Abstract
Over the last decade, pedicle fixation systems have evolved and modifications in spinal fusion techniques have been developed to increase fusion rates and improve clinical outcomes after lumbar interbody fusion (LIF). Regarding materials used for screw and rod manufacturing, metals, especially titanium alloys, are the most popular resources. In the case of pedicle screws, that biomaterial can be also doped with hydroxyapatite, CaP, ECM, or tantalum. Other materials used for rod fabrication include cobalt–chromium alloys and nitinol (nickel–titanium alloy). In terms of mechanical properties, the ideal implant used in LIF should have high tensile and fatigue strength, Young’s modulus similar to that of the bone, and should be 100% resistant to corrosion to avoid mechanical failures. On the other hand, a comprehensive understanding of cellular and molecular pathways is essential to identify preferable characteristics of implanted biomaterial to obtain fusion and avoid implant loosening. Implanted material elicits a biological response driven by immune cells at the site of insertion. These reactions are subdivided into innate (primary cellular response with no previous exposure) and adaptive (a specific type of reaction induced after earlier exposure to the antigen) and are responsible for wound healing, fusion, and also adverse reactions, i.e., hypersensitivity. The main purposes of this literature review are to summarize the physical and mechanical properties of metal alloys used for spinal instrumentation in LIF which include fatigue strength, Young’s modulus, and corrosion resistance. Moreover, we also focused on describing biological response after their implantation into the human body. Our review paper is mainly focused on titanium, cobalt–chromium, nickel–titanium (nitinol), and stainless steel alloys.
Keywords:
metal alloys; implants; inter body fusion; titanium; stainless steel; cobalt-chromium; nitinol 1. Introduction
Over the past few decades, lumbar spinal fusion (lumbar interbody fusion, LIF) has been recommended as a well-known, standard surgical treatment for degenerative disc disease (DDD) of the lumbar spine. DDD may cause low back pain and radicular symptoms, which can significantly decrease the quality of life. The prevalence of symptomatic DDD increases with age and occurs in 10% of the male population at the age of 50 and up to 50% at the age of 70 [1]. According to some reports, DDD may concern even 90% of the population including asymptomatic cases [2]. LIF effectively provides stabilization of painful motion segment, restores lordosis and disc height, corrects the deformity, and may provide indirect decompression of dural sac and nerve roots [3,4]. That allows immediate relief of DDD symptoms. Other indications for this procedure include traumatic injuries, degenerative or congenital deformities, spondylolisthesis, spinal stenosis, and tumors [3,5,6,7].
There are various approaches to a lumbar interbody fusion. However, there is a lack of sufficient and reliable evidence to establish one of them as a standard lumbar fusion method. Posterior lumbar interbody fusion (PLIF) and anterior lumbar interbody fusion (ALIF) are the most traditional techniques. Nowadays there are other, less invasive methods including lateral lumbar interbody fusion (LLIF), extreme lateral lumbar interbody fusion (XLIF), oblique lumbar interbody fusion (OLIF), and transforaminal lumbar interbody fusion (TLIF) [6]. Moreover, minimally invasive approaches such as minimally invasive TLIF or percutaneous pedicle screw fixation have gained popularity recently [8]. All lumbar spinal approaches require the use of proper instrumentation. The basic spinal fixation device consists of pedicle screws, connection rods, a cross-link device, and in some cases an interbody cage. Pedicle screws are placed into the vertebral bodies through the pedicles of vertebrae, the Harrington rods connect screws of adjacent vertebrae, and the cage is inserted into the intervertebral space (Figure 1). Such an interbody device enables distraction of disc space and successfully stabilizes the pathological segment.
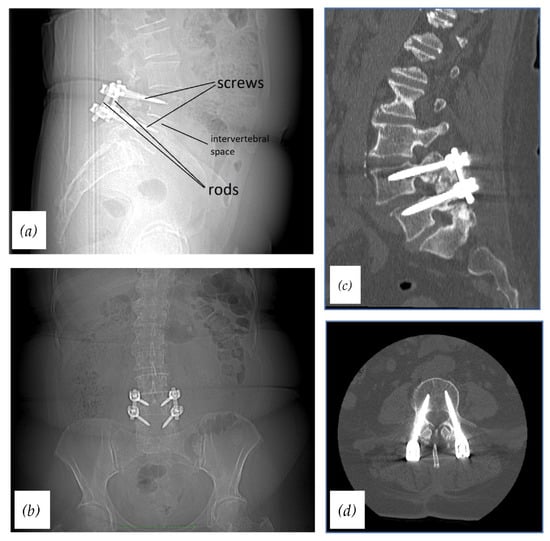
Figure 1.
Radiographs and CTs of stabilized lumbar spine using the standard spinal fixation device: (a) Radiograph, lateral view (with descriptions); (b) radiograph, AP view; (c) CT, sagittal plane; (d) CT, axial plane.
Over the last decade, pedicle fixation systems have evolved and modifications in spinal fusion techniques have been developed to increase fusion rates and improve clinical outcomes after LIF [3,7]. The new spinal fusion systems can vary in many strands including pedicle screw design (monoaxial or polyaxial), the method for the attachment to the spine, biomaterials used, and the screw–rod system connection (side-loading or top-loading) [7,9].
Spinal instrumentation efficiently improves interbody fusion rates and increases initial spinal stability. However, long-term increased stiffness of the stabilized segment provided by implanted screws and rods may lead to the development of adjacent segment degeneration (ASD) [10]. A possible pathogenic mechanism underlying that disease is the redistribution of stress at the adjacent levels, which results in increased intradiscal pressure and extended mobility in the neighboring segments [10,11]. These factors accelerate the degeneration of the adjacent segment intervertebral disc. ASD has been described mainly in the case of PLIF, but may also occur after other spinal fusion procedures. Recently, it has been noticed that ASD less frequently occurs after polyaxial pedicle screw fixation compared with monoaxial fixation [12].
The use of high-speed drilling, which is in some cases used during the LIF procedure, generates a lot of heat, which may cause thermal necrosis around the implant. To prevent this problem some authors showed that the use of CO2 as a coolant may be beneficial [13,14].
Regarding materials used for screw and rod manufacturing, metals, especially titanium alloys, are the most popular resources. Nowadays, many of them are made from titanium (mainly Ti-6Al-4V alloy). In the case of pedicle screws, that biomaterial can be also doped with hydroxyapatite, CaP, ECM, or tantalum. Regarding other materials used for rod fabrication, they include cobalt–chromium alloys and nitinol (nickel–titanium alloy) [9]. Furthermore, in the past few decades stainless steel (SS) has been commonly used in spinal instrumentation systems. Nowadays, SS is less often chosen as a biomaterial [10].
The purpose of this literature review is to summarize the physical and mechanical properties of metal alloys used for spinal instrumentation in LIF, which include fatigue strength, Young’s modulus, and corrosion resistance. Moreover, we also focused on describing biological response after their implantation into the human body.
Our article is mainly focused on titanium, cobalt–chromium, nickel–titanium (nitinol), and stainless steel alloys.
2. Physical and Mechanical Properties of Implant Important in LIF
The ideal biomaterial used in LIF should have high tensile and fatigue strength, Young’s modulus similar to that of the bone, and should be 100% resistant to corrosion to avoid mechanical failures. Therefore, the composition of the spinal rods and screws constitutes a crucial factor in defining the general functionality of the spinal instrumentation.
2.1. Fatigue Strength
One of the most important features of LIF implants is their fatigue strength. That property describes how long the spinal instrumentation can work without breaking down [15]. The cycling loading of the spine, which appears during daily activities, generates oscillating stresses on spinal instrumentation and may lead to a crack in the implant material. When the crack reaches a critical size, fatigue fracture of the material occurs, leading to the failure of the implant [15,16]. Remarkably, long cracks cause implant collapse slower than very small cracks, which are relative to the dimensions of the material micro-architecture [17]. Biomechanical performance and fatigue strength of spinal instrumentation significantly depend on the microstructure of the metal alloy of which it is made [18,19,20]. To increase the fatigue life of an alloy, many heat treatment techniques are implemented [21]. They include plasma-assisted microwave chemical vapor deposition, plasma nitriding, plasma etching, and deposition of amorphous diamond-like carbon (a-DLC) layers inoculated with nitrogen and silicon. It has been shown that these methods have a substantial influence on the surface characteristics and microarchitecture of alloys [22]. Furthermore, heat treatment allows for arranging an optimized balance of material features such as machinability, ductility, and stability [21].
Fatigue fractures almost always occur at stress concentration sites such as notches or the discontinuity of the geometrical structure of the material [16,17,23,24]. On the spinal rod’s surface, notches may be generated during manual contouring, which can impair the mechanical properties of the rod, especially its fatigue strength [16,25,26]. This phenomenon is known as the “notch effect”. Before metal rods are fixed to the patient’s spine, they are contoured to obtain optimal sagittal alignment of the spine [16,27]. Traditionally, contouring can be done by the surgeon with the use of the French bender. Alternatively, the rods can be anatomically designed and bent automatically by a machine before the surgical procedure. A biomechanical study by Yamada et al. [28] has shown that pre-contoured rods had a remarkably higher fatigue strength and ultimate load than intraoperative manually contoured rods at the same load condition. Moreover, pre-contoured rods not only reduce the risk of rod fracture but may also reduce operation time and bleeding and decrease the risk of infection in comparison to the manually bent rods [28,29]. This results from the manual contouring of the rods that requires a significant amount of time to adjust the proper shape of the rod. Furthermore, tightening of the screw leaves surface defects on the rods and may contribute to the notch effect formation, which has been described by many authors [16,17,18]. Therefore, avoiding severe tightening of the screws is recommended.
Recently, some studies have shown the negative influence of direct electrocautery use on the mechanical features of metal alloys [30,31,32]. According to a biomechanical study by Zobel et al. [31], electrocautery contact with the material was found to significantly decrease the fatigue strength of the Ti-6Al-4V titanium alloy. Even after a short contact with the electrode, the fatigue strength reduced remarkably. When electrocautery contact is applied at high-stress concentrations areas of instrumentation, it can be a notable problem for the mechanical properties of the implant and in extreme cases, it may lead to implant breakage [31,32]. Moreover, this problem was reported by many case studies in hip replacement surgery [32,33,34]. However, it remains unclear whether a decrease in fatigue strength after electrocautery contact depends on the material and whether it is determined by the type of the implant [31]. Furthermore, there is a lack of reports describing that issue in the case of other metal alloys. Regardless of that, spine surgeons should pay special attention and avoid any contact of the active electrocautery electrode with implants during the revision surgery, especially in the case of titanium implants and areas of the implant with high-stress concentrations.
