Sustained Delivery of Lactoferrin Using Poloxamer Gels for Local Bone Regeneration in a Rat Calvarial Defect Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Preparation of the Poloxamer Gels
2.2. Sol-to-Gel Transition Temperature
Gelation Time
2.3. Mechanical Properties
2.4. Rheological Analysis
2.5. In Vitro Gel Degradation Studies
2.6. In Vitro Drug Release Studies
2.7. Osteoblast Viability
2.8. Immune Response with THP-1 Cells
2.9. Rat Calvarial Defect Model
2.10. MicroCT
2.11. Histology
2.12. Statistical Analysis
3. Results
3.1. In Vitro Characterisation of Poloxamer Gels
3.1.1. Sol-to-Gel Transition Temperature and Time
3.1.2. Mechanical Properties
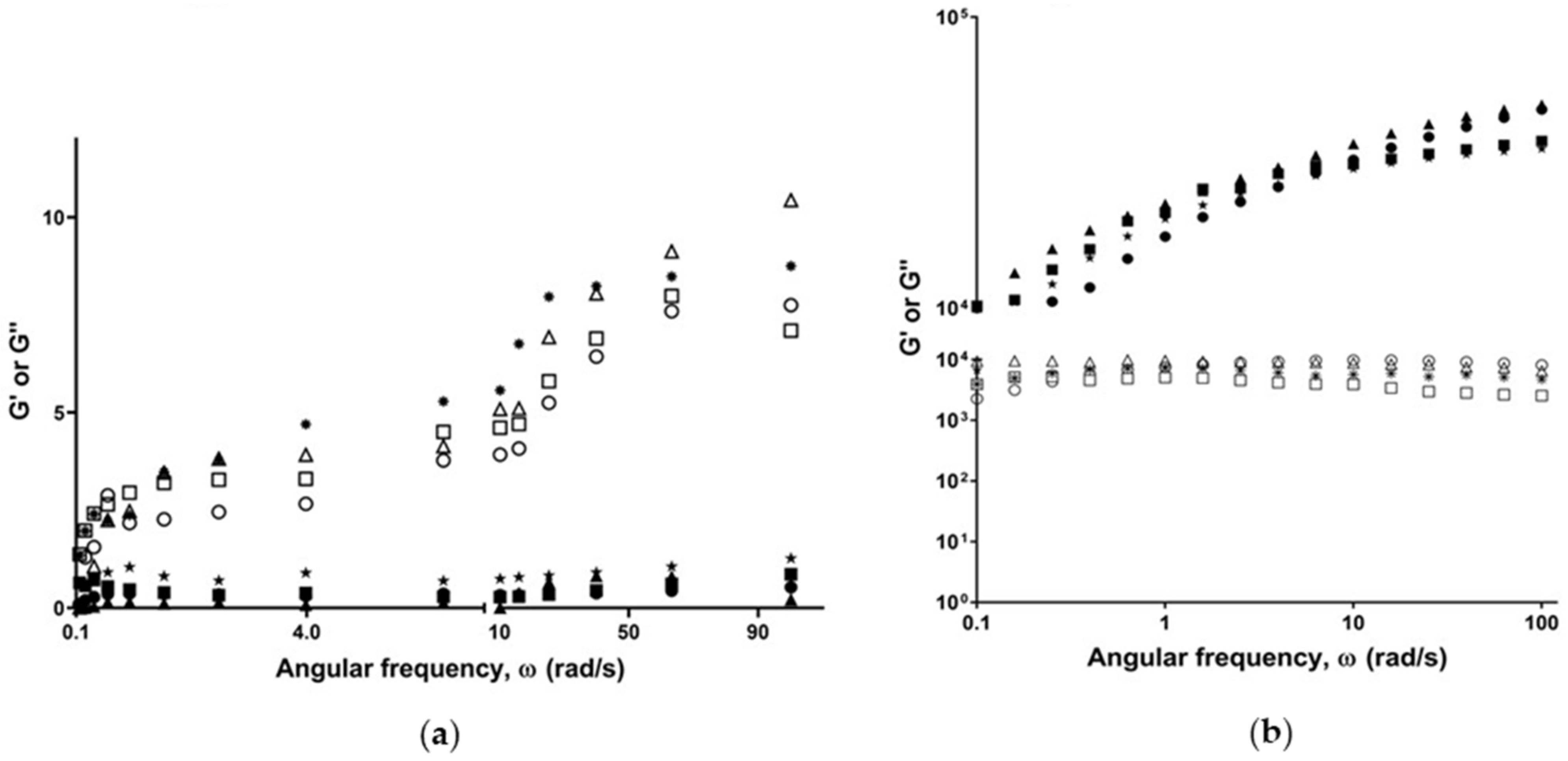
3.1.3. Rheological Properties
3.1.4. In Vitro Gel Degradation Profile
3.1.5. In Vitro Release Profile of LF from Poloxamers
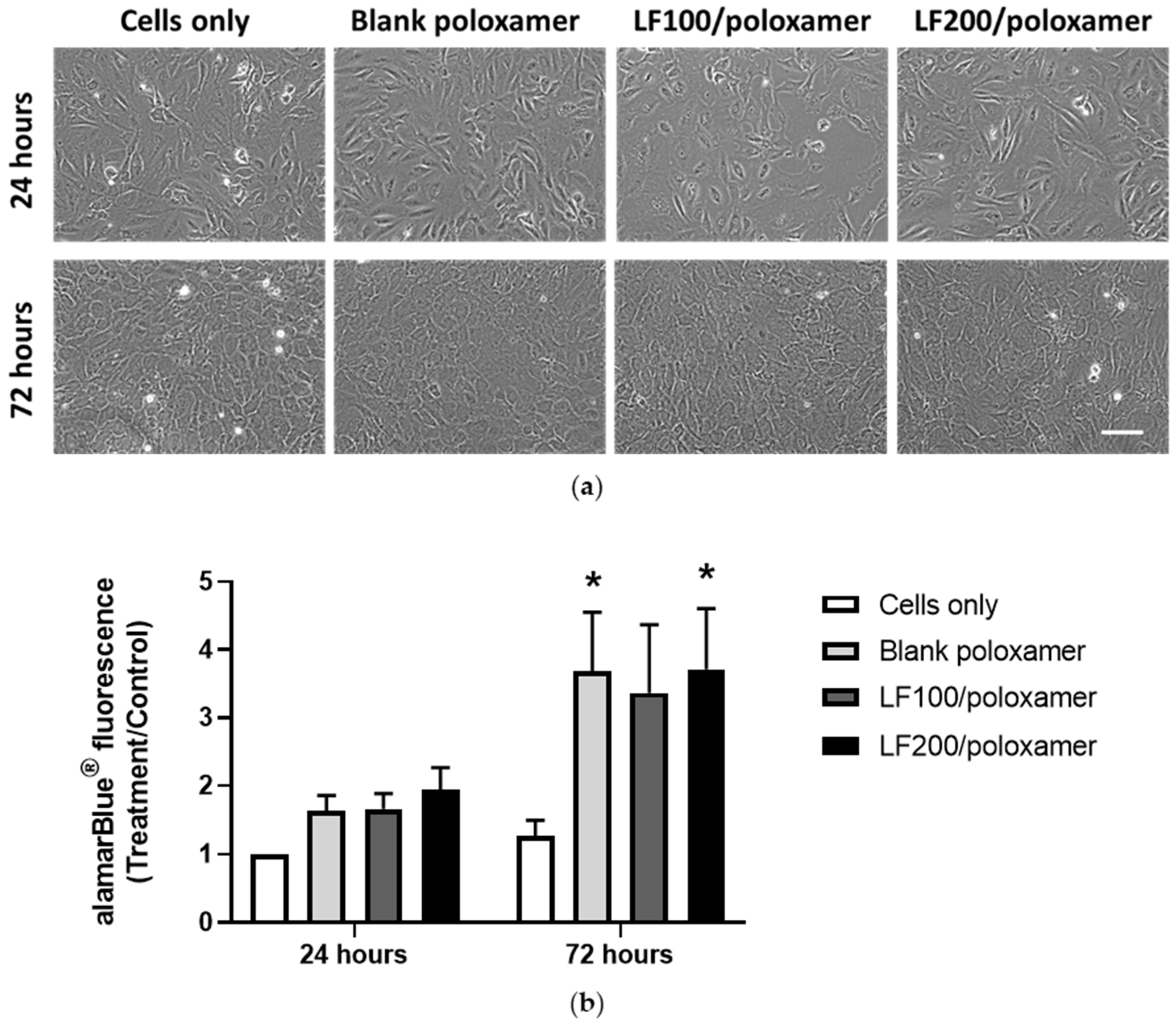
3.2. Effect of Poloxamers on Osteoblast Viability
3.3. Immune Response to Poloxamers
3.4. In Vivo Results
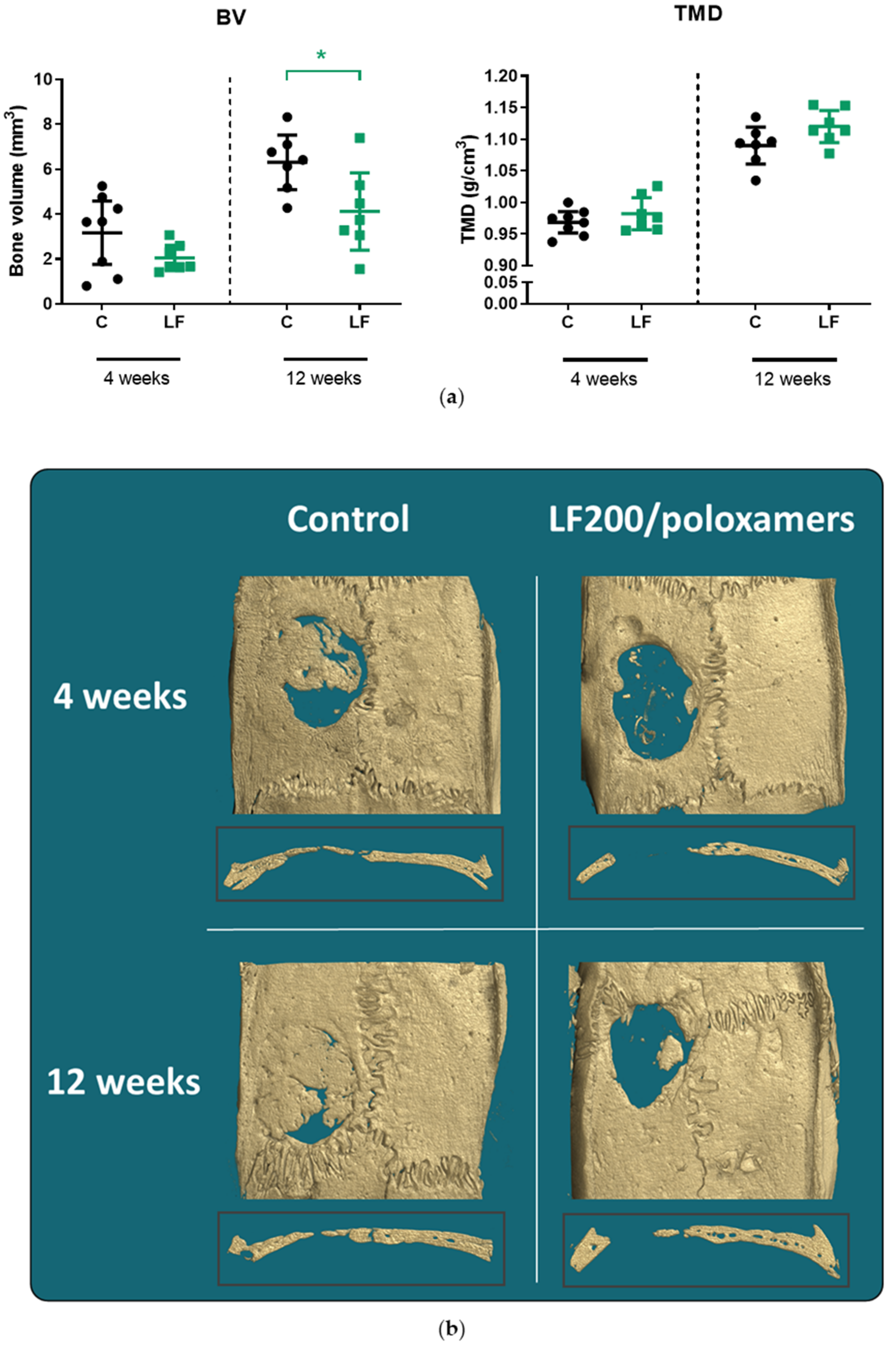
3.4.1. MicroCT
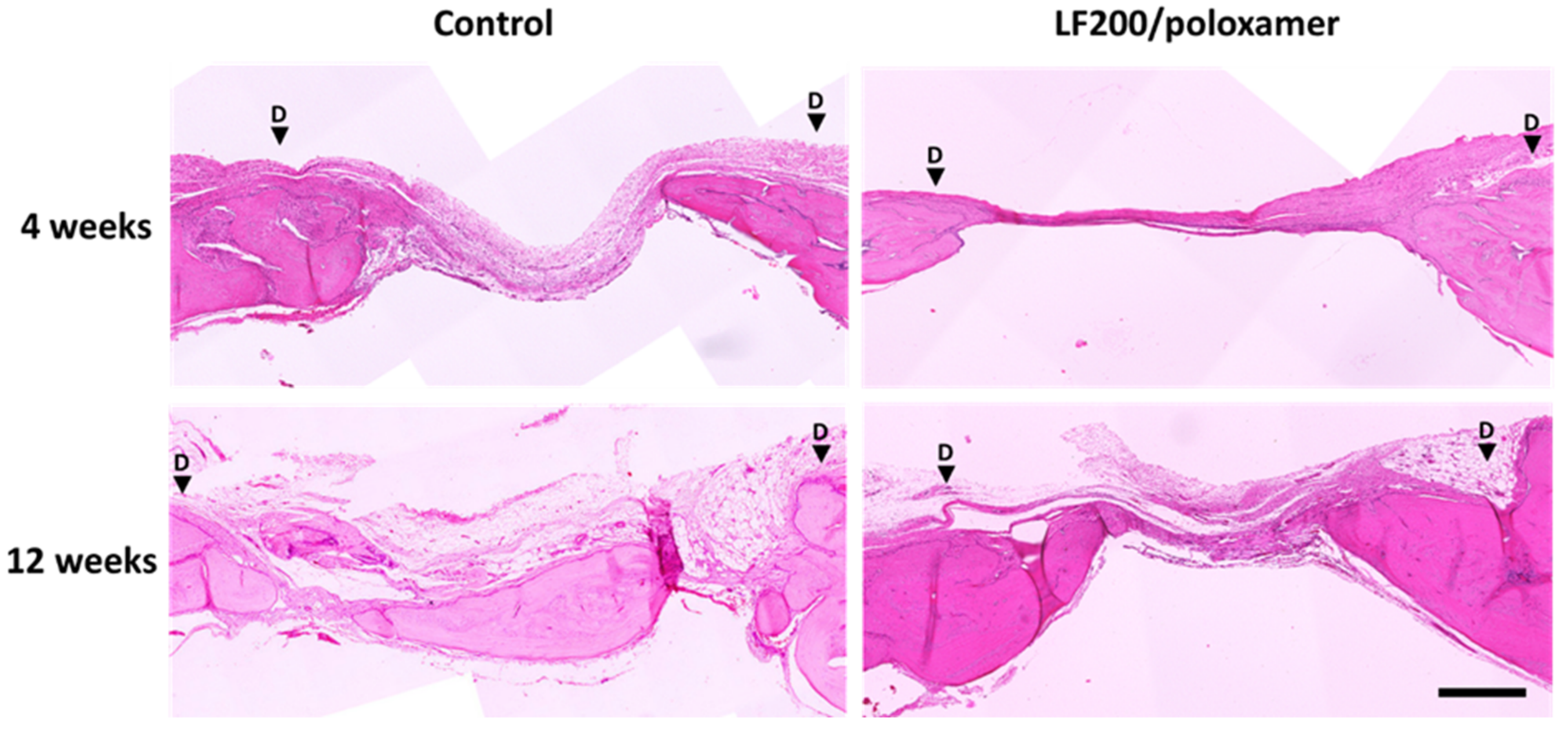
3.4.2. Histology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naot, D.; Grey, A.; Reid, I.R.; Cornish, J. Lactoferrin—A novel bone growth factor. Clin. Med. Res. 2005, 3, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.M.; Tong, P.C.; Chen, Q.; et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimaki, T.; Sato, S.; Tsunori, K.; Shino, H.; Iguchi, S.; Arai, Y.; Ito, K.; Ogiso, B. Bone regeneration with systemic administration of lactoferrin in non-critical-sized rat calvarial bone defects. J. Oral Sci. 2013, 55, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaoka, R.; Hikasa, Y.; Hayashi, K.; Tabata, Y. Bone regeneration by lactoferrin released from a gelatin hydrogel. J. Biomater. Sci. 2011, 22, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Gormez, U.; Kurkcu, M.; Benlidayi, M.E.; Ulubayram, K.; Sertdemir, Y.; Daglioglu, K. Effects of bovine lactoferrin in surgically created bone defects on bone regeneration around implants. J. Oral Sci. 2015, 57, 7–15. [Google Scholar] [CrossRef]
- Gao, R.; Watson, M.; Callon, K.E.; Tuari, D.; Dray, M.; Naot, D.; Amirapu, S.; Munro, J.T.; Cornish, J.; Musson, D.S. Local application of lactoferrin promotes bone regeneration in a rat critical-sized calvarial defect model as demonstrated by micro-CT and histological analysis. J. Tissue Eng. Regen. Med. 2018, 12, e620–e626. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 A resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef]
- Dorit Naot, K.P.; Cornish, J. Lactoferrin—A Potential Anabolic Intervention in Osteoporosis. In Osteoporosis; Dionyssiotis, Y., Ed.; IntechOpen: London, UK, 2012; pp. 803–820. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, E.K.; Holm, H.; Jensen, E.; Aaboe, R.; Devold, T.G.; Jacobsen, M.; Vegarud, G.E. Different digestion of caprine whey proteins by human and porcine gastrointestinal enzymes. Br. J. Nutr. 2010, 104, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Furlund, C.B.; Ulleberg, E.K.; Devold, T.G.; Flengsrud, R.; Jacobsen, M.; Sekse, C.; Holm, H.; Vegarud, G.E. Identification of lactoferrin peptides generated by digestion with human gastrointestinal enzymes. J. Dairy Sci. 2013, 96, 75–88. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef]
- Shiga, Y.; Oshima, Y.; Kojima, Y.; Sugimoto, A.; Tamaki, N.; Murata, D.; Takeuchi, T.; Sato, A. Recombinant human lactoferrin-Fc fusion with an improved plasma half-life. Eur. J. Pharm. Sci. 2015, 67, 136–143. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Boyd, B.J.; Hill, A.; Sharma, M. Injectable thermoresponsive gels offer sustained dual release of bupivacaine hydrochloride and ketorolac tromethamine for up to two weeks. Int. J. Pharm. 2021, 604, 120748. [Google Scholar] [CrossRef]
