A Tribological and Ion Released Research of Ti-Materials for Medical Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Structural and Microstructural Characterization
2.3. Tribological Characterization
2.4. Exposure
2.5. Surface Analysis
2.6. Ionic Dissolution Characterization
3. Results and Discussion
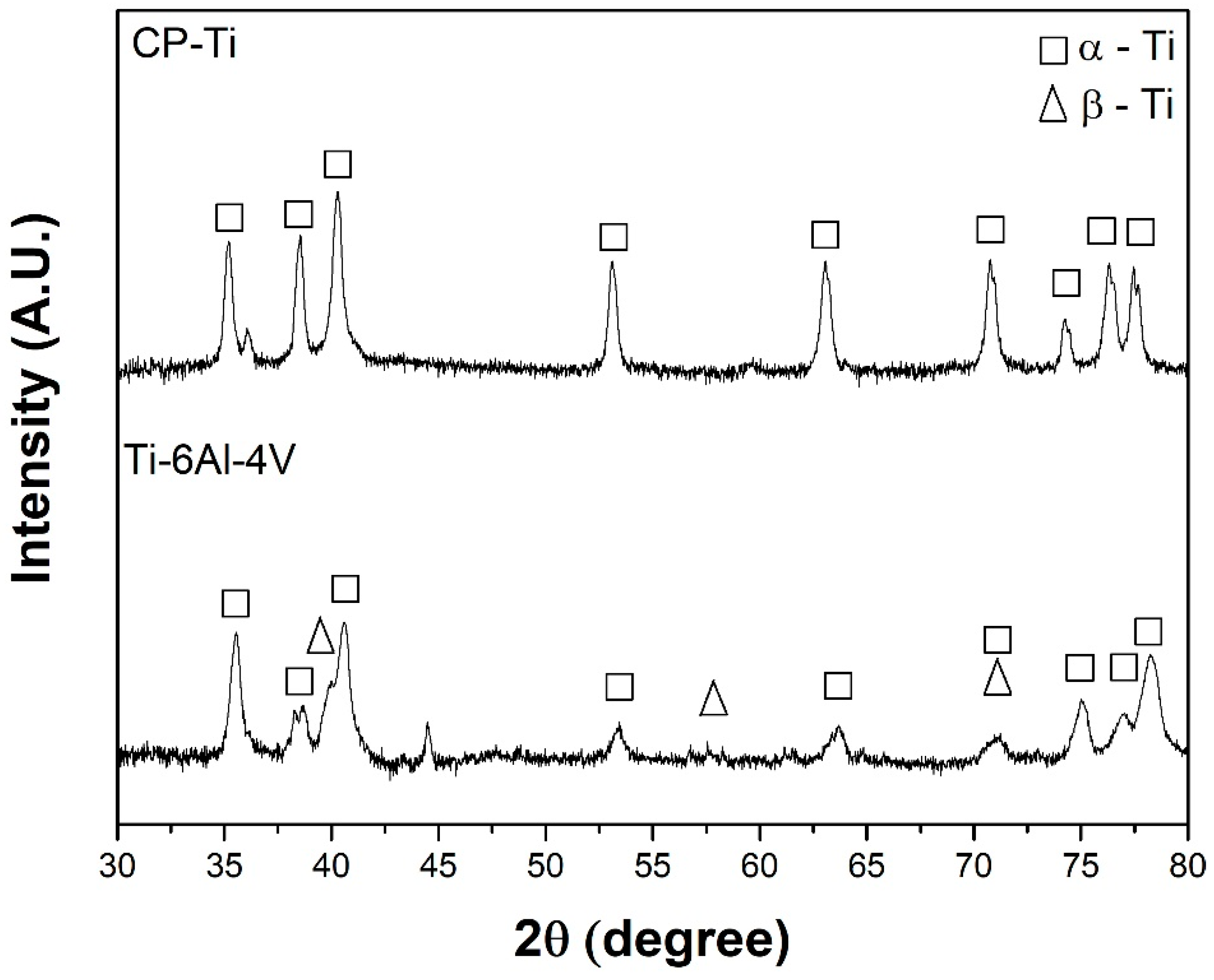
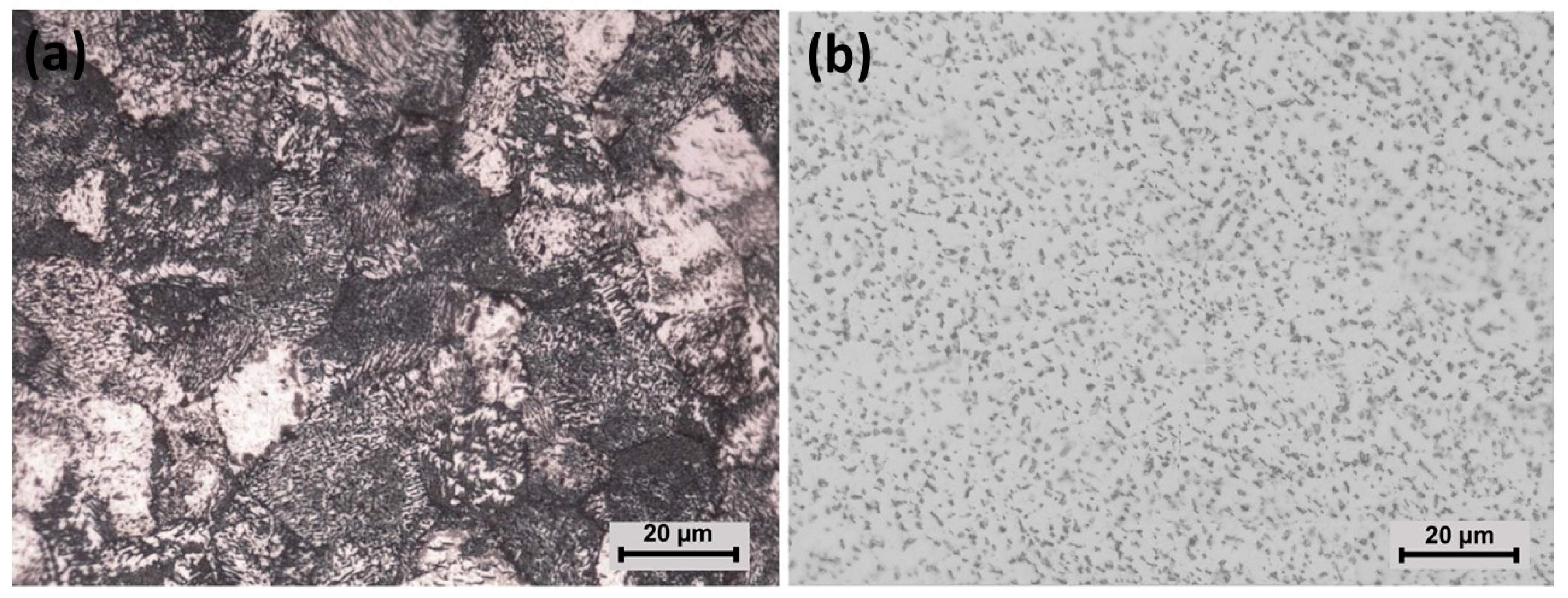
3.1. Structural and Microstructural Analysis
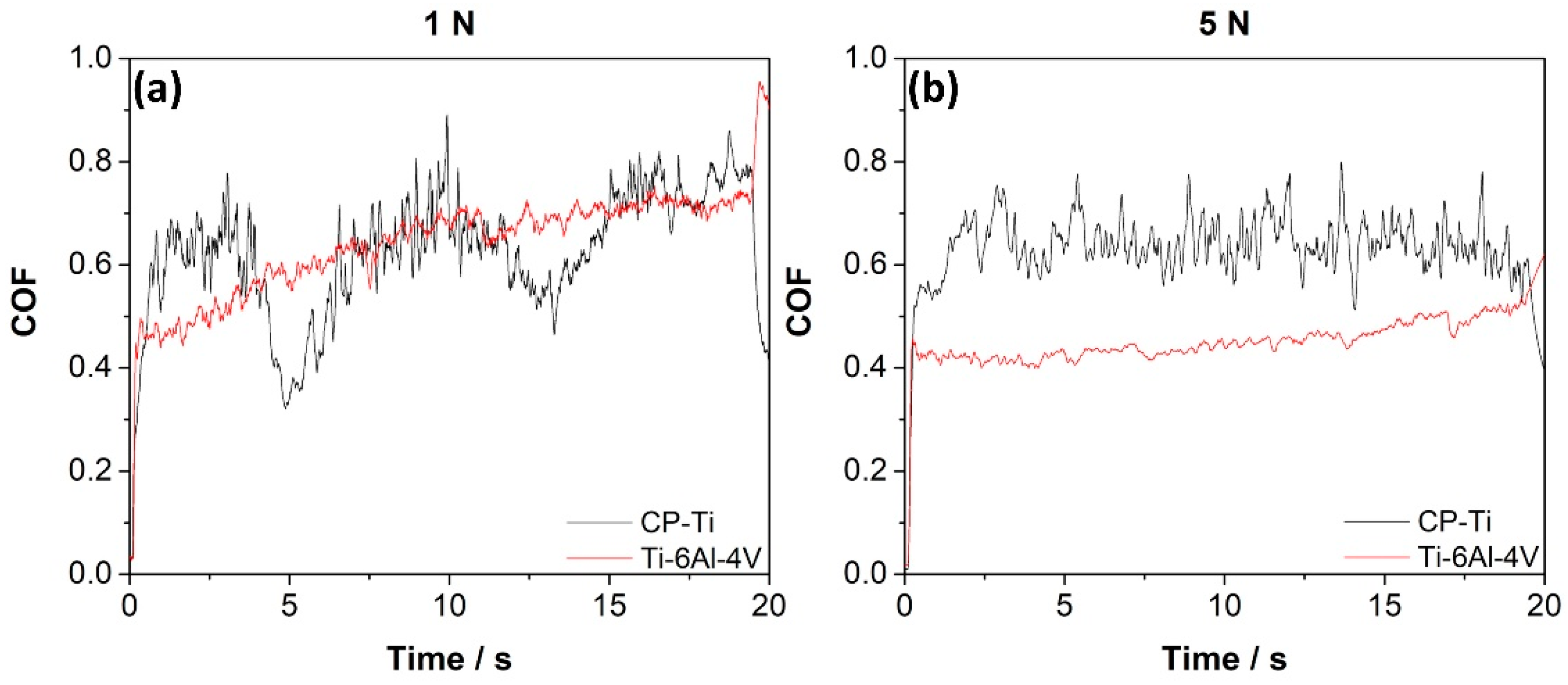
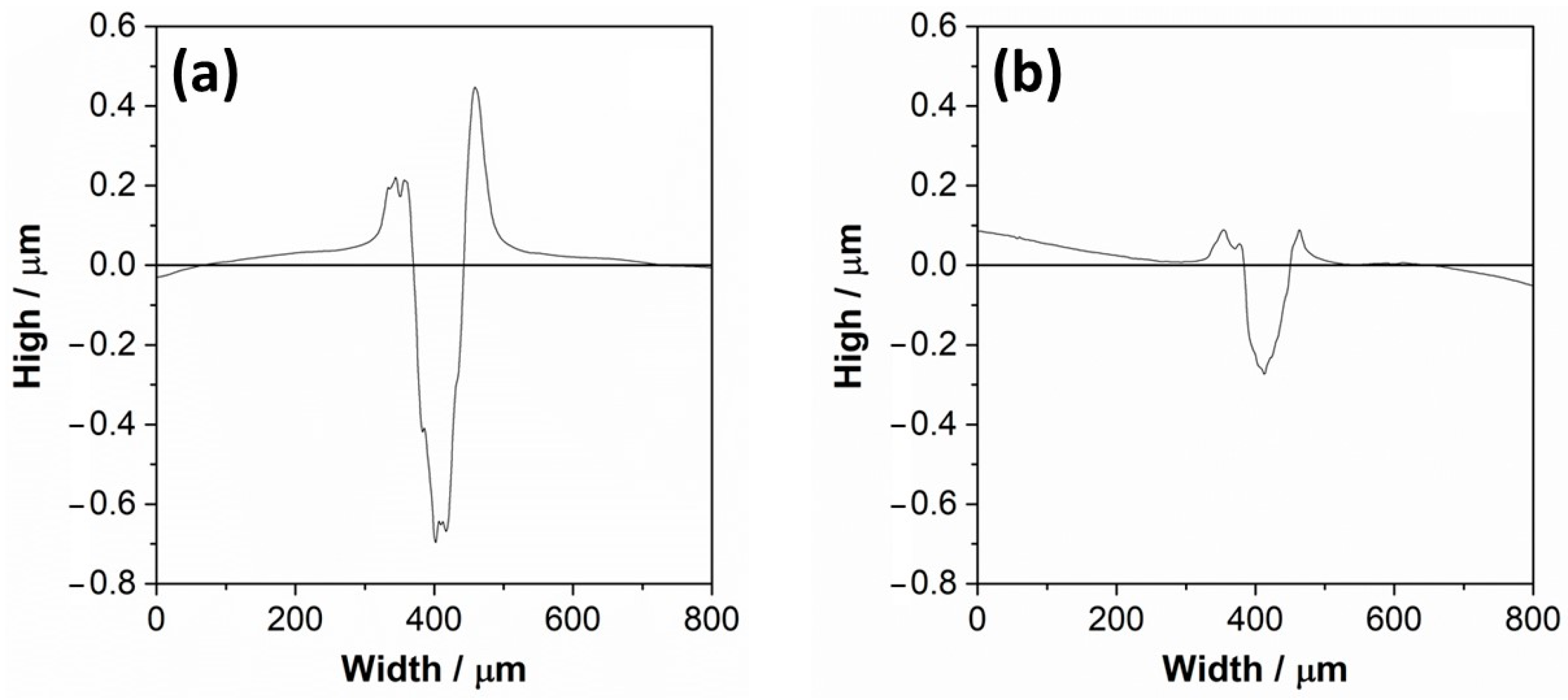
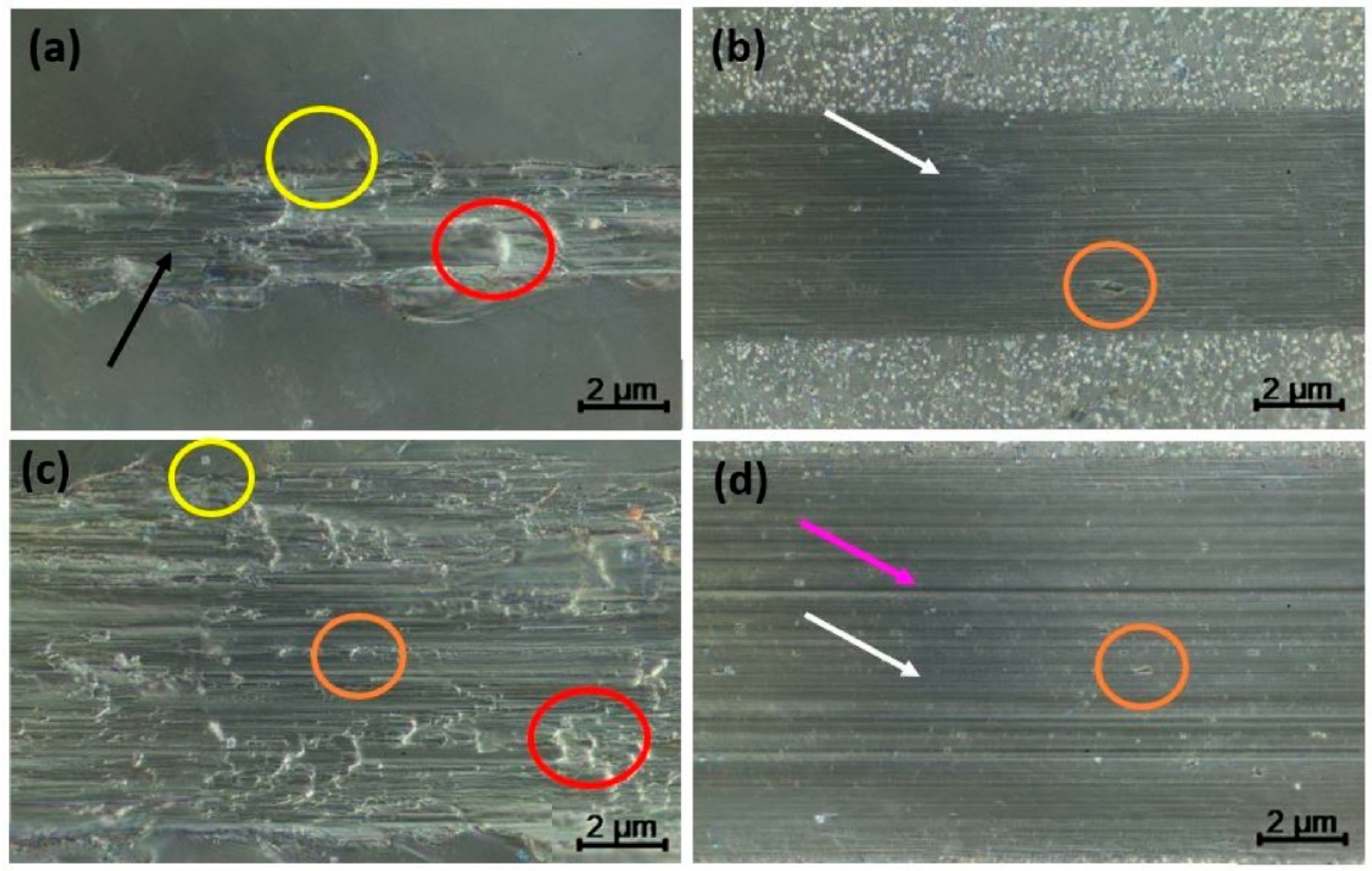
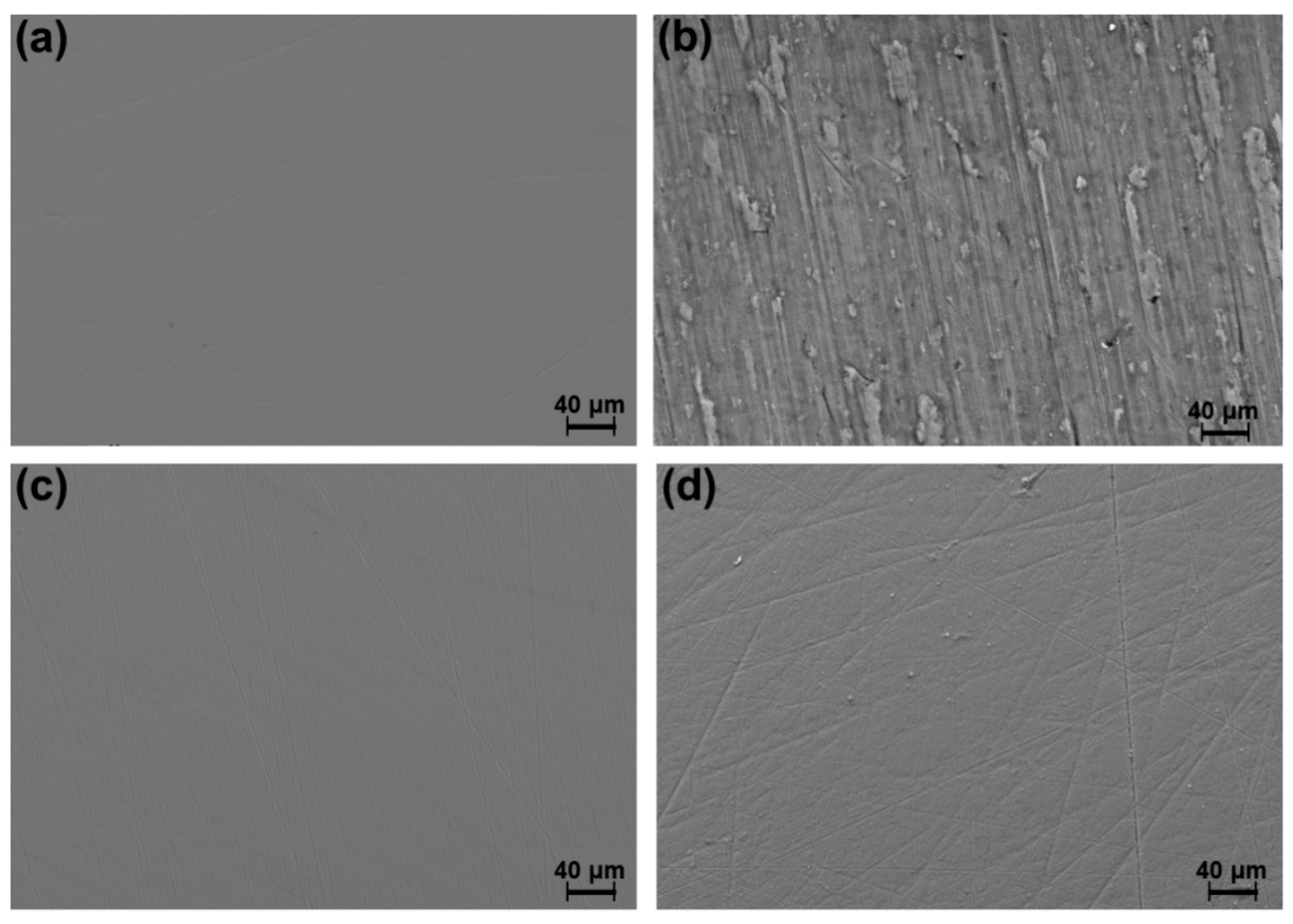
3.2. Tribological Characterization
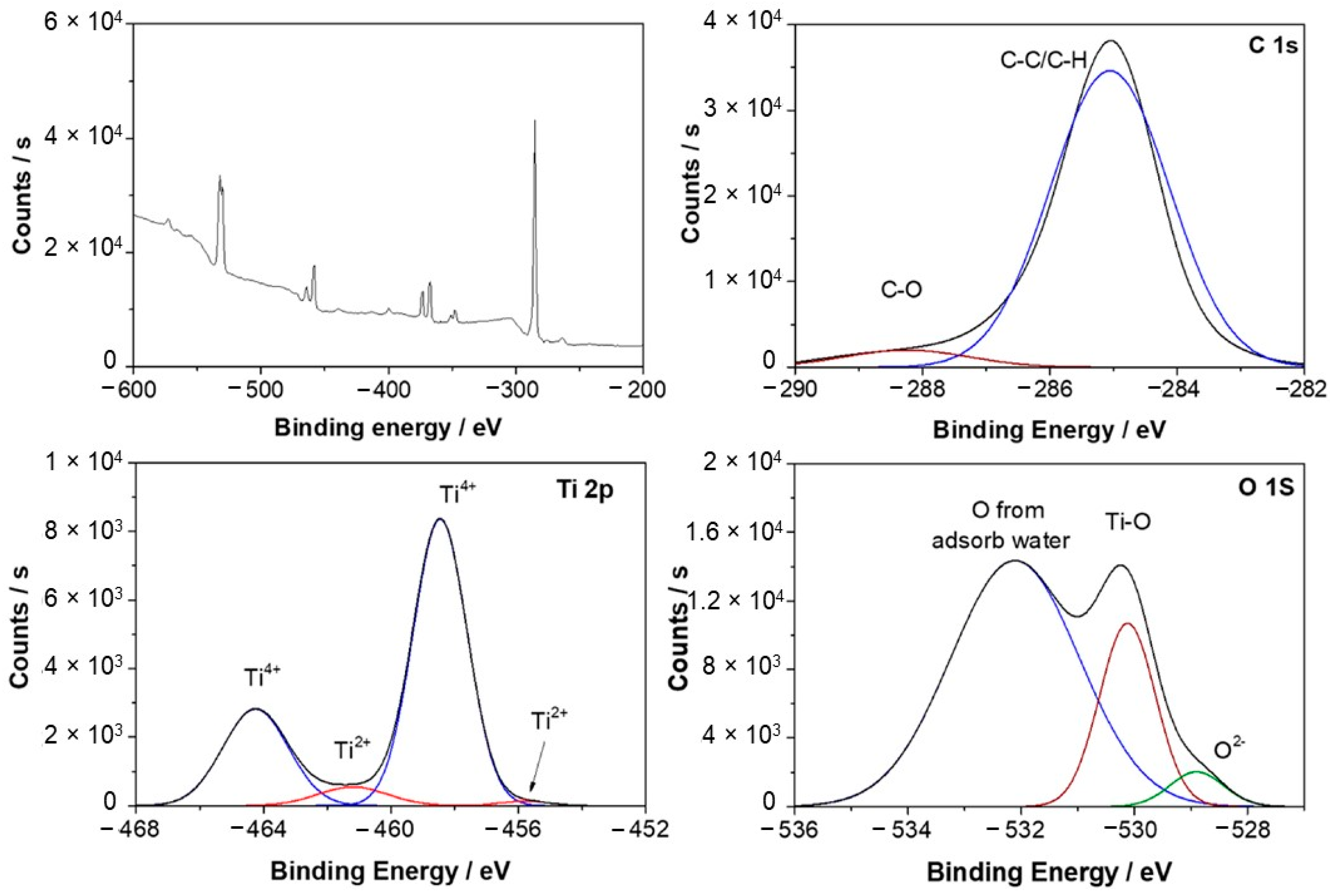
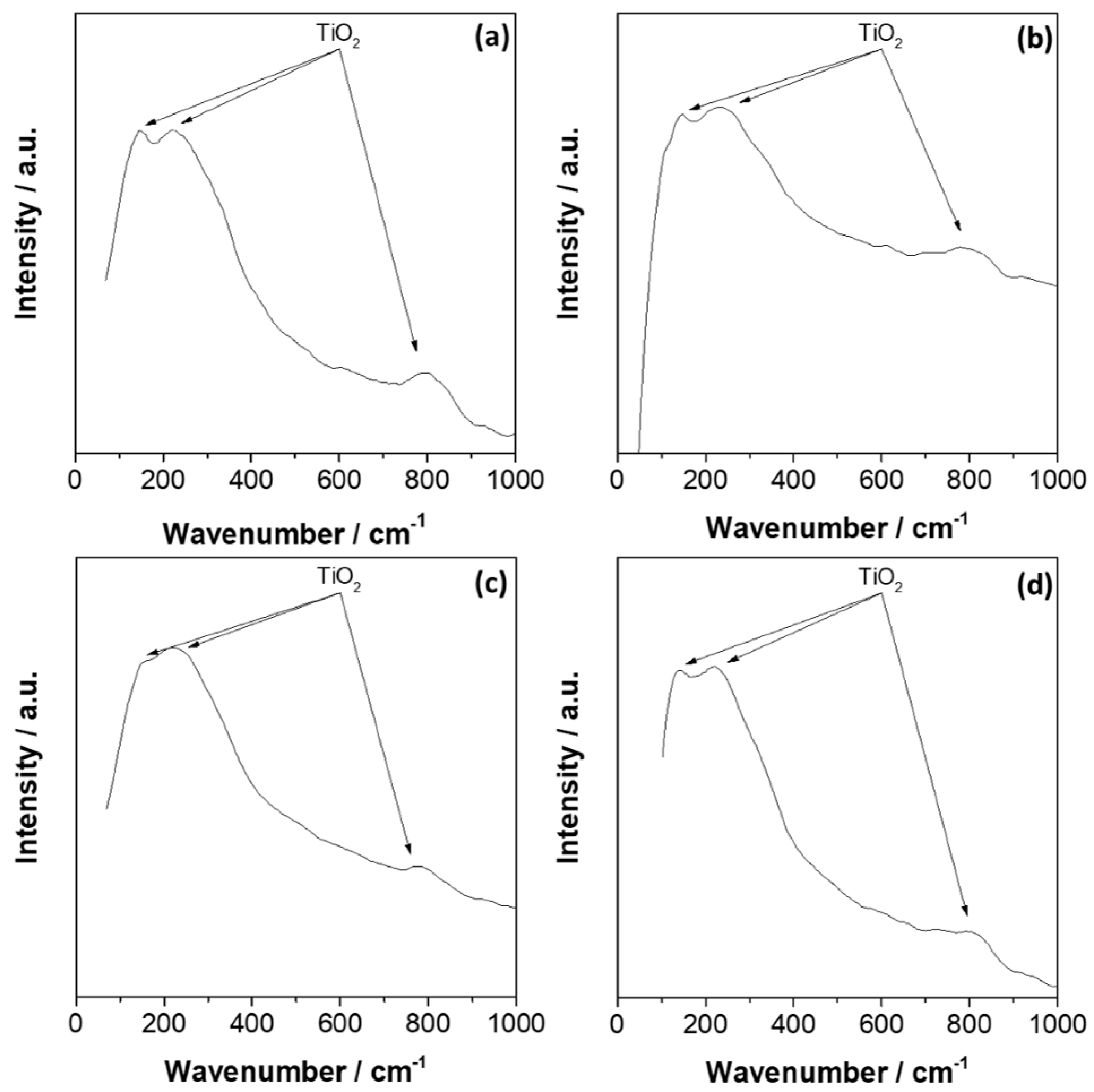
3.3. Surface Analysis
3.4. Real-Time Elemental Dissolution Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ke, Z.; Yi, C.; Zhang, L.; He, Z.; Tan, J.; Jiang, Y. Characterization of a new Ti-13Nb-13Zr-10Cu alloy with enhanced antibacterial activity for biomedical applications. Mater. Lett. 2019, 253, 335–338. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Toptan, F.; Alves, A.C.; Carvalho, Ó.; Bartolomeu, F.; Pinto, A.M.P.; Silva, F.; Miranda, G. Corrosion and tribocorrosion behaviour of Ti6Al4V produced by selective laser melting and hot pressing in comparison with the commercial alloy. J. Mater. Process. Technol. 2019, 266, 239–245. [Google Scholar] [CrossRef]
- Chávez, J.; Jimenez, O.; Olmos, L.; Farias, I.; Flores-Jimenez, M.; Suárez-Martínez, R.; Cabezas-Villa, J.L.; Lemus-Ruiz, J. Tribocorrosion behavior of Ti64-xTa alloys fabricated through powder metallurgy. Mater. Lett. 2020, 128590. [Google Scholar] [CrossRef]
- Pechancova, R.; Pluháček, T.; Gallo, J.; Milde, D. Study of chromium species release from metal implants in blood and joint effusion: Utilization of HPLC-ICP-MS. Talanta 2018, 185, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical behaviour and galvanic effects of titanium implants coupled to metallic suprastructures in artificial saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C. 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Lin, C.-W.; Ju, C.-P.; Chern Lin, J.-H. A comparison of the fatigue behavior of cast Ti–7.5Mo with c.p. titanium, Ti–6Al–4V and Ti–13Nb–13Zr alloys. Biomaterials 2005, 26, 2899–2907. [Google Scholar] [CrossRef]
- Gomes, C.C.; Moreira, L.M.; Santos, V.J.S.V.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet. Mol. Biol. 2011, 34, 116–121. [Google Scholar] [CrossRef]
