Mine Clay Washing Residues as a Source for Alkali-Activated Binders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Alkali Activation Procedure
2.3. Hardened Geopolymers Characterization
3. Results and Discussion
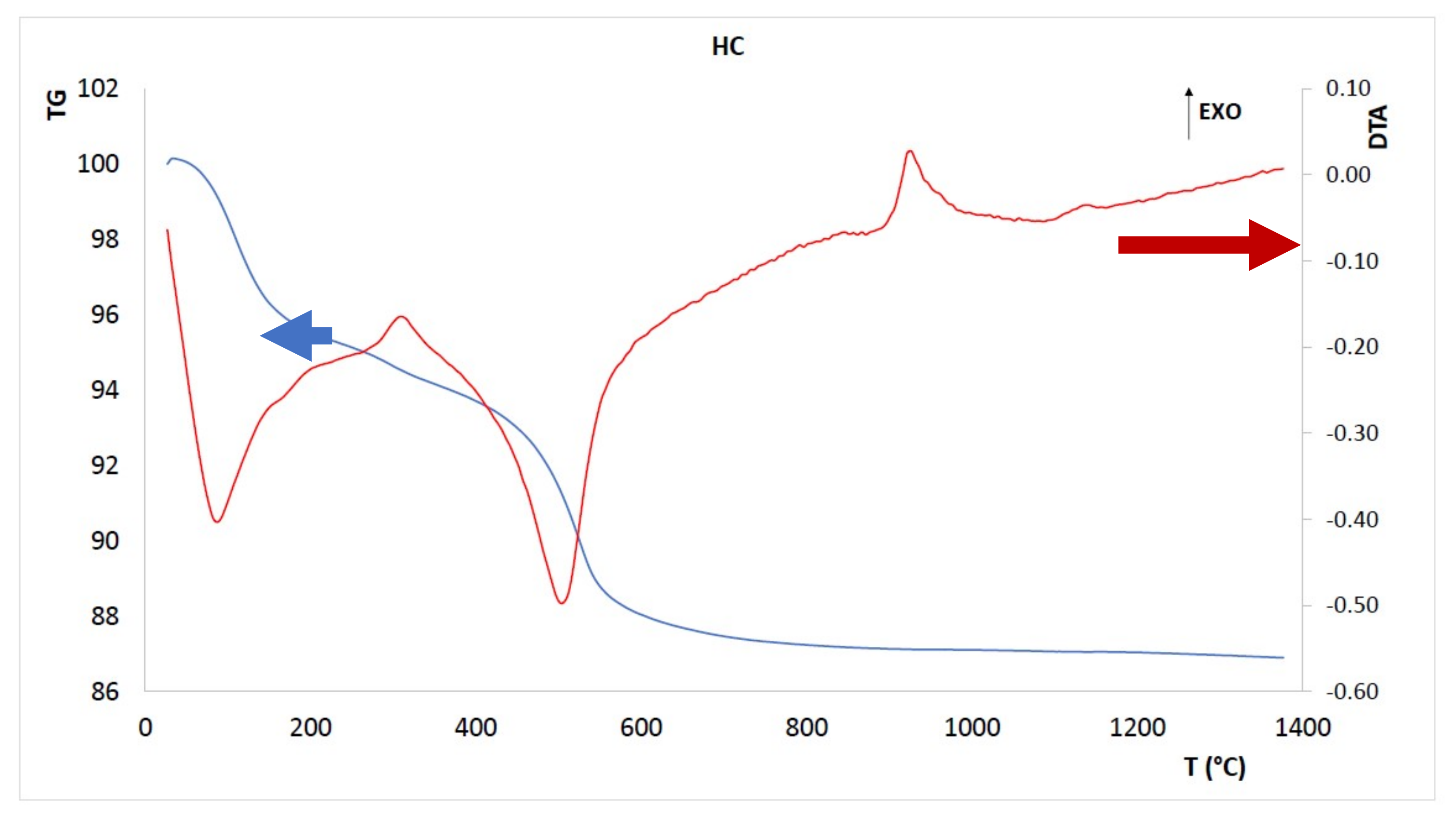
3.1. Thermal Characterization of the Clayey Co-Product
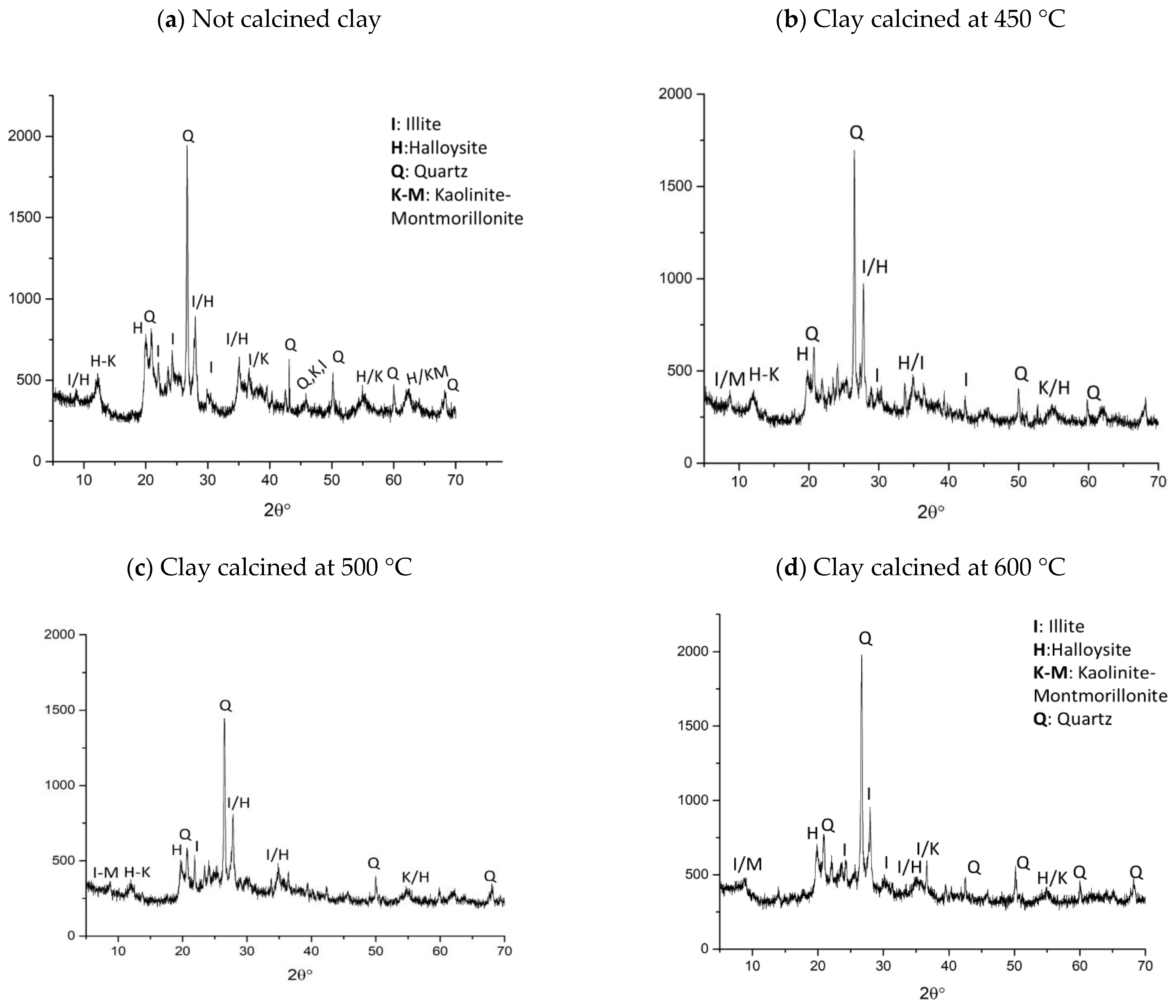
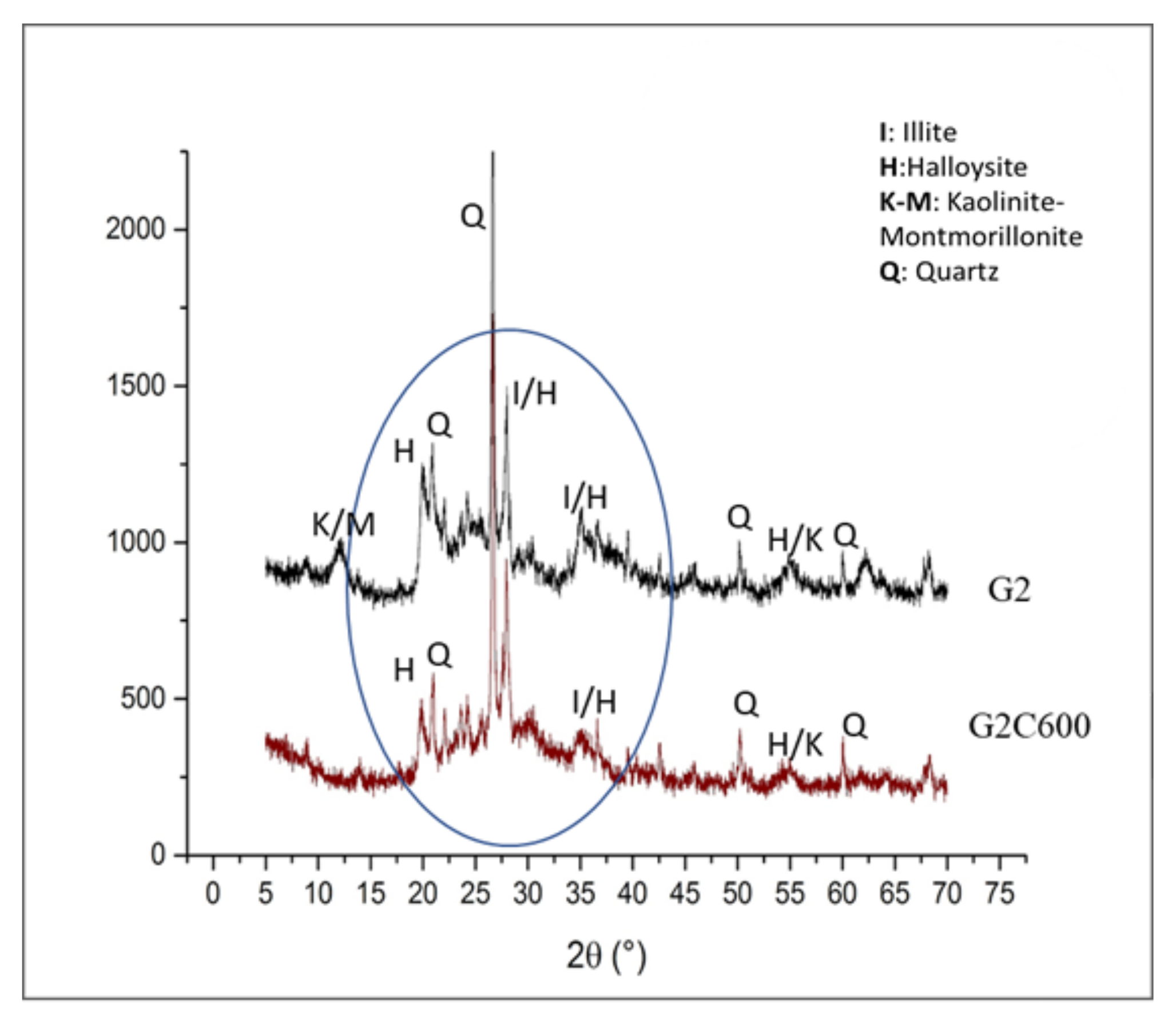
3.2. X-Ray Diffraction of Clay after Thermal Treatment
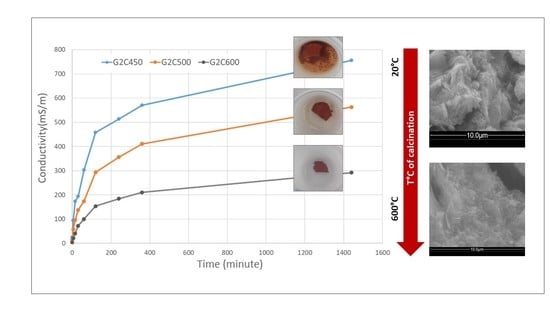
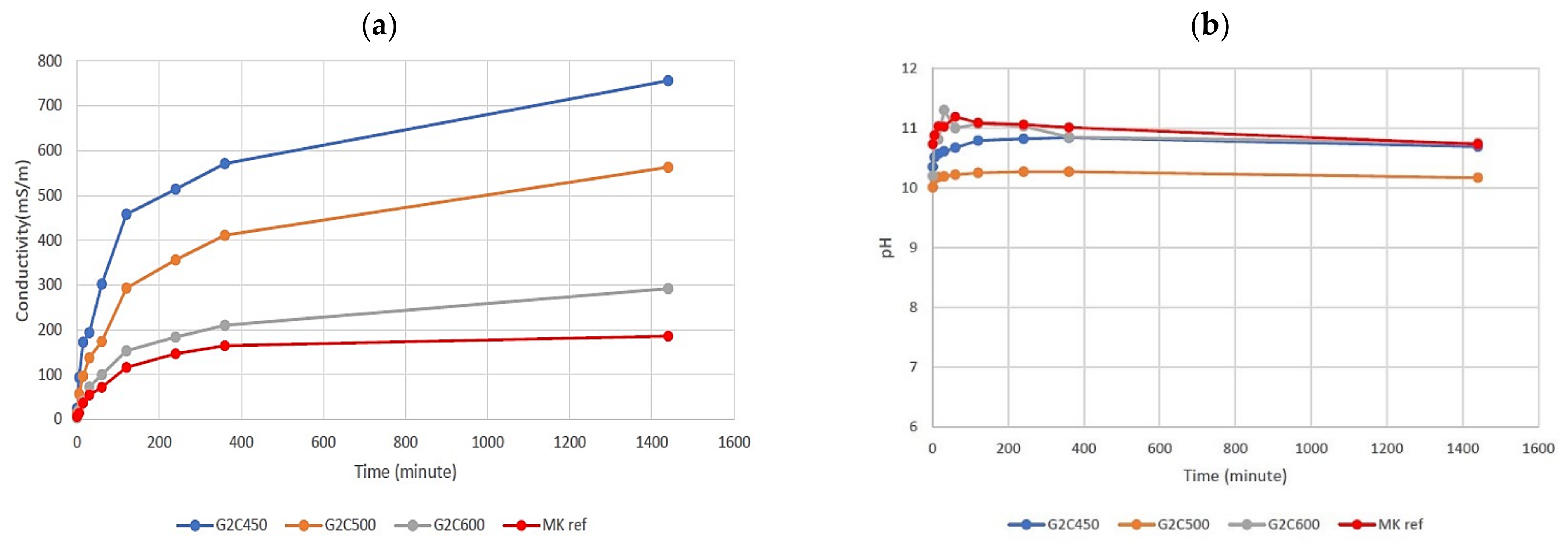
3.3. Chemical Stability of the Alkaline Activated Materials
3.4. SEM and XRD of Clay and Geopolymer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rakhimova, N.R.; Rakhimov, R.Z.; Bikmukhametov, A.R.; Morozov, V.P.; Eskin, A.A.; Gubaidullina, A.M. Calcined Low-Grade Multimineral Clays as Supplementary Cementitious Materials: A Feasibility Study. Geosyst. Eng. 2020, 23, 168–173. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in Alkali-Activation of Clay Minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Ababneh, A.; Matalkah, F.; Aqel, R. Synthesis of Kaolin-Based Alkali-Activated Cement: Carbon Footprint, Cost and Energy Assessment. J. Mater. Res. Technol. 2020, 9, 8367–8378. [Google Scholar] [CrossRef]
- Davidovits, J. GEOPOLYMERS: Synthesis of New High-Temperature Geo-Polymers for Reinforced Plastics/Composites. In Proceedings of the PACTEC’79, Society of Plastic Engineers, Brookfield Center, CA, USA; 1979; pp. 151–154. [Google Scholar]
- He, C.; Makovicky, E.; Osbæck, B. Thermal Stability and Pozzolanic Activity of Raw and Calcined Mixed-Layer Mica/Smectite. Appl. Clay Sci. 2000, 17, 141–161. [Google Scholar] [CrossRef]
