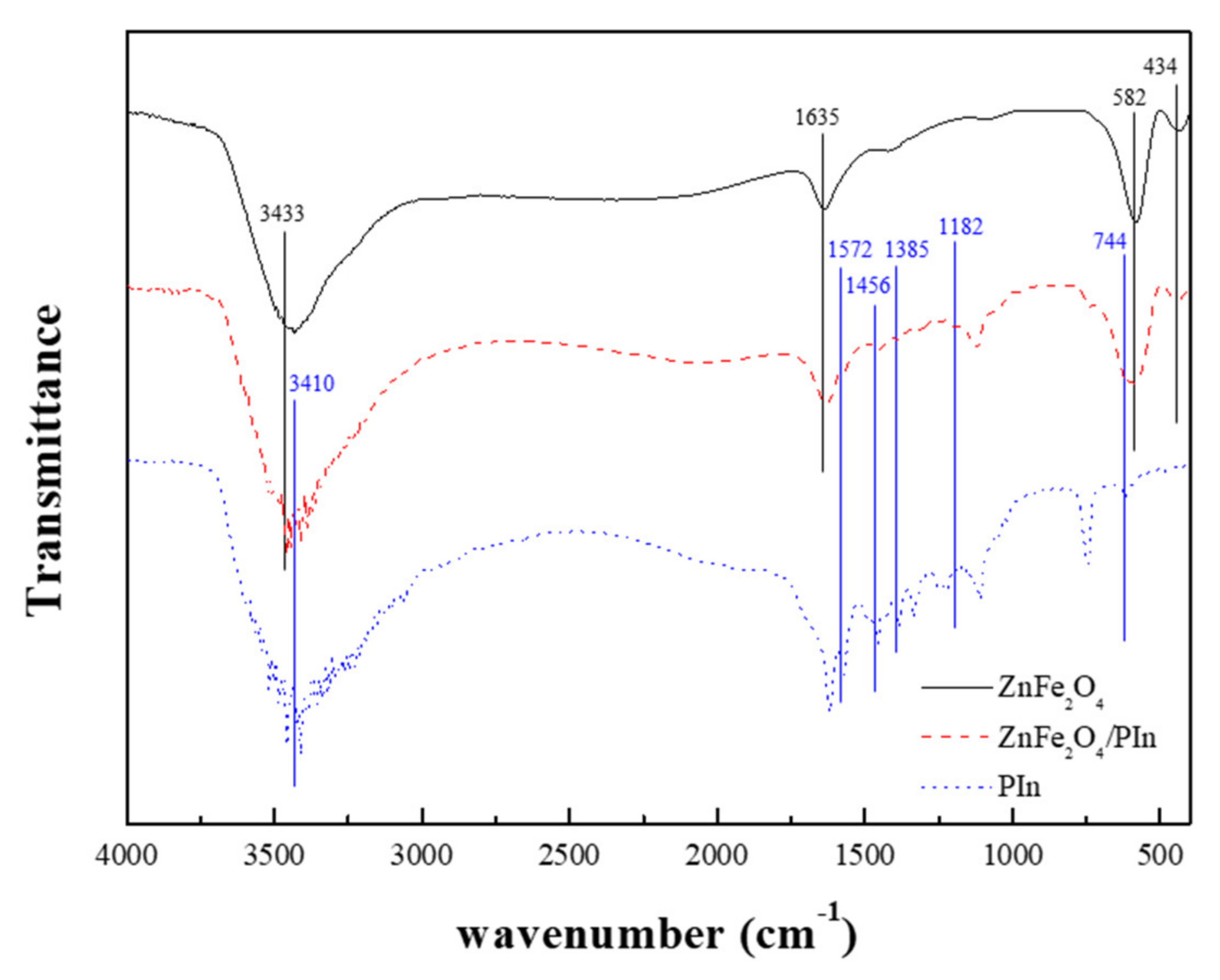
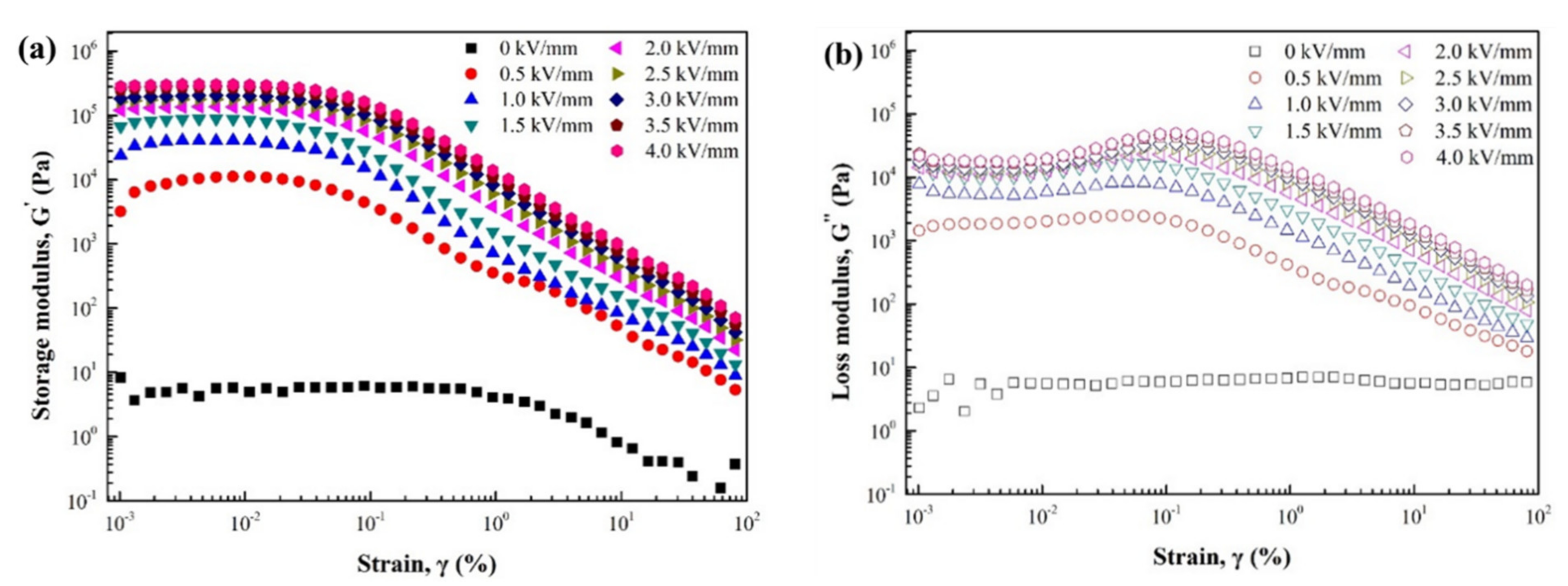
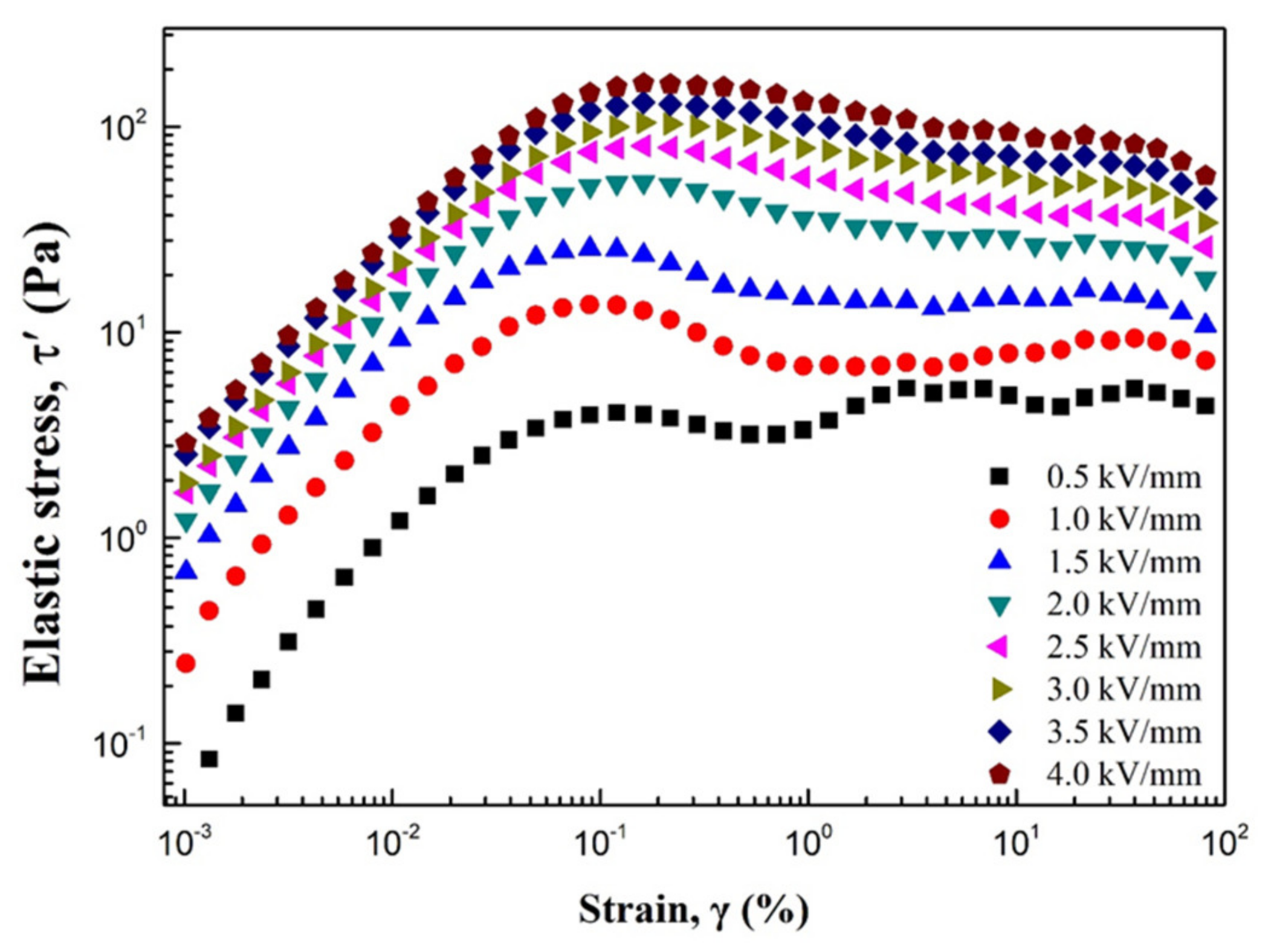
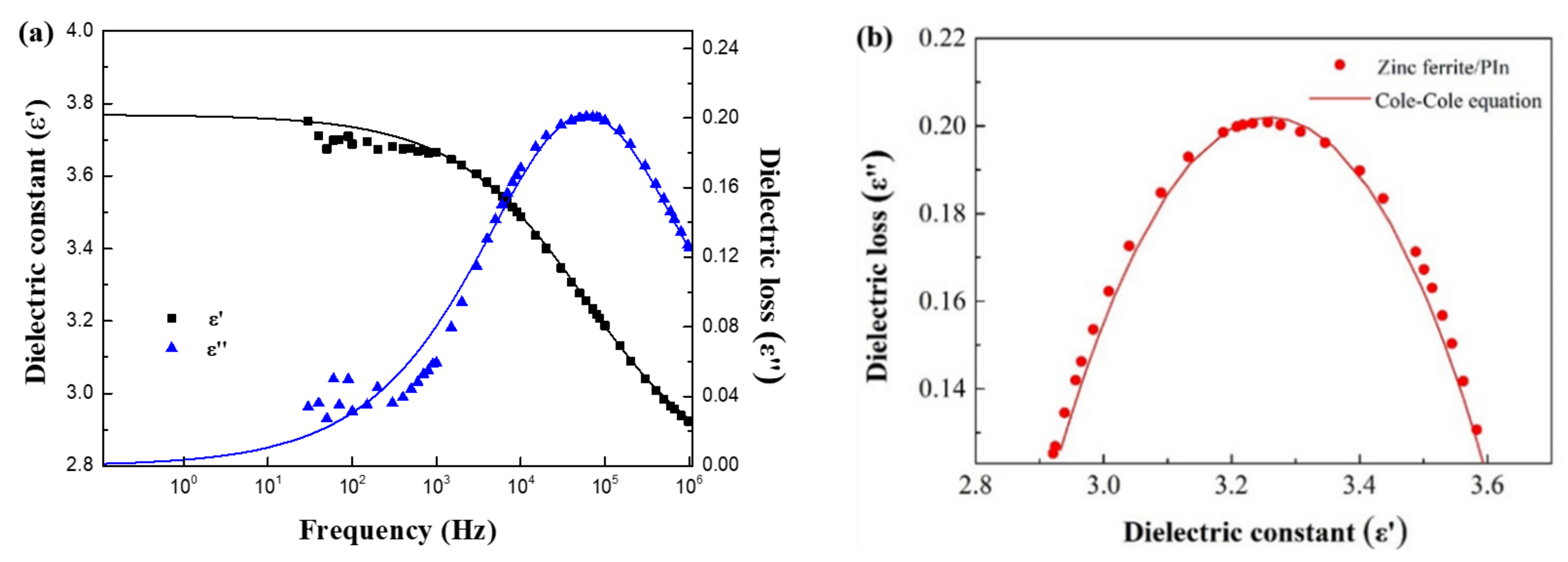
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qader, İ.N.; Mediha, K.; Dagdelen, F.; Aydogdu, Y. A review of smart materials: Researches and applications. El-Cezerî J. Sci. Eng. 2019, 6, 755–788. [Google Scholar]
- Gozdalik, A.; Wyciślik, H.; Płocharski, J. Electrorheological effect in suspensions of polyaniline. Synth. Met. 2000, 109, 147–150. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.D. Electrorheological properties of polypyrrole and its composite ER fluids. J. Ind. Eng. Chem. 2007, 13, 879–894. [Google Scholar]
- Brennan, M.; Day, M.; Randall, R. An electrorheological fluid vibration damper. Smart Mater. Struct. 1995, 4, 83. [Google Scholar] [CrossRef]
- Unal, H.I.; Sahan, B.; Erol, O. Effect of surfactant on electrokinetic properties of polyindole/TiO2-conducting nanocomposites in aqueous and nonaqueous media. Colloid Polym. Sci. 2014, 292, 499–509. [Google Scholar] [CrossRef]
- Unal, H.I.; Sahan, B.; Erol, O. Investigation of electrokinetic and electrorheological properties of polyindole prepared in the presence of a surfactant. Mater. Chem. Phys. 2012, 134, 382–391. [Google Scholar] [CrossRef]