- Ahmed, G.A.; Khalil, S.K.H.; El hotaby, W.; Abbas, L.; Sherif, H.H.A.; Abdel-Rahman, E.A.; Saber, S.H.; Hassan, M.; Hassan, M.H.; Ali, S.S. ATR-IR and EPR spectroscopy for detecting the alterations in cortical synaptosomes induced by aluminium stress. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117535. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers. Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef]
- Kumar, V.; Gill, K.D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology 2014, 41, 154–166. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, P.; Gharbi, O.; Zeng, Z.; Chen, X.; Volovitch, P.; Ogle, K.; Birbilis, N. Investigating ion release using inline ICP during in situ scratch testing of an Mg-Li(-Al-Y-Zr) alloy. Electrochem. Commun. 2019, 99, 46–50. [Google Scholar] [CrossRef]
- Hutchison, M.J.; Zhou, P.; Ogle, K.; Scully, J.R. Enhanced Electrochemical Cu Release from Commercial Cu-Sn Alloys: Fate of the Alloying Elements in Artificial Perspiration. Electrochim. Acta 2017, 241, 73–88. [Google Scholar] [CrossRef]
- Vu, T.N.; Volovitch, P.; Ogle, K. The effect of pH on the selective dissolution of Zn and Al from Zn-Al coatings on steel. Corros. Sci. 2013, 67, 42–49. [Google Scholar] [CrossRef]
- Liang, L.; Huang, Q.; Wu, H.; Ouyang, Z.; Liu, T.; He, H.; Xiao, J.; Lei, G.; Zhou, K. Stimulation of in vitro and in vivo osteogenesis by Ti-Mg alloys with the sustained-release function of magnesium ions. Colloids Surf. B Biointerfaces 2021, 197. [Google Scholar] [CrossRef]
- Guerra, C.; Sancy, M.; Walczak, M.; Martínez, C.; Ringuedé, A.; Cassir, M.; Han, J.; Ogle, K.; de Melo, H.G.; Salinas, V.; et al. Effect of added porosity on a novel porous Ti-Nb-Ta-Fe-Mn alloy exposed to simulated body fluid. Mater. Sci. Eng. C 2020, 111. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Tan, J.; Wang, D.; Qiu, J.; Liu, X. Surface alloyed Ti-Zr layer constructed on titanium by Zr ion implantation for improving physicochemical and osteogenic properties. Prog. Nat. Sci. Mater. Int. 2020, 30, 635–641. [Google Scholar] [CrossRef]
- Yamazoe, M. Study of corrosion of combinations of titanium/Ti-6Al-4V implants and dental alloys. Dent. Mater. J. 2010, 29, 542–553. [Google Scholar] [CrossRef]
- Hu, N.; Xie, L.; Liao, Q.; Gao, A.; Zheng, Y.; Pan, H.; Tong, L.; Yang, D.; Gao, N.; Starink, M.J.; et al. A more defective substrate leads to a less defective passive layer: Enhancing the mechanical strength, corrosion resistance and anti-inflammatory response of the low-modulus Ti-45Nb alloy by grain refinement. Acta Biomater. 2021, 126, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Ciapetti, G. Biology of implant wear. In Wear of Orthopaedic Implants and Artificial Joints; Woodhead Publishing Series in Biomaterials: Bologna, Italy, 2012; pp. 27–55. [Google Scholar] [CrossRef]
