Amine Plasma-Polymerization of 3D Polycaprolactone/β-Tricalcium Phosphate Scaffold to Improving Osteogenic Differentiation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of 3D PCL and PCL/β-TCP Scaffolds and PCL Films
2.3. Amine Plasma-Polymerization on the 3D Scaffolds
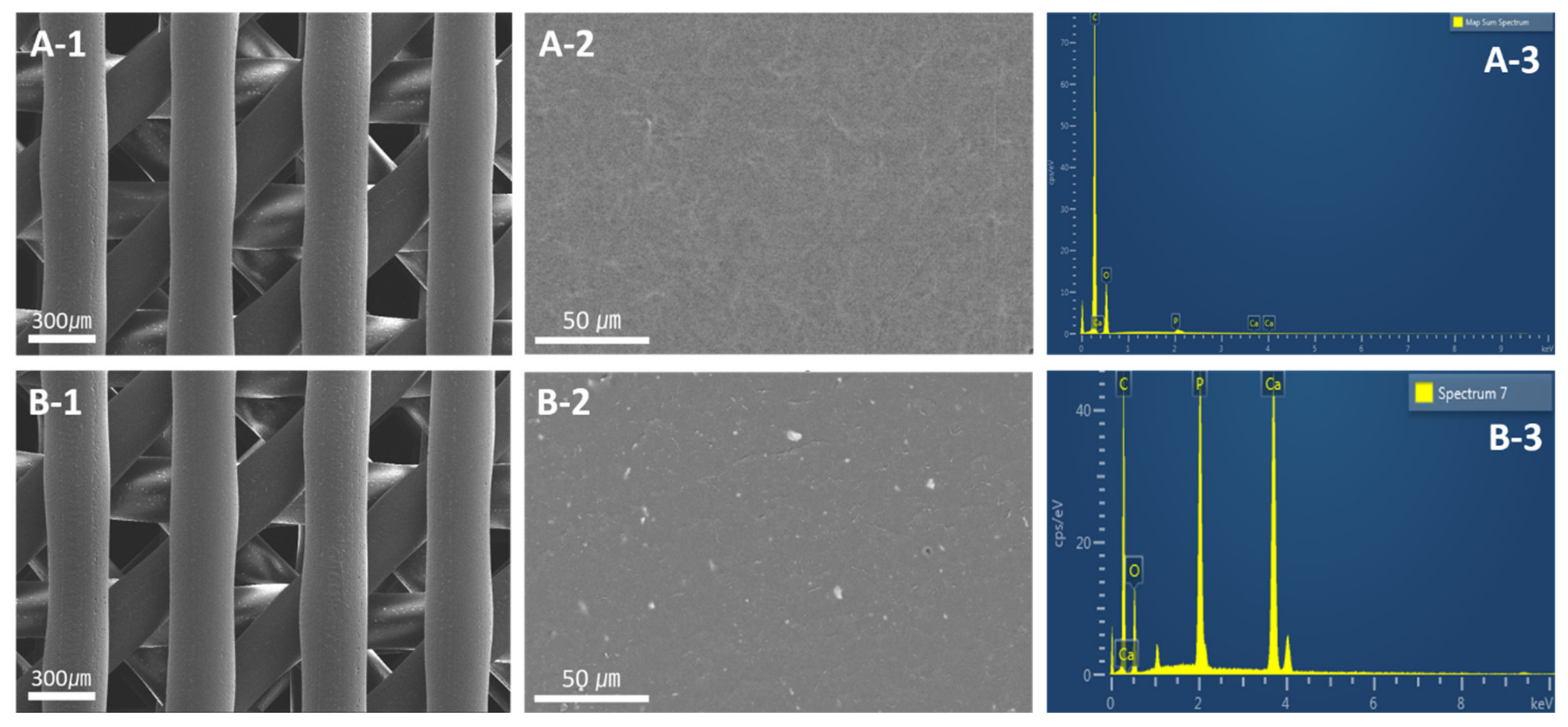
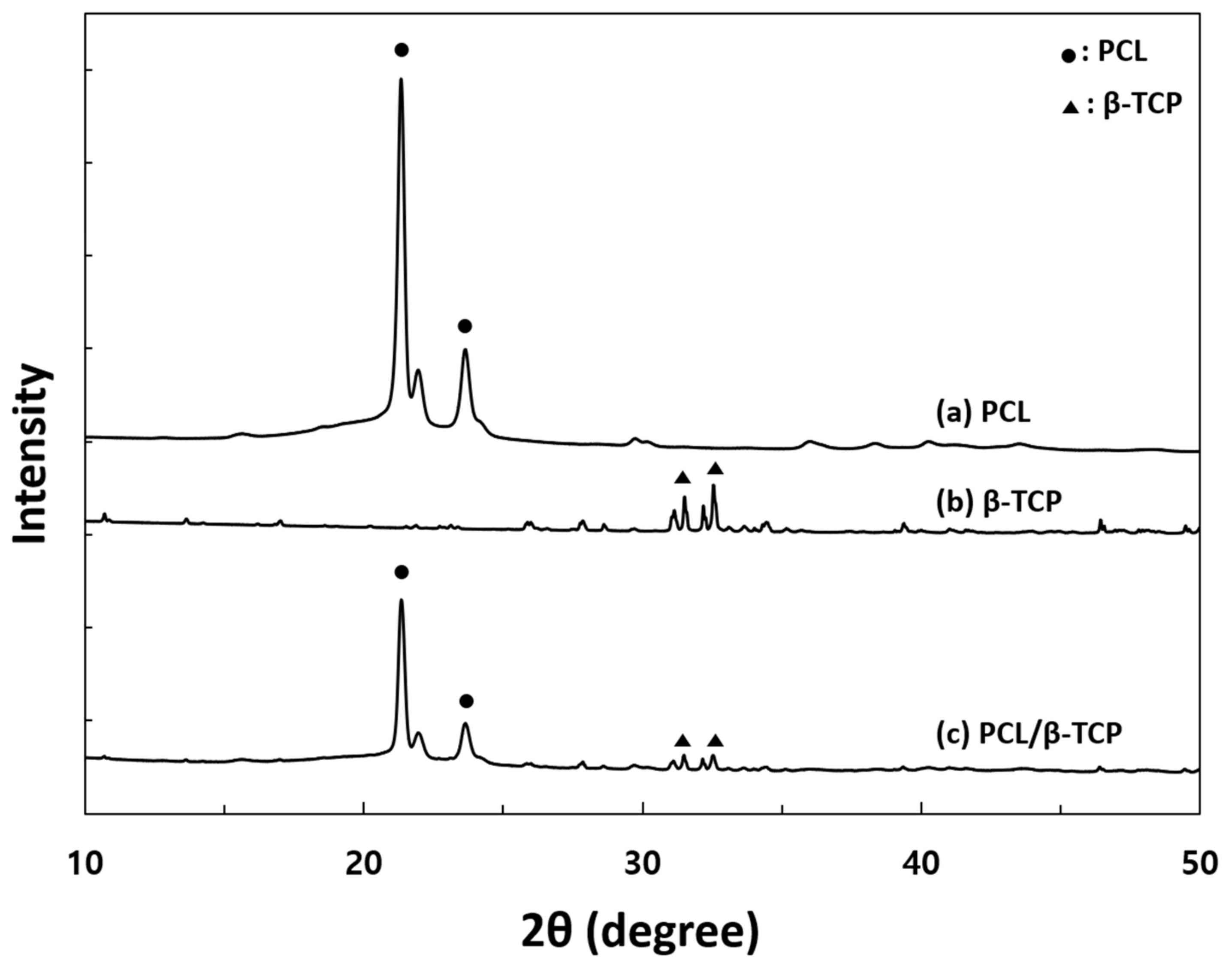
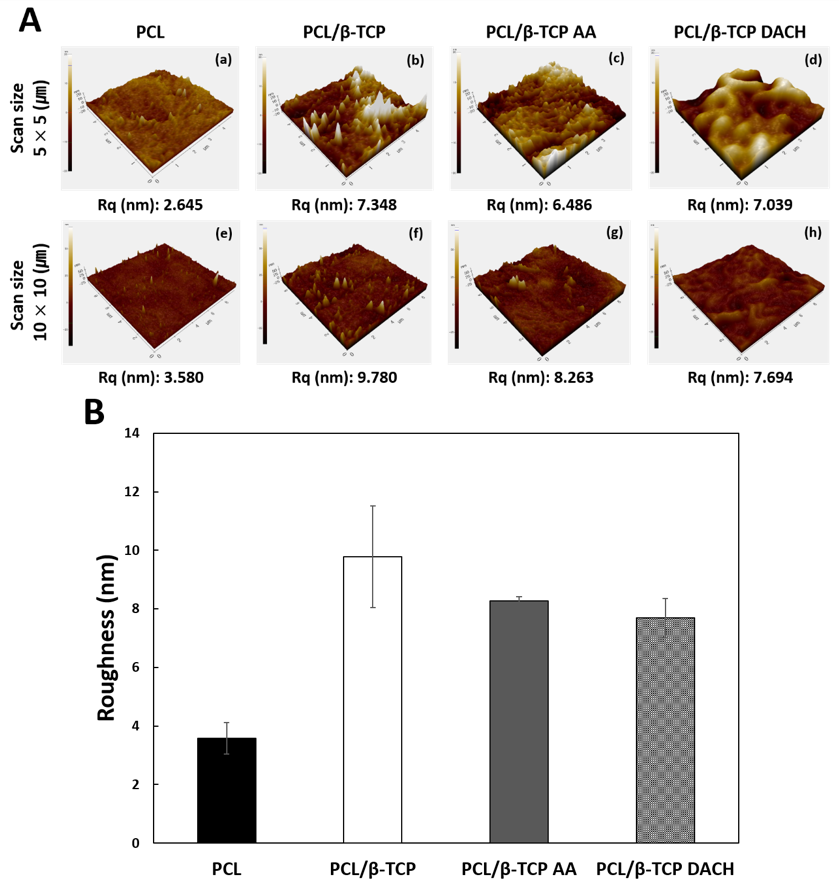
2.4. Surface Characterization of 3D Scaffolds
2.5. Mechanical Strength Evaluation of 3D Scaffolds
2.6. In Vitro Pre-Osteoblast Evaluation
2.6.1. Cell Culture
2.6.2. Cell Seeding into 3D Scaffolds
2.6.3. Cell Proliferation
2.6.4. Cell Viability
2.6.5. Cell Focal Adhesion
2.6.6. Cell Differentiation
2.6.7. Bone Mineralization (Alizarin Red Staining)
2.6.8. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Surface Characterization of Scaffold
3.2. In Vitro Pre-Osteoblast Evaluation
3.3. Live and Dead Cell
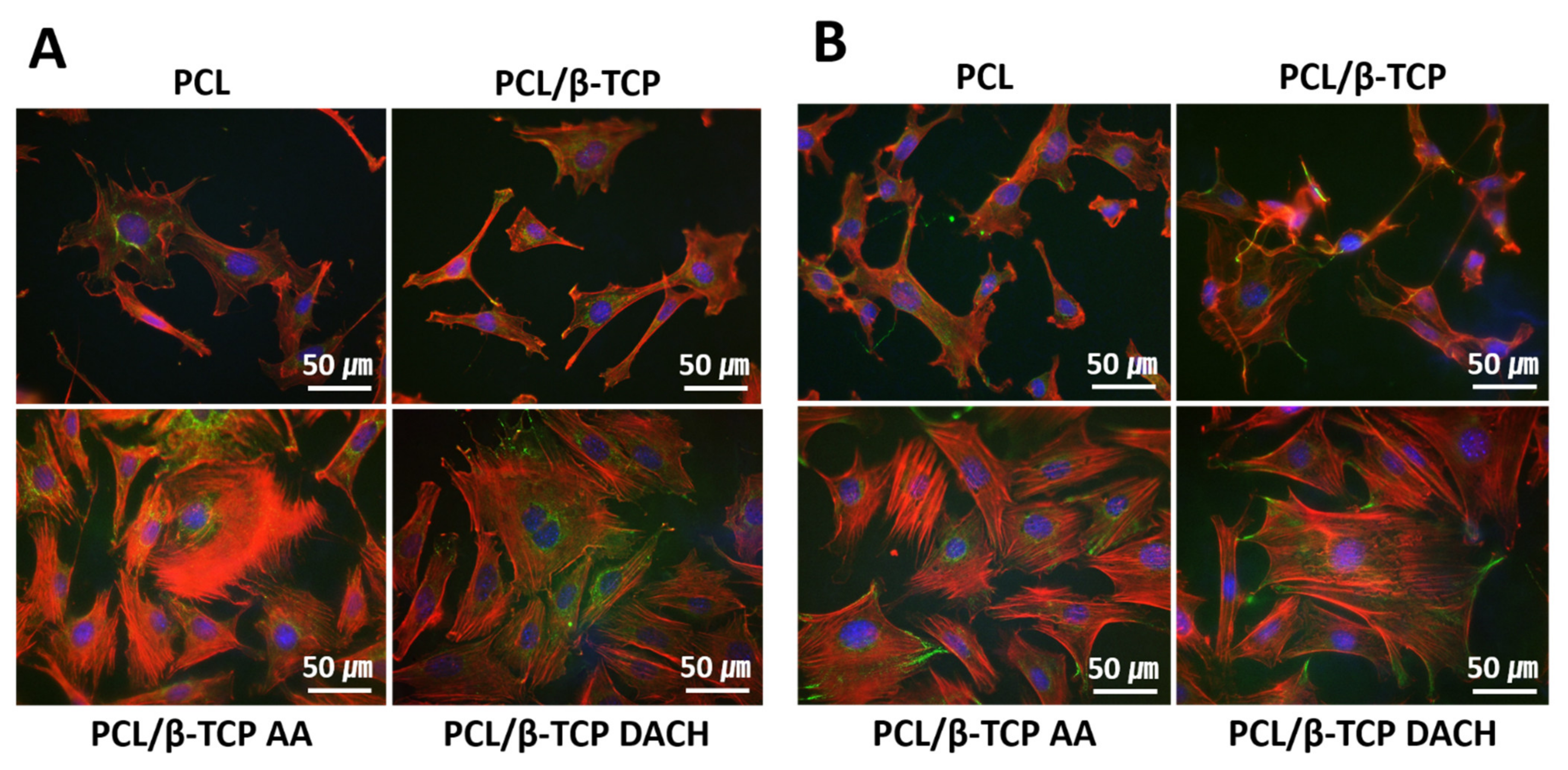
3.4. Cell Focal Adhesion
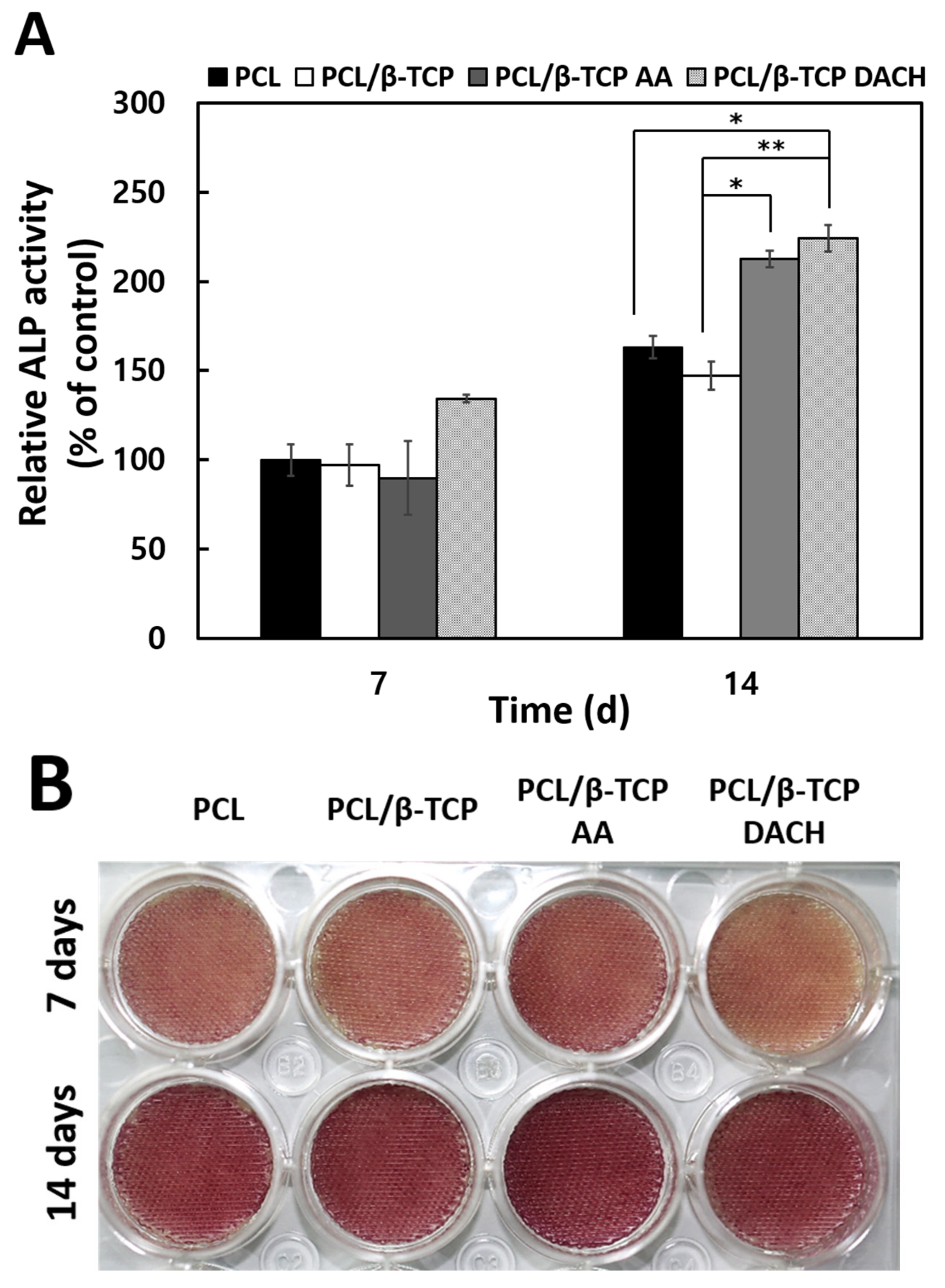
3.5. ALP Activity
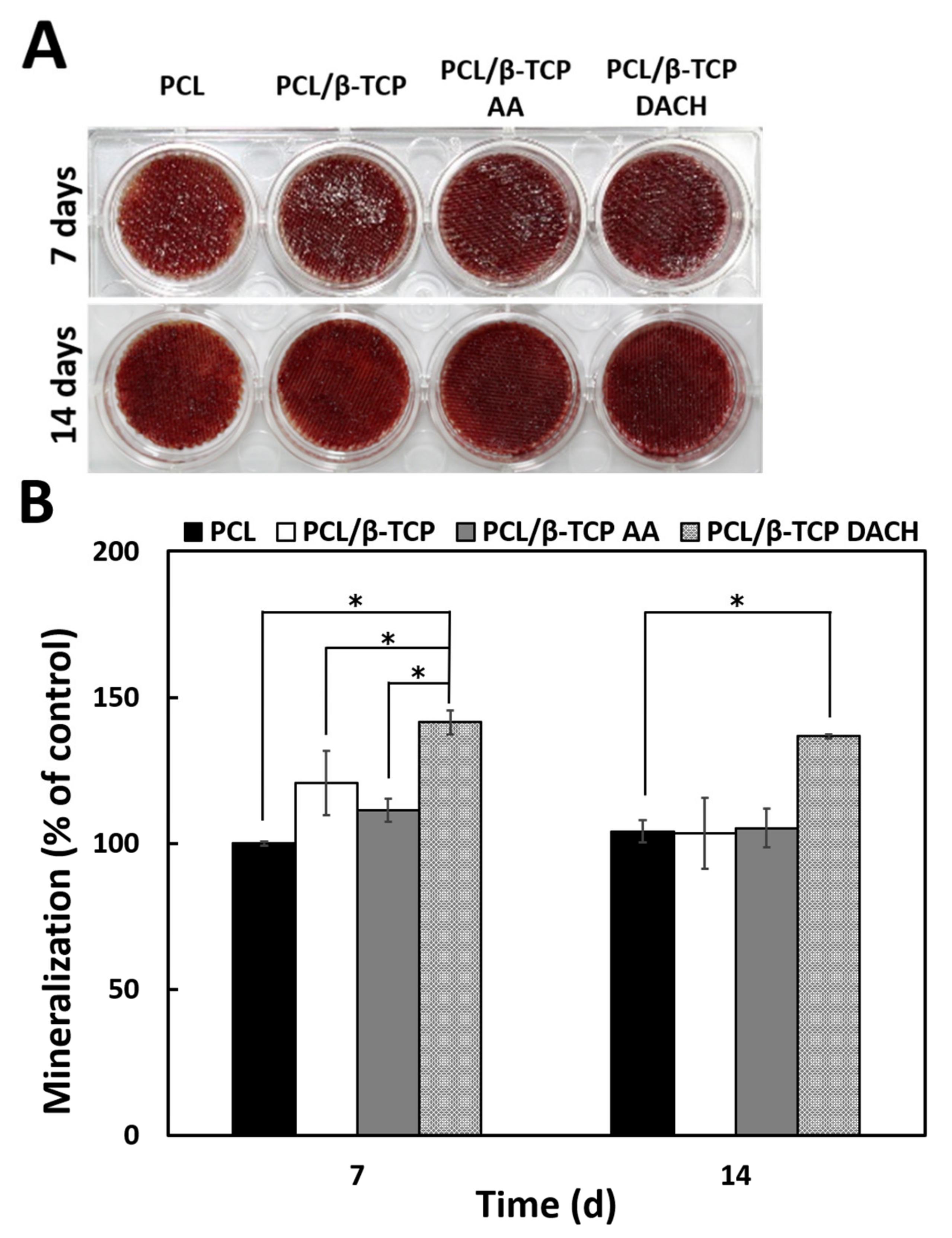
3.6. Alizarin Red Staining
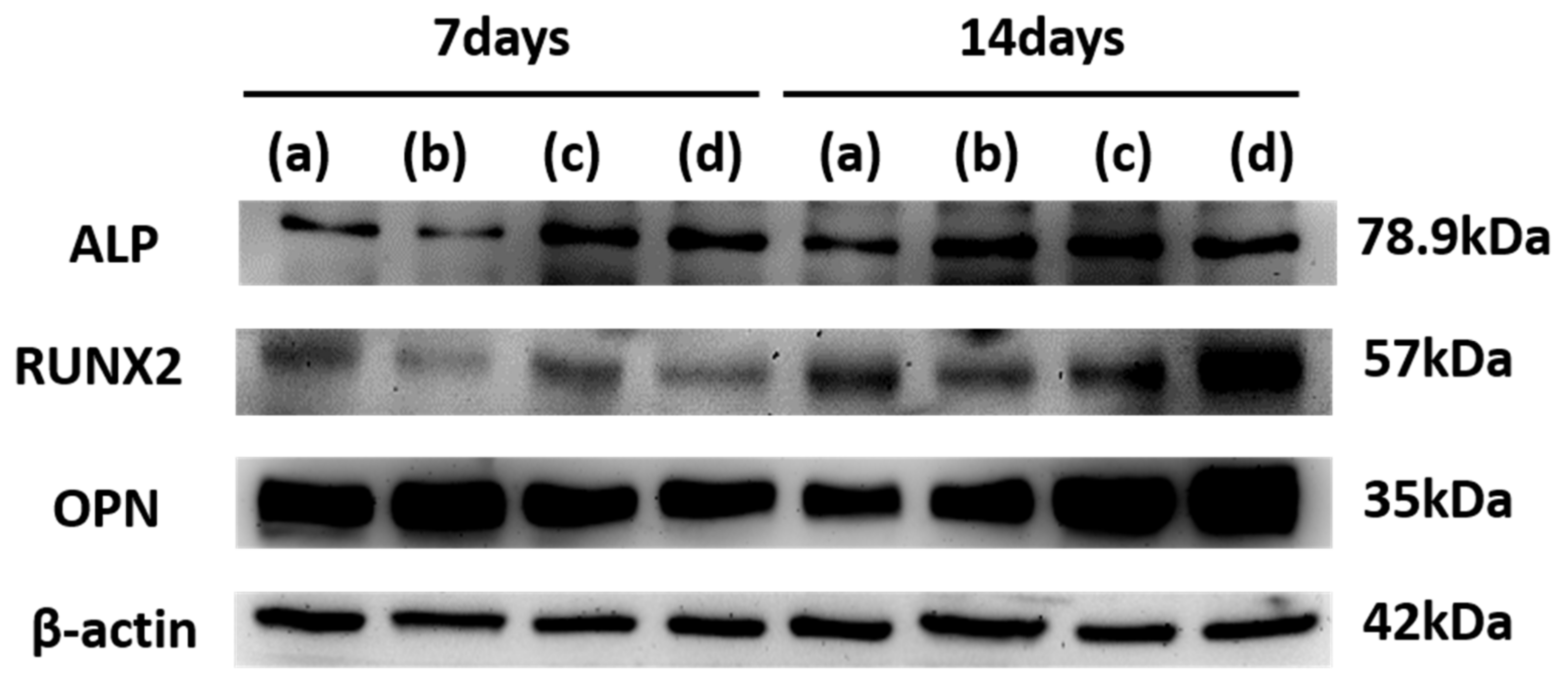
3.7. Western Blot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonitz, A.; Wieding, J.; Lochner, K.; Cornelsen, M.; Seitz, H.; Hansmann, D.; Bader, R. Migration Capacity and Viability of Human Primary Osteoblasts in Synthetic Three-Dimensional Bone Scaffolds Made of Tricalciumphosphate. Materials 2011, 4, 1249–1259. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The Use of Bone-Graft Substitutes in Large Bone Defects: Any Specific Needs? Injury 2011, 42, S56–S63. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J. Bone Tissue Engineering and Regeneration: From Discovery to the Clinic—An Overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Handoll, H.H.G.; Watts, A.C. Bone Grafts and Bone Substitutes for Treating Distal Radial Fractures in Adults. Cochrane Database Syst. Rev. 2008, CD006836. