Influence of Silica Fume on High-Calcium Fly Ash Expansion during Hydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
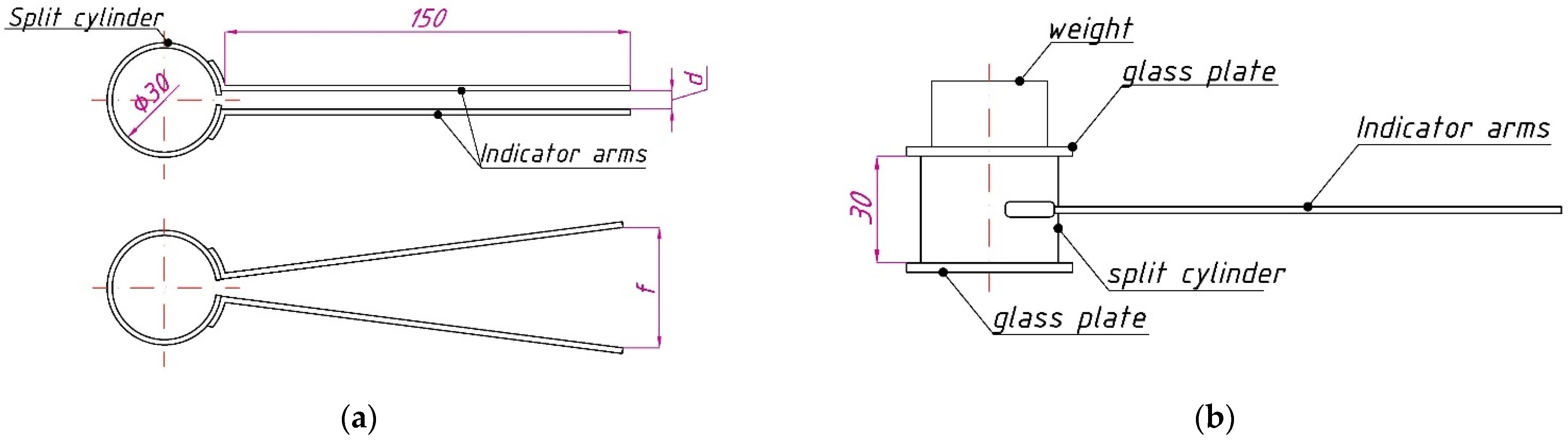
2.2. Fly Ash Expansion Test in Le Chatelier Molds
2.3. X-ray Diffraction Analysis and Differential Thermal Analysis
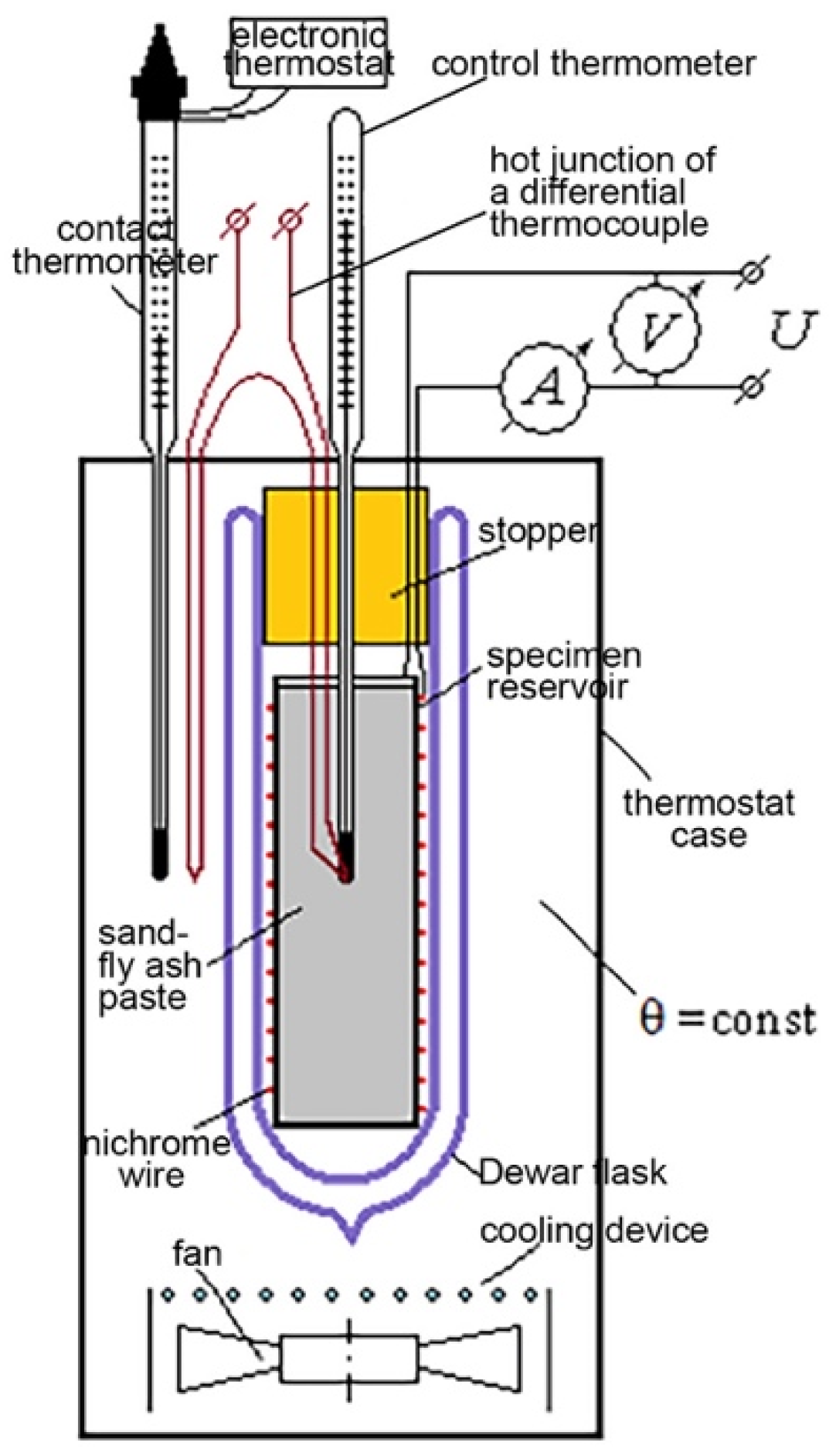
2.4. Heat of Hydration by Means of Semi-Adiabatic Calorimetry
2.4.1. Determination Method
2.4.2. Heat of Hydration Determination
2.4.3. Additive Heat Capacity Determination
2.4.4. Calculation Reduction of the Semi-Adiabatic Calorimetric Test Results to the Isothermal Regime
2.4.5. Specimens
2.5. Compressive Strength
3. Results
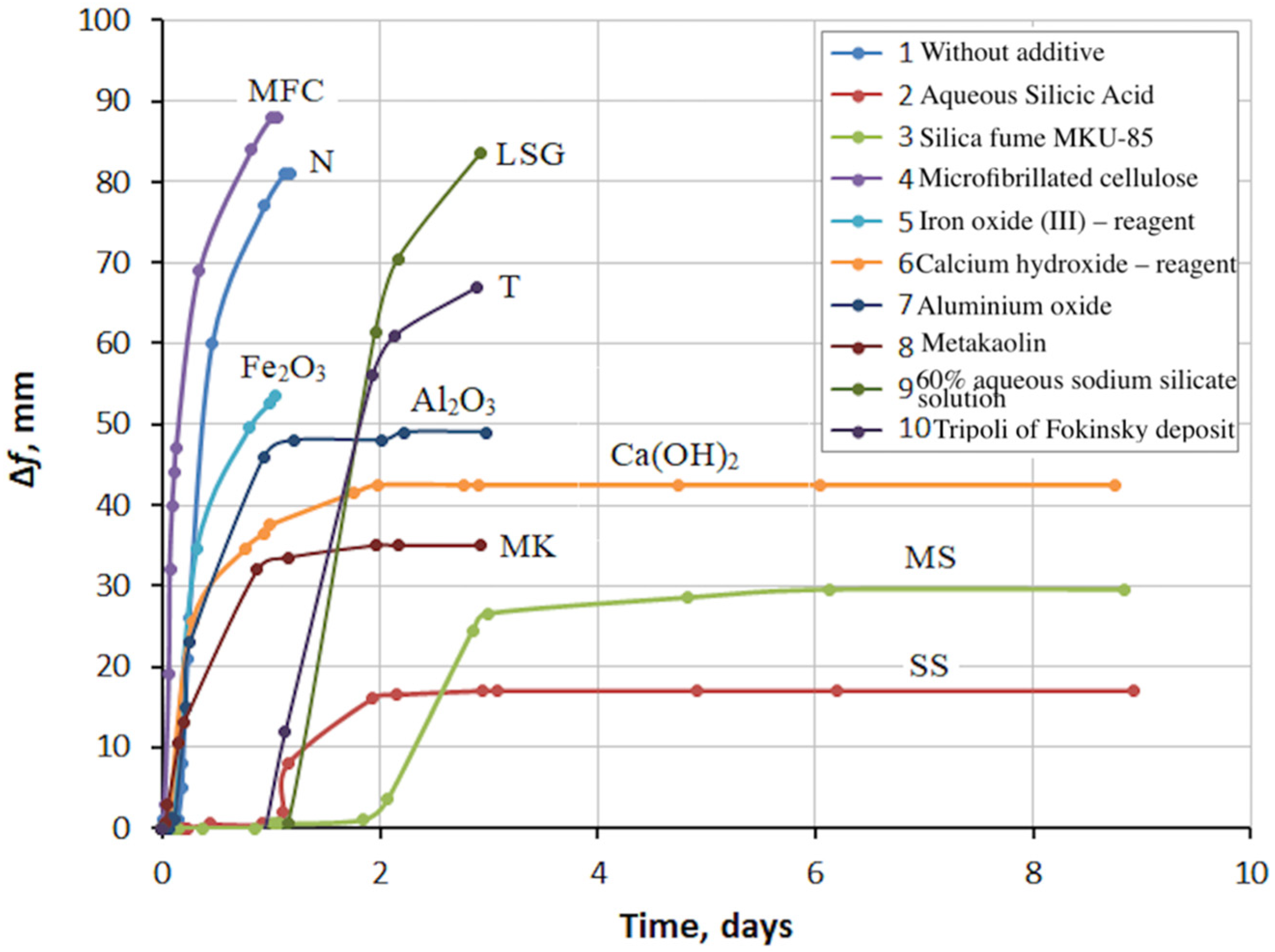