2.2. Young’s Elastic Modulus
A further property, which is crucial for the metal alloy to be useful as the material for the spinal implant, is its elasticity, which is the ability of a metal to resist distorting influence and return to its initial shape. This feature of a material can be described by a physical quantity known as Young’s elastic modulus. The value of the elastic modulus of human cortical bone ranges from 10 to 30 GPa [35]. The perfect alloy for use in spinal fusion systems should have Young’s modulus as similar as possible to the bone. That prevents a phenomenon called a “stress shielding effect”. That term refers to the reduction in bone density around the implant due to bearing of the majority of the mechanical load by the instrumentation. Normally, the bone experience stresses and remodels in response to the loadings. Therefore, due to decreased load, bone atrophy progressively occurs and it may result in implant loosening and failure [36].
Contemporary material engineering enables the development of metallic biomaterials, which have a modulus of elasticity more similar to human bone. They include different compositions of metals in alloys, which impact their mechanical properties and also materials with various porosity. Creating pores in the alloys not only improves osteointegration of the implant due to the better ingrowth of bone tissue into them but can also affect the value of Young’s modulus of the material. When the porosity of alloy increases, strength and elastic modulus of alloy decrease linearly [37,38]. Therefore, designing and manufacturing implants with the value of the elastic modulus close to that of human bone is possible.
2.3. Corrosion Resistance
The perfect biomaterial should also be corrosion resistant in any environment, especially in the internal environment of the body over any period of time. Generally, corrosion is a progressive degradation of a material resulting from its interaction with the extracellular body environment [39]. That environment contains a lot of ions of sodium, calcium, potassium, magnesium, chloride, phosphate, and bicarbonate, which may be potentially very corrosive factors [15,39].
In the literature, there are three different types of corrosion—fretting corrosion, crevice corrosion, and galvanic corrosion. Each of them has been observed in metallic spinal fusion systems [40,41]. Fretting corrosion develops as a result of mechanical damage from repeated micromotion and friction over time, occurring during the patient’s daily activity. It leads to the release of debris into the surrounding tissue [40]. This type of corrosion is determined by multiple factors such as the design of spinal instrumentation, used metal alloy, electrochemical environment, and load conditions [42]. Crevice corrosion results from exposing the metal to a surrounding tissue fluid, which can induce a local corrosion process by the point destruction of the passive oxide film [43]. Galvanic corrosion is the result of the presence of two different metals in contact with each other in the fluid environment [40]. The use of dissimilar metal alloys in the same spinal instrumentation systems could improve its mechanical features. On the other hand, mixing dissimilar metals in spinal implants brings with it an increased risk of inducing galvanic corrosion [40,42,44]. However, biomechanical studies conducted in 0.9% sodium chloride at 37 °C and retrieval analyses of spinal instrumentation have shown no evidence of galvanic corrosion in spinal constructs made of different metals [42,45,46]. After the literature review, we compared breakdown potential to assess corrosion resistance of each discussed metal alloy (Table 1). Materials with breakdown potential below 300 mV are regarded as unacceptable. The value of breakdown potential above 600 mV is considered corrosion resistance. Materials with marginal breakdown potential which ranges from 300 mV to 600 mV should be tested under the indicated use [47].
Most metallic alloys used in LIF are passive metals, which means that they have a stable oxide film on their surface [48]. That layer plays an important role in corrosion protection and the loss of its stability results in inducing the corrosion. In the presence of the above-mentioned ions in the surrounding environment, especially chloride ions, the passive film may be damaged [49]. Mean chloride ion concentration in interstitial body fluids is 113 mEq/L, which can induce corrosion in metallic implants [50]. Moreover, cycling loading, micromotion resulting from fretting, and other mechanical factors may also discontinue the passive layer on the surface of the implant [51].
Corrosion has a negative impact and not only leads to failure of the implant but also may leach debris and metal ions that could be harmful to the surrounding tissue. Moreover, some studies of spinal implants have detected elevated serum metal ion levels [43,52,53]. Other studies have found metal debris in lymph and organs such as the liver, spleen, and kidneys [54,55]. Metal ion release induces biological complications such as toxicity, hypersensitivity, and also cancerogenicity [39]. This phenomenon has been correlated with the output of cytokines and metallic proteases by activated macrophages, neutrophils, and T lymphocytes [56]. Other noted complications include pseudotumor and particle-induced osteolysis [57,58]. Localized neurological damage associated with rod breakdown has also been noted in several case reports [59,60,61].

Table 1.
Quantitative comparison of mechanical properties of titanium alloys, cobalt–chromium, nitinol alloys, and stainless steel 316 L.
Table 1.
Quantitative comparison of mechanical properties of titanium alloys, cobalt–chromium, nitinol alloys, and stainless steel 316 L.
| Alloy | Ultimate Tensile Strength [MPa] | Yield Strength [MPa] | Fatigue Strength [MPa] | Young Modulus [GPa] | Corrosion Resistance (Breakdown Potential) [mV] | References |
|---|---|---|---|---|---|---|
| Commercial Pure Titanium (CP-Ti) | 240–550 | 170–480 | 430 | 115 | 9000 | [21,62] |
| Ti-6Al-4V | 930 | 860 | 500 | 110 | 25,000 | [21,62,63] |
| Ti-24Nb-4Zr-8Sn (Ti2448) | 665 ± 18 | 563 ± 38 | 375–500 | 53 ± 1 | nd | [21,64,65] |
| Cobalt–Chromium | 655 | 450 | 310 | 210 | 870 | [30,62,63,66] |
| Nickel–Titanium | 895 | 195–690 (austenitic phase) 70–140 (martensitic phase) | nd | 40–75 | >1000 | [63,65] |
| Nickel–Titanium (CS 64% porous) | nd | ~700 | nd | 1 | 772 | [67] |
| 316L Stainless Steel | 490–1350 | 190–690 | 146 | 210 | 400–600 | [21,47,62,67,68] |
3. Mechanical Characteristics of the Most Frequently Used Metal Alloys in LIF
3.1. Titanium
Among all the metallic alloys used in the manufacturing of spinal instrumentation for LIF, titanium alloys are the most common materials. They owe their popularity to their excellent biocompatibility, superior mechanical properties, great corrosion resistance, and appropriately low Young’s modulus and generate minimal artefacts on computed tomography or magnetic resonance imaging [17,37,69,70]. These properties are highly preferable for biomedical applications. Due to the low elastic moduli and quite often observed notch effect of the titanium rods, titanium alloys are more often used in spinal screw fabrication than the spinal rods [9,16]. In contact with the air, a passive oxide film (TiO2) forms on the surface of the titanium. This layer is probably responsible for resistance to corrosion, chemical inertness, and stability of that metal [37].
There exist two well-known allotrophic phases of titanium—α and β phases. The type of alloy depends on the allotrophic phase of titanium which has been applied. Thus, we distinguish between α, near-α, α–β, and β alloys of titanium (Table 2).

Table 2.
Composition of titanium alloys used in lumbar interbody fusion.
Ti-6Al-4V alloy (α–β type alloy) is the most frequently used titanium alloy for spinal fixation devices [48,72,76]. Despite biocompatibility, excellent corrosion, and mechanical resistance of that alloy, its elastic modulus (~110 GPa), which is higher in comparison to human bone, may induce a stress-shielding effect and result in pedicle screw loosening and bone absorption [15,76,77,78]. Moreover, some studies have shown that Ti-6Al-4V accelerates the development of adjacent segment disease [78]. However, compared with other non-titanium metallic alloys used in spine fusion systems, Ti-6Al-4V has a relatively low modulus of elasticity and the stress-shielding effect is not as strong. Coating the pedicle screws with various materials such as PMMA, hydroxyapatite, extracellular matrix, and titanium plasma spray in tantalum was developed to improve the fixation and pull-out strength of the Ti-6Al-4V screws [79]. Many studies have successfully shown that coated screws may significantly increase resistance against pull-out force in comparison to uncoated screws [80,81,82]. Regarding potential toxicity associated with the leaching of the vanadium and aluminum ions from the Ti-6Al-4V implants, the amount of these metals released is minimal and does not induce suspected health problems, such as neurological or enzymatic disorders [83].
To better adjust the elastic modulus of titanium biomaterials to the cortical bone, increasing the β phase percentage in the alloy is an effective way [69]. One of them, in which this method was applied, is Ti-24Nb-4Zr-8Sn (Ti2448 alloy, α–β type). This material, drawing the attention of many researchers, has a lower Young’s modulus (~49 GPa) than Ti-6Al-4V and shows no toxic features [84]. Therefore, due to the elastic modulus value being more similar to human bone, the stress-shielding effect may be significantly less observed. It has been confirmed in a study conducted by Qu et al. [85] on a porcine model, which compared stress-shielding effects between Ti-24Nb-4Zr-8Sn alloy and Ti-6Al-4V alloy. Another low-modulus titanium alloy is the Ti-45Nb alloy (β type alloy) [73,74,75]. Besides the decreased value of Young’s elastic modulus, Ti-45Nb presents beneficial osteogenic features, which result from a high content of niobium [86]. Additionally, titanium alloys with increased content of β phase show increased corrosion resistance [87]. On the other hand, titanium alloys with low elastic modulus, such as β type alloys, usually also have low mechanical strength [78]. One of the well-known effective methods to increase the mechanical resistance of metals is precipitation hardening. However, due to irreversible changes in the crystalline structure, Young’s modulus increases, and corrosion resistance reduces due to this technique [88]. On the other hand, many studies have shown that severe plastic deformation (SPD) techniques, which include high-pressure torsion (HPT) [74,89,90,91], hydrostatic extrusion (HE) [92], and rolling and folding (R&F) [75], may improve the strength of β type titanium alloys without changing Young’s modulus. SPD techniques also improve corrosion resistance through the thickening of passive film [91]. However, alloys containing niobium, molybdenum, wolfram, or tantalum are expensive to manufacture due to the rarity and high melting points of these metals [48]. Thus, β type alloys such as Ti-24Nb-4Zr-8Sn and Ti-45Nb, despite their appropriately low Young’s modulus, excellent corrosion resistance, and sufficient mechanical properties after SPD processing, may have problems with spread of their use in LIF devices due to the very high costs of production.