- Moloughney, J.G.; Weisleder, N. Poloxamer 188 (p188) as a membrane resealing reagent in biomedical applications. Recent Pat. Biotechnol. 2012, 6, 200–211. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2018, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Veyries, M.L.; Couarraze, G.; Geiger, S.; Agnely, F.; Massias, L.; Kunzli, B.; Faurisson, F.; Rouveix, B. Controlled release of vancomycin from poloxamer 407 gels. Int. J. Pharm. 1999, 192, 183–193. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ha, J.C.; Lee, Y.M. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide)/poly(epsilon-caprolactone) (PCL) amphiphilic block copolymeric nanospheres. II. Thermo-responsive drug release behaviors. J. Control. Release 2000, 65, 345–358. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef]
- Dhanikula, R.S.; Dhanikula, A.B.; Panchagnula, R. Thermoreversible liposomal poloxamer gel for the delivery of paclitaxel: Dose proportionality and hematological toxicity studies. Die Pharm. 2008, 63, 439–445. [Google Scholar]
- Akash, M.S.H.; Rehman, K.; Chen, S. Pluronic F127-Based Thermosensitive Gels for Delivery of Therapeutic Proteins and Peptides. Polym. Rev. 2014, 54, 573–597. [Google Scholar] [CrossRef]
- Svirskis, D.; Chandramouli, K.; Bhusal, P.; Wu, Z.; Alphonso, J.; Chow, J.; Patel, D.; Ramakrishna, R.; Yeo, S.J.; Stowers, R.; et al. Injectable thermosensitive gelling delivery system for the sustained release of lidocaine. Ther. Deliv. 2016, 7, 359–368. [Google Scholar] [CrossRef]