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Zhu, L.; Huang, X.; Tang, D.; He, D.; Shi, J.; Xu, G. 3D Biomimetic Artificial Bone Scaffolds with Dual-Cytokines Spatiotemporal Delivery for Large Weight-Bearing Bone Defect Repair. Sci. Rep. 2017, 7, 7814. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Tsang, V.L.; Bhatia, S.N. Three-Dimensional Tissue Fabrication. Adv. Drug Deliv. Rev. 2004, 56, 1635–1647. [Google Scholar] [CrossRef]
- Farag, M.M.; Yun, H.-S. Effect of Gelatin Addition on Fabrication of Magnesium Phosphate-Based Scaffolds Prepared by Additive Manufacturing System. Mater. Lett. 2014, 132, 111–115. [Google Scholar] [CrossRef]
- Ang, T.H.; Sultana, F.S.A.; Hutmacher, D.W.; Wong, Y.S.; Fuh, J.Y.H.; Mo, X.M.; Loh, H.T.; Burdet, E.; Teoh, S.H. Fabrication of 3D Chitosan–Hydroxyapatite Scaffolds Using a Robotic Dispensing System. Mater. Sci. Eng. C Mater. Biol. Appl. 2002, 20, 35–42. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone Tissue Engineering Graft Substitutes: What Is the Future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone Tissue Engineering Scaffolding: Computer-Aided Scaffolding Techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered Three-Dimensional Scaffolds for Enhanced Bone Regeneration in Osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for Tissue Fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [Green Version]
- Roach, P.; Eglin, D.; Rohde, K.; Perry, C.C. Modern Biomaterials: A Review—Bulk Properties and Implications of Surface Modifications. J. Mater. Sci. Mater. Med. 2007, 18, 1263–1277. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biomedical Polymers: Influence on Cell-Material Interaction. Plasma Chem. Plasma Process. 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Arefi, F.; Andre, V.; Montazer-Rahmati, P.; Amouroux, J. Plasma Polymerization and Surface Treatment of Polymers. Pure Appl. Chem. 1992, 64, 715–723. [Google Scholar] [CrossRef]