- Hollanders, S. Mineralogical Study of the Pozzolanic Properties of Calcined Clays; Belgium Publisher: Leuven, Belgium, 2017; p. 236. [Google Scholar]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The Origin of the Pozzolanic Activity of Calcined Clay Minerals: A Comparison between Kaolinite, Illite and Montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.; Irassar, E.; Scian, A. Thermal Activation of Bentonites for Their Use as Pozzolan. Rev. Constr. 2012, 11, 44–53. [Google Scholar] [CrossRef]
- Taylor-Lange, S.C.; Lamon, E.L.; Riding, K.A.; Juenger, M.C.G. Calcined Kaolinite–Bentonite Clay Blends as Supplementary Cementitious Materials. Appl. Clay Sci. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays: Factors affecting its performance as pozzolans. Constr. Build. Mater. 2012, 28, 276–281. [Google Scholar] [CrossRef]
- Yamchelou, M.T.; Law, D.W.; Patnaikuni, I.; Li, J. Alkali Activation of Mechanically Activated Low-Grade Clay. J. Sustain. Cem.-Based Mater. 2021, 10, 272–288. [Google Scholar] [CrossRef]
- Mucsi, G.; Halyag Papn, N.; Ulsen, C.; Figueiredo, P.O.; Kristaily, F. Mechanical Activation of Construction and Demolition Waste in Order to Improve Its Pozzolanic Reactivity. ACS Sustain. Chem. Eng. 2021, 9, 3416–3427. [Google Scholar] [CrossRef]
- He, C.; Osbaeck, B.; Makovicky, E. Pozzolanic Reactions of Six Principal Clay Minerals: Activation, Reactivity Assessments and Technological Effects. Cem. Concr. Res. 1995, 25, 1691–1702. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lan, G.S.; Tuan, W.H. Microstructural Evolution of Mullite during the Sintering of Kaolin Powder Compacts. Ceram. Int. 2000, 26, 715–720. [Google Scholar] [CrossRef]