- Sharma, M.; Chandramouli, K.; Curley, L.; Pontre, B.; Reilly, K.; Munro, J.; Hill, A.; Young, S.; Svirskis, D. In vitro and ex vivo characterisation of an in situ gelling formulation for sustained lidocaine release with potential use following knee arthroplasty. Drug Deliv. Transl. Res. 2018, 8, 820–829. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Tavelin, S.; Gröbner, G. Effect of DMSO on micellization, gelation and drug release profile of Poloxamer 407. Int. J. Pharm. 2010, 394, 92–98. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/ poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules 2013, 18, 12415–12425. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kloxin, A.M.; Kiick, K.L. Dually degradable click hydrogels for controlled degradation and protein release. J. Mater. Chem. B 2014, 2, 5511–5521. [Google Scholar] [CrossRef]
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Ricci, E.J.; Bentley, M.V.L.B.; Farah, M.; Bretas, R.E.S.; Marchetti, J.M. Rheological characterization of Poloxamer 407 lidocaine hydrochloride gels. Eur. J. Pharm. Sci. 2002, 17, 161–167. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Lin, C.Q.-X.; Xiao, C.L.; Mulvey, T.B.; Cooper, G.J.S.; Reid, I.R. Trifluoroacetate, a contaminant in purified proteins, inhibits proliferation of osteoblasts and chondrocytes. Am. J. Physiol. -Endocrinol. Metab. 1999, 277, E779–E783. [Google Scholar] [CrossRef]
- Musson, D.S.; Gao, R.; Watson, M.; Lin, J.M.; Park, Y.E.; Tuari, D.; Callon, K.E.; Zhu, M.; Dalbeth, N.; Naot, D.; et al. Bovine bone particulates containing bone anabolic factors as a potential xenogenic bone graft substitute. J. Orthop. Surg. Res. 2019, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Wilson, A.N.; Blenner, M.; Guiseppi-Elie, A. Polyplex Formation Influences Release Mechanism of Mono- and Di-Valent Ions from Phosphorylcholine Group Bearing Hydrogels. Polymers 2014, 6, 2451–2472. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gilbert, J.R.; Zhang, X.; Zhao, B.; Ker, D.F.E.; Cooper, G.M. Calvarial Versus Long Bone: Implications for Tailoring Skeletal Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26, 46–63. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Parandeh, M.; Akbari, V.; Ebrahimi, Z.; Taheri, A. Incorporation of rosuvastatin-loaded chitosan/chondroitin sulfate nanoparticles into a thermosensitive hydrogel for bone tissue engineering: Preparation, characterization, and cellular behavior. Pharm. Dev. Technol. 2019, 24, 357–367. [Google Scholar] [CrossRef]
- Issa, J.P.M.; Nascimento, C.d.; Iyomasa, M.M.; Siéssere, S.; Regalo, S.C.H.; Defino, H.L.A.; Sebald, W. Bone healing process in critical-sized defects by rhBMP-2 using poloxamer gel and collagen sponge as carriers. Micron 2008, 39, 17–24. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.-W.; Kim, U.-K.; Oh, S.H.; June-Kim, S.; Park, B.-W.; Kim, J.-H.; Hah, Y.-S.; Kim, D.R.; Rho, G.-J.; et al. Generation of osteogenic construct using periosteal-derived osteoblasts and polydioxanone/pluronic F127 scaffold with periosteal-derived CD146 positive endothelial-like cells. J. Biomed. Mater. Res. Part A 2013, 101A, 942–953. [Google Scholar] [CrossRef]
- Lee, J.H.; Baek, H.R.; Lee, K.M.; Lee, H.K.; Im, S.B.; Kim, Y.S.; Lee, J.H.; Chang, B.S.; Lee, C.K. The effect of poloxamer 407-based hydrogel on the osteoinductivity of demineralized bone matrix. Clin. Orthop. Surg. 2014, 6, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Clokie, C.M.L.; Moghadam, H.; Jackson, M.T.; Sandor, G.K.B. Closure of Critical Sized Defects With Allogenic and Alloplastic Bone Substitutes. J. Craniofacial Surg. 2002, 13, 111–121. [Google Scholar] [CrossRef]