3.1. Evaluation of the Possibility of Regulating the Fly Ash Expansion Using Chemical Additives
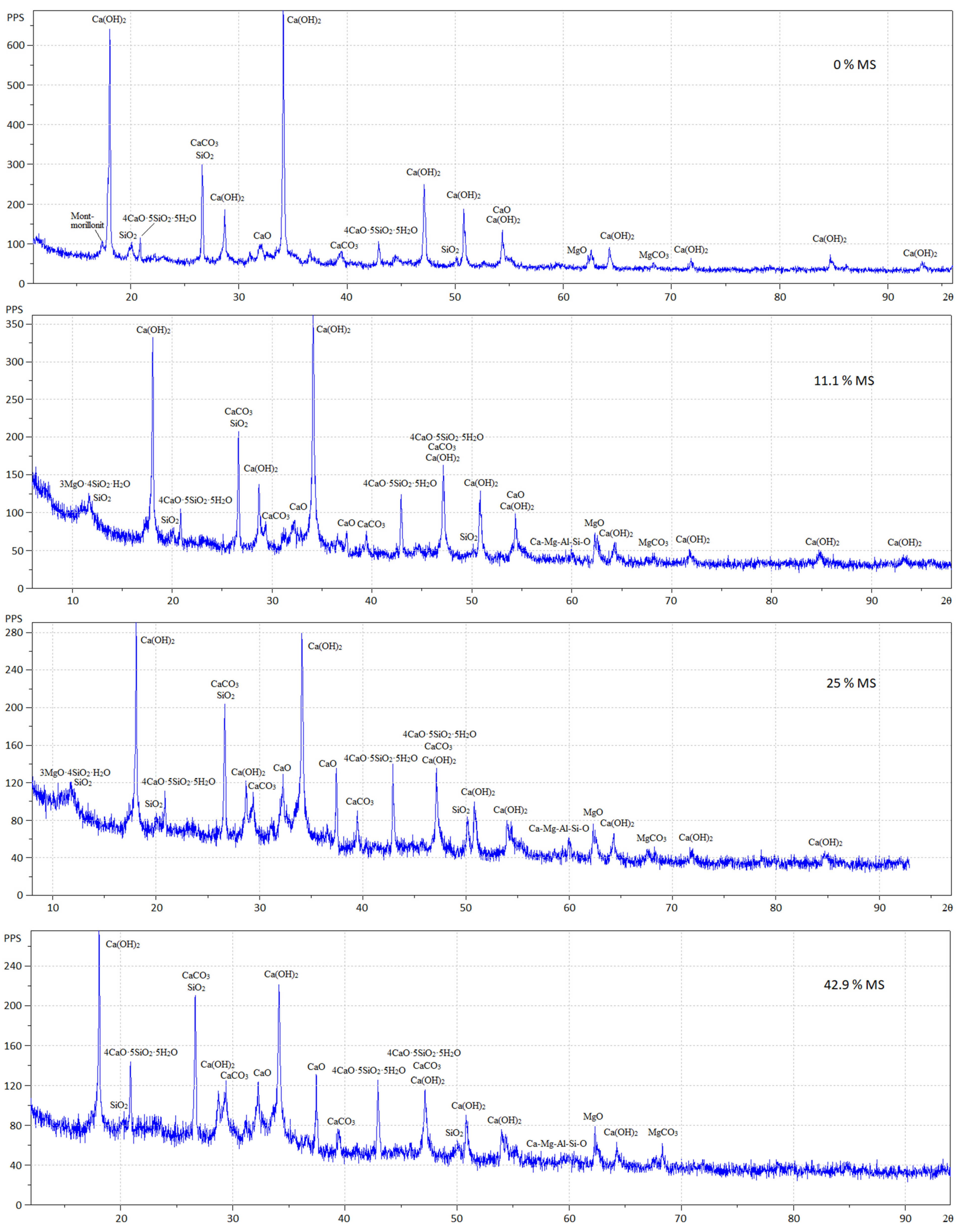
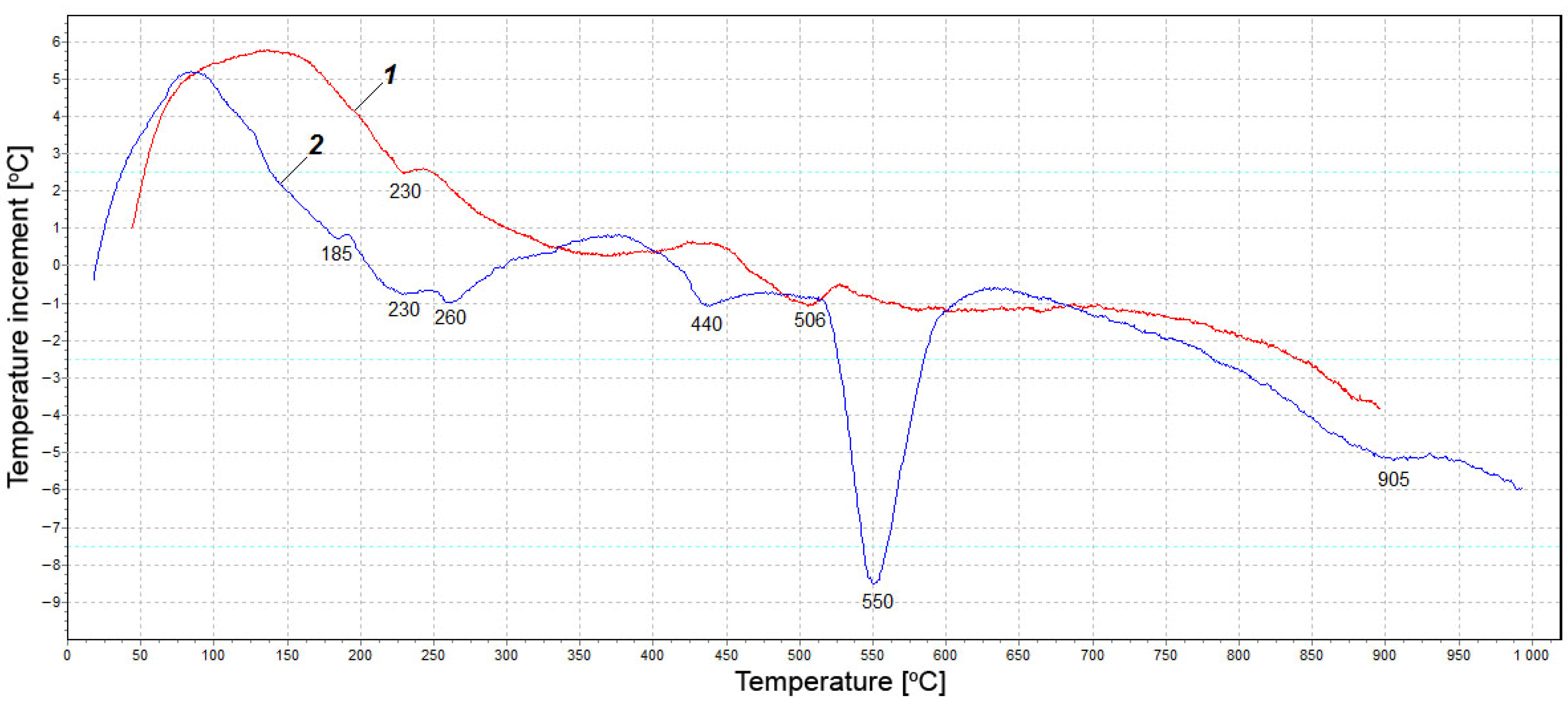
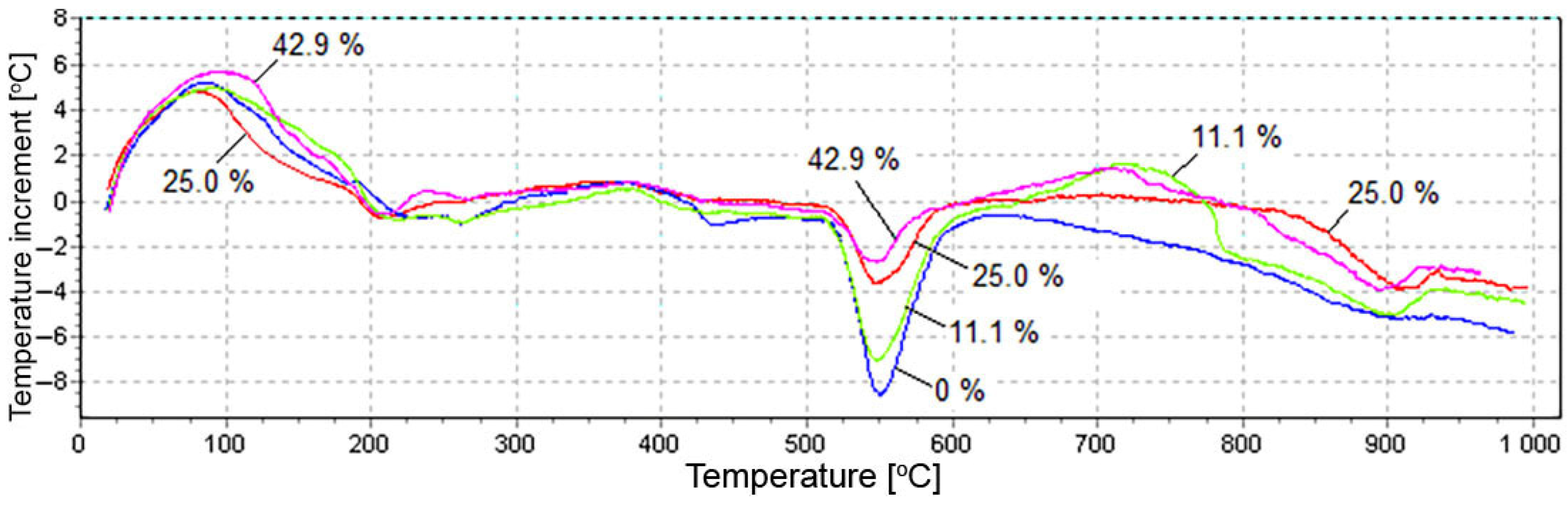
3.2. Test Results of X-ray Diffraction Analysis and Differential Thermal Analysis
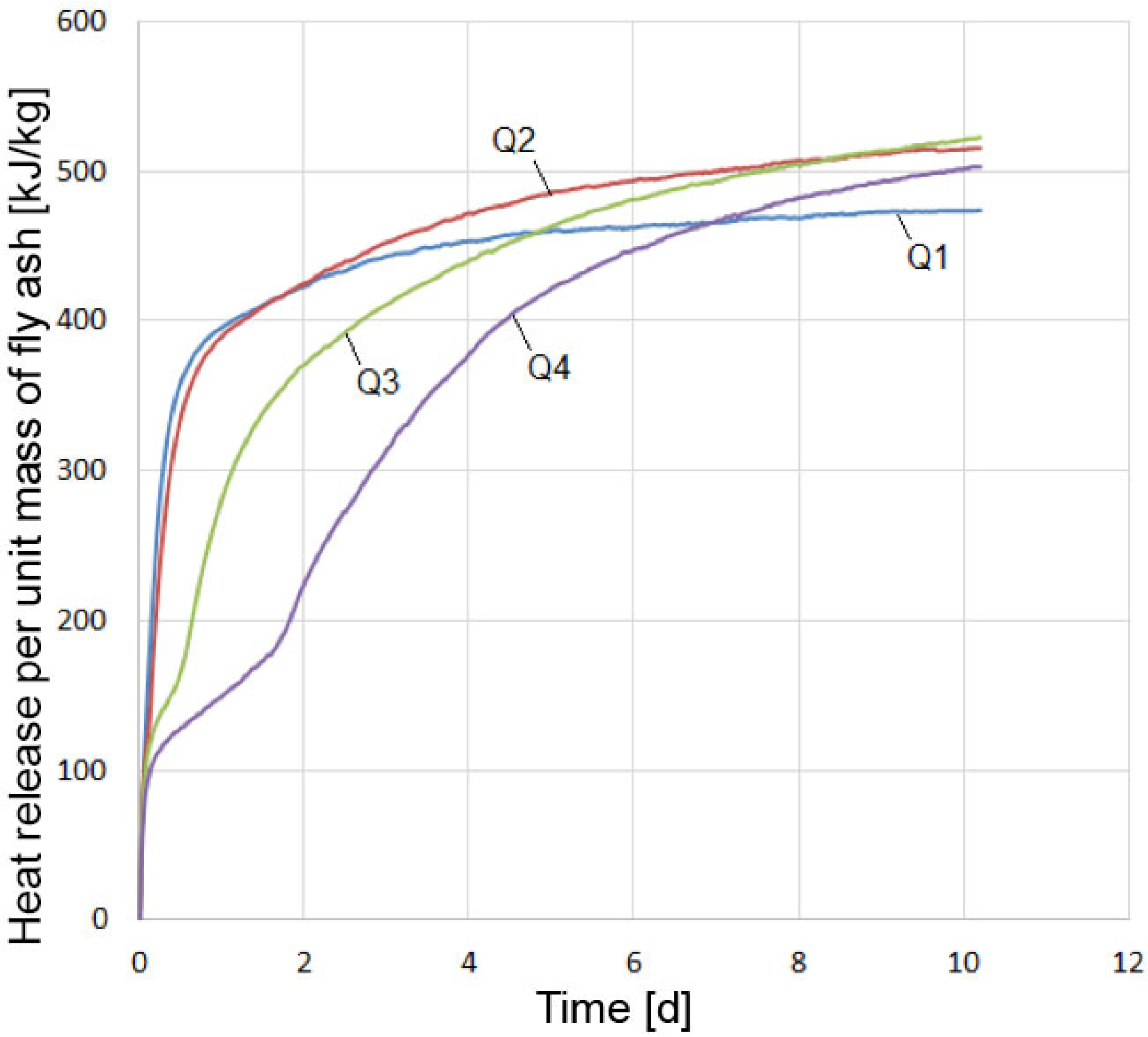
3.3. Heat of Hydration Test Results
3.4. Compressive Strength Test Results
4. Conclusions
- The fly ash of Berezovskaya GRES, captured by electrostatic precipitators, is capable of self-setting and hardening due to its chemical composition. However, the hardening of Beryozovskaya fly ash is accompanied by strong expansion and