New Solid Solution and Phase Equilibria in the Subsolidus Area of the Three-Component CuO–V2O5–Ta2O5 Oxide System
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Reactivity of CuO and V2O5 with Ta2O5 in the Air
3.2. Phase Equilibria in the Subsolidus Region of the CuO–Ta2O5–V2O5 System
- 1.
- V2O5–TaVO5–CuV2O6
- 2.
- CuV2O6–TaVO5–Cu2V2O7
- 3.
- Cu2V2O7–TaVO5–Ta9VO25(s.s)
- 4.
- Ta9VO25(s.s.)–CuTa2O6(s.s.)–Cu2V2O7
- 5.
- CuTa2O6(s.s.)–Ta9VO25(s.s.)
- 6.
- CuTa2O6(s.s.)–Ta9VO25–Ta2O5
- 7.
- CuTa2O6(s.s.)–Ta2O5
- 8.
- Cu2V2O7–CuTa2O6(s.s.)–Cu3V2O8
- 9.
- Cu3V2O8–CuTa2O6(s.s.)–Cu11V6O26
- 10.
- Cu11V6O26–CuTa2O6(s.s.)–Cu5V2O10
- 11.
- Cu5V2O10–CuTa2O6(s.s.)–CuO
- 12.
- CuO–CuTa2O6(s.s.)
4. Conclusions
- 1.
- In the three-component system of CuO–V2O5–Ta2O5 oxides, a substitution solid solution is formed with a limited range of homogeneity and the general formula CuTa2−xVxO6 for 0 < x ≤ 0.3.
- 2.
- The new solid solution is formed by the incorporation of V5+ ions in the CuTa2O6 crystal lattice in place of Ta5+ ions. The maximum degree of V5+ ion incorporation is 15 mol%.
- 3.
- CuTa2−xVxO6 for 0 < x ≤ 0.3 crystallizes in the tetragonal system, and with the increase of the degree of incorporation of V5+ ions in place of Ta5+ ions into the CuTa2O6 crystal lattice, the parameters a = b and c and the volume of unit cells decrease, and the crystal lattice contracts.
- 4.
- The CuTa2−xVxO6 solid solution is stable, depending on its composition, from a temperature of 1350 °C for x = 0.00 to 1270 °C for x = 0.30.
- 5.
- The IR spectra of solid solution CuTa2−xVxO6 (0 < x ≤ 0.30) and its matrix CuTa2O6 are very similar what corroborates their isostructural character. IR spectra of these phases contain bands in the boundaries of 950–850 cm−1, indicating that crystal lattices of these phases are built up of considerably distorted polyhedra. The incorporation of vanadium ions in the crystal lattice of CuTa2O6 mainly affects the position of the band with a maximum at 600 cm−1, which shifts gradually reaching 640 cm−1 in the spectrum of CuTa2−xVxO6 for x = 0.30.
- 6.
- The CuTa2−xVxO6 solid solution is a semiconductor, and the value of the energy gap for the solid solution ranges from 2.75 to 2.47 eV for 0.00 ≤ x ≤ 0.30.
- 7.
- The three-component system of metal oxides CuO–V2O5–Ta2O5 consists of 12 partial systems, i.e., I. V2O5–TaVO5–CuV2O6, II. CuV2O6–TaVO5–Cu2V2O7, III. Cu2V2O7–TaVO5–Ta9VO25(s.s), IV. Ta9VO25(s.s.)–CuTa2O6(s.s.)–Cu2V2O7, V. CuTa2O6(s.s.)–Ta9VO25(s.s.), VI. CuTa2O6(s.s.)–Ta9VO25–Ta2O5, VII. CuTa2O6(s.s.)–Ta2O5, VIII. Cu2V2O7–CuTa2O6(s.s.)–Cu3V2O8, IX. Cu3V2O8–CuTa2O6(s.s.)–Cu11V6O26, X. Cu11V6O26–CuTa2O6(s.s.)–Cu5V2O10, XI. Cu5V2O10–CuTa2O6(s.s.)–CuO, XII. CuO–CuTa2O6(s.s.).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, B.J.; Kouklin, N.; Lu, G.; Lin, I.K.; Chen, J.; Zhang, X. Transport, analyte detection, and opto-electronic response of p-type CuO nanowires. J. Phys. Chem C 2010, 114, 2440–2447. [Google Scholar] [CrossRef]
