Water Corrosion of Tungsten Target for Accelerator-Driven Neutron Source
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Corrosion Experiments
2.3. Physicochemical Characterizations
3. Results and Discussion
3.1. ICP-MS
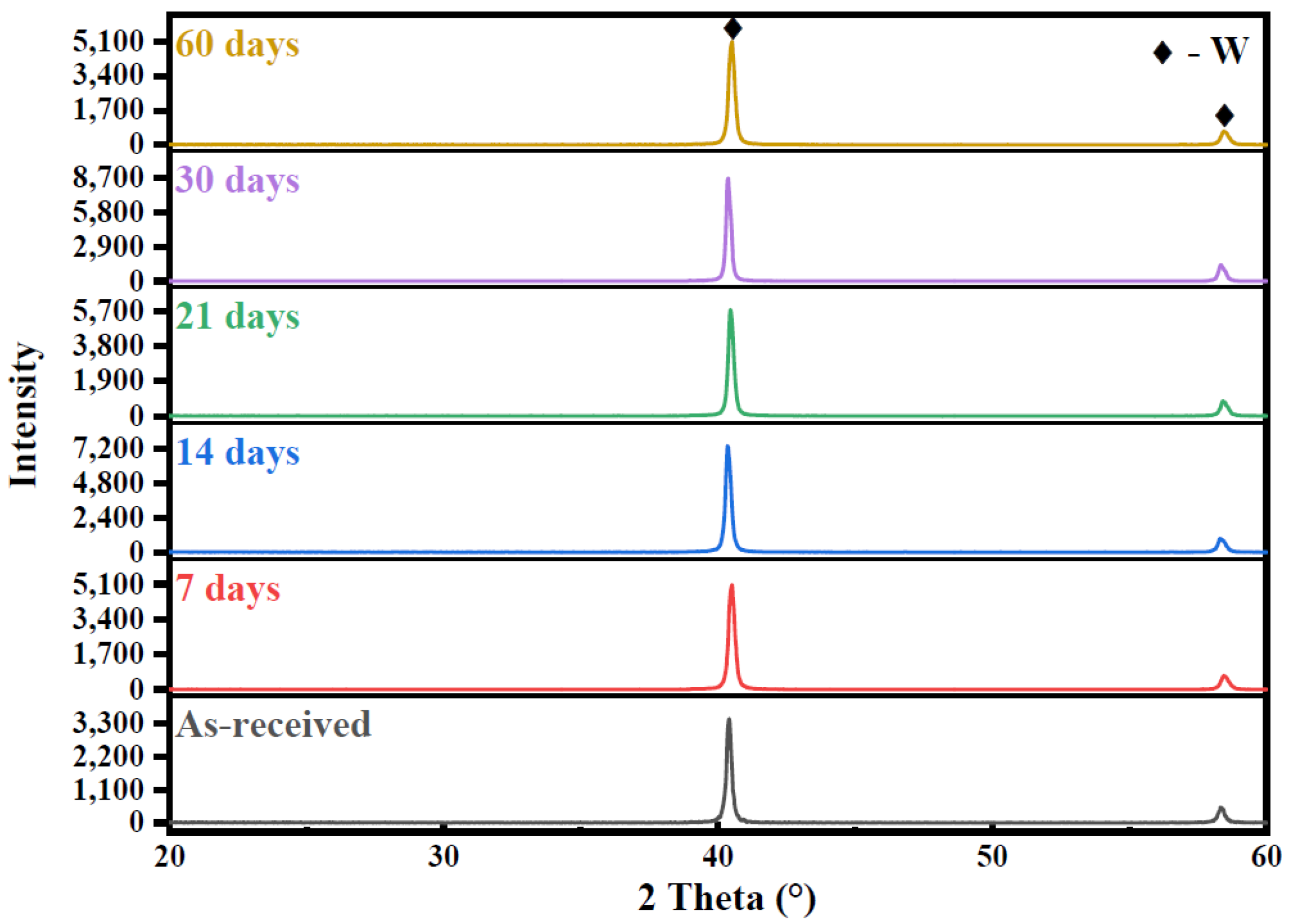
3.2. XRD
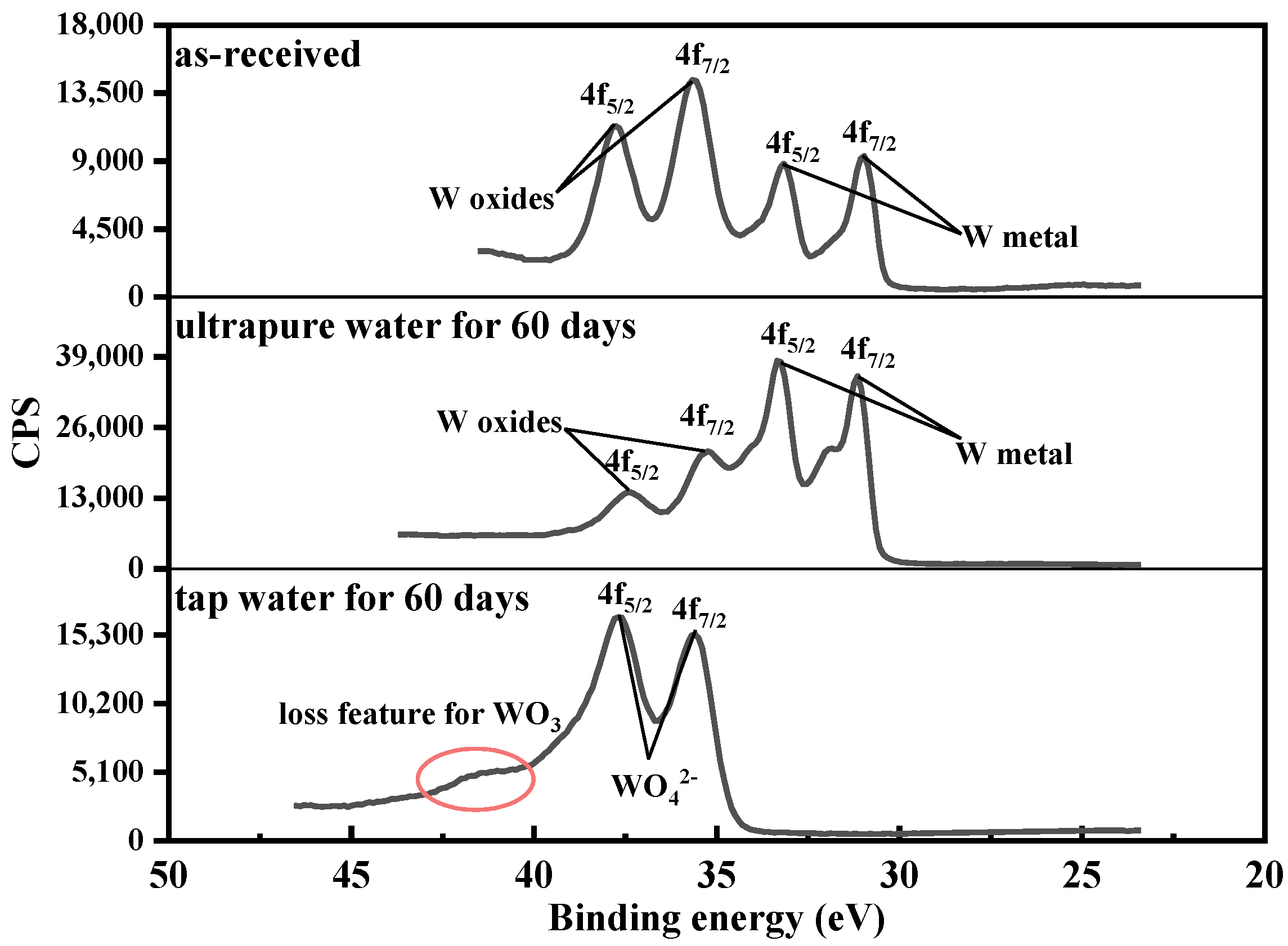