- Inal, O.; Yapar, E.A. Effect of mechanical properties on the release of meloxicam from poloxamer gel bases. Indian J. Pharm. Sci. 2013, 75, 700–706. [Google Scholar]
- Rangabhatla, A.S.L.; Tantishaiyakul, V.; Oungbho, K.; Boonrat, O. Fabrication of pluronic and methylcellulose for etidronate delivery and their application for osteogenesis. Int. J. Pharm. 2016, 499, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]


| Score |
Bone Defect Coverage % of Defect Filled (Volume) by New Bone within Defect Area |
New Bone Type Nature of New Bone within Defect |
Neo-Vascularisation Presence of Vascularisation within the Newly Formed Bone |
Inflammation Presence of Inflammatory Cells around the Newly Formed Bone |
|---|---|---|---|---|
| 0 | 0% | No new bone | No evidence of neovascularisation | Abundant inflammation and evidence of encapsulation |
| 1 | 1–24% | Predominantly woven | Few new vessels (<10) | Relatively few or normal amount of inflammatory cells present |
| 2 | 25–49% | 1:1 mix of woven and lamella | Abundant neovascularisation | - |
| 3 | 50–74% | Predominantly lamella remodelled | - | - |
| 4 | 75–100% | - | - | - |
| Formulation Code | LF Conc. (μg/mL) | Gelation Temperature (°C) | Gelation Time (sec) | Gel Strength (g·s) | Gel Hardness (g) |
|---|---|---|---|---|---|
| LF10/poloxamer | 10 | 28.5 ± 0.5 | 35 | 74.95 ± 6.87 | 34.46 ± 2.00 |
| LF50/poloxamer | 50 | 29.0 ± 0.5 | 45 | 52.61 ± 3.54 | 26.96 ± 1.72 |
| LF100/poloxamer | 100 | 29.0 ± 0.0 | 50 | 45.37 ± 2.39 | 21.10 ± 3.89 |
| LF200/poloxamer | 200 | 29.5 ± 0.5 | 55 | 34.41 ± 0.99 | 16.36 ± 0.50 |
| Formulation Code | r2 | n | K |
|---|---|---|---|
| LF100/poloxamer | 0.9732 | 0.2305 | 87.9631 |
| LF200/poloxamer | 0.9716 | 0.2604 | 72.4465 |