- Feng, L.; Yan, H.; Li, H.; Zhang, R.; Li, Z.; Chi, R.; Yang, S.; Ma, Y.; Fu, B.; Liu, J. Excellent field emission properties of vertically oriented CuO nanowire films. AIP Adv. 2018, 8, 045109. [Google Scholar] [CrossRef]
- Xin Ch Zhang, N.; Sun, K. Facile fabrication of CuO mesoporous nanosheet cluster array electrodes with super lithium-storage properties. J. Mater. Chem. 2012, 22, 13637–13642. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, G.; Rawat, M. A Brief Review on Synthesis and Characterization of Copper Oxide Nanoparticles and its Applications. J. Bioelectron. Nanotechnol. 2016, 1, 9. [Google Scholar] [CrossRef]
- Kim, G.T.; Muster, J.; Krstic, V. Field-effect transistor made of individual V2O5 nanofibers. Appl. Phys. Lett. 2000, 76, 1875–1877. [Google Scholar] [CrossRef]
- Schneider, K.; Lubecka, M.; Czapla, A. V2O5 thin films for gas sensor applications. Sens. Actuator B Chem. 2016, 236, 970–977. [Google Scholar] [CrossRef]
- Deepak, P.; Gupta, S.; Sridharan, M. Nanostructured V2O5 thin films deposited at low sputtering power. Mat. Sci. Semicon. Proc. 2015, 36, 426–432. [Google Scholar] [CrossRef]
- Vo, P.N.X.; Le-Phuc, N.; Tran, T.V.; Ngo, P.T.; Luong, T.N. Oxidative regeneration study of spent V2O5 catalyst from sulfuric acid manufacture. Reac. Kinet. Mech. Cat. 2018, 125, 887–900. [Google Scholar] [CrossRef]
- Rogers, O.; Pattisson, S.; Engel, R.V.; Jenkins, R.L.; Whiston, K.; Taylor, S.H.; Hutchings, G.J. Adipic acid formation from cyclohexanediol using vanadium catalysts: Elucidating the role of homogeneous species. Catal. Sci. Technol. 2020, 10, 4210–4218. [Google Scholar] [CrossRef]
- Gimeno, M.P.; Gascón, J.; Téllez, C.; Herguido, J.; Menéndez, M. Selective oxidation of o-xylene to phthalic anhydride over V2O5/TiO2: Kinetic study in a fluidized bed reactor. Chem. Eng. Process. 2008, 9, 1844–1852. [Google Scholar] [CrossRef]
- De Sousa, B.P.; Marcondes, L.M.; Maestri, S.A.; da Cunha, C.R.; Cassanjes, F.C.; Poirier, G.Y. Phosphate glasses with high tantalum oxide contents: Thermal, structural and optical properties. Mat. Chem. Phys. 2020, 239, 12199. [Google Scholar] [CrossRef]
- Kosiel, K.; Pągowska, K.; Kozubal, M.; Guziewicz, M.; Lawniczak-Jablonska, K.; Jakieła, R.; Syryanyy, Y.; Gabler, T.; Smietana, M. Compositional, structural, and optical properties of atomic layer deposited tantalum oxide for optical fiber sensor overlays. J. Vac. Sci. Technol. 2018, 36, 031505. [Google Scholar] [CrossRef]
- Calvo, C.; Manolescu, D. Refinement of the structure of CuV2O6. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 1743–1745. [Google Scholar] [CrossRef]
- Lavaud, D.; Galy, J. Structure crystalline de CuV2O6. Bull. Soc. Fr. Mineral. Cristallogr. 1972, 95, 134–135. [Google Scholar]
- Dąbrowska, G.; Filipek, E. Reactivity of the oxides in the ternary V2O5–CuO–α-Sb2O4 system in air. J. Therm. Anal. Cal. 2008, 93, 839–845. [Google Scholar] [CrossRef]
- Prokofiev, A.V.; Kremer, R.K.; Assmus, W. Crystal growth and magnetic properties of α-CuV2O6. J. Cryst. Growth 2001, 231, 498–505. [Google Scholar] [CrossRef]