- Chakchouk, A.; Samet, B.; Mnif, T. Study on the Potential Use of Tunisian Clays as Pozzolanic Material. Appl. Clay Sci. 2006, 33, 79–88. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic Reactivity of Low Grade Kaolinitic Clays: Influence of Calcination Temperature and Impact of Calcination Products on OPC Hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal Treatment of Kaolin: The Effect of Mineralogy on the Pozzolanic Activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Zhu, Z. Thermal Properties of Halloysite Nanotubes (HNTs) Intercalation Complexes-A Review. E3S Web Conf. 2019, 131, 01055. [Google Scholar] [CrossRef]
- Kaze, C.R.; Alomayri, T.; Hasan, A.; Tome, S.; Lecomte-Nana, G.L.; Nemaleu, J.G.D.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Rahier, H. Reaction Kinetics and Rheological Behaviour of Meta-Halloysite Based Geopolymer Cured at Room Temperature: Effect of Thermal Activation on Physicochemical and Microstructural Properties. Appl. Clay Sci. 2020, 196, 105773. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Yuan, P.; Deng, L.; Zhong, X.; Li, Y.; Wang, Q.; Liu, D. Novel Acid-Based Geopolymer Synthesized from Nanosized Tubular Halloysite: The Role of Precalcination Temperature and Phosphoric Acid Concentration. Cem. Concr. Compos. 2020, 110, 103601. [Google Scholar] [CrossRef]
- Garg, N.; Skibsted, J. Thermal Activation of a Pure Montmorillonite Clay and Its Reactivity in Cementitious Systems. J. Phys. Chem. C 2014, 118, 11464–11477. [Google Scholar] [CrossRef]