Decreasing Young’s modulus of titanium alloys may be also achieved by creating pores in them. A biomechanical study by Skolakova et al. [83] has shown that Ti alloy with the addition of 30 wt.% pore-forming agent (PA) obtained with self-propagating high-temperature synthesis (SHS) exhibits a very similar elastic modulus (~9 GPa) to human bone with good corrosion resistance. However, mechanical strength decreased after the SHS procedure and further studies are necessary to evaluate the usefulness of Ti with 30 wt.% PA in LIF.
3.2. Cobalt–Chromium
Another metal alloy which may be used in LIF systems is cobalt–chromium (CoCr) alloy. This biomaterial usually consists of 63% cobalt, 28% chromium, 5% molybdenum, and minor amounts of other metals [48]. Well-known applications of CoCr alloy as biomaterial include hip and knee joint implants, as well as crowns and implant abutments in dentistry [48,83,93]. CoCr is characterized by higher fatigue life and strength, increased stiffness, and better resistance to notch effects in comparison to titanium alloys [18,23,94,95,96,97]. Due to its higher Young’s modulus (~210 GPa) [30] than titanium, CoCr spinal rods more effectively stabilize the spine and correct abnormalities of spinal curvatures such as scoliosis [96,98,99]. In the study by Willson et al. [100], CoCr rods demonstrated the least amount of shape loss in a radius of curvature compared with commercially pure titanium rods throughout the study.
However, high stiffness of CoCr rods may result in acceleration development of adjacent segment disease [99,101]. According to a comparative study by Han et al. [102], breakages of CoCr rods have been less observed than for titanium rods, but in the case of CoCr, they observed a more frequent occurrence of proximal junctional kyphosis (PJK), which is a form of adjacent segment degeneration. Moreover, a Young’s modulus significantly higher than that of human bone disqualifies CoCr alloy as a biomaterial for screw manufacturing due to the increased risk of stress-shielding effects. Furthermore, compared with titanium alloys, CoCr has lower corrosion resistance, which results in higher overall metal ion release from CoCr implants. Leaching cobalt ions from the alloy due to fatigue and biocorrosion may cause metallosis, neurological-related symptoms (such as deafness and blindness), hypothyroidism, cardiological and hematological problems, and also cancers [103,104,105,106]. To prevent these issues, coating of CoCr implants with ceramics such as calcium phosphate can decrease cobalt ion release and improve biocompatibility, which has been proven in a study by Bandyopadhyay et al. [107] with the use of the surface melting (LSM) technique for tribofilm formation.
3.3. Nitinol
Nickel–titanium (nitinol) is a metal alloy which consists of titanium and nickel in equal atomic percentages [48]. Among all the alloys used in spinal fusion devices, nitinol is characterized by a unique feature which is its superelasticity [48,83,108]. This phenomenon enables the nitinol implant to immediately return to an undeformed shape after removal of external load, even after large deformations. In this way, the use of this super-elastic alloy in LIF systems as rod material makes stabilization more dynamic and may prevent ASD occurrence [63]. Moreover, nitinol is used clinically in intravascular stents, osteosynthesis staples, and orthodontic wires [63,67,83].
Additionally, nitinol has Young’s modulus ranging from 40 to 75 GPa, which is optimal for biomedical applications. Moreover, nitinol fabrication by combustion synthesis (CS) enables tailoring its elastic modulus to that of human bone with great accuracy. After that procedure, metal alloy achieves high compressive strength with appropriately low Young’s modulus and excellent corrosion resistance [67]. According to Aihara et al. [67], general porosity of nitinol to obtain the best elasticity was found to be 64%.
Due to the formation of a passive titanium oxide film (TiO2) on the surface of the nitinol, it is considered a long-term corrosion-resistant and biocompatible alloy [63]. Therefore, coupling nitinol rods with titanium pedicle screws may be considered the best combination for spinal fusion devices due to its high resistance to galvanic corrosion [109]. The corrosion resistance of nitinol alloy is better than CoCr and 316L stainless steel, but inferior to that of Ti-6Al-4V [48,63]. However, the in vitro study combined with retrieval analysis of the nitinol, CoCr, and Ti-6Al-4V rods by Lukina et al. [63] has also shown that nitinol fretting corrosion patterns were worse compared with CoCr. That result may be an effect of lower resistance to fretting corrosion of the nitinol due to higher mobility of the rod. Moreover, intensive fretting may damage the passive oxide layer, whose restoration is relatively low. Thus, it may induce galvanic corrosion and deteriorate its overall corrosion resistance. As result, it affects the fatigue strength of nitinol and may release nickel ions into the blood. However, some studies have shown that the nickel ion levels in blood and tissues were not higher compared with the control group. In any case, to prevent fretting corrosion, coating nitinol rods with protective layers and enhancing the locking mechanism of the pedicle screws would be beneficial solutions [63].
3.4. Stainless Steel
Before introducing titanium alloy as a biomaterial for spinal construct manufacturing, stainless steel (SS) was the most popular metal alloy in this field. It is widely used for other biomedical applications such as bone fixation, cardiovascular systems, catheters, surgical instruments, or dental crowns. Surgical 316 L SS is the most common form of stainless steel for biomedical uses. This specific composition consists of 0.02% carbon, 10–14% nickel, 16–18% chromium, 2% manganese, 2–3% molybdenum, with the rest being iron. The high mechanical properties of this alloy are great advantages for use in spinal fixation. However, SS exhibits a significantly higher elastic modulus (210 GPa) [67] in comparison to human bone. Thus, the stress-shielding effect is strongly observed after SS implant application [110]. Regarding corrosion resistance, many studies have shown that it is significantly inferior compared with CoCr and titanium alloys [40,42,45]. Long-term biomechanical tests by Singh et al. [45] have shown that both CoCr and titanium constructs were more resistant to the fretting corrosion compared with SS. Moreover, during the corrosion process, SS constructs have produced a noticeably greater volume of debris than titanium or CoCr instrumentation systems [45]. Therefore, stainless steel should no longer be in used in spinal surgery.
4. Biological Response to Metal Implants Used in LIF
Implanted material elicits a biological response driven by immune cells at the site of insertion as well as systematically [111]. These reactions are subdivided into innate (primary cellular response with no previous exposure) and adaptive (a specific type of reaction induced after earlier exposure to the antigen) and are responsible for wound healing, fusion, and also adverse reactions, i.e., hypersensitivity [112,113,114]. A comprehensive understanding of cellular and molecular pathways is essential to identify preferable characteristics of implanted biomaterial to obtain fusion and avoid implant loosening.
4.1. Wound Healing
Bone decortication and bleeding are caused by the inserted implant triggering intramembranous ossification, a process essential for successful bone remodeling and incorporation, leading to arthrodesis [115,116,117]. Implantation of the metal screw is followed by adsorption of a proteinaceous layer [118] (made of serum molecules, water, and proteins) on the implant surface followed by the formation of a blood clot that recruits inflammatory cells on the side of the instalment and initiates provisional matrix formation [119]. Complex molecular processes following wound healing and interbody fusion are characterized by three main phases described initially by Boden et al.: inflammatory, reparative, and remodeling phases [120]. The acute inflammatory phase lasts up to three weeks and is defined by the migration of inflammatory cells including lymphocytes, leukocytes, and macrophages and secretion of cytokines at a site of merging [121]. Pro-inflammatory IL-6 and C reactive protein are the key components of this stage. Here, it is vital to distinguish the healing process from complications such as surgical site infection [122]. According to Thalander and Larsson, the levels of CRP and IL-6 reach their peak on post-operative day 3 and then decrease with time [123]. If otherwise, the patient should be suspected as having infectious process development [124]. The prolonged, unresolved inflammatory response may lead to chronic inflammation, the development of granulation tissue, and the formation of a fibrous capsule—a host of reactions that lead to implant dysfunction. The main process following the second, reparative stage is the differentiation of progenitor cells, neovascularization, and resorption of necrotic tissue [125]. The fusion mass subsequently matures at the entry point (transverse processes in the case of lumbar fusion) which is followed by the migration of the ossification process to the central zone [126]. Consequently, the last, remodeling phase occurs, which is defined by further maturation of new bone tissue and an increase in cortical to cancellous bone ratio [127]. The evolution of these processes is regulated by the expression of various genes responsible for the translation of bone morphogenic proteins—BMPs 2, 4, and 6 [128].
The success and intensification of the mentioned processes are largely dependent on the properties of the implanted material. In recent years, instead of producing materials diminishing host responses, designed implants are made of biomaterials that aim to modulate immunologic reactions towards enhanced fusion [129]. Desirable attributes which define biocompatibility leading to osteointegration include osteogenicity, the capacity to provide stem cells and osteoblast enabling new bone formation [130], osteoinductivity, recruitment of osteogenic growth factors [131], and osteoconductivity, ensuring appropriate conditions for the ingrowth of bone-forming elements and providing a scaffold for osteogenic cells and neovascularization [132,133].
The opposite, undesirable chronic inflammatory reaction on the implant/bone interface caused by metal debris or ions results in peri-implant bone osteolysis (PPOL) [134], a process that threatens permanent implant endurance.
4.2. Foreign Body Reaction
Acceptance of implanted instrumentation is dependent on various innate reactions that are collectively described as foreign body response (FBR) reactions that begin immediately after insertion [135]. Injury to the bone tissue results in the recruitment of immune cells and activates coagulation and complement pathways [136]. Subsequently, extravasated proteins, i.e., fibronectin, fibrinogen, and vitronectin, are adsorbed on the metal–tissue interface, which contributes to the formation of a provisional matrix in the vicinity of the implanted material and, after migration of macrophages, contributes to the formation of foreign body giant cells [137]. Due to potency variation of the Vroman effect [138], a process of competitive protein adsorption and desorption on a metal surface, foreign body response differs among materials used in fusion [139]. This event is followed by neutrophil recruitment which further enhances the inflammatory process, i.e., activation of mast cells and attraction of monocytes and, consequently, the transformation of monocytes to macrophages [140]. Accumulation of cells and proteins on biomaterial through integrins, particularly aMB2 [141,142], creates a privileged microenvironment between the implant and host tissues. Due to the process called “frustrated phagocytosis”, macrophages release degradation enzymes and ROI that aim to break down implanted biomaterial [135]. Unsuccessful degradation leads to the transition from an acute to chronic phase hallmarked by a switch from M1 to M2 macrophage polymerization [143]. Whereas M1 macrophages, referred to as classically activated, are pro-inflammatory, M2 macrophages, which are alternatively activated, are responsible for anti-inflammatory reactions that participate in wound healing. M1 macrophages produce TNF1, IL-1, and IL-6 and are linked to the Th1 type of immune response [144]. M2 macrophages produce anti-inflammatory cytokines including IL-4, IL-10, and IL-13 and are associated with Th2 immune response [145,146]. Although, naturally, the preponderance of M2 polymerized macrophages heralds a typical wound healing process, in the case of biomaterial implantation their predominance marks a shift from elimination to the tissue healing process. In the chronic phase, macrophages fuse to create foreign body giant cells on the implant’s surface [147]. This process is followed by neovascularization mediated by VEGF and PDGF and terminates when the created capsule becomes entirely isolated from neighboring tissues [148].