- Plachy, T.; Zitka, J.; Mrlik, M.; Bazant, P.; Kadleckova, M.; Trchova, M.; Stejskal, J. Electrorheology of polyindole. Polymer 2021, 217, 123448. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Synthesis of nano-sized polyindole via emulsion polymerization and doping. Synth. Met. 2016, 219, 142–153. [Google Scholar] [CrossRef]
- Susan-Resiga, D.; Vékás, L. Ferrofluid based composite fluids: Magnetorheological properties correlated by Mason and Casson numbers. J. Rheol. 2017, 61, 401–408. [Google Scholar] [CrossRef]
- Zubarev, A.; Iskakova, L.; López-López, M.T.; Kuzhir, P.; Bossis, G. On the theory of magnetoviscous effect in magnetorheological suspensions. J. Rheol. 2014, 58, 1673–1692. [Google Scholar] [CrossRef]
- Sim, B.M.; Chae, H.S.; Choi, H.J. Fabrication of polyaniline coated iron oxide hybrid particles and their dual stimuli-response under electric and magnetic fields. Express Polym. Lett. 2015, 9, 736–743. [Google Scholar] [CrossRef]
- Park, I.H.; Kwon, S.H.; Choi, H.J.; Kim, N.H.; You, C.Y. Polyindole-Coated Soft-Magnetic Particles and their Viscoelastic Behaviors under Applied Magnetic Field. J. Magn. 2019, 24, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Mrlik, M.; Ilcikova, M.; Plachy, T.; Moucka, R.; Pavlinek, V.; Mosnacek, J. Tunable electrorheological performance of silicone oil suspensions based on controllably reduced graphene oxide by surface initiated atom transfer radical polymerization of spoly(glycidyl methacrylate). J. Ind. Eng. Chem. 2018, 57, 104–112. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Sedlacik, M.; Mosnacek, J.; Peer, P.; Filip, P. Cholesteryl-coated carbonyl iron particles with improved anti-corrosion stability and their viscoelastic behaviour under magnetic field. Colloid Polym. Sci. 2014, 292, 2137–2143. [Google Scholar] [CrossRef]
- Han, J.K.; Lee, J.Y.; Choi, H.J. Rheological effect of Zn-doped ferrite nanoparticle additive with enhanced magnetism on micro-spherical carbonyl iron based magnetorheological suspension. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 168–173. [Google Scholar] [CrossRef]
- Wang, G.; Ma, Y.; Tong, Y.; Dong, X.; Li, M. Solvothermal synthesis, characterization, and magnetorheological study of zinc ferrite nanocrystal clusters. J. Intell. Mater. Syst. Struct. 2017, 28, 2331–2338. [Google Scholar] [CrossRef]