- Barry, J.J.; Silva, M.M.; Shakesheff, K.M.; Howdle, S.M.; Alexander, M.R. Using Plasma Deposits to Promote Cell Population of the Porous Interior of Three-dimensional Poly (D, L-lactic Acid) Tissue-engineering Scaffolds. Adv. Funct. Mater. 2005, 15, 1134–1140. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface Modification by Allylamine Plasma Polymerization Promotes Osteogenic Differentiation of Human Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2014, 6, 9733–9741. [Google Scholar] [CrossRef]
- Mehdikhani, B.; Borhani, G.H. Densification and Mechanical Behavior of β-Tricalcium Phosphate Bioceramics. Int. Lett. Chem. Phys. Astron. 2014, 36, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-J.; Kim, K.S.; Kim, K.H.; Li, H.J.; Kim, J.-H.; Jeong, M.H.; Kim, B.H.; Ko, Y.M.; Cho, D.L. Preparation of a Biocompatible Stent Surface by Plasma Polymerization Followed by Chemical Grafting of Drug Compounds. J. Mater. Chem. 2009, 19, 3248. [Google Scholar] [CrossRef]
- Shin, G.S.; Kim, B.H.; Hwang, Y.H.; Ko, Y.M. Plasma Polymerization of 1,2-Diaminocyclohexane for Covalent Bonding of Bone Morphogenic Protein-2 on Titanium Surface. J. Nanosci. Nanotechnol. 2015, 15, 5624–5627. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Sasmazel, H.T. Novel Hybrid Scaffolds for the Cultivation of Osteoblast Cells. Int. J. Biol. Macromol. 2011, 49, 838–846. [Google Scholar] [CrossRef]