- Eger, M.; Sterer, N.; Liron, T.; Kohavi, D.; Gabet, Y. Scaling of titanium implants entrains inflammation-induced osteolysis. Sci. Rep. 2017, 7, 39612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, M.; Li, Z.; Zhu, M.; Fan, Y.; Chu, B.; Yuan, C.; Chen, L.; Lv, H.; Hong, Z.; et al. Bulleyaconitine A prevents Ti particle-induced osteolysis via suppressing NF-κB signal pathway during osteoclastogenesis and osteoblastogenesis. J. Cell. Physiol. 2018, 233, 7067–7079. [Google Scholar] [CrossRef] [PubMed]
- Reports and Data. Titanium-Based Alloys Market Share, Trends—Industry Analysis, 2028. 2019. Available online: https://www.reportsanddata.com/report-detail/titanium-based-alloys-market (accessed on 26 October 2021).
- Mordor Intelligence Titanium Alloy Market—2021–26—Industry Share, Size, Growth—Mordor Intelligence. 2020. Available online: https://www.mordorintelligence.com/industry-reports/titanium-alloy-market (accessed on 26 October 2021).
- Martínez, C.; Briones, F.; Araya, N.; Aguilar, C.; Machado, I.; Guerra, C.; Medina, A.; Sancy, M. Influence of the synthesis technique on tribological behavior of a Ti-6Al-4V alloy. Mater. Lett. 2020, 281, 4–7. [Google Scholar] [CrossRef]
- Silva, D.; Guerra, C.; Muñoz, H.; Aguilar, C.; Walter, M.; Azocar, M.; Muñoz, L.; Gürbüz, E.; Ringuedé, A.; Cassir, M.; et al. The effect of Staphylococcus aureus on the electrochemical behavior of porous Ti-6Al-4V alloy. Bioelectrochemistry 2020, 136, 107622. [Google Scholar] [CrossRef] [PubMed]
- Ogle, K. Atomic Emission Spectroelectrochemistry: Real-Time Rate Measurements of Dissolution, Corrosion, and Passivation. Corrosion 2019, 75, 1398–1419. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 8–12. [Google Scholar] [CrossRef]
- ASM International. Titanium: Physical Metallurgy, Processing and Applications; ASM International: Geauga County, OH, USA, 2015; ISBN 9781627080798. [Google Scholar] [CrossRef]
- Mindivan, F.; Mindivan, H.; Darcan, C. Electroless Ni–B Coating of Pure Titanium Surface for Enhanced Tribocorrosion Performance in Artificial Saliva and Antibacterial Activity. Tribol. Ind. 2017, 39, 270–276. [Google Scholar] [CrossRef]
- Lee, K.; Choe, H.C.; Ko, Y.M.; Brentley, W.A. Nanotubular Structure Formation on Ti-6Al-4V and Ti-Ta Alloy Surfaces by Electrochemical Methods. Korean J. Met. Mater. 2012, 50, 164–170. [Google Scholar] [CrossRef]
- Li, H.; Jia, D.; Yang, Z.; Liao, X.; Jin, H.; Cai, D.; Zhou, Y. Effect of heat treatment on microstructure evolution and mechanical properties of selective laser melted Ti–6Al–4V and TiB/Ti–6Al–4V composite: A comparative study. Mater. Sci. Eng. A 2021, 801, 140415. [Google Scholar] [CrossRef]