- Ferrari, S.; Kumar, R.; Grinblat, F.; Aphesteguy, J.C.; Saccone, F.D.; Errandonea, D. In-situ high-pressure x-ray diffraction study of zinc ferrite nanoparticles. Solid State Sci. 2016, 56, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Huang, Y.; Wang, D.; Zhao, Y.; Wang, M.; Chen, X.; Qin, X.; Li, S. Preparation and application of hollow ZnFe2O4@ PANI hybrids as high performance anode materials for lithium-ion batteries. RSC Adv. 2015, 5, 107247–107253. [Google Scholar] [CrossRef]
- Qin, M.; Shuai, Q.; Wu, G.; Zheng, B.; Wang, Z.; Wu, H. Zinc ferrite composite material with controllable morphology and its applications. Mater. Sci. Eng. B 2017, 224, 125–138. [Google Scholar] [CrossRef]
- Rafienia, M.; Bigham, A.; Hassanzadeh-Tabrizi, S.A. Solvothermal synthesis of magnetic spinel ferrites. J. Signals Sens. 2018, 8, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Choi, H.J. Fabrication of Polyindole Coated Zinc Ferrite Particles and Their Dual Rheological Response under Magnetic and Electric Fields. IEEE Trans. Magn. 2021. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Swamy, P.P.; Basavaraja, S.; Lagashetty, A.; Rao, N.S.; Nijagunappa, R.; Venkataraman, A. Synthesis and characterization of zinc ferrite nanoparticles obtained by self-propagating low-temperature combustion method. Bull. Mater. Sci. 2011, 34, 1325–1330. [Google Scholar] [CrossRef]