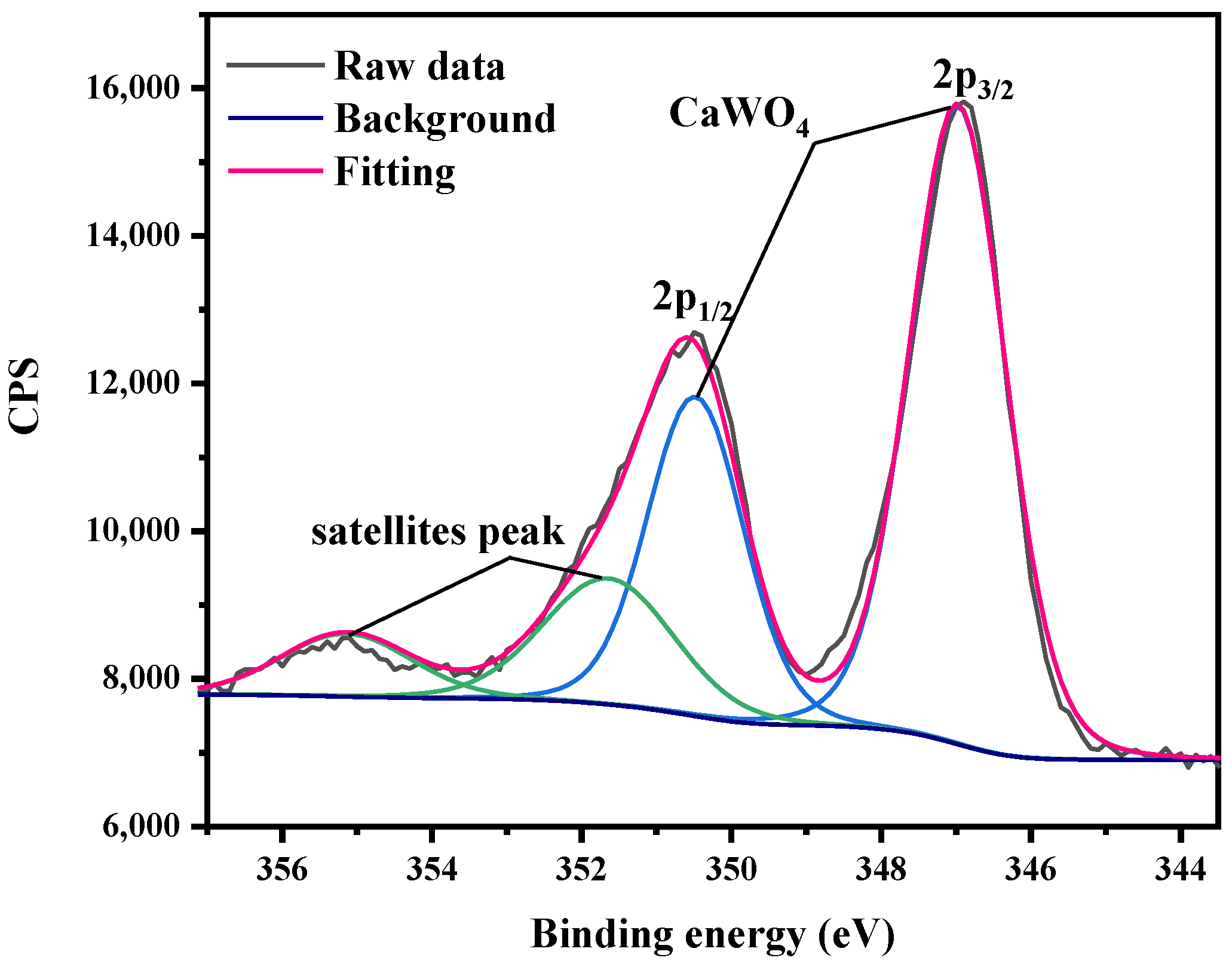
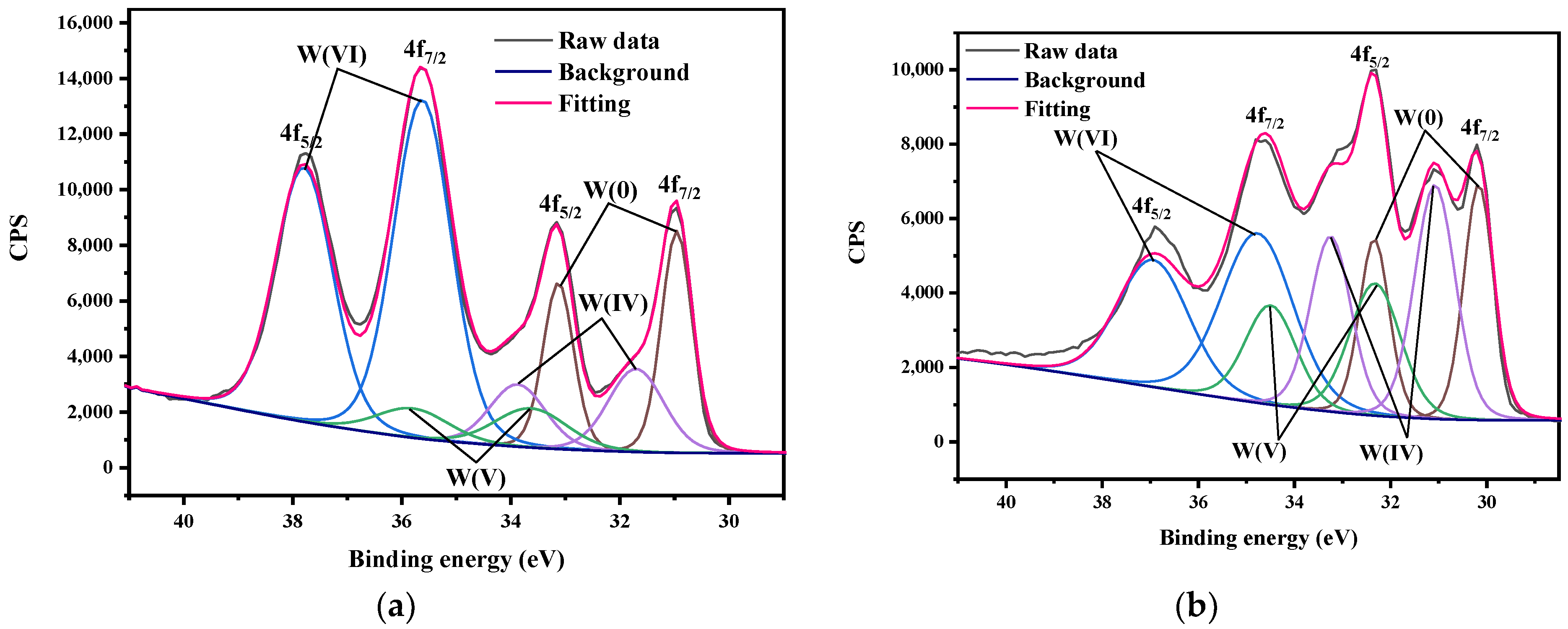
3.3. XPS
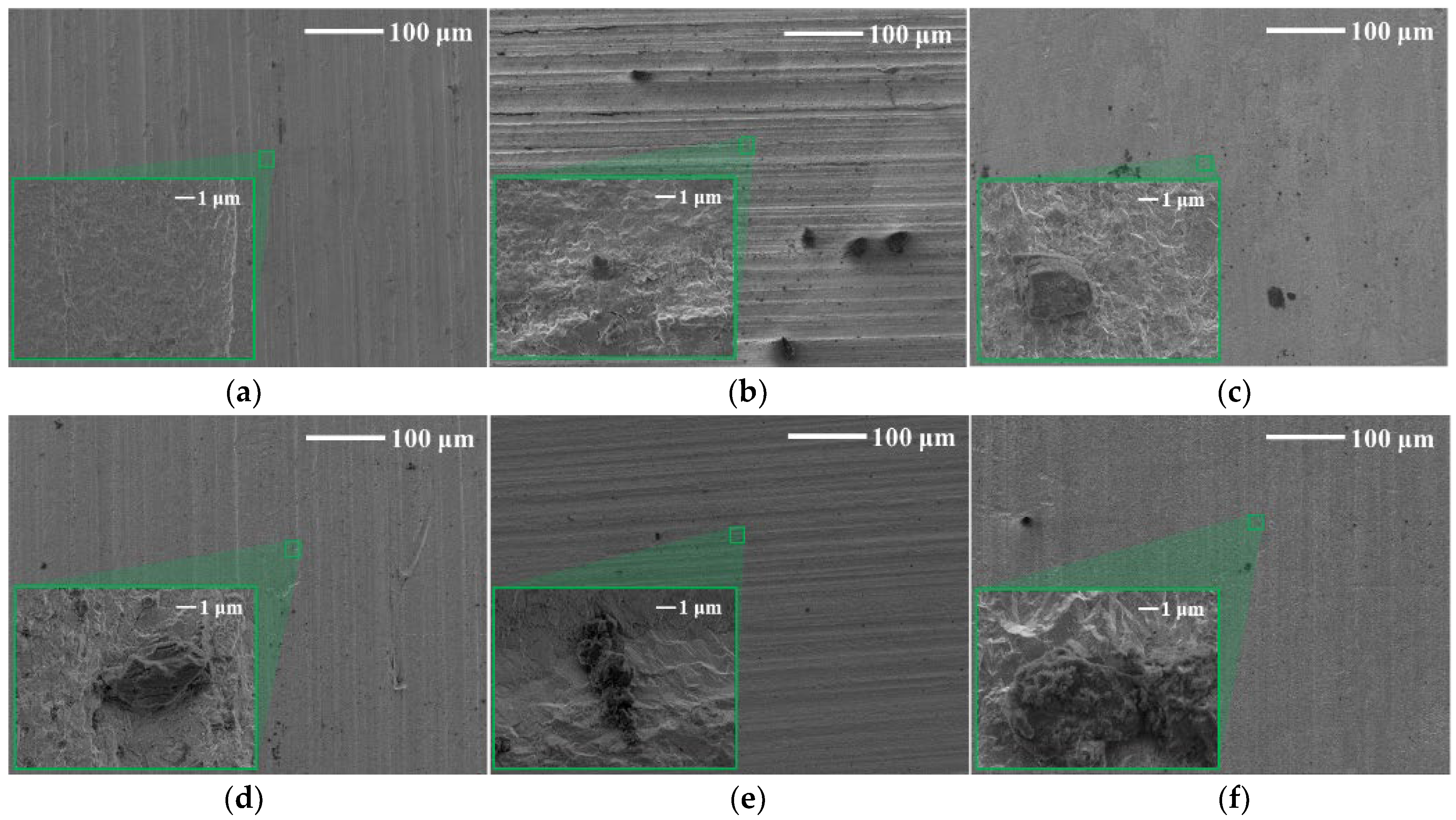
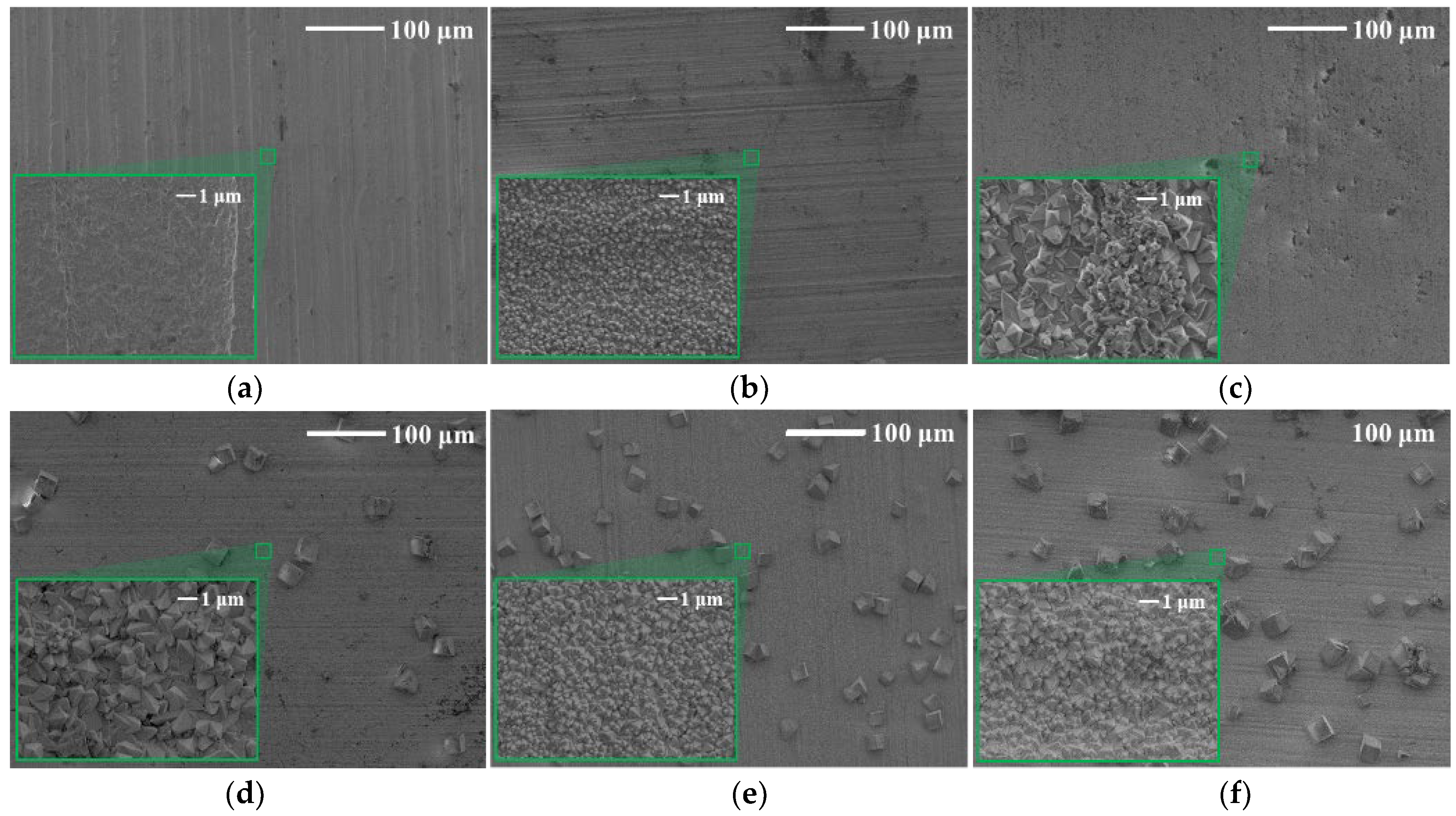