- Calvo, C.; Faggiani, R. α Cupric Divanadate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 603–605. [Google Scholar] [CrossRef]
- Mercurio, D.; Frit, M. Structure crystalline de la variete haute temperature dy pyrovanadate de cuivre: Cu2V2O7 β. CR Acad. Sci. Paris 1973, 277, 1001–1104. [Google Scholar]
- Krivovichev, S.V.; Filatov, S.K.; Cherepanksy, P.N.; Armbruster, T.; Pankratova, O.Y. Crystal structure of γ-Cu2V2O7 and its comparison to blossite (α-Cu2V2O7) and ziesite (β-Cu2V2O7). Can. Mineral. 2005, 43, 671–677. [Google Scholar] [CrossRef]
- Fleury, P. Etudes sur les systems V2O5–CuO ou Ag2O ou Tl2O3 et sur les combinaisons interoxydes correspondantes. Rev. Chim. Min. 1969, 6, 819–830. [Google Scholar]
- Clark, G.M.; Garlick, R. Formation and properties of copper(II) divanadate(V). J. Inorg. Nucl. Chem. 1978, 40, 1347–1349. [Google Scholar] [CrossRef]
- Coing-Boyat, J. Structure de la variete ordinaire, Triclinique, de l`orthovanadate de cuivre(II), Cu3(VO4)2. Acta Cryst. B 1982, 38, 1546–1548. [Google Scholar] [CrossRef]
- Shannon, R.; Calvo, C. Crystal structure of a new form of Cu3V2O8. Can. J. Chem. 1972, 50, 3944–3949. [Google Scholar] [CrossRef]
- Birnie, R.; Stoiberite, H.M. Cu5V2O10, a new copper vanadate from Izalco Volcano El Salvador. Am. Mineral. 1979, 64, 941–944. [Google Scholar]
- Finger, L. Fingerite, Cu11O2(VO4)6, a new vanadium sublimate from Izalco, El Salvador: Crystal structure. Am. Mineral. 1985, 70, 197–199. [Google Scholar]
- Brisi, C.; Moliniari, M. Il sistema ossido ramico-anidrive vanadica. Ann. Chim. Rome 1958, 48, 263–269. [Google Scholar]
- Cao, X.; Xie, J.; Zhan, H.; Zhou, Y. Synthesis of CuV2O6 as a cathode material for rechargeable lithium batteries from V2O5 gel. Mat. Chem. Phys. 2006, 98, 71–75. [Google Scholar] [CrossRef]
- Kawada, T.; Hinokuma, S.; Machida, M. Structure and SO3 decomposition acitivity of nCuO–V2O5/SiO2 (n = 0, 1, 2, 3 and 5) catalyst for solar thermochemical water splitting cycles. Catal. Today 2015, 242, 268–273. [Google Scholar] [CrossRef]
- Kim, M.; Joshi, B.; Ohm, T.; Kim, K.; Al-Deyab, S.; Yoon, S. Electrosprayed copper hexaoxidivanadate (CuV2O6) and pyrovanadate (Cu2V2O7) photoanodes for efficient solar water splitting. J. Alloys Compd. 2017, 708, 444–450. [Google Scholar] [CrossRef]
- Schadow, H.; Oppermann, H.; Wehner, B. Investigations on the Quasi-binary System V2O5-Ta2O5. Crys. Res. Technol. 1992, 27, 691–695. [Google Scholar] [CrossRef]
- Zuev, M.G. Phase Formation in the V2O5–Ta2O5–MoO3 System. Rus. J. Inorg. Chem. 2010, 55, 93–95. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Mukaida, Y.; Shigeta, H. Preparation of Alkoxy-Derived Tantalum Vanadate. J. Am. Ceram. Soc. 1989, 72, 1914–1917. [Google Scholar] [CrossRef]
- Trunov, V.K.; Kovba, L.M.; Sirotkina, E.I. X-ray Study of the Double Oxides of Some Transition Metals. Dokl. Akad. Nauk SSSR 1963, 153, 1083–1088. Available online: http://mi.mathnet.ru/eng/dan/v153/i5/p1085 (accessed on 24 November 2021).