- Fan, W.; Tan, H.; Zhang, F.; Feng, Z.; Wang, Y.; Zhang, L.C.; Lin, X.; Huang, W. Overcoming the limitation of in-situ microstructural control in laser additive manufactured Ti–6Al–4V alloy to enhanced mechanical performance by integration of synchronous induction heating. J. Mater. Sci. Technol. 2021, 94, 32–46. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, B.; Cao, L.; Liu, K.Y.; Li, S.; Jia, L.; Kondoh, K.; Li, J.S. Improved mechanical properties in titanium matrix composites reinforced with quasi-continuously networked graphene nanosheets and in-situ formed carbides. J. Mater. Sci. Technol. 2022, 96, 85–93. [Google Scholar] [CrossRef]
- Dumontet, N.; Connétable, D.; Malard, B.; Viguier, B. Elastic properties of the α’ martensitic phase in the Ti-6Al-4V alloy obtained by additive manufacturing. Scr. Mater. 2019, 167, 115–119. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C. Titanium; Engineering Materials and Processes Book Series; Springer: Berlin, Germany, 2007; p. 341. ISBN 978-3-540-71397-5. [Google Scholar]
- Wang, S.; Ma, Z.; Liao, Z.; Song, J.; Yang, K.; Liu, W. Study on improved tribological properties by alloying copper to CP-Ti and Ti-6Al-4V alloy. Mater. Sci. Eng. C 2015, 57, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, D.; Niu, Z.; Zhou, W.; Chen, G.; Li, Z.; Fu, X. The role of pyramidal <c + a> dislocations in the grain refinement mechanism in Ti-6Al-4V alloy processed by severe plastic deformation. Acta Mater. 2020, 200, 101–115. [Google Scholar] [CrossRef]
- Beladi, H.; Chao, Q.; Rohrer, G.S. Variant selection and intervariant crystallographic planes distribution in martensite in a Ti-6Al-4V alloy. Acta Mater. 2014, 80, 478–489. [Google Scholar] [CrossRef]
- Amanov, A.; Cho, I.S.; Kim, D.E.; Pyun, Y.S. Fretting wear and friction reduction of CP titanium and Ti-6Al-4V alloy by ultrasonic nanocrystalline surface modification. Surf. Coat. Technol. 2012, 207, 135–142. [Google Scholar] [CrossRef]
- Javier Gil, F.; Planell, J.A.; Padrós, A.; Aparicio, C. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar] [CrossRef]
- Greger, M.; Kander, L.; Snasel, V.; Cerny, M. Microstructure evolution of pure titanium during ECAP. Mater. Des. 2011, 18, 97–104. [Google Scholar]
- Vander Voort, G. Metallographic Preparation of Titanium and its Alloys. In Proceedings of the Ninth World Concerence on Titanium, Central Research Institute of Structural Materials (CRISM) “Prometey”, Saint-Petersburg, Russia, 7–11 June 1999. [Google Scholar]