- Kaminskas, R.; Monstvilaite, D.; Valanciene, V. Influence of Low-Pozzolanic Activity Calcined Mica Clay on Hydration and Hardening of Portland Cement. Adv. Cem. Res. 2017, 30, 231–239. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary Cementitious Materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Mielenz, R.C.; White, L.P.; Glantz, O.J. Effect of Calcination on Natural Pozzolanz. Symp on Use of Pozzolanic Materials in Mortars and Concrete. ASTM Spec. Tech. Publ. 1950, 99, 43–92. [Google Scholar]
- Saad, M.N.; De Andrade, W.P.; Paulon, V.A. Properties of Mass Concrete Containing an Active Pozzolan Made from Clay. Concr. Int. 1982, 4, 59–65. [Google Scholar]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Haha, M.B.; Scrivener, K. Development of a New Rapid, Relevant and Reliable (R3) Test Method to Evaluate the Pozzolanic Reactivity of Calcined Kaolinitic Clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Yao, X.; Zhu, Y. Effects of Halloysite in Kaolin on the Formation and Properties of Geopolymers. Cem. Concr. Compos. 2012, 34, 709–715. [Google Scholar] [CrossRef]
- Churchman, G.J.; Pontifex, I.R.; McClure, S.G. Factors Influencing the Formation and Characteristics of Halloysites or Kaolinites in Granitic and Tuffaceous Saprolites in Hong Kong. Clays Clay Miner. 2010, 58, 220–237. [Google Scholar] [CrossRef]
- Yanguatin, H.; Ramírez, J.H.; Tironi, A.; Tobón, J.I. Effect of Thermal Treatment on Pozzolanic Activity of Excavated Waste Clays. Constr. Build. Mater. 2019, 211, 814–823. [Google Scholar] [CrossRef]
- Tironi, A.; Cravero, F.; Scian, A.N.; Irassar, E.F. Pozzolanic Activity of Calcined Halloysite-Rich Kaolinitic Clays. Appl. Clay Sci. 2017, 147, 11–18. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Application; Institute Geopolymer: Saint Quentin, France, 2011. [Google Scholar]