- Kook, M.S.; Roh, H.S.; Kim, B.H. Effect of oxygen plasma etching on pore size-controlled 3D polycaprolactone scaffolds for enhancing the early new bone formation in rabbit calvaria. Dent. Mater. J. 2018, 37, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.S.; Jung, S.C.; Kook, M.S.; Kim, B.H. In vitro study of 3D PLGA/n-HAp/β-TCP composite scaffolds with etched oxygen plasma surface modification in bone tissue engineering. Appl. Surf. Sci. 2016, 388, 321–330. [Google Scholar] [CrossRef]
- Wang, L.; Abedalwafa, M.; Wang, F.; Li, C. Biodegradable Poly-Epsilon-Caprolactone (PCL) for Tissue Engineering Applications: A Review. Rev. Adv. Mater. Sci 2013, 34, 123–140. [Google Scholar]
- Saltzman, W.M.; Kyriakides, T.R. Chapter 20—Cell Interactions with Polymers. In Principles of Tissue Engineering; Lanza, R.P., Langer, R., Vacanti, J., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 385–406. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. A Porous Hydroxyapatite Scaffold for Bone Tissue Engineering: Physico-Mechanical and Biological Evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Yeo, M.G.; Kim, G.H. Preparation and Characterization of 3D Composite Scaffolds Based on Rapid-Prototyped PCL/β-TCP Struts and Electrospun PCL Coated with Collagen and HA for Bone Regeneration. Chem. Mater. 2012, 24, 903–913. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Wootton, D.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.; Zhou, J. Biocompatibility and Biodegradation Studies of PCL/β-TCP Bone Tissue Scaffold Fabricated by Structural Porogen Method. J. Mater. Sci. Mater. Med. 2012, 23, 2217–2226. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Kawai, T.; Shanjani, Y.; Fazeli, S.; Behn, A.W.; Okuzu, Y.; Goodman, S.B.; Yang, Y.P. Customized, Degradable, Functionally Graded Scaffold for Potential Treatment of Early Stage Osteonecrosis of the Femoral Head: Treatment Of Early Stage Osteonecrosis Of The Femoral Head. J. Orthop. Res. 2018, 36, 1002–1011. [Google Scholar] [CrossRef] [Green Version]