- Han, X.; Gai, L.; Jiang, H.; Zhao, L.; Liu, H.; Zhang, W. Core–shell structured Fe3O4/PANI microspheres and their Cr (VI) ion removal properties. Synth. Met. 2013, 171, 1–6. [Google Scholar] [CrossRef]
- Xing, H.; Liu, Y.; Liu, Z.; Wang, H.; Jia, H. Structure and microwave absorption properties of polyaniline/Zn ferrite composites. Nano 2018, 13, 1850105. [Google Scholar] [CrossRef] [Green Version]
- Shaterian, M.; Rezvani, A.; Abbasian, A.R. Controlled synthesis and self-assembly of ZnFe2O4 nanoparticles into microspheres by solvothermal method. Mater. Res. Express 2020, 6, 1250e5. [Google Scholar] [CrossRef]
- Park, I.H.; Choi, H.J. Fabrication of p-aminobenzoic acid grafted carbonyl iron/polyindole composite particles and their magnetorheological response. J. Ind. Eng. Chem. 2018, 64, 102–106. [Google Scholar] [CrossRef]
- Chhattise, P.; Handore, K.; Horne, A.; Mohite, K.; Chaskar, A.; Dallavalle, S.; Chabukswar, V. Synthesis and characterization of Polyindole and its catalytic performance study as a heterogeneous catalyst. J. Chem. Sci. 2016, 128, 467–475. [Google Scholar] [CrossRef]