- Dupuy, C.; Gharzouni, A.; Texier-Mandoki, N.; Bourbon, X.; Rossignol, S. Alkali-Activated Materials Based on Callovo-Oxfordian Argillite: Formation, Structure and Mechanical Properties. J. Ceram. Sci. Technol. 2018, 9, 127–140. [Google Scholar] [CrossRef]
- Belmokhtar, N.; Ammari, M.; Brigui, J.; Ben Allal, L. Comparison of the Microstructure and the Compressive Strength of Two Geopolymers Derived from Metakaolin and an Industrial Sludge. Constr. Build. Mater. 2017, 146, 621–629. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and Properties of Clay-Based Geopolymer Cements: A Review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from Kaolin in China: An Overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the Calcined Kaolinite Content on the Hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Bratoev, B.; Doykov, I.; Ninov, J.; Lenchev, A. Pozzolanic Activity Assessment of Calcined Clays with Complex Minerals Content. Adv. Cem. Res. 2018, 30, 103–112. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhou, Z.; Zhang, Y.; Yang, H. High Morphological Stability and Structural Transition of Halloysite (Hunan, China) in Heat Treatment. Appl. Clay Sci. 2014, 101, 16–22. [Google Scholar] [CrossRef]
- Argın, G.; Uzal, B. Enhancement of Pozzolanic Activity of Calcined Clays by Limestone Powder Addition. Constr. Build. Mater. 2021, 284, 122789. [Google Scholar] [CrossRef]
- Bellotto, M.; Gualtieri, A.; Artioli, G.; Clark, S.M. Kinetic Study of the Kaolinite-Mullite Reaction Sequence. Part I: Kaolinite Dehydroxylation. Phys. Chem. Miner. 1995, 22, 207–217. [Google Scholar] [CrossRef]
- Gualtieri, A.; Bellotto, M.; Artioli, G.; Clark, S.M. Kinetic Study of the Kaolinite-Mullite Reaction Sequence. Part II: Mullite Formation. Phys. Chem. Miner. 1995, 22, 215–222. [Google Scholar] [CrossRef]