cracking due to the large amount of free lime. Therefore, this fly ash is not used as a binder or as a concrete additive and is also not suitable for granulation.
- A search for additives to the fly ash, which could prevent the expansion, was carried out. Aqueous silicic acid SiO2∙nH2O, silica fume MKU-85, micro-fibrillated cellulose, Fe2O3 (reagent, pure), calcium hydroxide Ca(OH)2 (reagent, pure), Al2O3 (AR), metakaolin Al2O3·2SiO2, 60% aqueous sodium silicate solution Na2O(SiO2)n, and tripoli of the Fokinsky deposit were tested in the Le Chatelier mold. The greatest effect was obtained from silicic acid and silica fume, and the smallest effect was obtained from 60% aqueous sodium silicate solution, Tripoli, and microcellulose.
- The expansion and cracking of the fly ash could be completely prevented by the addition of silica fume in an amount of about 40% by weight of the fly ash. At lower silica fume values, expansion was observed. The expansion increased with a decrease in the silica fume content.
- Silica fume allowed the fly ash to harden normally and gain strength in air, but did not provide sufficient water resistance to the hardened paste. Specimens swelled and cracked when placed in water.
- The results of XRD and DTA showed that free lime in the fly ash was completely hydrated in 11 days in the absence of silica and gave the highest degree of expansion. In the presence of silica fume, the hydration of lime by the specified time was not complete, and the expansion was reduced. Unslaked free lime remained in the system. Its amount increased with an increase in the silica fume content, while the content of Ca(OH)2 decreased. This circumstance could explain the low water resistance by the lack of water for the complete redemption of free lime, since water is additionally spent on the formation of calcium hydrosilicates, the amount of which increases with an increase in the dosage of silica fume. However, exothermic data showed that silica fume inhibited CaO hydration from the first seconds of the reaction.