- Çaha, I.; Alves, A.C.; Chirico, C.; Tsipas, S.A.; Rodrigues, I.R.; Pinto, A.M.P.; Grandini, C.R.; Rocha, L.A.; Gordo, E.; Toptan, F. Interactions between wear and corrosion on cast and sintered Ti-12Nb alloy in comparison with the commercial Ti-6Al-4V alloy. Corros. Sci. 2020, 176, 108925. [Google Scholar] [CrossRef]
- Dabrowski, J.R. The Kinetics of Phase Transformations during Continuous Cooling of Ti6Al4V Alloy from the Diphase alfa + beta Range. Arch. Metall. Mater. 2011, 56, 5–9. [Google Scholar] [CrossRef]
- Sherif, H.A.; Kossa, S.S. Relationship between normal and tangential contact stiffness of nominally flat surfaces. Wear 1991, 151, 49–62. [Google Scholar] [CrossRef]
- Lee, S.M.; Shin, M.W.; Lee, W.K.; Jang, H. The correlation between contact stiffness and stick-slip of brake friction materials. Wear 2013, 302, 1414–1420. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L.; Lim, S. Tribological behavior of Ti–6Al–4V alloy: Subsurface structure, damage mechanism and mechanical properties. Wear 2021, 464–465, 203551. [Google Scholar] [CrossRef]
- Yazdi, R.; Ghasemi, H.M.; Abedini, M.; Monshi, M. Interplay between mechanical wear and electrochemical corrosion during tribocorrosion of oxygen diffusion layer on Ti–6Al–4V in PBS solution. Appl. Surf. Sci. 2020, 518, 146048. [Google Scholar] [CrossRef]
- Li, X.X.; Zhou, Y.; Ji, X.L.; Li, Y.X.; Wang, S.Q. Effects of sliding velocity on tribo-oxides and wear behavior of Ti–6Al–4V alloy. Tribol. Int. 2015, 91, 228–234. [Google Scholar] [CrossRef]
- Vencl, A.; Manić, N.; Popovic, V.; Mrdak, M. Possibility of the abrasive wear resistance determination with scratch tester. Tribol. Lett. 2010, 37, 591–604. [Google Scholar] [CrossRef]
- Patil, S.; Kekade, S.; Phapale, K.; Jadhav, S.; Powar, A.; Supare, A.; Singh, R. Effect of α and β Phase Volume Fraction on Machining Characteristics of Titanium Alloy Ti6Al4V. Procedia Manuf. 2016, 6, 63–70. [Google Scholar] [CrossRef]
- Hutchings, I.M. Tribology: Friction and Wear of Engineering Materials; Butterworth-Heinemann: Oxford, UK, 1992; Volume 13, ISBN 9780081009109. [Google Scholar] [CrossRef]
- Rajendhran, N.; De Baets, P.; Huang, S.; Vleugels, J.; Sukumaran, J. Single-point scratch testing for understanding particle engagement in abrasion of multiphase materials. Wear 2021, 476, 203689. [Google Scholar] [CrossRef]
- Alam, M.O.; Haseeb, A.S.M.A. Response of Ti–6Al–4V and Ti–24Al–11Nb alloys to dry sliding wear against hardened steel. Tribol. Int. 2002, 35, 357–362. [Google Scholar] [CrossRef]