3.4. Surface Morphology
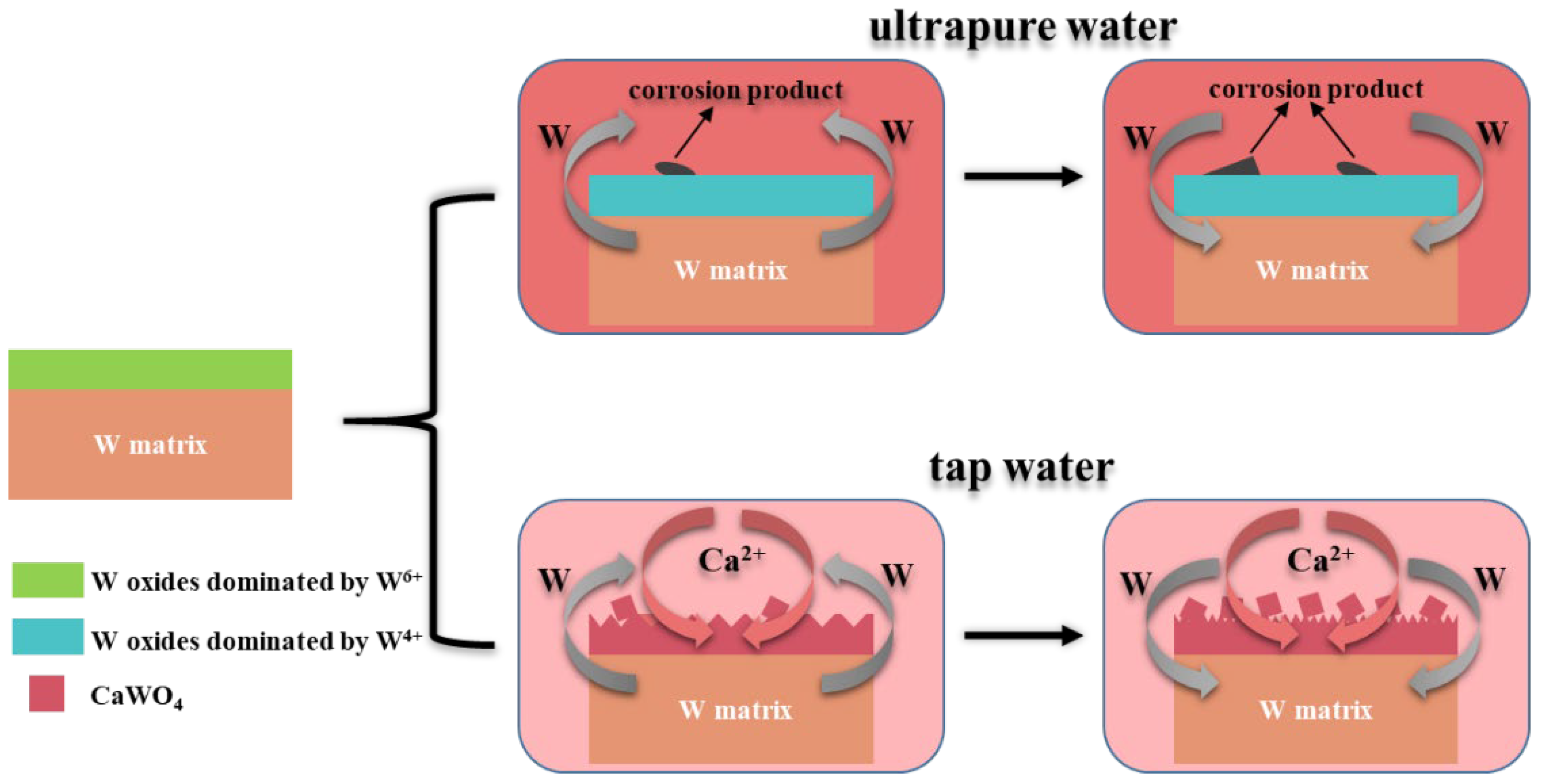
3.5. Corrosion Mechanism
4. Conclusions
- (1)
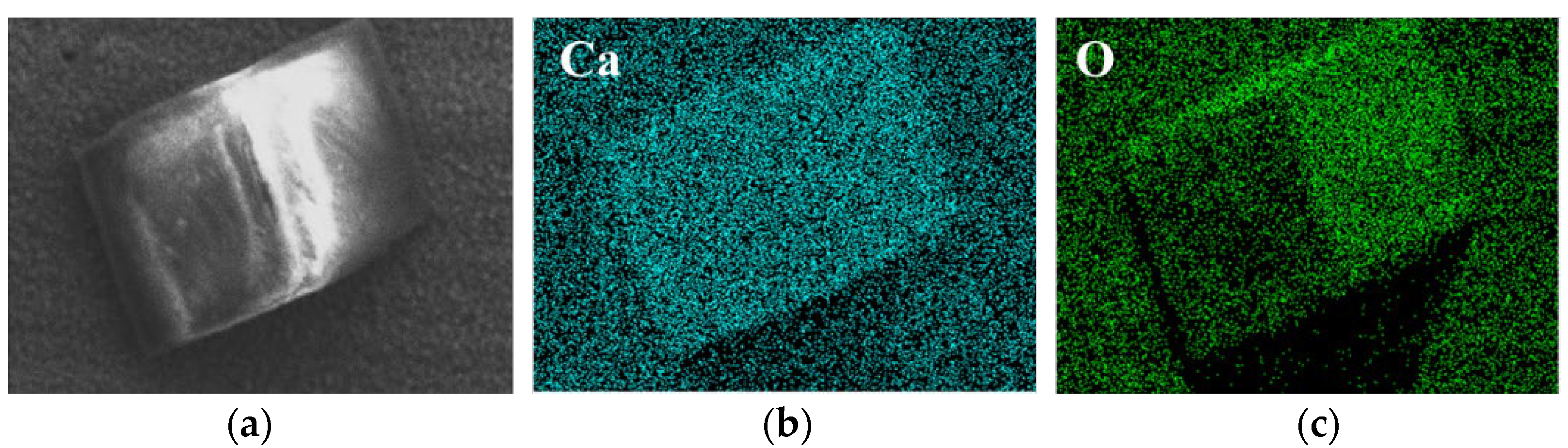
- The corrosion-caused dissolution of tungsten reached its maximum between 30 days and 60 days in both solutions. Compared with other elements (Mg, Fe and Na), Ca in tap water was more easily adsorbed on the surface of tungsten, indicating the Ca-containing substance was the main corrosion product in tap water.