4.3. Response to Implant Wear Debris and Metal Ions
Biological reactivity to metal implant debris is the main factor that determinates successful spinal implant fusion and is the leading cause of undesirable implant rejection. There are two main types of immunologic reactions induced by implanted metals: innate and adaptive [149,150].
Resident macrophages are responsible for the slow elimination of metal wear debris particles due to subtle innate, non-antigen-specific immune responses [151]. As a result, no immunologic memory is preserved after exposure. Apart from that, their activation inaugurates a response to metal ions in a hypersensitivity reaction, a delayed hypersensitivity response (DTH), which is a type of adaptive T lymphocyte and antigen-dependent reaction resulting in immunologic memory development. Both processes cause pain, implant loosening, and, in consequence, aseptic implant failure [152].
4.4. Innate Reaction
Innate reactions directed to metal implant wear debris are central immune responses that lead to implant failure. Identification and uptake of wear particles activate macrophage pattern recognition receptors (PRRs) and initiate the release of pro-inflammatory cytokines (IL-1, IL-6, TNFα, PGE-2), chemokines (monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein (MIP-1a)) and pro-osteoclastic factors (receptor activator of nuclear factor kappa B ligand (RANKL)) [153,154]. PPRs recognize stimuli composing pathogen- or danger/damage-associated molecular patterns (PAMPs and DAMPs) and are divided into Toll-like (TLRs) and C-type leptin receptors (CLRs) [155]. Metal particles directly activate TLRs resulting in activation of NLRP3 inflammasome and promotion of interleukins such as IL-1B secretion [156] and recruitment of myeloid-lineage cells [157,158]. Inflammasomes function as the main regulator of the wound healing process and cause the production of various immune mediators including IL-1B, IL-6, CXCL8/L8, CCL/MCP-1, TNFα, nitric oxide, etc. [159,160].
Pro-inflammatory cytokines, i.e., IL-1B and TNFα, enhance the expression of RANKL and inhibit the expression of suppressors of osteoclastogenesis (i.e., osteoprotegerin), impede mesenchymal stem cell differentiation into osteoblasts, and even cause osteoblast apoptosis [161,162]. RANKL binds to RANK receptors on osteoclast precursors (OCPs) and activates nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and mitogen activated protein kinase (MAPK) signaling pathways, resulting in augmentation of bone resorption and consequent implant failure [163,164].
4.5. Adaptive Response
Adaptive immunity depends on the activity of lymphocytes, is antigen dependent, and results in the formation of immunologic memory following exposure. Metal particles, i.e., ions, act as haptens with high immunogenic potential. Some, especially when present at excessive levels, are able to initiate an adaptive immune response, in the form of antigen-dependent metal allergy or type IV delayed type hypersensitivity (DTH) [165]. Due to the preponderance of leukocytes among macrophages, giant cells, and other cells within peri-implant pseudotumor/granule tissues, it has been proposed that adaptive reactions play an important role in metal implant failure. These reactions are characterized by vasculitis and infiltration of the vessel wall, perivascular space, endothelium edema, and necrosis.
Among known metals, beryllium [166], chromium [167], cobalt [168], nickel [169], tantalum [170], titanium [171], and vanadium [172] belong to metals considered as sensitizers. While nickel is known as the most common allergen in humans, chromium and cobalt are frequent hypersensitivity inducers [173]. The available literature provides data that demonstrate relevant dependence between the amount and size of metal debris and initiation of hypersensitivity reactions [152,174]. In an event of suspicion of metal allergy, a lymphocyte transformation test is advised.
4.6. Biocompatibility of the Most Frequently Used Metal Alloys in LIF
4.6.1. Titanium
Out of all available alloys, commercially pure titanium possesses the distinctive feature of osteointegration, an ability to create a direct structural and functional connection between the implant and bone tissue without the production of any soft tissues in between. Following implantation, on a micro- and nanometer scale, titanium is covered by a thin Ti oxide layer, proteinaceous layer, a slender cell layer, calcified region, and bone tissue [175]. The efficacy and rate of the osteointegration process are enhanced by modification of Ti properties by altering surface micro- and nanostructure, roughness, hydrophilicity, biological surface treatment, or chemical addition [176,177,178,179,180]. Titanium presents early macrophage polarization into M2 macrophages, resulting in early anti-inflammatory/reparative (ARG1, CD4+) response [181]. Moreover, the change in surface structure modulates the degree of polarization into pro-healing M2 macrophages [179]. Through that mechanism, ceramic coatings with the use of hydroxyapatite enhance osteogenic bone response [182]. In a study conducted by Trinidade et al., during the first 10 days following titanium implantation, bone suppression markers were downregulated in an in vivo rabbit model [183]. In a further study on an animal model that investigated the osteointegration process in the first 4 weeks following implantation, the genetic evaluation revealed suppression of bone resorptive genes including ANKL, OPG, TRAP, and CathK compared to a sham control group [183,184].
4.6.2. Titanium Alloys
Commercially pure titanium (CP-Ti) and titanium alloy Ti-6Al-4V are both widely utilized in the operative field as metal implants. Although there are growing concerns about toxicity of vanadium [185], in a study conducted by Doe et al., despite achieving highest concentrations after 4 weeks of implantation, toxic levels have not been reached in animal models [186]. The search for alternatives has driven adaptation of other alloys such as Ti-6Al-7Nb, Ti-5Al-2.5Fe, Ti-15Mo, Ti-13Nb-13Zr, Ti-12Mo-6Zr-2Fe, Ti-35Nb-5Ta-7Z, and Ti-29Nb-13Ta-4.6Zr. Some components present great biocompatibility (i.e., Au, Ca, Mg, Mo, Nb, Pt, Pd, P, Sr, Sn, Si, Ta, Ti, and Zr) but others, i.e., Al, Ag, Be, Cr, Co, Cu, Cr, Fe, Mn, Ni, V, and Zn, display toxic reactions both in in vitro and in vivo studies [187,188,189]. Hence, alloys comprising biocompatible elements—Ti-39Nb-6Zr (TNZ) and Ti-39Nb-6Zr + 0.45Al (TNZA)—started to gain growing attention [190]. Nevertheless, some studies suggest a detrimental effect of Al and its involvement in neurodegenerative disorders or metabolic diseases [104,191] which might necessitate additional research that would evaluate its employment in spinal surgery.
4.6.3. Cobalt–Chromium
As reported in recent studies, metal ions and wear particles have the potential to leach from metallic implants, eliciting adverse immunologic reactions [192,193,194]. In in vitro studies conducted by Moeed Akbar et al., Cr (6+) and Co (2+) ions affected primary human lymphocytes by inducing apoptosis, inhibiting T lymphocytes and impeding the release of IL-2 through yet unknown mechanisms [195]. This is also viable with low circulating ion levels [196]. Cobalt and chromium alloy particles as well as their ions activate the NLR family pyrin domain containing 3 (NLPR3) inflammasome and caspase-1 mediated pathway, leading to activation of pro-interleukin IL-1B and pro-IL-18 as a part of an innate immunologic response [197,198,199]. This in turn activates NFκB that stimulates various pro-inflammatory responses [156].
4.6.4. Nitinol
Nickel is the most common contact allergen and affects up to 10% of representatives of the Caucasian population [200,201].
Nitinol has similar biocompatibility to titanium and it is better than that of stainless steel, therefore it shows promising potential in clinical application. It has similar fibroblast and osteoblast proliferation potential to Ti and SS [202]. In a study conducted by Haider et al., Ni ions exhibited greater toxicity on HUVECs than Cr and Ta [203]. Biocompatibility of nitinol then depends on Ni which is advised not to exceed 50% [204]. Its clinical use is limited by Ni toxicity, which can cause inflammation, DNA damage, ROI formation, etc. Although the level of tolerance is not established yet, due to a lack of evidence in in vitro studies, even low concentrations are proved to limit proliferation in in vitro experiments [185].
Studies have shown that due to the passive titanium oxide layer coating the surface of the nitinol implant, the likelihood of releasing the ions into the recipient’s tissues is similar to that of stainless steel and cobalt-based alloys. Instances of Ni ions being released have been noted as more frequent in parts of the implant covered with a thicker oxide layer, and they are more prone to breaking [205]. In a study performed by Nagaraja et al., on healthy lab minipigs with implanted optimally surfaced nitinol stents, no adverse effects of nitinol on kidneys or the hematopoietic system were observed. Nevertheless, non-optimally surfaced stents caused vessel stenosis and inflammation [206]. This outcome is coherent with other studies and proves that avoidance of wear debris and adverse effect of nitinol is feasible by surface processing, i.e., DLC coating [207,208].
4.6.5. Stainless Steel
The most commonly used types of stainless steel implants are ones made from the SAE 316L alloy. This specific composition consists of 0.02% carbon, 10–14% nickel, 16–18% chromium, 2% manganese, 2–3% molybdenum, with the rest being iron and it shall be treated as a whole, as well as the sum of its’ metals and as such. All of the possible reactions of the aforementioned metals shall be taken into consideration, as they have been reported to dissociate into the recipient’s tissue [209,210]. Cytotoxicity of these metal particles as well as immediate hypersensitivity caused by them have been characterized by peri-implant infiltration of macrophages and lymphocytes with CD68+, CD14+, and HLA-DR+ macrophages, as well as formation of CD3+ T cell and CD20+ B cell congregates [209,211].