- Shanjani, Y.; Kang, Y.; Zarnescu, L.; Ellerbee Bowden, A.K.; Koh, J.T.; Ker, D.F.E.; Yang, Y. Endothelial Pattern Formation in Hybrid Constructs of Additive Manufactured Porous Rigid Scaffolds and Cell Laden Hydrogels for Orthopedic Applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 356–372. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.M.; Park, J.-S.; Jeong, S.I.; An, S.-J.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C.; Kim, C.-Y. Promotion of Human Mesenchymal Stem Cell Differentiation on Bioresorbable Polycaprolactone/Biphasic Calcium Phosphate Composite Scaffolds for Bone Tissue Engineering. Biotechnol. Bioprocess Eng. 2014, 19, 341–349. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Zhang, S.-S.; Liu, C.-G.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Low-Temperature Extrusion-Based 3D Printing of Icariin-Laden Scaffolds for Osteogenesis Enrichment. Regen. Ther. 2021, 16, 53–62. [Google Scholar] [CrossRef]
- Chu, P. Plasma-Surface Modification of Biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.C.; St-Georges-Robillard, A.; Thérésy, C.; Lerouge, S.; Wertheimer, M.R. Fabrication and Characterisation of Amine-Rich Organic Tin Films: Focus on Stability. Plasma Process. Polym. 2010, 7, 737–753. [Google Scholar] [CrossRef]
- Qi, P.; Yang, Y.; Zhao, S.; Wang, J.; Li, X.; Tu, Q.; Yang, Z.; Huang, N. Improvement of Corrosion Resistance and Biocompatibility of Biodegradable Metallic Vascular Stent via Plasma Allylamine Polymerized Coating. Mater. Des. 2016, 96, 341–349. [Google Scholar] [CrossRef]
- Javid, A. Surface Energy and Wettability Control in Bio-Inspired PEG like Thin Flms. Mater. Des. 2016, 92, 405–413. [Google Scholar] [CrossRef]