- Staus, P.; Dąbrowska, G. Synthesis and properties of compounds from the photocatalytic system of Ta2O5–V2O5 oxides. In Advances in Technology and Chemical Engineering; West Pomeranian University of Technology, Publishing House: Szczecin, Poland, 2021; pp. 250–260. ISBN 978-83-7663-326-8. [Google Scholar]
- Casais, M.T.; Gutierrez-Puebla, E.; Monge, M.A.; Rasines, I.; Ruiz-Valero, C. VM9O25 (M = Nb, Ta), a combination of tetrahedral VO4 and octahedral MO6 Units. J. Solid State Chem. 1993, 102, 261–266. [Google Scholar] [CrossRef]
- Salke, N.P.; Rao, R.; Achary, S.N.; Nayak Ch Garg, A.B.; Krishna, P.S.R.; Shinde, A.B.; Jha, S.N.; Bhattacharyya, D.; Jagannath Tyagi, A.K. Negative thermal expansion: Mechanisms and materials. Inorg. Chem. 2018, 57, 6973–6980. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Q.; Deng, J.; Yu, R.; Chen, J.; Xing, X. Phase Transformation and Negative Thermal Expansion in TaVO5. Inorg. Chem. 2011, 50, 2685–2690. [Google Scholar] [CrossRef]
- Golubev, A.; Dinnebier, R.E.; Schulz, A.; Kremer, R.K.; Langbein, H.; Senyshyn, A.; Law, J.M.; Hansen, T.C.; Koo, H.J.; Whangbo, M.H. Structural and magnetic properties of the trirutile-type 1D-Heisenberg anti-ferromagnet CuTa2O6. Inorg. Chem. 2017, 56, 6318–6329. [Google Scholar] [CrossRef] [PubMed]
- Krabbes, I.; Langbein, H. Herstellung von CuTa2O6 von der Trirutil- zur Perowskit-Struktur. Z. Naturforsch. B 1996, 51, 1605–1610. [Google Scholar] [CrossRef]
- Longo, J.M.; Sleight, A.W. CuTa2O6–crystal growth and characterization. Mater. Res. Bull. 1975, 10, 1273–1277. [Google Scholar] [CrossRef]
- Vincent, H.; Bochu, B.; Aubert, J.J.; Joubert, J.C.; Marezio, M. Structure cristalline de CuTa2O6. J. Solid State Chem. 1978, 24, 245–253. [Google Scholar] [CrossRef]
- Miura, K.; Yokota, Y. Preparation of CuO–Ta2O5 composites using a simple Co-sputtering method. J. Chem. Eng. Mater. Sci. 2015, 3, 47–51. [Google Scholar] [CrossRef][Green Version]