Other than direct primal inflammation, stainless steel implants have been shown to cause apoptosis and chronic inflammation dependent on CD8+ cells [212]. As for metal particles, histological research has shown a high expression of HLA-DR active cells proximal to the SS area. T cell lymphocytes have been detected up to 6 months after initial surgery, suggesting chronic inflammation; it is worth noting that this behavior is consistent with both SS and titanium implants [212]. There have been reported cases of type IV delayed allergic reaction to implanted stainless steel plates. It is mediated by antigen-presenting cells and T lymphocytes causing the buildup of lymphocytes, histiocytes, and foreign body giant cells as well as inflammation of the implant area [213,214].
As for specific components of the alloy, iron exhibits an important role in modulating immune response from lymphocytes, NK cells, T cells, monocytes, and macrophages. Research done on mice has shown that iron oxide, depending on doses and particle size, either suppresses or enhances immune responsiveness. There are studies suggesting that the surface texture and roughness also play a role in the severity of the reaction, as macrophages tend to adhere more consistently to grooved surfaces of the metal and, in turn, induce a more significant inflammation [215].
Stainless steel implants that undergo corrosion have also been found to release hexavalent chromium into the recipients’ tissues and bloodstreams [216]. There is a small concentration of manganese ions that could be released from the implant, and there have been cases of type IV allergic reaction to this component [217]. However, some research has shown that stimulation of anti-viral immune responses is achievable by implementing manganese ions. They have been reported to stimulate M1 macrophages and CD8+ T cells as well as boost the host’s adaptive immunity [218]. Molybdenum has been reported to induce an inflammatory response by activating the NLRP3 inflammasome in macrophages by stimulating the secretion of IL-1β, which is hypothesized to be one of the reasons behind peri-implant tissue inflammation [219].
The biocompatible characteristics of above-mentioned metal alloys have been compared in Table 3.

Table 3.
Comparison of biocompatible characteristics of titanium, cobalt–chromium, nitinol, and stainless steel alloys.
5. Summary
Both mechanical features of metal alloy and the biological response induced by the metal implant are essential for the efficiency of LIF. An appropriate fatigue strength, mainly determined by the microstructure of the metal alloy, decreases the risk of implant fractures. Corrosion resistance has an influence not only on implants’ mechanical performance but also can prevent releasing metal ions into the surrounding tissue and bloodstream. Furthermore, decreasing the Young’s modulus may avoid the “stress-shielding” effect. On the other hand, implanted material elicits a biological response driven by immune cells at the site of insertion. That response is determined by wound healing, foreign body reaction, response to implant wear debris and metal ions, innate reactions, and adaptive immunity. All these properties are crucial to avoiding implant failure and obtaining spinal fusion.
Every metal alloy discussed in this paper has its advantages and disadvantages. However, high Young’s elastic modulus, poor corrosion resistance, and allergic and inflammation reactions disqualify stainless steel from use in LIF instrumentation. Cobalt–chromium alloy as a material eliciting adverse immunologic reactions and demonstrating high elastic modulus does not appear to be a good alternative. The use of superelastic and biocompatible nitinol may reduce the rate of adjacent segment disease. Unfortunately, its clinical use is limited by the toxicity of nickel. However, surface processing (e.g., DLC coating) may prevent this limitation and in the future enhance the popularity of this alloy in spinal instrumentation. Despite decreased, but still relatively high, elastic modulus, titanium alloys, especially the most popular Ti-6Al-4V alloy, remain a standard biomaterial for LIF instrumentation. Moreover, some manufacturing techniques (e.g., surface processing) can decrease Young’s modulus while preserving the mechanical and distinctive osteointegration properties of these alloys. On the other hand, the osteogenicity of titanium can be enhanced by ceramic coatings. Furthermore, adverse immunologic reactions are not frequent in comparison to other alloys. Therefore, titanium alloys currently represent the safest and the most effective materials among the discussed metal alloys for implants in LIF.
There are some more unknown aspects in designing implants and their material properties that can connect lumbar interbody fusion to other spine disorders such as cerebrospinal fluid leakage and CM-I that can be studied in future works [223]. In the future, further achievements in biomaterial engineering may help to obtain desirable biological and mechanical features of spinal implants to provide effective and safe spinal fusion.
Author Contributions
Conceptualization, P.K. and J.L. (Jakub Litak); methodology, M.S., Z.H. and J.L. (Joanna Litak); software, M.S.; validation, J.L. (Jakub Litak), P.K. and W.C.; formal analysis, W.C.; investigation, J.L. (Jakub Litak); resources, Z.H. and J.L. (Joanna Litak); data curation, L.S.; writing—original draft preparation, M.S.; writing—review and editing, W.C. and L.S.; visualization, J.L. (Jakub Litak); supervision, P.K.; project administration, P.K.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kos, N.; Gradisnik, L.; Velnar, T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med. Arch. 2019, 73, 421. [Google Scholar] [CrossRef]
- Kalichman, L.; Kim, D.H.; Li, L.; Guermazi, A.; Hunter, D.J. Computed tomography–evaluated features of spinal degeneration: Prevalence, intercorrelation, and association with self-reported low back pain. Spine J. 2010, 10, 200. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef]
- Baliga, S.; Treon, K.; Craig, N.J.A. Low Back Pain: Current Surgical Approaches. Asian Spine J. 2015, 9, 645–657. [Google Scholar] [CrossRef]
- Provaggi, E.; Capelli, C.; Leong, J.J.H.; Kalaskar, D.M. A UK-based pilot study of current surgical practice and implant preferences in lumbar fusion surgery. Medicine 2018, 97, e11169. [Google Scholar] [CrossRef]
- Meng, B.; Bunch, J.; Burton, D.; Wang, J. Lumbar interbody fusion: Recent advances in surgical techniques and bone healing strategies. Eur. Spine J. 2020, 30, 22–33. [Google Scholar] [CrossRef]
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion—A literature review. J. Spine Surg. 2020, 6, 752–776. [Google Scholar] [CrossRef]
- Momin, A.A.; Steinmetz, M.P. Evolution of Minimally Invasive Lumbar Spine Surgery. World Neurosurg. 2020, 140, 622–626. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101. [Google Scholar] [CrossRef]
- Mesregah, M.K.; Yoshida, B.; Lashkari, N.; Abedi, A.; Meisel, H.-J.; Diwan, A.; Hsieh, P.; Wang, J.C.; Buser, Z.; Yoon, S.T. Demographic, clinical, and operative risk factors associated with postoperative adjacent segment disease in patients undergoing lumbar spine fusions: A systematic review and meta-analysis. Spine J. 2021. [Google Scholar] [CrossRef]
- Mannion, A.F.; Leivseth, G.; Brox, J.I.; Fritzell, P.; Hägg, O.; Fairbank, J.C.T. ISSLS Prize winner: Long-term follow-up suggests spinal fusion is associated with increased adjacent segment disc degeneration but without influence on clinical outcome: Results of a combined follow-up from 4 randomized controlled trials. Spine 2014, 39, 1373–1383. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Mo, Z.; Han, J.; Chen, Y.; Yu, H.; Wang, Q.; Liu, J.; Li, C.; Zhou, Y.; et al. Comparison of short-segment monoaxial and polyaxial pedicle screw fixation combined with intermediate screws in traumatic thoracolumbar fractures: A finite element study and clinical radiographic review. Clinics 2017, 72, 609–617. [Google Scholar] [CrossRef]
- Gholampour, S.; Shakouri, E.; Deh, H.H.H. Effect of drilling direction and depth on thermal necrosis during tibia drilling: An in vitro study. Technol. Health Care 2018, 26, 687–697. [Google Scholar] [CrossRef]
- Gholampour, S.; Deh, H.H.H. The effect of spatial distances between holes and time delays between bone drillings based on examination of heat accumulation and risk of bone thermal necrosis. Biomed. Eng. Online 2019, 18, 65. [Google Scholar] [CrossRef]
- Antunes, R.A.; De Oliveira, M.C.L. Corrosion fatigue of biomedical metallic alloys: Mechanisms and mitigation. Acta Biomater. 2012, 8, 937–962. [Google Scholar] [CrossRef]
- Lindsey, C.; Deviren, V.; Xu, Z.; Yeh, R.F.; Puttlitz, C.M. The effects of rod contouring on spinal construct fatigue strength. Spine 2006, 31, 1680–1687. [Google Scholar] [CrossRef]
- Yamanaka, K.; Mori, M.; Yamazaki, K.; Kumagai, R.; Doita, M.; Chiba, A. Analysis of the fracture mechanism of Ti-6Al-4V alloy rods that failed clinically after spinal instrumentation surgery. Spine 2015, 40, E767–E773. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Buckley, J.M.; Ames, C.; Deviren, V. The fatigue life of contoured cobalt chrome posterior spinal fusion rods. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 194–198. [Google Scholar] [CrossRef]
- Chan, K.S. Changes in fatigue life mechanism due to soft grains and hard particles. Int. J. Fatigue 2010, 32, 526–534. [Google Scholar] [CrossRef]
- Ghonem, H. Microstructure and fatigue crack growth mechanisms in high temperature titanium alloys. Int. J. Fatigue 2010, 32, 1448–1460. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Kyzioł, K.; Kaczmarek, Ł.; Brzezinka, G.; Kyzioł, A. Structure, characterization and cytotoxicity study on plasma surface modified Ti–6Al–4V and γ-TiAl alloys. Chem. Eng. J. 2014, 240, 516–526. [Google Scholar] [CrossRef]