- This work is a preliminary assessment of the effectiveness of silica fume for Berezovskaya GRES fly ash in relation to its expansion during hydration, due to the high content of free lime. On the basis of some data not included in the article, it was established that the strength and water resistance of mixes with fly ash from Berezovskaya GRES and silica fume can be increased by adding some accelerating agents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Jafer, H.M.; Atherton, W.; Sadique, M.; Ruddock, F.; Loffill, E. Development of a new ternary blended cementitious binder produced from waste materials for use in soft soil stabilisation. J. Clean. Prod. 2018, 172, 516–528. [Google Scholar] [CrossRef]
- Coudert, E.; Paris, M.; Deneele, D.; Russo, G.; Tarantino, A. Use of alkali activated high-calcium fly ash binder for kaolin clay soil stabilisation: Physicochemical evolution. Constr. Build. Mater. 2019, 201, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.N.; Khan, K.; Aslam, F.; Shah, M.I.; Javed, M.F.; Musarat, M.A.; Usanova, K. Multigene Expression Programming Based Forecasting the Hardened Properties of Sustainable Bagasse Ash Concrete. Materials 2021, 14, 5659. [Google Scholar] [CrossRef] [PubMed]
- Phoo-Ngernkham, T.; Hanjitsuwan, S.; Damrongwiriyanupap, N.; Chindaprasirt, P. Effect of sodium hydroxide and sodium silicate solutions on strengths of alkali activated high calcium fly ash containing Portland cement. KSCE J. Civ. Eng. 2016, 21, 2202–2210. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Characterization of the high-calcium fly ash geopolymer mortar with hot-weather curing systems for sustainable application. Adv. Powder Technol. 2017, 28, 2317–2324. [Google Scholar] [CrossRef]
- Vatin, N.; Barabanshchikov, Y.; Usanova, K.; Akimov, S.; Kalachev, A.; Uhanov, A. Cement-based materials with oil shale fly ash additives. IOP Conf. Ser. Earth Environ. Sci. 2020, 578, 12043. [Google Scholar] [CrossRef]
- Barabanshchikov, Y.; Usanova, K. Influence of High-Calcium Oil Shale Ash Additive on Concrete Properties. Lect. Notes Civ. Eng. 2021, 150, 23–34. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Mateus, R.; Camões, A.; Bragança, L.; Branco, F.G. Comparative environmental life-cycle analysis of concretes using biomass and coal fly ashes as partial cement replacement material. J. Clean. Prod. 2016, 112, 2221–2230. [Google Scholar] [CrossRef] [Green Version]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Gholampour, A. Fly Ash-Based Eco-Efficient Concretes: A Comprehensive Review of the Short-Term Properties. Materials 2021, 14, 4264. [Google Scholar] [CrossRef] [PubMed]
- Barabanshchikov, Y.; Usanova, K.; Akimov, S.; Uhanov, A.; Kalachev, A. Influence of Electrostatic Precipitator Ash “Zolest-Bet” and Silica Fume on Sulfate Resistance of Portland Cement. Materials 2020, 13, 4917. [Google Scholar] [CrossRef] [PubMed]
- Usanova, K. Properties of Cold-Bonded Fly Ash Lightweight Aggregate Concretes. Lect. Notes Civ. Eng. 2020, 70, 507–516. [Google Scholar]
- Narattha, C.; Chaipanich, A. Effect of curing time on the hydration and material properties of cold-bonded high-calcium fly ash–Portland cement lightweight aggregate. J. Therm. Anal. 2020, 145, 2277–2286. [Google Scholar] [CrossRef]
- Korniejenko, K.; Halyag, N.; Mucsi, G. Fly ash as a raw material for geopolymerisation–chemical composition and physical properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 12002. [Google Scholar] [CrossRef]
- Korniejenko, K.; Łach, M.; Marczyk, J.; Ziejewska, C.; Halyag, N.P.; Mucsi, G. Fly ash as a raw material for geopolymerisa-tion-mineralogical composition and morphology. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 12002. [Google Scholar]
- Yang, H.M.; Zhang, S.M.; Wang, L.; Chen, P.; Shao, D.K.; Tang, S.W.; Li, J.Z. High-ferrite Portland cement with slag: Hydration, microstructure, and resistance to sulfate attack at elevated temperature. Cem. Concr. Compos. 2022, 130, 104560. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Influence of recycled aggregate on fly ash geopolymer concrete properties. J. Clean. Prod. 2016, 112, 2300–2307. [Google Scholar] [CrossRef]
- Valanciunaite, J.; Poderys, V.; Bagdonas, S.; Dabrowski, B.; Zuchowska, A.; Kasprzak, A.; Shen, C.; Chen, L.; Jia, X. The use of fly ash the thermal power plants in the construction. IOP Conf. Ser. Mater. Sci. Eng. 2015, 93, 12070. [Google Scholar]