- Weng, C.-M.; Tsai, C.-C.; Hong, C.-S.; Lin, C.-C.; Chen, C.-C.; Chu, S.-Y.; Sheen, J.; Chen, Z.-Y.; Su, H.-H. of Non-Stoichiometry on the Microstructure, Oxygen Vacancies, and Electrical Properties of KNN-Based Thin Films. J. Solid State Sci. Technol. 2016, 5, 49. [Google Scholar] [CrossRef]
- Yang, S.; Chen, S.; Tsai, C.; Hong, C. Fabrication of high-power piezoelectric transformers using lead-free ceramics for application in electronic ballasts. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Azmirad, R.; Safa, S.; Akhavan, O. Hydrothermally Synthesized CuO Powders for Photocatalytic Inactivation of Bacteria. Acta Phys. Pol. A 2015, 127, 1727–1731. [Google Scholar] [CrossRef]
- Hristea, A.; Popovici, E.J.; Muresana, L.; Stefana, M.; Grecua, R.; Johanssonc, A.; Boman, M. Morpho-structural and luminescent investigations of niobium activated yttrium tantalate powders. J. Alloys Compd. 2009, 471, 524–529. [Google Scholar] [CrossRef]
- Tabero, P. Formation and properties of the new Al8V10W16O85 and Fe8-xAlxV10W16O85 phases with the M-Nb2O5 structure. J. Therm. Anal. Calorim. 2010, 101, 560–566. [Google Scholar] [CrossRef]
- Tabero, P.; Frackowiak, A.; Filipek, E.; Dąbrowska, G.; Homonnay, Z.; Szilágyi, P.Á. Synthesis, thermal stability and unknown properties of Fe1-xAlxVO4 solid solution. Ceram. Int. 2018, 44, 17759–17766. [Google Scholar] [CrossRef]
- Vaz, T.; Salker, A.V. Comparatively Low Temperature Synthesis, Characterization and Some Physical Studies on Transition Metal Vanadates. J. Sci. Res. 2021, 13, 571–578. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, X.; Zhu, Y. Synthesis of hexagonal BaTa2O6 nanorods and influence of defects on the photocatalytic activity. J. Phys. Chem. B 2007, 110, 5825–5832. [Google Scholar] [CrossRef]
- Mukherjee, R.; Duttay, A.; Sinhaz, T.P. Octahedral distortion-driven electrical and vibrational properties of A2ErTaO6 (A ¼ Sr and Ca) double perovskite oxides. J. Adv. Dielectr. 2018, 8, 1850025. [Google Scholar] [CrossRef]