- Slivka, M.A.; Fan, Y.K.; Eck, J.C. The Effect of Contouring on Fatigue Strength of Spinal Rods: Is it Okay to Re-bend and Which Materials Are Best? Spine Deform. 2013, 1, 395–400. [Google Scholar] [CrossRef]
- Tang, J.A.; Leasure, J.M.; Smith, J.S.; Buckley, J.M.; Kondrashov, D.; Ames, C.P. Effect of Severity of Rod Contour on Posterior Rod Failure in the Setting of Lumbar Pedicle Subtraction Osteotomy (PSO)A Biomechanical Study. Neurosurgery 2013, 72, 276–283. [Google Scholar] [CrossRef]
- Demura, S.; Murakami, H.; Hayashi, H.; Kato, S.; Yoshioka, K.; Yokogawa, N.; Ishii, T.; Igarashi, T.; Fang, X.; Tsuchiya, H. Influence of Rod Contouring on Rod Strength and Stiffness in Spine Surgery. Orthopedics 2015, 38, e520–e523. [Google Scholar] [CrossRef]
- Ohrt-Nissen, S.; Dahl, B.; Gehrchen, M. Choice of Rods in Surgical Treatment of Adolescent Idiopathic Scoliosis: What Are the Clinical Implications of Biomechanical Properties?—A Review of the Literature. Neurospine 2018, 15, 123–130. [Google Scholar] [CrossRef]
- Yoshihara, H. Rods in spinal surgery: A review of the literature. Spine J. 2013, 13, 1350–1358. [Google Scholar] [CrossRef]
- Yamada, K.; Sudo, H.; Iwasaki, N.; Chiba, A. Mechanical Analysis of Notch-Free Pre-Bent Rods for Spinal Deformity Surgery. Spine 2020, 45, E312–E318. [Google Scholar] [CrossRef]
- Kokabu, T.; Kanai, S.; Abe, Y.; Iwasaki, N.; Sudo, H. Identification of optimized rod shapes to guide anatomical spinal reconstruction for adolescent thoracic idiopathic scoliosis. J. Orthop. Res. 2018, 36, 3219–3224. [Google Scholar] [CrossRef]
- Almansour, H.; Sonntag, R.; Pepke, W.; Bruckner, T.; Kretzer, J.P.; Akbar, M. Impact of Electrocautery on Fatigue Life of Spinal Fusion Constructs-An In Vitro Biomechanical Study. Mater 2019, 12, 2471. [Google Scholar] [CrossRef]
- Zobel, S.M.; Morlock, M.M.; Huber, G. Fatigue strength reduction of Ti-6Al-4V titanium alloy after contact with high-frequency cauterising instruments. Med. Eng. Phys. 2020, 81, 58–67. [Google Scholar] [CrossRef]
- Sonntag, R.; Gibmeier, J.; Pulvermacher, S.; Mueller, U.; Eckert, J.; Braun, S.; Reichkendler, M.; Kretzer, J.P. Electrocautery Damage Can Reduce Implant Fatigue Strength. J. Bone Jt. Surg. 2019, 101, 868–878. [Google Scholar] [CrossRef]
- Huber, G.; Weik, T.; Morlock, M.M. Schädigung eines hüftendoprothesenschafts durch einsatz eines hochfrequenzmessers. Orthopade 2009, 38, 622–625. [Google Scholar] [CrossRef]
- Konrads, C.; Wente, M.N.; Plitz, W.; Rudert, M.; Hoberg, M. Damage to implants due to high-frequency electrocautery: Analysis of four fractured hip endoprostheses shafts. Orthopade 2014, 43, 1106–1111. [Google Scholar] [CrossRef]
- Rho, J.Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325–1330. [Google Scholar] [CrossRef]
- Teles, A.R.; Yavin, D.; Zafeiris, C.P.; Thomas, K.C.; Lewkonia, P.; Nicholls, F.H.; Swamy, G.; Jacobs, W.B. Fractures After Removal of Spinal Instrumentation: Revisiting the Stress-Shielding Effect of Instrumentation in Spine Fusion. World Neurosurg. 2018, 116, e1137–e1143. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.-E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 1–21. [Google Scholar] [CrossRef]
- Jha, N.; Mondal, D.P.; Dutta Majumdar, J.; Badkul, A.; Jha, A.K.; Khare, A.K. Highly porous open cell Ti-foam using NaCl as temporary space holder through powder metallurgy route. Mater. Des. 2013, 47, 810–819. [Google Scholar] [CrossRef]
- Hansen, D.C. Metal corrosion in the human body: The ultimate bio-corrosion scenario. Electrochem. Soc. Interface. 2008, 17, 31–34. [Google Scholar] [CrossRef]
- Kirkpatrick, J.S.; Venugopalan, R.; Beck, P.; Lemons, J. Corrosion on spinal implants. J. Spinal Disord. Tech. 2005, 18, 247–251. [Google Scholar] [CrossRef]
- Peterson, H.A. Metallic implant removal in children. J. Pediatr. Orthop. 2005, 25, 107–115. [Google Scholar] [CrossRef]
- Mali, S.A.; Singh, V.; Gilbert, J.L. Effect of mixed alloy combinations on fretting corrosion performance of spinal screw and rod implants. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1169–1177. [Google Scholar] [CrossRef]
- Cundy, T.P.; Delaney, C.L.; Rackham, M.D.; Antoniou, G.; Oakley, A.P.; Freeman, B.J.C.; Sutherland, L.M.; Cundy, P.J. Chromium Ion Release From Stainless Steel Pediatric Scoliosis Instrumentation. Spine 2010, 35, 967–974. [Google Scholar] [CrossRef]
- Del Rio, J.; Beguiristain, J.; Duart, J. Metal levels in corrosion of spinal implants. Eur. Spine J. 2007, 16, 1055–1061. [Google Scholar] [CrossRef]
- Singh, V.; Shorez, J.P.; Mali, S.A.; Hallab, N.J.; Gilbert, J.L. Material dependent fretting corrosion in spinal fusion devices: Evaluation of onset and long-term response. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2858–2868. [Google Scholar] [CrossRef]
- Panagiotopoulou, V.C.; Hothi, H.S.; Anwar, H.A.; Molloy, S.; Noordeen, H.; Rezajooi, K.; Sutcliffe, J.; Skinner, J.; Hart, A. Assessment of corrosion in retrieved spine implants. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 632–638. [Google Scholar] [CrossRef]
- Rosenbloom, S.N.; Corbett, R.A. An assessment of ASTMF 2129 electrochemical testing ofsmall medical implants—Lessons learned. In Proceedings of the CORROSION 2007, Nashville, Tennessee, 11–15 March 2007. [Google Scholar]
- Tahal, D.; Madhavan, K.; Chieng, L.O.; Ghobrial, G.M.; Wang, M.Y. Metals in Spine. World Neurosurg. 2017, 100, 619–627. [Google Scholar] [CrossRef]
- Garbacz, H.; Królikowski, A. Corrosion resistance of nanocrystalline titanium. Nanocryst. Titan. 2019, 145–173. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- MacDonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 4, 1761–1772. [Google Scholar] [CrossRef]
- Cundy, W.J.; Mascarenhas, A.R.; Antoniou, G.; Freeman, B.J.C.; Cundy, P.J. Local and systemic metal ion release occurs intraoperatively during correction and instrumented spinal fusion for scoliosis. J. Child. Orthop. 2015, 9, 39–43. [Google Scholar] [CrossRef]
- Sherman, B.; Crowell, T. Corrosion of Harrington rod in idiopathic scoliosis: Long-term effects. Eur. Spine J. 2018, 27, 298–302. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Joint Surg. Am. 2000, 82, 457–477. [Google Scholar] [CrossRef]
- Wang, J.C.; Yu, W.D.; Sandhu, H.S.; Betts, F.; Bhuta, S.; Delamarter, R.B. Metal debris from titanium spinal implants. Spine 1999, 24, 899–903. [Google Scholar] [CrossRef]
- Kumazawa, R.; Watari, F.; Takashi, N.; Tanimura, Y.; Uo, M.; Totsuka, Y. Effects of Ti ions and particles on neutrophil function and morphology. Biomaterials 2002, 23, 3757–3764. [Google Scholar] [CrossRef]
- Campbell, P.; Ebramzadeh, E.; Nelson, S.; Takamura, K.; De Smet, K.; Amstutz, H.C. Histological Features of Pseudotumor-like Tissues From Metal-on-Metal Hips. Clin. Orthop. Relat. Res. 2010, 468, 2321. [Google Scholar] [CrossRef]
- Maloney, W.J.; Smith, R.L. Periprosthetic osteolysis in total hip arthroplasty: The role of particulate wear debris. Medicine 1995, 77, 1448–1461. [Google Scholar] [CrossRef]
- Takahashi, S.; Delécrin, J.; Passuti, N. Intraspinal metallosis causing delayed neurologic symptoms after spinal instrumentation surgery. Spine 2001, 26, 1495–1498. [Google Scholar] [CrossRef]
- Tezer, M.; Kuzgun, U.; Hamzaoglu, A.; Ozturk, C.; Kabukcuoglu Sirvanci, M. Intraspinal metalloma resulting in late paraparesis. Arch. Orthop. Trauma Surg. 2005, 125, 417–421. [Google Scholar] [CrossRef]
- Beguiristain, J.; Del Río, J.; Duart, J.; Barroso, J.; Silva, A.; Villas, C. Corrosion and late infection causing delayed paraparesis after spinal instrumentation. J. Pediatr. Orthop. B 2006, 15, 320–323. [Google Scholar] [CrossRef]
- Gotman, I. Characteristics of Metals Used in Implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef]
- Lukina, E.; Kollerov, M.; Meswania, J.; Khon, A.; Panin, P.; Blunn, G.W. Fretting corrosion behavior of nitinol spinal rods in conjunction with titanium pedicle screws. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 601–610. [Google Scholar] [CrossRef]
- Zhang, L.; Klemm, D.; Eckert, J.; Hao, Y.; Sercombe, T. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti–24Nb–4Zr–8Sn alloy. Scr. Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Cui, C. Biocompatibility and fabrication of in situ bioceramic coating/titanium alloy biocomposites. Met. Biomed. Devices 2010, 202–232. [Google Scholar] [CrossRef]
- Park, J.; Lakes, R.S. Biomaterials: An Introduction. Available online: https://books.google.com/books/about/Biomaterials.html?id=bb68wb0R_EAC (accessed on 23 April 2022).
- Aihara, H.; Zider, J.; Fanton, G.; Duerig, T. Combustion Synthesis Porous Nitinol for Biomedical Applications. Int. J. Biomater. 2019, 2019, 4307461. [Google Scholar] [CrossRef]
- A Mohammad, K.; Ali, A.; Sahari, B.B.; Abdullah, S. Fatigue behavior of Austenitic Type 316L Stainless Steel. IOP Conf. Ser. Mater. Sci. Eng. 2012, 36, 012012. [Google Scholar] [CrossRef]
- Li, X.; Ye, S.; Yuan, X.P. Fabrication of biomedical Ti-24Nb-4Zr-8Sn alloy with high strength and low elastic modulus by powder metallurgy. J. Alloys Compd. 2019, 772, 968–977. [Google Scholar] [CrossRef]
- Etemadifar, M.R.; Andalib, A.; Rahimian, A.; Nodushan, S.M.H.T. Cobalt chromium-Titanium rods versus Titanium-Titanium rods for treatment of adolescent idiopathic scoliosis; which type of rod has better postoperative outcomes? Rev. Assoc. Med. Bras. 2018, 64, 1085–1090. [Google Scholar] [CrossRef]
- Gottstein, G.; Goerdeler, M.; Prasad, G.V.S.S. Encyclopedia of Condensed Matter Physics. Encycl. Condens. Matter Phys. 2005, 298–305. Available online: http://www.sciencedirect.com/science/article/pii/B0123694019005696 (accessed on 25 April 2022).