- Qiu, G.; Jiang, T.; Li, G.; Fan, X.; Huang, Z. Activation and Removal of Silicon in Kaolinite by Thermochemical Process. Scand. J. Metall. 2004, 33, 121–128. [Google Scholar] [CrossRef]
- Schulze, D.G.; Schwertmann, U. The Influence of Aluminium on Iron Oxides: X. Properties of Al-Substituted Goethites. Clay Miner. 1984, 19, 521–539. [Google Scholar] [CrossRef]
- Obonyo, E.A.; Kamseu, E.; Lemougna, P.N.; Tchamba, A.B.; Melo, U.C.; Leonelli, C. A Sustainable Approach for the Geopolymerization of Natural Iron-Rich Aluminosilicate Materials. Sustainability 2014, 6, 5535–5553. [Google Scholar] [CrossRef] [Green Version]
- Cara, S.; Carcangiu, G.; Massidda, L.; Paola, M.; Sanna, U.; Tamanini, M. Assessment of Pozzolanic Potential in Lime–Water Systems of Raw and Calcined Kaolinic Clays from the Donnigazza Mine (Sardinia–Italy). Appl. Clay Sci. 2006, 33, 66–72. [Google Scholar] [CrossRef]
- Slaty, F.; Khoury, H.; Wastiels, J.; Rahier, H. Characterization of Alkali Activated Kaolinitic Clay. Appl. Clay Sci. 2013, 75–76, 120–125. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali Activation Processes for Incinerator Residues Management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef]
- Aly, Z.; Vance, E.R.; Perera, D.S.; Hanna, J.V.; Griffith, C.S.; Davis, J.; Durce, D. Aqueous Leachability of Metakaolin-Based Geopolymers with Molar Ratios of Si/Al = 1.5–4. J. Nucl. Mater. 2008, 378, 172–179. [Google Scholar] [CrossRef]
- Luna Galiano, Y.; Fernández Pereira, C.; Vale, J. Stabilization/Solidification of a Municipal Solid Waste Incineration Residue Using Fly Ash-Based Geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef]