- Solovieva, A. Immobilization of Platelet-Rich Plasma onto COOH Plasma-Coated PCL Nanofbers Boost Viability and Proliferation of Human Mesenchymal Stem Cells. Polymers 2017, 9, 736. [Google Scholar] [CrossRef] [Green Version]
- Štrbková, L.; Manakhov, A.; Zajíčková, L.; Stoica, A.; Veselý, P.; Chmelík, R. The Adhesion of Normal Human Dermal Fibroblasts to the Cyclopropylamine Plasma Polymers Studied by Holographic Microscopy. Surf. Coat. Technol. 2016, 295, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Mager, M.D.; LaPointe, V.; Stevens, M.M. Exploring and Exploiting Chemistry at the Cell Surface. Nat. Chem. 2011, 3, 582–589. [Google Scholar] [CrossRef]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability Influences Cell Behavior on Superhydrophobic Surfaces with Different Topographies. Biointerphases 2012, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of Surface Wettability on Competitive Protein Adsorption and Initial Attachment of Osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of Hydrophilic and Hydrophobic Titanium Discs in Rat Tibia: Cellular Reactions on the Surfaces during the First 3 Weeks in Bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Smith, J.; Fabry, B.; Goldmann, W.H. Mechano-Coupling and Regulation of Contractility by the Vinculin Tail Domain. Biophys. J. 2008, 94, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.E.; Glenney, J.R., Jr.; Burridge, K. Paxillin: A New Vinculin-Binding Protein Present in Focal Adhesions. J. Cell Biol. 1990, 111, 1059–1068. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Integrin Binding Specificity Regulates Biomaterial Surface Chemistry Effects on Cell Differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5953–5957. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Ducheyne, P.; Lynch, L.; Boettiger, D.; Composto, R.J. Effect of Biomaterial Surface Properties on Fibronectin Alpha5beta1 Integrin Interaction and Cellular Attachment. Biomaterials 2006, 27, 1907–1916. [Google Scholar] [CrossRef]