- Farokhzadeh, K.; Qian, J.; Edrisy, A. Effect of SPD surface layer on plasma nitriding of Ti–6Al–4V alloy. Mater. Sci. Eng. A 2014, 589, 199–208. [Google Scholar] [CrossRef]
- Li, S.J.; Yang, R.; Li, S.; Hao, Y.L.; Cui, Y.Y.; Niinomi, M.; Guo, Z.X. Wear characteristics of Ti-Nb-Ta-Zr and Ti-6Al-4V alloys for biomedical applications. Wear 2004, 257, 869–876. [Google Scholar] [CrossRef]
- Yildiz, F.; Yetim, A.F.; Alsaran, A.; Efeoglu, I. Wear and corrosion behaviour of various surface treated medical grade titanium alloy in bio-simulated environment. Wear 2009, 267, 695–701. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Wang, L.; Dong, C.; Wang, L.; Kong, D.; Wang, X. Different corrosion behaviors between α and β phases of Ti6Al4V in fluoride-containing solutions: Influence of alloying element Al. Corros. Sci. 2020, 169, 108605. [Google Scholar] [CrossRef]
- Hasan, J.; Li, H.; Tian, G.; Qin, C. Fabrication of Cr2S3-GO-TiO2 composite with high visible-light-driven photocatalytic activity on degradation of organic dyes. Chem. Phys. 2020, 539, 110950. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Tian, J.; Huang, C.; Chen, M.; Yan, Y.; Wang, L.; Qu, X.; Wen, C. Microstructure, wear resistance, and corrosion performance of Ti35Zr28Nb alloy fabricated by powder metallurgy for orthopedic applications. J. Mater. Sci. Technol. 2020, 41, 191–198. [Google Scholar] [CrossRef]
- Mendis, S.; Xu, W.; Tang, H.P.; Jones, L.A.; Liang, D.; Thompson, R.; Choong, P.; Brandt, M.; Qian, M. Characteristics of oxide films on Ti-(10–75)Ta alloys and their corrosion performance in an aerated Hank’s balanced salt solution. Appl. Surf. Sci. 2020, 506, 145013. [Google Scholar] [CrossRef]
- Xu, Y.F.; Xiao, Y.F.; Yi, D.Q.; Liu, H.Q.; Wu, L.; Wen, J. Corrosion behavior of Ti-Nb-Ta-Zr-Fe alloy for biomedical applications in Ringer’s solution. Trans. Nonferrous Met. Soc. China 2015, 25, 2556–2563. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, Y.; Zhu, J.; Tu, J.; Jiao, S. Surface treatment functionalization of sodium hydroxide onto 3D printed porous Ti6Al4V for improved biological activities and osteogenic potencies. J. Mater. Res. Technol. 2020, 9, 13661–13670. [Google Scholar] [CrossRef]
- El Mel, A.A.; Angleraud, B.; Gautron, E.; Granier, A.; Tessier, P.Y. XPS study of the surface composition modification of nc-TiC/C nanocomposite films under in situ argon ion bombardment. Thin Solid Films 2011, 519, 3982–3985. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Xu, H.; Feng, L.; Zhang, T. Dynamic evolution of oxide film on selective laser melted Ti–6Al–4V alloy. J. Alloys Compd. 2020, 849, 156622. [Google Scholar] [CrossRef]