- Husson, E.; Repelin, Y.; Brusset, H.; Cerez, A. Spectres de vibration et calcul du champ de force des antimoniates et des tantalates de structure trirutile. Spectrochim. Acta A 1979, 35, 1177–1187. [Google Scholar] [CrossRef]
- Dąbrowska, G.; Kurzawa, M.; Tabero, P. Phase Relations in the Al2O3–V2O5–MoO3 system in the solid state. The crystal structure of AlVO4. J. Phase Equilibria Diffus. 2009, 30, 220–229. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]

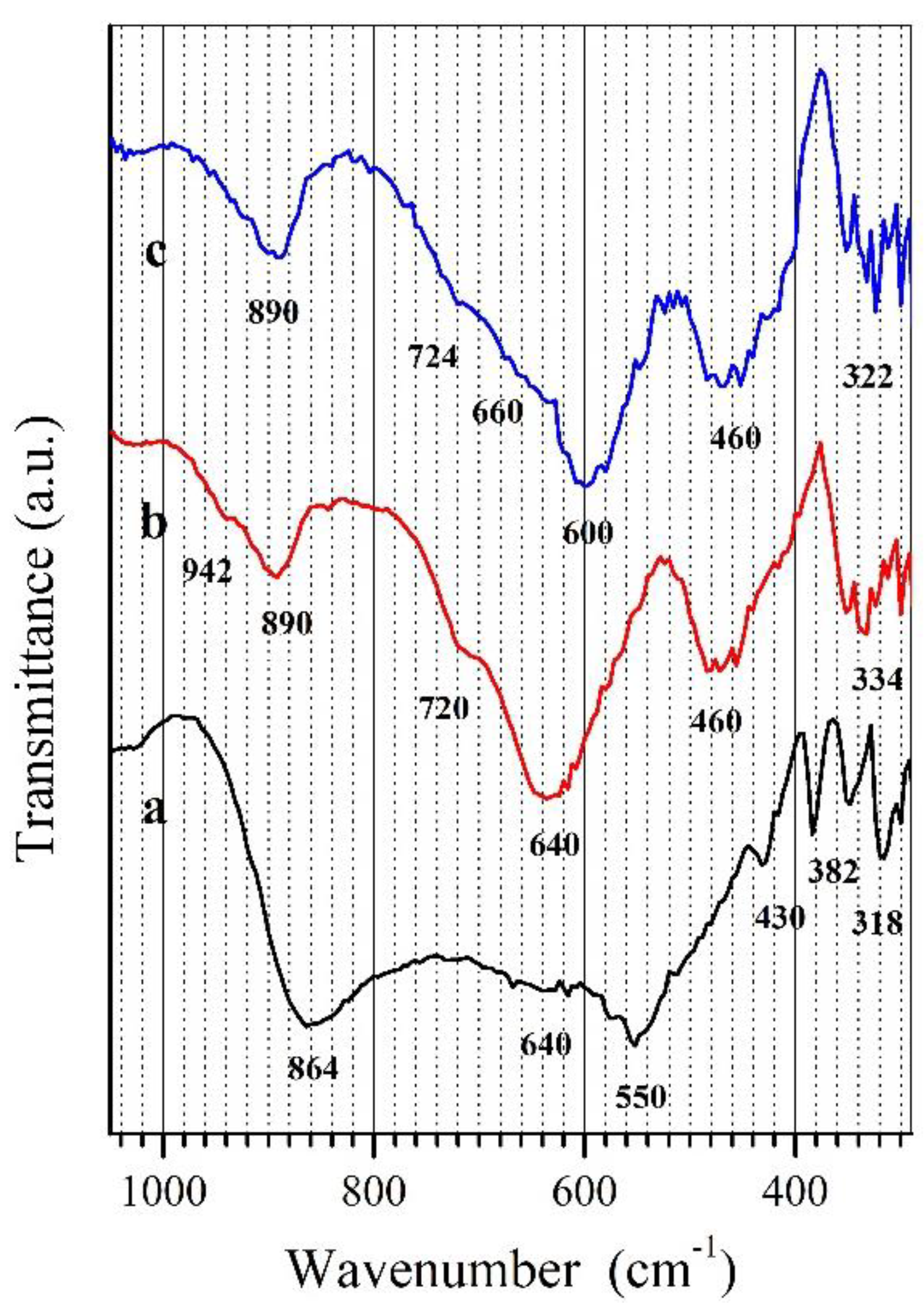


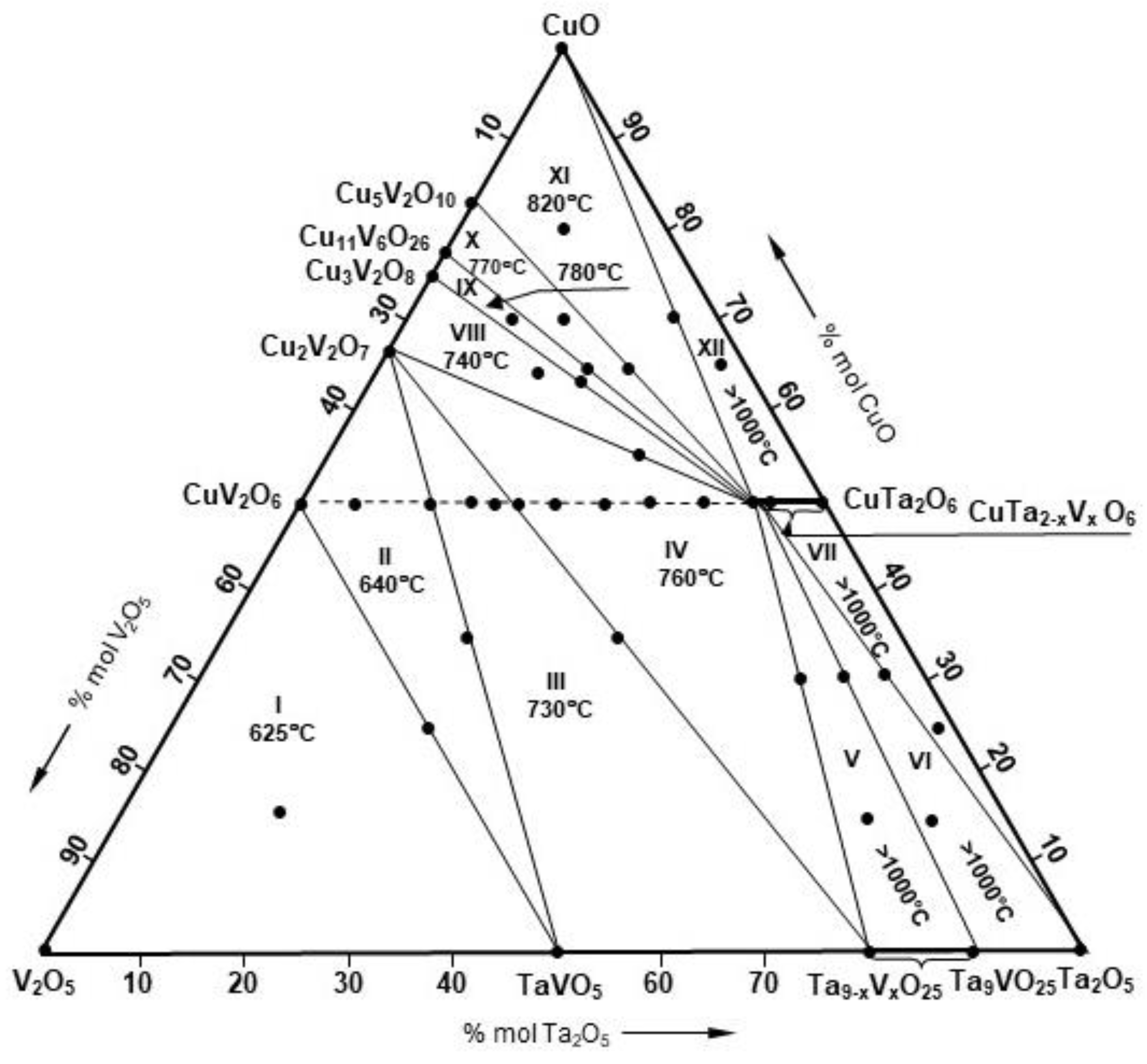

| No. | Composition of Initial Mixtures [Mol%] | Composition of Initial Mixtures Calculated as Oxides [Mol%] | Final Heating Temperature [°C] | Composition of Samples at Equilibrium | |||
|---|---|---|---|---|---|---|---|
| CuV2O6 | CuTa2O6 | CuO | V2O5 | Ta2O5 | |||
| 1 | 90.00 | 10.00 | 50.00 | 45.00 | 5.00 | 620 | CuV2O6, TaVO5, Cu2V2O7 |
| 2 | 75.00 | 25.00 | 50.00 | 37.50 | 12.50 | Cu2V2O7, TaVO5 | |
| 3 | 66.67 | 33.33 | 50.00 | 33.33 | 16.67 | 720 | Ta9VO25(s.s.), Cu2V2O7, TaVO5 |
| 4 | 63.64 | 36.36 | 50.00 | 31.82 | 18.18 | ||
| 5 | 60.00 | 40.00 | 50.00 | 30.00 | 20.00 | 700 | CuTa2O6(s.s.), Ta9VO25(s.s.), Cu2V2O7 |
| 6 | 50.00 | 50.00 | 50.00 | 25.00 | 25.00 | ||
| 7 | 40.00 | 60.00 | 50.00 | 20.00 | 30.00 | 725 | |
| 8 | 33.33 | 66.67 | 50.00 | 16.67 | 33.33 | ||
| 9 | 20.00 | 80.00 | 50.00 | 10.00 | 40.00 | ||
| 10 | 10.00 | 90.00 | 50.00 | 5.00 | 45.00 | 900 | CuTa2O6(s.s.) |
| No. | Composition of Initial Mixtures [Mol%] | Composition of Initial Mixtures Calculated as Oxides [Mol%] | Parameter X in the Obtained Solid Solution with Formula CuTa2−xVxO6(s.s) | Composition of Samples at Equilibrium | |||
|---|---|---|---|---|---|---|---|
| CuV2O6 | CuTa2O6 | CuO | V2O5 | Ta2O5 | |||
| 1. | 10.00 | 90.00 | 50.00 | 5.00 | 45.00 | 0.20 | CuTa1.80V0.20O6 |