- Moursi, A.M.; Globus, R.K.; Damsky, C.H. Interactions between Integrin Receptors and Fibronectin Are Required for Calvarial Osteoblast Differentiation in Vitro. J. Cell Sci. 1997, 110, 2187–2196. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface Chemistry Modulates Fibronectin Conformation and Directs Integrin Binding and Specificity to Control Cell Adhesion: Surface Chemistry Alters Integrin Binding. J. Biomed. Mater. Res. A 2003, 66, 247–259. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface Chemistry Modulates Focal Adhesion Composition and Signaling through Changes in Integrin Binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef]
- Michael, K.E.; Vernekar, V.N.; Keselowsky, B.G.; Meredith, J.C.; Latour, R.A.; García, A.J. Adsorption-Induced Conformational Changes in Fibronectin Due to Interactions with Well-Defined Surface Chemistries. Langmuir 2003, 19, 8033–8040. [Google Scholar] [CrossRef]
- Kornberg, L.; Earp, H.S.; Parsons, J.T.; Schaller, M.; Juliano, R.L. Cell Adhesion or Integrin Clustering Increases Phosphorylation of a Focal Adhesion-Associated Tyrosine Kinase. J. Biol. Chem. 1992, 267, 23439–23442. [Google Scholar] [CrossRef]
- Biggs, M.J.P.; Dalby, M.J. Focal Adhesions in Osteoneogenesis. Proc. Inst. Mech. Eng. H 2010, 224, 1441–1453. [Google Scholar] [CrossRef] [Green Version]
- Biggs, M.J.P.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.W.; Oreffo, R.O.C.; Dalby, M.J. The Use of Nanoscale Topography to Modulate the Dynamics of Adhesion Formation in Primary Osteoblasts and ERK/MAPK Signalling in STRO-1+ Enriched Skeletal Stem Cells. Biomaterials 2009, 30, 5094–5103. [Google Scholar] [CrossRef]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical Role of the Extracellular Signal–Regulated Kinase–MAPK Pathway in Osteoblast Differentiation and Skeletal Development. J. Cell Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef]