- Sgarlata, C.; Formia, A.; Ferrari, F.; Leonelli, C. Effect of the Introduction of Reactive Fillers and Metakaolin in Waste Clay-Based Materials for Geopolymerization Processes. Molecules 2021, 26, 1325. [Google Scholar] [CrossRef] [PubMed]
- Walkley, B.; Kashani, A.; Sani, M.-A.; Ngo, T.D.; Mendis, P. Examination of Alkali-Activated Material Nanostructure during Thermal Treatment. J. Mater. Sci. 2018, 53, 9486–9503. [Google Scholar] [CrossRef] [Green Version]
- Do Rosario Pinheiro, D.; Gonçalves, L.R.; de Sena, R.L.P.; Martelli, M.C.; de Freitas Neves, R.; da Paixão Ribeiro, N.F. Industrial Kaolin Waste as Raw Material in the Synthesis of the SAPO-34 Molecular Sieve. Mater. Res. 2020, 23, 1–6. [Google Scholar] [CrossRef]



| Clay Type | Calcination T (°C) | Reference |
|---|---|---|
| Kaolinite | 550–950 | [13] |
| 650–800 | [14] | |
| 600–800 | [7] | |
| 500–700 | [15] | |
| 600–800 | [16] | |
| 650 | [17] | |
| Halloysite | 450–700 | [18] |
| 600–750 | [19] | |
| Kaolinite/halloysite | 600–800 | [1] |
| 600–700 | [20] | |
| Illite | 650–930 | [13] |
| 600–800 | [7] | |
| 600–800 | [16] | |
| 600–800 | [1] | |
| Illite/Montmorillonite | 730–920 | [13] |
| 600–800 | [21] | |
| 600–800 | [7] | |
| 500–800 | [15] | |
| 600–800 | [16] | |
| Mica clay | 560–960 | [13] |
| 700–1100 | [22] | |
| 600–800 | [1] |
| Oxide Composition | Clay | Phase | Clay |
|---|---|---|---|
| SiO2(wt%) | 51–55 | Quartz(wt%) | 22–24 |
| Al2O3 (wt%) | 26–30 | Alkali feldspar (wt%) | 10 |
| Fe2O3 * (wt%) | 4 | Illite (wt%) | 8–10 |
| TiO2 (wt%) | ≤1 | Illite/smectite (wt%) | 1–2 |
| CaO (wt%) | ≤0.5 | Plagioclase (wt%) | 22–23 |