- Asa, E.; Shrestha, M.; Baffoe-Twum, E.; Awuku, B. Development of sustainable construction material from fly ash class C. J. Eng. Des. Technol. 2020, 18, 1615–1640. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Malkawi, A.B.; Nuruddin, M.F.; Fauzi, A.; Al-Mattarneh, H.; Mohammed, B.S. Effects of Alkaline Solution on Properties of the HCFA Geopolymer Mortars. Procedia Eng. 2016, 148, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.; Gong, W.; Liu, T.; Wang, N.; Tan, Y.; Wu, C. Effects of low- and high-calcium fly ash on the water resistance of magnesium oxysulfate cement. Constr. Build. Mater. 2019, 230, 116951. [Google Scholar] [CrossRef]
- Fan, W.-J.; Wang, X.-Y.; Park, K.-B. Evaluation of the Chemical and Mechanical Properties of Hardening High-Calcium Fly Ash Blended Concrete. Materials 2015, 8, 5933–5952. [Google Scholar] [CrossRef] [Green Version]
- Goenawan, V.; Hardjito, D. A Preliminary Study on Cracking Tendency of Cement Paste Incorporating High Calcium Fly Ash. Appl. Mech. Mater. 2015, 815, 158–163. [Google Scholar] [CrossRef]
- Shehata, M.H.; Thomas, M.D.A. The effect of fly ash composition on the expansion of concrete due to alkali–silica reaction. Cem. Concr. Res. 2000, 30, 1063–1072. [Google Scholar] [CrossRef]
- Dhole, R.; Thomas, M.D.A.; Folliard, K.J.; Drimalas, T. Sulfate resistance of mortar mixtures of high-calcium fly ashes and other pozzolans. ACI Mater. J. 2011, 108, 645–654. [Google Scholar]
- Antiohos, S.; Tsimas, S. Investigating the role of reactive silica in the hydration mechanisms of high-calcium fly ash/cement systems. Cem. Concr. Compos. 2005, 27, 171–181. [Google Scholar] [CrossRef]
- Korpa, A.; Seiti, B.; Xhaxhiu, K.; Mele, A.; Trettin, R.; Arjan, K.; Bujar, S.; Kledi, X.; Altin, M.; Reinhard, T. Processing of high sulphate and free lime calcareous coal fly ash for producing high volume blended cements and complying grade products employed in civil engineering. Zastita Mater. 2014, 55, 251–258. [Google Scholar] [CrossRef]
- Tsimas, S.; Moutsatsou-Tsima, A. High-calcium fly ash as the fourth constituent in concrete: Problems, solutions and perspectives. Cem. Concr. Compos. 2005, 27, 231–237. [Google Scholar] [CrossRef]
- Klyuev, S.V.; Bratanovskiy, S.N.; Trukhanov, S.V.; Manukyan, H.A. Strengthening of Concrete Structures with Composite Based on Carbon Fiber. J. Comput. Theor. Nanosci. 2019, 16, 2810–2814. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J. Effect of fibers on expansion of concrete with a large amount of high f-CaO fly ash. Cem. Concr. Res. 2003, 33, 1549–1552. [Google Scholar] [CrossRef]
- Klyuev, S.V.; Khezhev, T.; Pukharenko, Y.; Klyuev, A. Fibers and their Properties for Concrete Reinforcement. Mater. Sci. Forum 2019, 945, 125–130. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bulewicz, E.M.; Dudek, K.; Kozak, A. The long term behaviour of CFBC ash–water systems. Waste Manag. 2002, 22, 99–111. [Google Scholar] [CrossRef]
- Iribarne, J.; Iribarne, A.; Blondin, J.; Anthony, E. Hydration of combustion ashes—A chemical and physical study. Fuel 2001, 80, 773–784. [Google Scholar] [CrossRef]
- Domanskaya, I.; Oleynik, V.; Minyazev, D. ICSC Problems and Perspectives of high-calcium fly ash from heat power plants in the composition of “green” building materials. E3S Web Conf. 2016, 6, 1014. [Google Scholar] [CrossRef] [Green Version]
- Sheng, G.; Li, Q.; Zhai, J.; Li, F. Self-cementitious properties of fly ashes from CFBC boilers co-firing coal and high-sulphur petroleum coke. Cem. Concr. Res. 2007, 37, 871–876. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Li, X.; Guo, F.; Tang, S.; Lu, X.; Hanif, A. Influence of reactivity and dosage of MgO expansive agent on shrinkage and crack resistance of face slab concrete. Cem. Concr. Compos. 2021, 126, 104333. [Google Scholar] [CrossRef]
- Zaporozhets, I.D.; Okorokov, S.D.; Pariyskiy, A.A. Heat Liberation by Concrete; Stroyizdat: Moscow, Russia, 1966. [Google Scholar]
- Geiker, N.; Thaulow, M. Effect of fly ash and silica fume on alkali silica reactions. Proc. Eng. Found. Conf. 1994, 123–136. [Google Scholar]
- Lobo, K.; Cohen, M.D. Hydration of type K expansive cement paste and the effect of silica fume: I. Expansion and solid phase analysis. Cem. Concr. Res. 1992, 22, 961–969. [Google Scholar] [CrossRef]
- Dongyun, T.; Xiaoli, X.; Hua, L.; Huaqiang, C. Quantitative Study on Hydration Process of f-CaO in Cement Paste. J. Build. Mater. 2020, 23, 18–24. [Google Scholar]