- (2)
- CaWO4, the main corrosion product, was detected on the surface of tungsten after exposure to tap water. Moreover, the surface oxide valence of tungsten changed (from W6+ to W4+) during corrosion in ultrapure water.
- (3)
- The corrosion product in ultrapure water was blocky on the tungsten surface, while CaWO4 was in a cube shape on the surface after corrosion in the tap water, and the surface was completely destroyed, featuring a dense diamond shape.
- (4)
- During the construction of the neutron source, the use of ultrapure water with the resistivity of 18.25 MΩ•cm can lead to high costs. According to the results of this study, Ca2+ in tap water is the precursor of the main corrosion products (CaWO4). In order to reduce the construction cost, it is only necessary to remove Ca from tap water and use it as cooling medium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, L.; Rinaldi, R.; Schober, H. Neutron Applications in Earth, Energy and Environmental Sciences; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 2021, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Harling, O.K.; Riley, K.J. Fission reactor neutron sources for neutron capture therapy—A critical review. J. Neuro-Oncol. 2003, 62, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.; Andreani, C.; Carpenter, J.; Festa, G.; Gorini, G.; Loong, C.-K.; Senesi, R. Research opportunities with compact accelerator-driven neutron sources. Phys. Rep. 2016, 654, 1–58. [Google Scholar] [CrossRef] [Green Version]
- Akkaya, G. The calculation of self-shielding correction factors for large samples in 241Am–Be isotopic neutron source. Appl. Radiat. Isot. 2022, 179, 109990. [Google Scholar] [CrossRef] [PubMed]
- Kiyanagi, Y. Neutron applications developing at compact accelerator-driven neutron sources. AAPPS Bull. 2021, 31, 22. [Google Scholar] [CrossRef]
- Kawai, M.; Kikuchi, K.; Kurishita, H.; Li, J.-F.; Furusaka, M. Fabrication of a tantalum-clad tungsten target for KENS. J. Nucl. Mater. 2001, 296, 312–320. [Google Scholar] [CrossRef]
- Garoby, R.; Vergara, A.; Danared, H.; Alonso, I.; Bargallo, E.; Cheymol, B.; Darve, C.; Eshraqi, M.; Hassanzadegan, H.; Jansson, A. The European spallation source design. Phys. Scr. 2017, 93, 014001. [Google Scholar] [CrossRef]
- Wei, J.; Fu, S.-N.; Tang, J.-Y.; Tao, J.-Z.; Wang, D.-S.; Wang, F.-W.; Wang, S. China Spallation Neutron Source-an overview of application prospects. Chin. Phys. C 2009, 33, 1033. [Google Scholar]
- Li, M.; Werner, E.; You, J.-H. Fracture mechanical analysis of tungsten armor failure of a water-cooled divertor target. Fusion Eng. Des. 2014, 89, 2716–2725. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, J.; Xie, Z.; Fang, Q. Enhanced Erosion–Corrosion Resistance of Tungsten by Carburizing Using Spark Plasma Sintering Technique. Materials 2020, 13, 2719. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.T. Erosion/Corrosion of Machinable Tungsten in Water; Argonne National Lab.: Argonne, IL, USA, 2000. [Google Scholar]
- Nelson, A.; O’Toole, J.; Valicenti, R.; Maloy, S. Fabrication of a tantalum-clad tungsten target for LANSCE. J. Nucl. Mater. 2012, 431, 172–184. [Google Scholar] [CrossRef]
- Gohar, M.Y.; Sofu, T.; Zhong, Z.; Belch, H.; Naberezhnev, D. Target Design Optimization for an Electron Accelerator Driven Subcritical Facility with Circular and Square Beam Profiles; Argonne National Lab. (ANL): Argonne, IL, USA, 2008. [Google Scholar]