| Bone Defect Coverage | New Bone Type | Neo-Vascularisation | Inflammation | |||||
|---|---|---|---|---|---|---|---|---|
| 4 Weeks | 12 Weeks | 4 Weeks | 12 Weeks | 4 Weeks | 12 Weeks | 4 Weeks | 12 Weeks | |
| Control | 2.2 ± 0.40 | 3.15 ± 0.38 | 1.45 ± 0.20 | 2 ± 0.17 | 1.8 ± 0.11 | 1.65 ± 0.13 | 0.55 ± 0.16 | 0.45 ± 0.12 |
| LF200/poloxamer | 1.7 ± 0.28 | 1.95 * ± 0.32 | 1.25 ± 0.15 | 1.7 ± 0.20 | 1.7 ± 0.11 | 1.35 ± 0.15 | 0.65 ± 0.13 | 0.3 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.E.; Chandramouli, K.; Watson, M.; Zhu, M.; Callon, K.E.; Tuari, D.; Abdeltawab, H.; Svirskis, D.; Musson, D.S.; Sharma, M.; et al. Sustained Delivery of Lactoferrin Using Poloxamer Gels for Local Bone Regeneration in a Rat Calvarial Defect Model. Materials 2022, 15, 212. https://doi.org/10.3390/ma15010212
Park YE, Chandramouli K, Watson M, Zhu M, Callon KE, Tuari D, Abdeltawab H, Svirskis D, Musson DS, Sharma M, et al. Sustained Delivery of Lactoferrin Using Poloxamer Gels for Local Bone Regeneration in a Rat Calvarial Defect Model. Materials. 2022; 15(1):212. https://doi.org/10.3390/ma15010212
Chicago/Turabian StylePark, Young Eun, Kaushik Chandramouli, Maureen Watson, Mark Zhu, Karen E. Callon, Donna Tuari, Hani Abdeltawab, Darren Svirskis, David Shaun Musson, Manisha Sharma, and et al. 2022. "Sustained Delivery of Lactoferrin Using Poloxamer Gels for Local Bone Regeneration in a Rat Calvarial Defect Model" Materials 15, no. 1: 212. https://doi.org/10.3390/ma15010212
APA StylePark, Y. E., Chandramouli, K., Watson, M., Zhu, M., Callon, K. E., Tuari, D., Abdeltawab, H., Svirskis, D., Musson, D. S., Sharma, M., & Cornish, J. (2022). Sustained Delivery of Lactoferrin Using Poloxamer Gels for Local Bone Regeneration in a Rat Calvarial Defect Model. Materials, 15(1), 212. https://doi.org/10.3390/ma15010212