- Kanno, T.; Takahashi, T.; Tsujisawa, T.; Ariyoshi, W.; Nishihara, T. Mechanical Stress-Mediated Runx2 Activation Is Dependent on Ras/ERK1/2 MAPK Signaling in Osteoblasts. J. Cell. Biochem. 2007, 101, 1266–1277. [Google Scholar] [CrossRef]
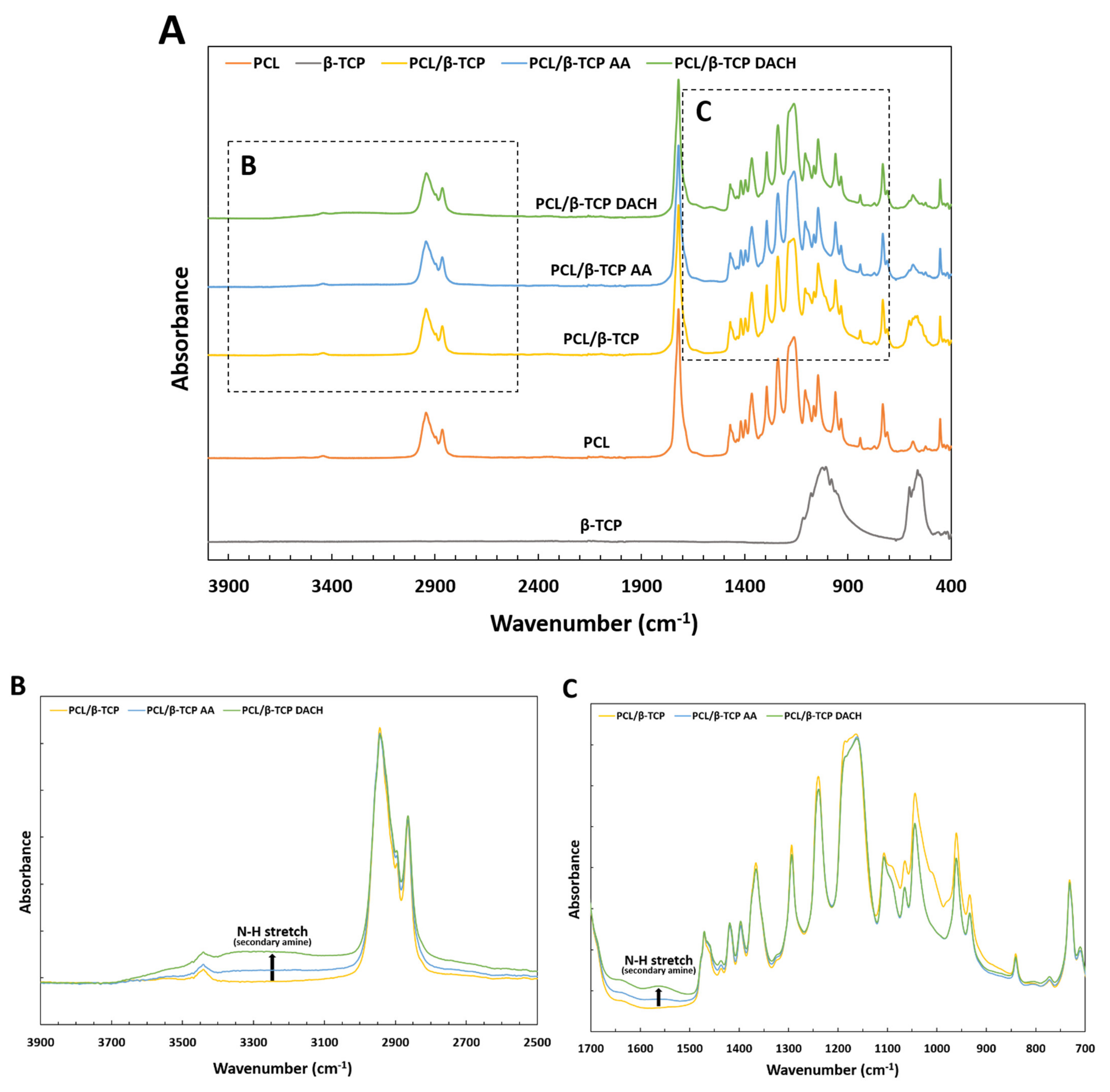




| Pre-Treatment and Post-Treatment | Gas Flow Rate (sccm) | Pressure (mTorr) | Power (W) | Time (s) |
|---|---|---|---|---|
| Argon gas | 20 | 100 | 30 | 10 |
| Plasma-Polymerization | Precursor | Pressure (mTorr) | Power (W) | Time (s) |
| AA | Allylamine | 30 | 60 | 60 |
| DACH | 1,2-diaminocyclohexane | 10 | 80 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Kim, B.-H.; Kim, M.-S. Amine Plasma-Polymerization of 3D Polycaprolactone/β-Tricalcium Phosphate Scaffold to Improving Osteogenic Differentiation In Vitro. Materials 2022, 15, 366. https://doi.org/10.3390/ma15010366
Kim H-Y, Kim B-H, Kim M-S. Amine Plasma-Polymerization of 3D Polycaprolactone/β-Tricalcium Phosphate Scaffold to Improving Osteogenic Differentiation In Vitro. Materials. 2022; 15(1):366. https://doi.org/10.3390/ma15010366
Chicago/Turabian StyleKim, Hee-Yeon, Byung-Hoon Kim, and Myung-Sun Kim. 2022. "Amine Plasma-Polymerization of 3D Polycaprolactone/β-Tricalcium Phosphate Scaffold to Improving Osteogenic Differentiation In Vitro" Materials 15, no. 1: 366. https://doi.org/10.3390/ma15010366
APA StyleKim, H.-Y., Kim, B.-H., & Kim, M.-S. (2022). Amine Plasma-Polymerization of 3D Polycaprolactone/β-Tricalcium Phosphate Scaffold to Improving Osteogenic Differentiation In Vitro. Materials, 15(1), 366. https://doi.org/10.3390/ma15010366