- Bolokang, A.S.; Phasha, M.J.; Oliphant, C.; Motaung, D. XRD analysis and microstructure of milled and sintered V, W, C, and Co powders. Int. J. Refract. Met. Hard Mater. 2011, 29, 108–111. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Tao, J.; Zhu, X.; Zhou, J.; Zhao, Z.; Xie, L.; Tian, H. Synthesis of CaWO4 nanoparticles by a molten salt method-ScienceDirect. Mater. Lett. 2006, 60, 291–293. [Google Scholar] [CrossRef]
- Inoue, M.; Hirasawa, I. The relationship between crystal morphology and XRD peak intensity on CaSO4· 2H2O. J. Cryst. Growth 2013, 380, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Adamsen, K.C.; Li, Z.; Lammich, L.; Lauritsen, J.V.; Wendt, S. WO3 Monomers Supported on Anatase TiO2 (101),−(001), and Rutile TiO2 (110): A Comparative STM and XPS Study. J. Phys. Chem. C 2022, 126, 2493–2502. [Google Scholar] [CrossRef]
- Ryu, J.H.; Bang, S.Y.; Kim, W.S.; Park, G.S.; Kim, K.M.; Yoon, J.-W.; Shim, K.B.; Koshizaki, N. Microstructure and optical properties of nanocrystalline CaWO4 thin films deposited by pulsed laser ablation in room temperature. J. Alloy. Compd. 2007, 441, 146–151. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Sun, B.; Liu, J.; Cao, Y.; Qiao, H.; Huang, Y.; Pang, X. Photo-Induced Multiple-State Memory Behaviour in Non-Volatile Bipolar Resistive-Switching Devices. J. Nanosci. Nanotechnol. 2018, 18, 2650–2656. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J. The Elements, 2nd ed.; Clarendon Press: Oxford, UK, 1991. [Google Scholar]
- Rieck, G.D. Tungsten and Its Compounds; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Khan, A.; Al-Muhaish, N.; Mohamedkhair, A.; Khan, M.Y.; Qamar, M.; Yamani, Z.; Drmosh, Q. Oxygen-deficient non-crystalline tungsten oxide thin films for solar-driven water oxidation. J. Non-Cryst. Solids 2022, 580, 121409. [Google Scholar] [CrossRef]
- Atuchin, V.; Pokrovsky, L.; Khyzhun, O.Y.; Sinelnichenko, A.; Ramana, C. Surface crystallography and electronic structure of potassium yttrium tungstate. J. Appl. Phys. 2008, 104, 033518. [Google Scholar] [CrossRef]




| Mg | Fe | Na | Ca | |
|---|---|---|---|---|
| Concentration, mg/L | 12.3 | 0.404 | 81.9 | 34.5 |
| W(VI), eV | W(V), eV | W(IV), eV | W(0), eV | |||||
|---|---|---|---|---|---|---|---|---|
| 4f5/2 | 4f7/2 | 4f5/2 | 4f7/2 | 4f5/2 | 4f7/2 | 4f5/2 | 4f7/2 | |
| As-received | 37.79 | 35.62 | 35.80 | 33.63 | 33.88 | 31.71 | 33.13 | 30.96 |
| Ultrapure water for 60 days | 37.55 | 35.38 | 35.29 | 33.12 | 34.04 | 31.87 | 33.28 | 31.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Sun, Q.; Hu, Y.; Li, X.; Qiao, Z.; Wang, J.; Wang, S. Water Corrosion of Tungsten Target for Accelerator-Driven Neutron Source. Materials 2022, 15, 3448. https://doi.org/10.3390/ma15103448
Xie Y, Sun Q, Hu Y, Li X, Qiao Z, Wang J, Wang S. Water Corrosion of Tungsten Target for Accelerator-Driven Neutron Source. Materials. 2022; 15(10):3448. https://doi.org/10.3390/ma15103448
Chicago/Turabian StyleXie, Yupeng, Qiuyu Sun, Yaocheng Hu, Xiaobo Li, Zhaopeng Qiao, Jie Wang, and Sheng Wang. 2022. "Water Corrosion of Tungsten Target for Accelerator-Driven Neutron Source" Materials 15, no. 10: 3448. https://doi.org/10.3390/ma15103448