- Kafkas, F.; Ebel, T. Metallurgical and mechanical properties of Ti–24Nb–4Zr–8Sn alloy fabricated by metal injection molding. J. Alloys Compd. 2014, 617, 359–366. [Google Scholar] [CrossRef]
- Völker, B.; Jäger, N.; Calin, M.; Zehetbauer, M.; Eckert, J.; Hohenwarter, A. Influence of testing orientation on mechanical properties of Ti45Nb deformed by high pressure torsion. Mater. Des. 2017, 114, 40–46. [Google Scholar] [CrossRef]
- Delshadmanesh, M.; Khatibi, G.; Ghomsheh, M.Z.; Lederer, M.; Zehetbauer, M.; Danninger, H. Influence of microstructure on fatigue of biocompatible β-phase Ti-45Nb. Mater. Sci. Eng. A 2017, 706, 83–94. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sulkowski, B.; Waitz, T.; Ozaltin, K.; Chrominski, W.; Pukenas, A.; Horky, J.; Lewandowska, M.; Skrotzki, W.; Zehetbauer, M. Mechanical properties, structural and texture evolution of biocompatible Ti–45Nb alloy processed by severe plastic deformation. J. Mech. Behav. Biomed. Mater. 2016, 62, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Niinomi, M.; Nakai, M.; Hieda, J.; Ishimoto, T.; Nakano, T. Optimization of Cr content of metastable β-type Ti-Cr alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2012, 8, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Nune, K.C.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Cellular response of osteoblasts to low modulus Ti-24Nb-4Zr-8Sn alloy mesh structure. J. Biomed. Mater. Res. Part A 2017, 105, 859–870. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Chen, C.H.; Tsuang, F.Y.; Wu, L.C.; Lin, S.C.; Chiang, C.J. Removal of fixation construct could mitigate adjacent segment stress after lumbosacral fusion: A finite element analysis. Clin. Biomech. 2017, 43, 115–120. [Google Scholar] [CrossRef]
- Litak, J.; Czyzewski, W.; Szymoniuk, M.; Pastuszak, B.; Litak, J.; Litak, G.; Grochowski, C.; Rahnama-Hezavah, M.; Kamieniak, P. Hydroxyapatite Use in Spine Surgery—Molecular and Clinical Aspect. Materials 2022, 15, 2906. [Google Scholar] [CrossRef]
- Liu, G.-M.; Kong, N.; Zhang, X.-Y.; Bai, H.-T.; Yao, Y.; Han, H.-Z.; Luo, Y.-G. Extracellular matrix-coating pedicle screws conduct and induce osteogenesis. Eur. J. Orthop. Surg. Traumatol. 2013, 24, 173–182. [Google Scholar] [CrossRef]
- Shi, L.Y.; Wang, A.; Zang, F.Z.; Wang, J.X.; Pan, X.W.; Chen, H.J. Tantalum-coated pedicle screws enhance implant integration. Coll. Surf. B Biointerfaces 2017, 160, 22–32. [Google Scholar] [CrossRef]
- Yi, S.; Rim, D.C.; Park, S.W.; Murovic, J.A.; Lim, J.; Park, J. Biomechanical Comparisons of Pull Out Strengths After Pedicle Screw Augmentation with Hydroxyapatite, Calcium Phosphate, or Polymethylmethacrylate in the Cadaveric Spine. World Neurosurg. 2015, 83, 976–981. [Google Scholar] [CrossRef]
- Školáková, A.; Körberová, J.; Málek, J.; Rohanová, D.; Jablonská, E.; Pinc, J.; Salvetr, P.; Gregorová, E.; Novák, P. Microstructural, Mechanical, Corrosion and Cytotoxicity Characterization of Porous Ti-Si Alloys with Pore-Forming Agent. Materials 2020, 13, 5607. [Google Scholar] [CrossRef] [PubMed]
- Nune, K.C.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Osteoblast cellular activity on low elastic modulus Ti–24Nb–4Zr–8Sn alloy. Dent. Mater. 2017, 33, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zheng, S.; Dong, R.; Kang, M.; Zhou, H.; Zhao, D.; Zhao, J. Ti-24Nb-4Zr-8Sn Alloy Pedicle Screw Improves Internal Vertebral Fixation by Reducing Stress-Shielding Effects in a Porcine Model. BioMed Res. Int. 2018, 2018, 8639648. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Xue, P.; Li, Y.; Li, K.; Zhang, D.; Zhou, C. Superelasticity, corrosion resistance and biocompatibility of the Ti-19Zr-10Nb-1Fe alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 179–186. [Google Scholar] [CrossRef]
- Atapour, M.; Pilchak, A.L.; Frankel, G.S.; Williams, J.C. Corrosion behavior of β titanium alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 885–891. [Google Scholar] [CrossRef]
- Kilmametov, A.; Ivanisenko, Y.; Mazilkin, A.; Straumal, B.; Gornakova, A.; Fabrichnaya, O.; Kriegel, M.; Rafaja, D.; Hahn, H. The α→ω and β→ω phase transformations in Ti–Fe alloys under high-pressure torsion. Acta Mater. 2018, 144, 337–351. [Google Scholar] [CrossRef]
- Völker, B.; Maier-Kiener, V.; Werbach, K.; Müller, T.; Pilz, S.; Calin, M.; Eckert, J.; Hohenwarter, A. Influence of annealing on microstructure and mechanical properties of ultrafine-grained Ti45Nb. Mater. Des. 2019, 179, 107864. [Google Scholar] [CrossRef]
- Hu, N.; Xie, L.; Liao, Q.; Gao, A.; Zheng, Y.; Pan, H.; Tong, H.; Yang, D.; Gao, N.; Starink, M.J.; et al. A more defective substrate leads to a less defective passive layer: Enhancing the mechanical strength, corrosion resistance and anti-inflammatory response of the low-modulus Ti-45Nb alloy by grain refinement. Acta Biomater. 2021, 126, 524–536. [Google Scholar] [CrossRef]
- Ozaltin, K.; Chrominski, W.; Kulczyk, M.; Panigrahi, A.; Horky, J.; Zehetbauer, M.; Lewandowska, M. Enhancement of mechanical properties of biocompatible Ti–45Nb alloy by hydrostatic extrusion. J. Mater. Sci. 2014, 49, 6930–6936. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Qian, B.; Shen, Z.; Virtanen, S.; Odnevall Wallinder, I. In vitro biocompatibility of CoCrMo dental alloys fabricated by selective laser melting. Dent. Mater. 2014, 30, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.S.; Baas, J.; Jakobsen, T.; Soballe, K. Biomechanical implant fixation of CoCrMo coating inferior to titanium coating in a canine implant model. J. Biomed. Mater. Res. Part A 2010, 94, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Mathew, M.T.; Wimmer, M.A.; Lesuer, R.J. Effect of Tribolayer Formation on Corrosion of CoCrMo Alloys Investigated Using Scanning Electrochemical Microscopy. Anal. Chem. 2013, 85, 7159–7166. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, C.I.; Ames, C.P.; Demakakos, J.; Fu, K.-M.G.; Keshavarzi, S.; Li, C.M.Y.; Deviren, V.; Schwab, F.J.; Lafage, V.; et al. Assessment of Symptomatic Rod Fracture After Posterior Instrumented Fusion for Adult Spinal Deformity. Neurosurgery 2012, 71, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Takigawa, T.; Tanaka, M.; Sugimoto, Y.; Arataki, S.; Yamane, K.; Watanabe, N.; Ozaki, T.; Sarai, T. Implant Failure of Titanium Versus Cobalt-Chromium Growing Rods in Early-onset Scoliosis. Spine 2016, 41, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, E.; Klineberg, E.; Shaffrey, C.I.; Lafage, V.; Schwab, F.J.; Protopsaltis, T.; Scheer, J.K.; Mundis, G.M.; Fu, K.-M.G.; et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J. Neurosurg. Spine 2014, 21, 994–1003. [Google Scholar] [CrossRef]
- Serhan, H.; Mhatre, D.; Newton, P.; Giorgio, P.; Sturm, P. Would CoCr rods provide better correctional forces than stainless steel or titanium for rigid scoliosis curves? J. Spinal. Disord. Tech. 2013, 26, E70–E74. [Google Scholar] [CrossRef]
- Willson, R.; Zhou, H.; Fulzele, S.; Mitchell, S.M.; Chutkan, N. Shape Loss of Autoclaved, Machine-Bent Cobalt-Chrome and Titanium Spine Surgery Rods. Glob. Spine J. 2021, 11, 509–514. [Google Scholar] [CrossRef]
- Han, S.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Comparative Study Between Cobalt Chrome and Titanium Alloy Rods for Multilevel Spinal Fusion: Proximal Junctional Kyphosis More Frequently Occurred in Patients Having Cobalt Chrome Rods. World Neurosurg. 2017, 103, 404–409. [Google Scholar] [CrossRef]
- Han, S.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Lee, S.; Rhim, S.C. Rod stiffness as a risk factor of proximal junctional kyphosis after adult spinal deformity surgery: Comparative study between cobalt chrome multiple-rod constructs and titanium alloy two-rod constructs. Spine J. 2017, 17, 962–968. [Google Scholar] [CrossRef]
- Heneghan, C.; Langton, D.; Thompson, M. Ongoing problems with metal-on-metal hip implants. BMJ 2012, 344, e1349. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Posada, O.M.; Tate, R.J.; Dominic Meek, R.M.; Helen Grant, M. In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants. Lubricants 2015, 3, 539–568. [Google Scholar] [CrossRef]
- Ke, D.; Robertson, S.F.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. Effects of MgO and SiO2 on Plasma-Sprayed Hydroxyapatite Coating: An in Vivo Study in Rat Distal Femoral Defects. ACS Appl. Mater. Interfaces. 2017, 9, 25731–25737. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Shivaram, A.; Isik, M.; Avila, J.D.; Dernell, W.S.; Bose, S. Additively manufactured calcium phosphate reinforced CoCrMo alloy: Bio-tribological and biocompatibility evaluation for load-bearing implants. Addit. Manuf. 2019, 28, 312–324. [Google Scholar] [CrossRef]
- Kok, D.; Firkins, P.J.; Wapstra, F.H.; Veldhuizen, A.G. A new lumbar posterior fixation system, the memory metal spinal system: An in-vitro mechanical evaluation. BMC Musculoskelet. Disord. 2013, 14, 269. [Google Scholar] [CrossRef]