| 2. | 12.50 | 87.50 | 50.00 | 6.25 | 43.75 | 0.25 | CuTa1.75V0.25O6 |
| 3. | 15.00 | 85.00 | 50.00 | 7.50 | 42.50 | 0.30 | CuTa1.70V0.30O6 |
| 4. | 17.50 | 82.50 | 50.00 | 8.75 | 41.25 | 0.30 (theoretical 0.35) | CuTa1.70V0.30O6, Cu2V2O7, Ta9VO25 |
| x | a = b [nm] | c [nm] | V [nm3] | dcal. [g/cm3] | dexp. [g/cm3] |
|---|---|---|---|---|---|
| 0.00 | 7.5221 | 3.7582 | 212.64643 | 8.14355 | 7.9775 |
| 0.20 | 7.5188 | 3.7572 | 212.40336 | 7.74633 | 7.9368 |
| 0.25 | 7.5172 | 3.7568 | 212.29037 | 7.64876 | 7.8163 |
| 0.30 | 7.5150 | 3.7560 | 212.12095 | 7.5531 | 7.5088 |
| No. | Composition of the Initial Mixtures [Mol%] | Final Stage of Heating [°C] | Phase Composition of the Samples in Equilibrium State | ||
|---|---|---|---|---|---|
| CuO | V2O5 | Ta2O5 | |||
| 11. | 15.00 | 70.00 | 15.00 | 620 | CuV2O6, TaVO5, V2O5 |
| 12. | 25.00 | 50.00 | 25.00 | 625 | CuV2O6, TaVO5 |
| 13 | 35.00 | 41.00 | 24.00 | 725 | Cu2V2O7, TaVO5 |
| 14. | 35.00 | 23.00 | 42.00 | Cu2V2O7, Ta9VO25(s.s.) | |
| 15. | 30.00 | 14.00 | 56.00 | 900 | CuTa2O6(s.s.), Ta9VO25(s.s.) |
| 16. | 15.00 | 13.00 | 72.00 | 900 | CuTa2O6(s.s.), Ta9VO25(s.s.) |
| 17. | 30.00 | 9.00 | 61.00 | 900 | CuTa2O6(s.s.), Ta9VO25 |
| 18. | 15.00 | 7.00 | 78.00 | 900 | Ta9VO25, CuTa2O6(s.s.), Ta2O5 |
| 19. | 30.00 | 4.00 | 66.00 | 900 | CuTa2O6(s.s.), Ta2O5 |
| 20. | 25.00 | 2.00 | 73.00 | 900 | CuTa2O6(s.s.), Ta2O5 |
| 21. | 54.00 | 11.00 | 35.00 | 725 | Cu2V2O7, CuTa2O6(s.s.) |
| 22. | 65.00 | 25.00 | 10.00 | 725 | Cu2V2O7, CuTa2O6(s.s.), Cu3V2O8 |
| 23. | 64.00 | 16.00 | 20.00 | 725 | Cu3V2O8, CuTa2O6(s.s.) |
| 24. | 70.00 | 18.00 | 12.00 | 750 | Cu3V2O8, CuTa2O6(s.s.), Cu11V6O26, |
| 25. | 65.00 | 15.00 | 20.00 | 750 | Cu11V6O26, CuTa2O6(s.s.) |
| 26. | 70.00 | 13.00 | 17.00 | 750 | Cu11V6O26, CuTa2O6(s.s.), Cu5V2O10 |
| 27 | 65.00 | 12.00 | 23.00 | 750 | Cu5V2O10, CuTa2O6(s.s.) |
| 28 | 80.00 | 10.00 | 10.00 | 750 | CuO, Cu5V2O10, CuTa2O6(s.s.) |