| MgO (wt%) | ≤1 | Halloysite (wt%) | 35 |
| K2O (wt%) | ≤2 | Goethite (wt%) | 1–2 |
| Na2O (wt%) | ≤2 | ||
| D90 (µm) | 18–20 | ||
| LOI (wt%) | 10 | B.E.T. (m2/g) | 35.90 ± 0.22 |
| Sample | Calcination of Clay | S/L | Si/Al | Na/Al | Alkaline Solution | |
|---|---|---|---|---|---|---|
| (°C) | (h) | |||||
| G1 | - | - | 1 | 2.3 | 1.33 | NaOH 8M+ Na2SiO3 |
| G2 | - | - | 1.46 | 2.04 | 2.12 | NaOH 8M+ Na2SiO3 |
| G2C600 | 600 °C | 2 | 1.66 | 2.04 | 2.12 | NaOH 8M+ Na2SiO3 |
| G2C500 | 500 °C | 2 | 1.66 | 2.04 | 2.12 | NaOH 8M+ Na2SiO3 |
| G2C450 | 450 °C | 2 | 1.66 | 2.04 | 2.12 | NaOH 8M+ Na2SiO3 |
| G1C450 | 600 °C | 2 | 1.06 | 2.3 | 1.33 | NaOH 8M+ Na2SiO3 |
| G1C500 | 500 °C | 2 | 1.06 | 2.3 | 1.33 | NaOH 8M+ Na2SiO3 |
| G1C600 | 450 °C | 2 | 1.06 | 2.3 | 1.33 | NaOH 8M+ Na2SiO3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgarlata, C.; Formia, A.; Siligardi, C.; Ferrari, F.; Leonelli, C. Mine Clay Washing Residues as a Source for Alkali-Activated Binders. Materials 2022, 15, 83. https://doi.org/10.3390/ma15010083
Sgarlata C, Formia A, Siligardi C, Ferrari F, Leonelli C. Mine Clay Washing Residues as a Source for Alkali-Activated Binders. Materials. 2022; 15(1):83. https://doi.org/10.3390/ma15010083
Chicago/Turabian StyleSgarlata, Caterina, Alessandra Formia, Cristina Siligardi, Francesco Ferrari, and Cristina Leonelli. 2022. "Mine Clay Washing Residues as a Source for Alkali-Activated Binders" Materials 15, no. 1: 83. https://doi.org/10.3390/ma15010083
APA StyleSgarlata, C., Formia, A., Siligardi, C., Ferrari, F., & Leonelli, C. (2022). Mine Clay Washing Residues as a Source for Alkali-Activated Binders. Materials, 15(1), 83. https://doi.org/10.3390/ma15010083