- Tanriverdi, E.E.; Uzumcu, A.T.; Kavas, H.; Demir, A.; Baykal, A. Conductivity Study of Polyaniline-Cobalt Ferrite (PANI-CoFe2O4) Nanocomposite. Nano-Micro Lett. 2011, 3, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Sim, B.M.; Zhang, W.L.; Choi, H.J. Graphene oxide/poly (2-methylaniline) composite particle suspension and its electro-response. J. Chem. Sci. 2015, 153, 443–449. [Google Scholar] [CrossRef]
- Pan, X.D.; McKinley, G.H. Structural limitation to the material strength of electrorheological fluids. Appl. Phys. Lett. 1997, 71, 333–335. [Google Scholar] [CrossRef]
- Fernández-Toledano, J.C.; Rodríguez-López, J.; Shahrivar, K.; Hidalgo-Álvarez, R.; Elvira, L.; Montero de Espinosa, F.; de Vicente, J. Two-step yielding in magnetorheology. J. Rheol. 2014, 58, 1507–1534. [Google Scholar] [CrossRef]
- Bonnecaze, R.; Brady, J. Yield stresses in electrorheological fluids. J. Rheol. 1992, 36, 73–115. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.C.; Scriven, L.; Macosko, C. Some rheological measurements on magnetic iron oxide suspensions in silicone oil. J. Rheol. 1986, 30, 1015–1029. [Google Scholar] [CrossRef]