- Turek, A.M.; Wachs, I.E.; Hirt, A.M. Physical and chemical characterization of surface vanadium oxide supported on titania: Influence of the titania phase (anatase, rutile, brookite and B) Goutam. Appl. Catal. A Gen. 1992, 91, 27–42. [Google Scholar] [CrossRef]
- Lukacevic, I.; Gupta, S.K.; Jha, P.K.; Kirin, D. Lattice dynamics and Raman spectrum of rutile TiO2: The role of soft phonon modes in pressure induced phase transition. Mater. Chem. Phys. 2012, 137, 282–289. [Google Scholar] [CrossRef]
- Ekoi, E.J.; Stallard, C.; Reid, I.; Dowling, D.P. Tailoring oxide-layer formation on titanium substrates using microwave plasma treatments. Surf. Coat. Technol. 2017, 325, 299–307. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Lib, Z.; Cao, X. Understanding the effect of surface/bulk defects on the photocatalytic activity of TiO2: Anatase versus rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978–10988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Harris, C.X.; Wallenmeyer, P.; Murowchick, J.; Chen, X. Asymmetric Lattice Vibrational Characteristics of Rutile TiO2 as Revealed by Laser Power Dependent Raman Spectroscopy. J. Phys. Chem. 2013, 117, 24015–24022. [Google Scholar] [CrossRef]
- Nouicer, E.; Benlahreche, F.Z.; Nouicer, A. Spectroscopic characterization of titanium alloy surface in a biological medium. Acta Phys. Pol. A 2017, 131, 28–31. [Google Scholar] [CrossRef]
- Shaikh, S.; Kedia, S.; Majumdar, A.G.; Subramanian, M.; Sinha, S. In vitro bioactivity and biocompatibility of femtosecond laser-modified Ti6Al4V alloy. Appl. Phys. A Mater. Sci. Process. 2018, 124, 821. [Google Scholar] [CrossRef]
- Gurappa, I. Characterization of different materials for corrosion resistance under simulated body fluid conditions. Mater. Charact. 2002, 49, 73–79. [Google Scholar] [CrossRef]
- Lee, M.H. Biocompatibility of a modified metallic surface of pure Ti and Ti-6Al-4V alloy with the connective tissue of mouse. Met. Mater. 2000, 6, 373–379. [Google Scholar] [CrossRef]
- Lee, M.H.; Yoon, D.J.; Won, D.H.; Bae, T.S.; Watari, F. Biocompatibility of surface treated pure titanium and titanium alloy byin vivo andin vitro test. Met. Mater. Int. 2003, 9, 35–42. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation Mechanism of Biomedical Titanium Alloy Surface and Experiment. Int. J. Corros. 2020, 2020, 1678615. [Google Scholar] [CrossRef]
- Chen, C.C. Phase Equilibria at Ti–Al Interface Under Low Oxygen Pressure. Atlas J. Mater. Sci. 2014, 1, 1–11. [Google Scholar] [CrossRef]