- Nguyen, T.B.T.; Chatchawan, R.; Saengsoy, W.; Tangtermsirikul, S.; Sugiyama, T. Influences of different types of fly ash and confinement on performances of expansive mortars and concretes. Constr. Build. Mater. 2019, 209, 176–186. [Google Scholar] [CrossRef]
- Opravil, T.; Ptáček, P.; Šoukal, F.; Havlica, J.; Brandštetr, J. The synthesis and characterization of an expansive admixture for M-type cements I. The influence of free CaO to the formation of ettringite. J. Therm. Anal. 2012, 111, 517–526. [Google Scholar] [CrossRef]
- He, S.; Zhu, X.Y.; Bao, W.Z.; Zhao, W.X.; Jin, D.M. Study on Expansion of Circulating Fluidized Bed Combustion Coal Ash. Adv. Mater. Res. 2012, 518–523, 3501–3506. [Google Scholar] [CrossRef]
- Deng, M.; Hong, D.; Lan, X.; Tang, M. Mechanism of expansion in hardened cement pastes with hard-burnt free lime. Cem. Concr. Res. 1995, 25, 440–448. [Google Scholar] [CrossRef]









| SiO2 | Al2O3 | FeO | Fe2O3 | MgO | CaO | SO3 | K2O | Na2O | CO2 |
|---|---|---|---|---|---|---|---|---|---|
| 15.8 ± 6.9 | 8.0 ± 0.2 | 0.7 ± 1.0 | 7.0 ± 1.7 | 4.9 ± 0.8 | 46.7 ± 5.4 | 7.2 ± 4.8 | 0.76 ± 0.4 | 1.28 ± 0.9 | 3.61 ± 1.88 |
| Physical Property | Unit Measure | Value |
|---|---|---|
| Residue on sieve No. 008 | % | 8.7 |
| Residue on sieve No. 005 | % | 16.7 |
| Bulk density | g/cm3 | 1094 |
| True density | g/cm3 | 3.09 |
| Specific surface area (Blaine) | cm2/g | 2815 |
| Volume median diameter | µm | 7.7 |
| Chemical Compound | Conditional Content (%) | Chemical Compound | Conditional Content (%) |
|---|---|---|---|
| CaO | 42.6 | 2CaO·Al2O3 | 3.4 |
| SiO2 | 9.7 | 4CaO·Al2O3· Fe2O3 | 0.9 |
| Al2O3 | 5.4 | 2CaO·0.7Al2O3·0.3Fe2O3 | 0.7 |
| MgO | 4.6 | 4CaO·2MgO·Al2O3·Fe2O3 | 2.2 |
| 2CaO·SiO2 | 5.6 | 2CaO·0.2MgO·0.5Al2O3·0.3Fe2O3·0.2SiO2 | 3.3 |
| 3CaO·MgO·2SiO2 | 2.6 | 3Al2O3·2SiO2 | 2.6 |
| CaO·2Al2O3 | 4.2 | TiO2 | 1.7 |
| 3CaO·Al2O3 | 2.8 | C | 7.7 |
| Mix Number | Additive Type | Mix Symbol | Additive Content by Weight of Fly Ash (%) | Water–Solid Ratio W/S |
|---|---|---|---|---|
| 1 | Without additive | N | - | 0.43 |
| 2 | Aqueous silicic acid, SiO2∙nH2O—AR | SS | 18.4 | 0.8 |
| 3 | Silica fume MKU-85 | MS | 20.0 | 0.42 |
| 4 | Micro-fibrillated cellulose | MFC | 32.0 | 0.39 |
| 5 | Iron oxide Fe2O3—reagent, pure | Fe2O3 | 20.0 | 0.35 |
| 6 | Calcium hydroxide Ca(OH)2—reagent, pure | Ca(OH)2 | 17.4 | 0.33 |
| 7 | Al2O3—AR | Al2O3 | 40.0 | 0.48 |
| 8 | Metakaolin Al2O3·2SiO2 | MK | 42.9 | 0.5 |
| 9 | 60% aqueous sodium silicate solution Na2O(SiO2)n | LSG | 6.6 | 0.51 |
| 10 | Tripoli of the Fokinsky deposit (Bryansk region, Russia) | T | 42.3 | 0.54 |
| 11 | Silica Fume MKU-85 | MS | 5.3 | 0.42 |
| 12 | Silica Fume MKU-85 | MS | 11.1 | 0.42 |
| 13 | Silica Fume MKU-85 | MS | 17.6 | 0.42 |
| 14 | Silica Fume MKU-85 | MS | 25.0 | 0.42 |
| 15 | Silica Fume MKU-85 | MS | 33.3 | 0.42 |
| 16 | Silica Fume MKU-85 | MS | 42.9 | 0.42 |
| Material | Material Consumption (kg/m3) | |||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Fly ash from Berezovskaya GRES | 210 | 210 | 210 | 210 |
| Silica fume MKU-85 | 0 | 23 (11.1%) | 53 (25%) | 90 (42.9%) |
| Polyfractional sand | 1645 | 1623 | 1558 | 1476 |
| Water | 318 | 316 | 327 | 340 |
| Total | 2173 | 2172 | 2147 | 2116 |
| Sample Type | Phase Content (%) | ||
|---|---|---|---|
| Ca(OH)2 | CaOfree | Hydrosilicates | |
| Fly ash original | - | 100 | - |
| Fly ash after hydration | 96.2 | - | 3.8 |
| Fly ash + 11.1% silica fume after hydration | 80.1 | 8.1 | 11.8 |
| Fly ash + 25% silica fume after hydration | 67.8 | 17.9 | 14.3 |
| Fly ash + 42.8% silica fume after hydration | 63.3 | 18.2 | 18.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barabanshchikov, Y.; Usanova, K. Influence of Silica Fume on High-Calcium Fly Ash Expansion during Hydration. Materials 2022, 15, 3544. https://doi.org/10.3390/ma15103544
Barabanshchikov Y, Usanova K. Influence of Silica Fume on High-Calcium Fly Ash Expansion during Hydration. Materials. 2022; 15(10):3544. https://doi.org/10.3390/ma15103544
Chicago/Turabian StyleBarabanshchikov, Yurii, and Kseniia Usanova. 2022. "Influence of Silica Fume on High-Calcium Fly Ash Expansion during Hydration" Materials 15, no. 10: 3544. https://doi.org/10.3390/ma15103544
APA StyleBarabanshchikov, Y., & Usanova, K. (2022). Influence of Silica Fume on High-Calcium Fly Ash Expansion during Hydration. Materials, 15(10), 3544. https://doi.org/10.3390/ma15103544