| 29 | 70.00 | 5.00 | 25.00 | 900 | CuO, CuTa2O6(s.s.) |
| 30 | 65.00 | 3.00 | 32.00 | 900 | CuO, CuTa2O6(s.s.) |
| No. | Phases at Equilibrium | Melting Point [°C] |
|---|---|---|
| 1. | V2O5–TaVO5–CuV2O6 | 625 |
| 2. | CuV2O6, TaVO5 | 650 |
| 3. | CuV2O6–TaVO5–Cu2V2O7 | 640 |
| 4. | Cu2V2O7, TaVO5 | 730 |
| 5. | Cu2V2O7–TaVO5–Ta9VO25(s.s.) | 730 |
| 6. | Cu2V2O7–Ta9VO25(s.s.) | 760 |
| 7. | Ta9VO25(s.s.)–CuTa2O6(s.s.)–Cu2V2O7 | 760 |
| 8. | CuTa2O6(s.s.)–Ta9VO25(s.s.) | >1000 |
| 9. | Cu2V2O7–CuTa2O6(s.s.) | 760 |
| 10. | Cu2V2O7–CuTa2O6(s.s.)–Cu3V2O8 | 740 |
| 11. | Cu3V2O8–CuTa2O6(s.s.) | 750 |
| 12. | Cu3V2O8–CuTa2O6(s.s.)–Cu11V6O26 | 780 |
| 13. | Cu11V6O26–CuTa2O6(s.s.) | 780 |
| 14. | Cu11V6O26–CuTa2O6(s.s.)–Cu5V2O10 | 770 |
| 15. | Cu5V2O10–CuTa2O6(s.s.) | 810 |
| 16. | Cu5V2O10–CuTa2O6(s.s.)–CuO | 820 |
| 17 | Ta9VO25(s.s.)–CuTa2O6(s.s.)–Ta2O5 | >1000 |
| 18. | CuO–CuTa2O6(s.s.) | >1000 |
| 19. | CuTa2O6(s.s.)–Ta2O5 | >1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, G.; Filipek, E.; Tabero, P. New Solid Solution and Phase Equilibria in the Subsolidus Area of the Three-Component CuO–V2O5–Ta2O5 Oxide System. Materials 2022, 15, 232. https://doi.org/10.3390/ma15010232
Dąbrowska G, Filipek E, Tabero P. New Solid Solution and Phase Equilibria in the Subsolidus Area of the Three-Component CuO–V2O5–Ta2O5 Oxide System. Materials. 2022; 15(1):232. https://doi.org/10.3390/ma15010232
Chicago/Turabian StyleDąbrowska, Grażyna, Elżbieta Filipek, and Piotr Tabero. 2022. "New Solid Solution and Phase Equilibria in the Subsolidus Area of the Three-Component CuO–V2O5–Ta2O5 Oxide System" Materials 15, no. 1: 232. https://doi.org/10.3390/ma15010232
APA StyleDąbrowska, G., Filipek, E., & Tabero, P. (2022). New Solid Solution and Phase Equilibria in the Subsolidus Area of the Three-Component CuO–V2O5–Ta2O5 Oxide System. Materials, 15(1), 232. https://doi.org/10.3390/ma15010232