- Kim, H.M.; Choi, H.J. Polyaniline coated ZnFe2O4 microsphere and its electrorheological and magnetorheological response. Colloid Surf. A Physicochem. Eng. Asp. 2021, 626, 127079. [Google Scholar] [CrossRef]
- Park, I.H.; Kwon, S.H.; Choi, H.J. Emulsion-polymerized polyindole nanoparticles and their electrorheology. J. Appl. Polym. Sci. 2018, 135, 46384. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, H.J. Core–shell structured semiconducting poly (diphenylamine)-coated polystyrene microspheres and their electrorheology. Polymer 2017, 131, 120–131. [Google Scholar] [CrossRef]
- Schwarzl, F. On the interconversion between viscoelastic material functions. Pure Appl. Chem. 1970, 23, 219–234. [Google Scholar] [CrossRef]
- Park, B.J.; Park, B.O.; Ryu, B.H.; Choi, Y.M.; Kwon, K.S.; Choi, H.J. Rheological properties of Ag suspended fluid for inkjet printing. J. Appl. Phys. 2010, 108, 102803. [Google Scholar] [CrossRef]
- Kutalkova, E.; Plachy, T.; Osicka, J.; Cvek, M.; Mrlik, M.; Sedlacik, M. Electrorheological behavior of iron(II) oxalate micro-rods. RSC Adv. 2018, 8, 24773–24779. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Nonmonotonic influence of size of quaternary ammonium countercations on micromorphology, polarization, and electroresponse of anionic poly (ionic liquid) s. J. Phys. Chem. B 2020, 124, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Gao, C.Y.; Nam, J.D.; Choi, H.J. Cellulose-based smart fluids under applied electric fields. Materials 2017, 10, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]




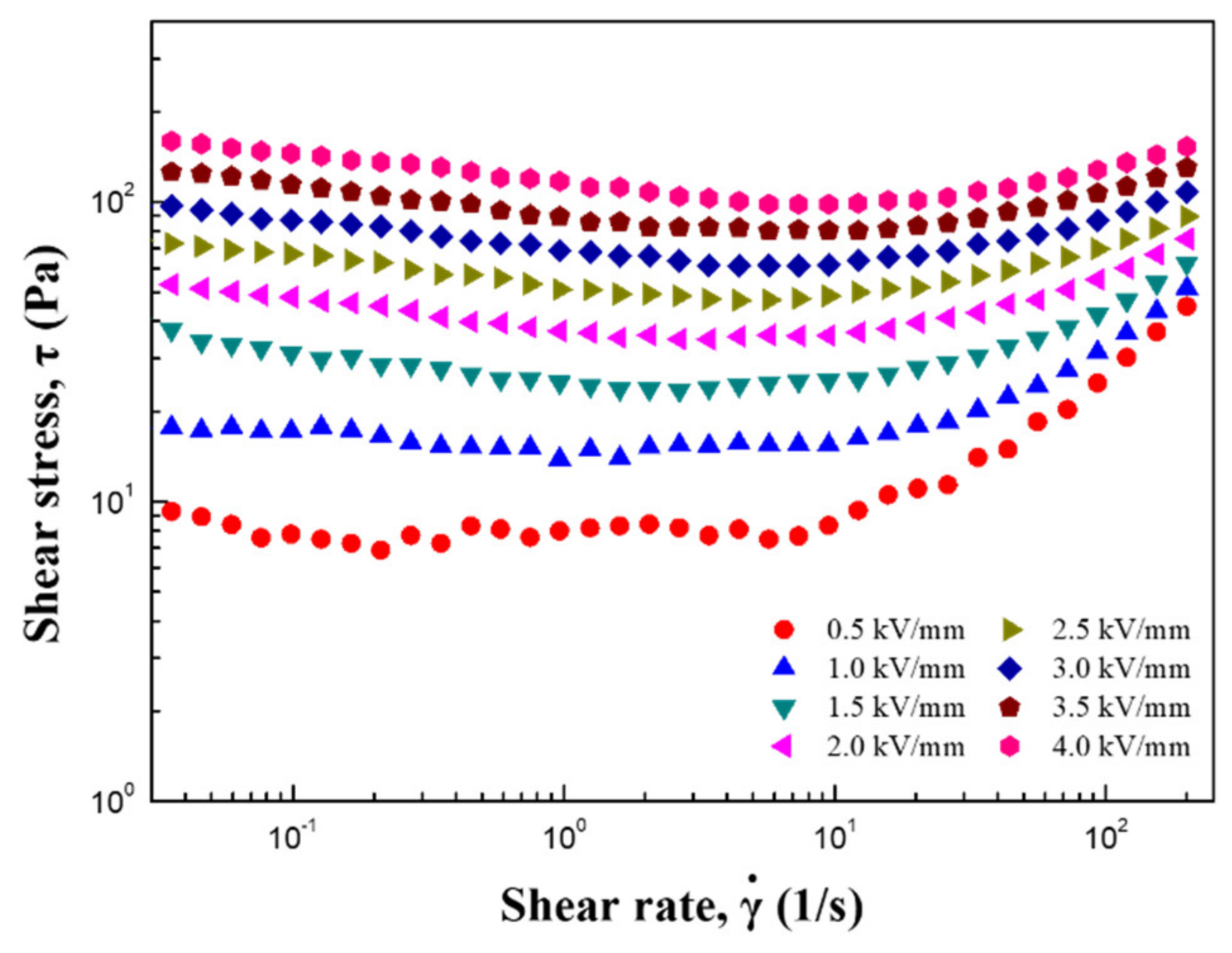
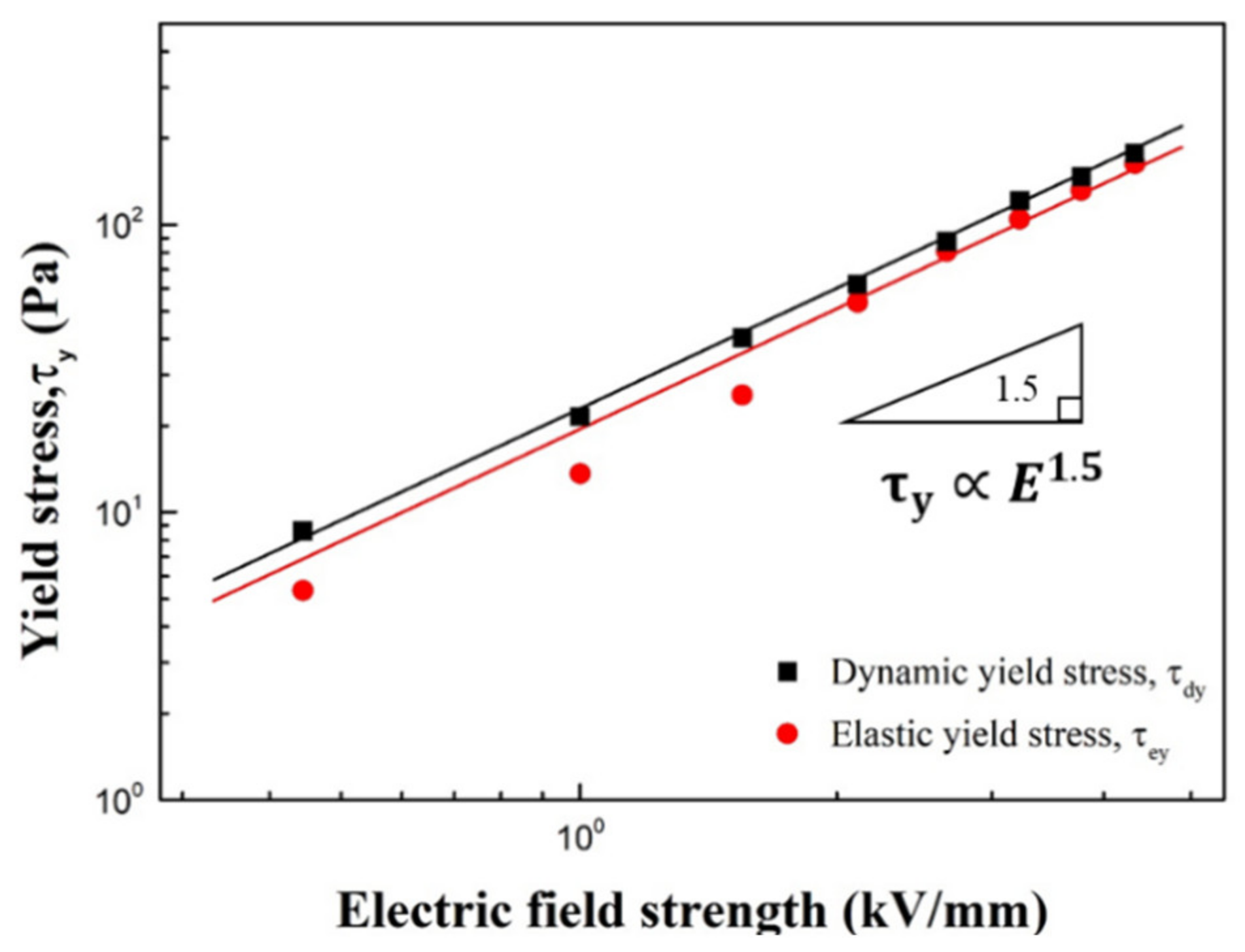
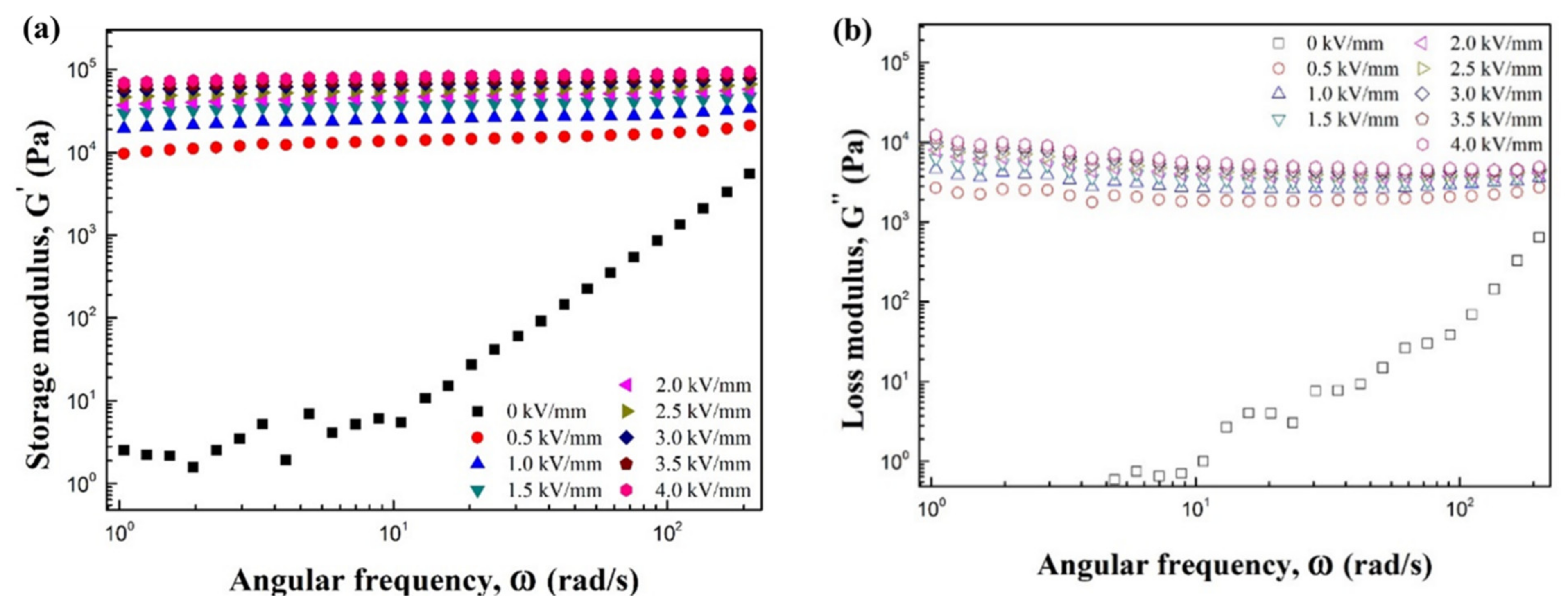
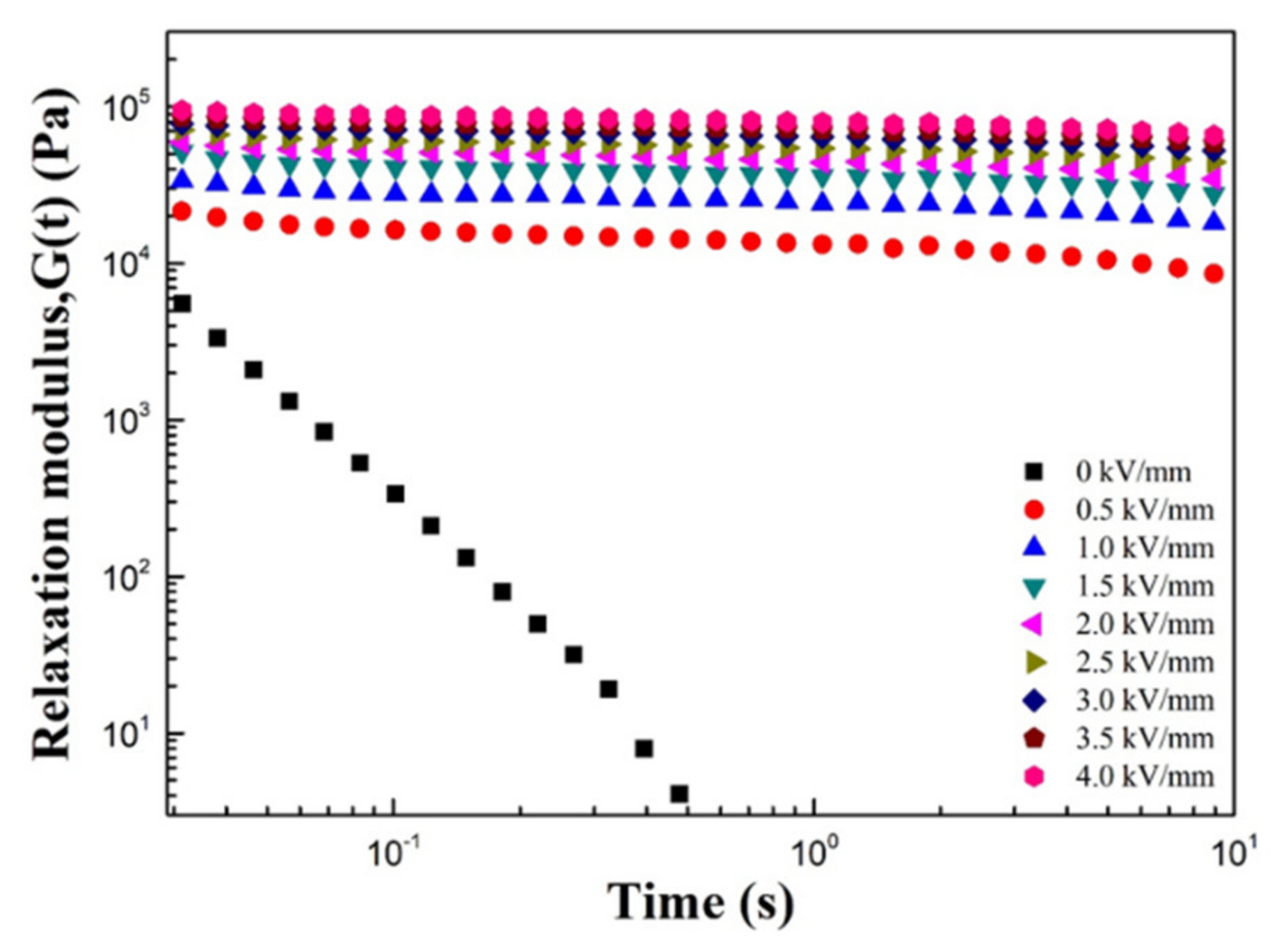
| Sample | Parameters | ||||
|---|---|---|---|---|---|
| (ms) | |||||
| ZnFe2O4/PIn | 3.77 | 2.75 | 1.02 | 0.52 | 0.0027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.H.; Choi, H.J. Dynamic Response of Polyindole Coated Zinc Ferrite Particle Suspension under an Electric Field. Materials 2022, 15, 101. https://doi.org/10.3390/ma15010101
Kang SH, Choi HJ. Dynamic Response of Polyindole Coated Zinc Ferrite Particle Suspension under an Electric Field. Materials. 2022; 15(1):101. https://doi.org/10.3390/ma15010101
Chicago/Turabian StyleKang, Su Hyung, and Hyoung Jin Choi. 2022. "Dynamic Response of Polyindole Coated Zinc Ferrite Particle Suspension under an Electric Field" Materials 15, no. 1: 101. https://doi.org/10.3390/ma15010101