- Prodana, M.; Caposi, M.; Iordachescu, D. Ions release from Ti implant alloys in simulated bioliquids. In Proceedings of the 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, Riga, Latvia, 16–20 June 2008; Volume 20, pp. 60–63. [Google Scholar] [CrossRef]
- Zhao, P.; Song, Y.; Dong, K.; Shan, D.; Han, E.-H. Corrosion behavior of dual-phase Ti–6Al–4V alloys: A discussion on the impact of Fe content. J. Alloys Compd. 2020, 858, 157708. [Google Scholar] [CrossRef]
- Su, B.; Luo, L.; Wang, B.; Su, Y.; Wang, L.; Ritchie, R.O.; Guo, E.; Li, T.; Yang, H.; Huang, H.; et al. Annealed microstructure dependent corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy. J. Mater. Sci. Technol. 2021, 62, 234–248. [Google Scholar] [CrossRef]
- Chen, X.; Shah, K.; Dong, S.; Peterson, L.; Callagon La Plante, E.; Sant, G. Elucidating the corrosion-related degradation mechanisms of a Ti-6Al-4V dental implant. Dent. Mater. 2020, 36, 431–441. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, R.L.; Majumdar, T.; Mantri, S.A.; Ravi, V.A.; Banerjee, R.; Birbilis, N. A closer look at the in vitro electrochemical characterisation of titanium alloys for biomedical applications using in-situ methods. Acta Biomater. 2017, 54, 469–478. [Google Scholar] [CrossRef]
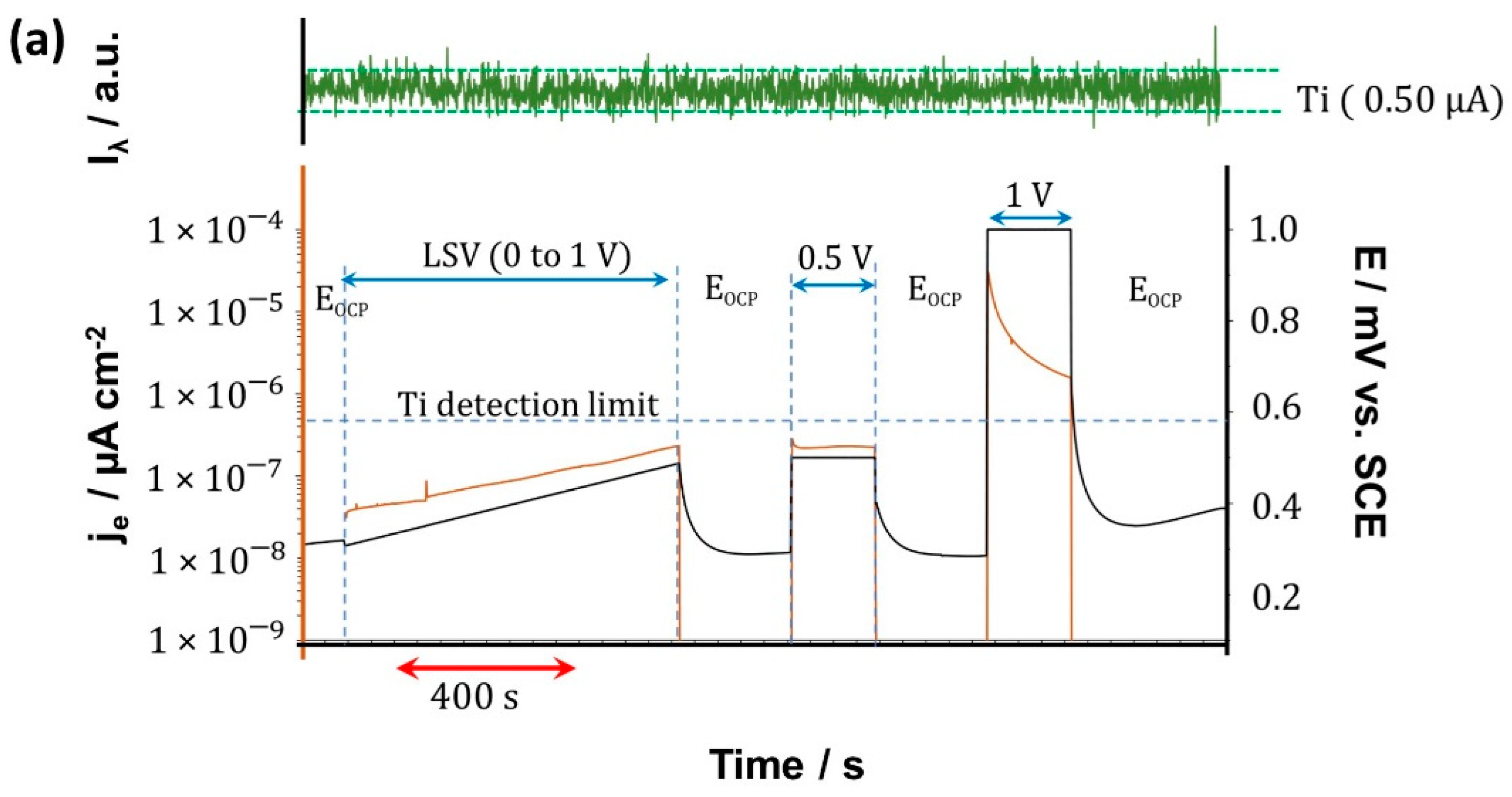
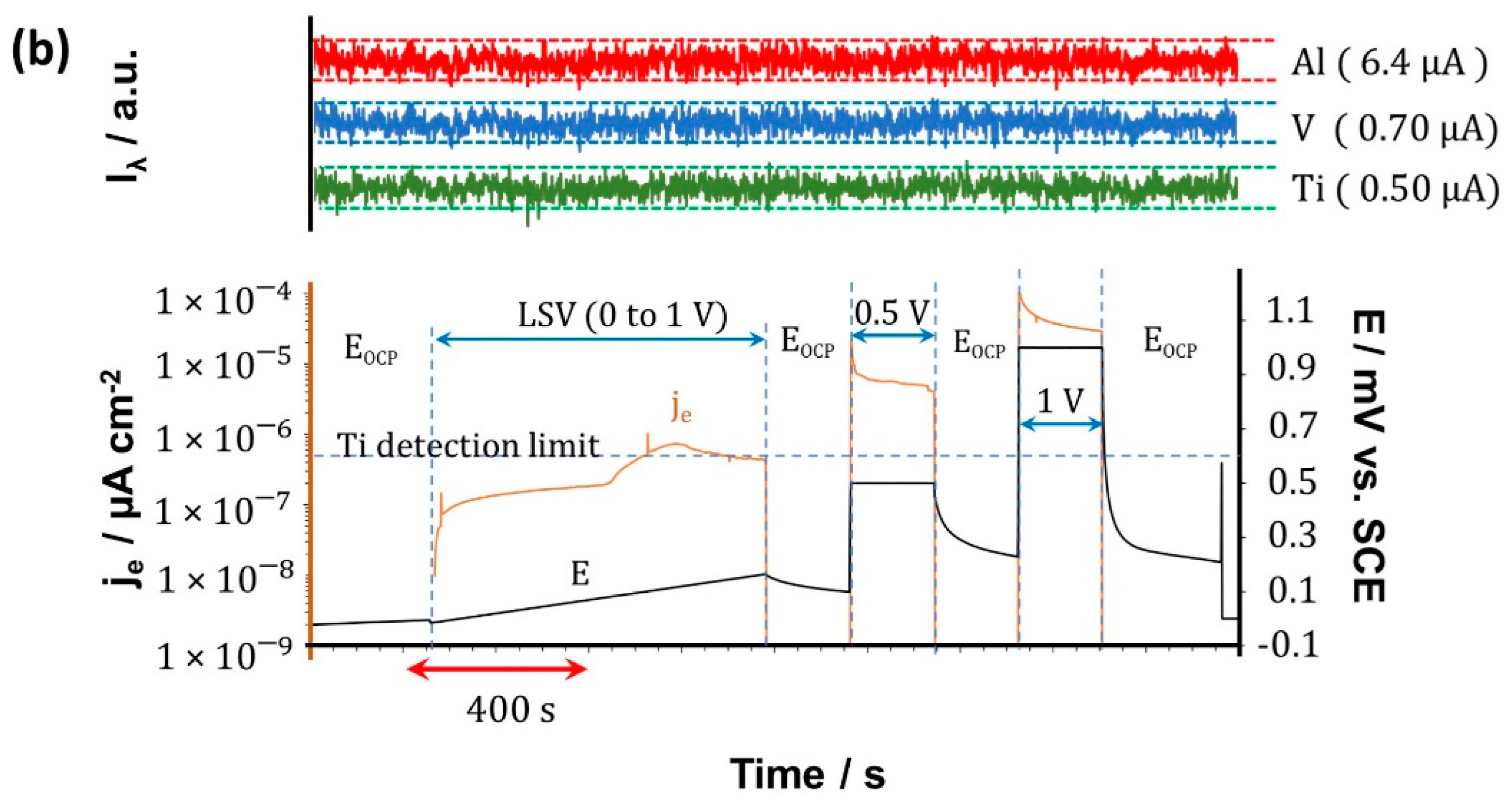
| ICP-AES | Accumulated Mass per Area/µg·cm−2 | |||
|---|---|---|---|---|
| CP-Ti | Ti-6Al-4V | |||
| Time of Exposure/days | Ti | Ti | Al | V |
| 7 | <DL | <DL | 5.37 | <DL |
| 14 | <DL | <DL | 6.96 | <DL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.; Arcos, C.; Montero, C.; Guerra, C.; Martínez, C.; Li, X.; Ringuedé, A.; Cassir, M.; Ogle, K.; Guzmán, D.; et al. A Tribological and Ion Released Research of Ti-Materials for Medical Devices. Materials 2022, 15, 131. https://doi.org/10.3390/ma15010131
Silva D, Arcos C, Montero C, Guerra C, Martínez C, Li X, Ringuedé A, Cassir M, Ogle K, Guzmán D, et al. A Tribological and Ion Released Research of Ti-Materials for Medical Devices. Materials. 2022; 15(1):131. https://doi.org/10.3390/ma15010131
Chicago/Turabian StyleSilva, Daniela, Camila Arcos, Cecilia Montero, Carolina Guerra, Carola Martínez, Xuejie Li, Armelle Ringuedé, Michel Cassir, Kevin Ogle, Danny Guzmán, and et al. 2022. "A Tribological and Ion Released Research of Ti-Materials for Medical Devices" Materials 15, no. 1: 131. https://doi.org/10.3390/ma15010131
APA StyleSilva, D., Arcos, C., Montero, C., Guerra, C., Martínez, C., Li, X., Ringuedé, A., Cassir, M., Ogle, K., Guzmán, D., Aguilar, C., Páez, M., & Sancy, M. (2022). A Tribological and Ion Released Research of Ti-Materials for Medical Devices. Materials, 15(1), 131. https://doi.org/10.3390/ma15010131