- Kassab, E.J.; Gomes, J.P. Assessment of nickel titanium and beta titanium corrosion resistance behavior in fluoride and chloride environments. Angle Orthod. 2013, 83, 864–869. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Oakes, R.S.; Froimchuk, E.; Jewell, C.M. Engineering Biomaterials to Direct Innate Immunity. Adv. Ther. 2019, 2, 1800157. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, Y.; Si, X.; Yao, H.; Ma, S.; Xu, Y.; Zhao, J.; Ma, C.; He, C.; Tang, Z.; et al. Biopolymer Immune Implants’ Sequential Activation of Innate and Adaptive Immunity for Colorectal Cancer Postoperative Immunotherapy. Adv. Mater. 2020, 33, e2004559. [Google Scholar] [CrossRef] [PubMed]
- Billing, F.; Jakobi, M.; Martin, D.; Gerlach, K.; Arefaine, E.; Weiss, M.; Schneiderhan-Marra, N.; Hartmann, H.; Shipp, C. The immune response to the SLActive titanium dental implant surface in vitro is predominantly driven by innate immune cells. J. Immunol. Regen. Med. 2021, 13, 100047. [Google Scholar] [CrossRef]
- Moran, M.M.; Sena, K.; A McNulty, M.; Sumner, D.; Virdi, A.S. Intramembranous bone regeneration and implant placement using mechanical femoral marrow ablation: Rodent models. BoneKEy Rep. 2016, 5, 837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical behaviours of the bone–implant interface: A review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef]
- Dewey, M.J.; Harley, B.A.C. Biomaterial design strategies to address obstacles in craniomaxillofacial bone repair. RSC Adv. 2021, 11, 17809–17827. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Anitua, E.; Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Influence of calcium ion-modified implant surfaces in protein adsorption and implant integration. Int. J. Implant Dent. 2021, 7, 32. [Google Scholar] [CrossRef]
- Smoljanovic, T.; Bojanic, I.; Cimic, M. Letters. Spine 2010, 35, E1010–E1011. [Google Scholar] [CrossRef]
- Dapunt, U.; Giese, T.; Lasitschka, F.; Reinders, J.; Lehner, B.; Kretzer, J.P.; Ewerbeck, V.; Hänsch, G.M. On the inflammatory response in metal-on-metal implants. J. Transl. Med. 2014, 12, 74. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Huang, Q.; Wang, L.Y.; Lei, Y.T.; Xu, H.; Shen, B.; Pei, F.X. Normal trajectory of Interleukin-6 and C-reactive protein in the perioperative period of total knee arthroplasty under an enhanced recovery after surgery scenario. BMC Musculoskelet. Disord. 2020, 21, 264. [Google Scholar] [CrossRef]
- Thelander, U.; Larsson, S. Quantitation of C-Reactive Protein Levels and Erythrocyte Sedimentation Rate After Spinal Surgery. Spine 1992, 17, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, G.; Piscopo, A.; Grubor, P.; Rollo, G.; Medici, A.; Pipola, V.; Bisaccia, M.; Caraffa, A.; Barron, E.M.; Nobile, F.; et al. Use of Common Inflammatory Markers in the Long-Term Screening of Total Hip Arthroprosthesis Infections: Our Experience. Adv. Orthop. 2017, 2017, 9679470. [Google Scholar] [CrossRef] [PubMed]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2021, 7, e10239. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2018, 84, 1–15. [Google Scholar] [CrossRef]
- Irandoust, S.; Müftü, S. The interplay between bone healing and remodeling around dental implants. Sci. Rep. 2020, 10, 4335. [Google Scholar] [CrossRef]
- Boden, S.D.; Zdeblick, T.A.; Sandhu, H.S.; Heim, S.E. The Use of rhBMP-2 in Interbody Fusion Cages. Spine 2000, 25, 376–381. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, L.; Tan, G. Fourth-generation biomedical materials. Mater. Today 2015, 19, 2–3. [Google Scholar] [CrossRef]
- Chen, W.; Xie, G.; Lu, Y.; Wang, J.; Feng, B.; Wang, Q.; Xu, K.; Bao, J. An improved osseointegration of metal implants by pitavastatin loaded multilayer films with osteogenic and angiogenic properties. Biomaterials 2021, 280, 121260. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; van Wijnen, A.J. Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef]
- Goto, M.; Matsumine, A.; Yamaguchi, S.; Takahashi, H.; Akeda, K.; Nakamura, T.; Asanuma, K.; Matsushita, T.; Kokubo, T.; Sudo, A. Osteoconductivity of bioactive Ti-6Al-4V implants with lattice-shaped interconnected large pores fabricated by electron beam melting. J. Biomater. Appl. 2020, 35, 1153–1167. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Eger, M.; Hiram-Bab, S.; Liron, T.; Sterer, N.; Carmi, Y.; Kohavi, D.; Gabet, Y. Mechanism and Prevention of Titanium Particle-Induced Inflammation and Osteolysis. Front. Immunol. 2018, 9, 2963. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Badylak, S.F.; Elisseeff, J. Immunomodulatory Biomaterials: Regulating the Immune Response with Biomaterials to Affect Clinical Outcome; Woodhead Publishing: Duxford, UK, 2021. [Google Scholar]
- Felgueiras, H.P.; Evans, M.D.; Migonney, V. Contribution of fibronectin and vitronectin to the adhesion and morphology of MC3T3-E1 osteoblastic cells to poly(NaSS) grafted Ti6Al4V. Acta Biomater. 2015, 28, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef]
- Saleh, L.S.; Bryant, S.J. In vitro and in vivo models for assessing the host response to biomaterials. Drug Discov. Today: Dis. Model. 2017, 24, 13–21. [Google Scholar] [CrossRef]
- Sun, H.; Zhi, K.; Hu, L.; Fan, Z. The Activation and Regulation of β2 Integrins in Phagocytes and Phagocytosis. Front. Immunol. 2021, 12, 978. [Google Scholar] [CrossRef]
- Zaveri, T.D.; Lewis, J.S.; Dolgova, N.V.; Clare-Salzler, M.J.; Keselowsky, B.G. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials 2014, 35, 3504–3515. [Google Scholar] [CrossRef]
- Kim, O.-H.; Kim, H.; Kang, J.; Yang, D.; Kang, Y.-H.; Lee, D.H.; Cheon, G.J.; Park, S.C.; Oh, B.-C. Impaired phagocytosis of apoptotic cells causes accumulation of bone marrow-derived macrophages in aged mice. BMB Rep. 2017, 50, 43–48. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; LaValle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Lucke, S.; Walschus, U.; Hoene, A.; Schnabelrauch, M.; Nebe, J.B.; Finke, B.; Schlosser, M. The in vivo inflammatory and foreign body giant cell response against different poly( l -lactide-co- d/l -lactide) implants is primarily determined by material morphology rather than surface chemistry. J. Biomed. Mater. Res. Part A 2018, 106, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Levey, R.E.; Coulter, F.B.; Scheiner, K.C.; Deotti, S.; Robinson, S.T.; McDonough, L.; Nguyen, T.T.; Steendam, R.; Canney, M.; Wylie, R.; et al. Assessing the Effects of VEGF Releasing Microspheres on the Angiogenic and Foreign Body Response to a 3D Printed Silicone-Based Macroencapsulation Device. Pharmaceutics 2021, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Chemokines Associated with Pathologic Responses to Orthopedic Implant Debris. Front. Endocrinol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Zhang, B.; Su, Y.; Zhou, J.; Zheng, Y.; Zhu, D. Toward a Better Regeneration through Implant-Mediated Immunomodulation: Harnessing the Immune Responses. Adv. Sci. 2021, 8, 2100446. [Google Scholar] [CrossRef]
- Zago, G.; Saavedra, P.H.V.; Keshari, K.R.; Perry, J.S.A. Immunometabolism of Tissue-Resident Macrophages—An Appraisal of the Current Knowledge and Cutting-Edge Methods and Technologies. Front. Immunol. 2021, 12, 665782. [Google Scholar] [CrossRef]
- Samelko, L.; Caicedo, M.; McAllister, K.; Jacobs, J.; Hallab, N.J. Metal-induced delayed type hypersensitivity responses potentiate particle induced osteolysis in a sex and age dependent manner. PLoS ONE 2021, 16, e0251885. [Google Scholar] [CrossRef]
- McKee, A.S.; Fontenot, A.P. Interplay of innate and adaptive immunity in metal-induced hypersensitivity. Curr. Opin. Immunol. 2016, 42, 25–30. [Google Scholar] [CrossRef]
- Couto, M.; Vasconcelos, D.; Sousa, D.M.; Sousa, B.; Conceição, F.; Neto, E.; Lamghari, M.; Alves, C.J. The Mechanisms Underlying the Biological Response to Wear Debris in Periprosthetic Inflammation. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Le Cann, S.; Tudisco, E.; Turunen, M.J.; Patera, A.; Mokso, R.; Tägil, M.; Belfrage, O.; Hall, S.A.; Isaksson, H. Investigating the Mechanical Characteristics of Bone-Metal Implant Interface Using in situ Synchrotron Tomographic Imaging. Front. Bioeng. Biotechnol. 2019, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Samelko, L.; Landgraeber, S.; McAllister, K.; Jacobs, J.; Hallab, N.J. Cobalt Alloy Implant Debris Induces Inflammation and Bone Loss Primarily through Danger Signaling, Not TLR4 Activation: Implications for DAMP-ening Implant Related Inflammation. PLoS ONE 2016, 11, e0160141. [Google Scholar] [CrossRef] [PubMed]
- Vinaik, R.; Abdullahi, A.; Barayan, D.; Jeschke, M.G. NLRP3 inflammasome activity is required for wound healing after burns. Transl. Res. 2019, 217, 47–60. [Google Scholar] [CrossRef]
- Baron, L.; Gombault, A.; Fanny, M.; Villeret, B.; Savigny, F.; Guillou, N.; Panek, C.; Le Bert, M.; Lagente, V.; Rassendren, F.; et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015, 6, e1629. [Google Scholar] [CrossRef]
- Boro, M.; Balaji, K.N. CXCL1 and CXCL2 Regulate NLRP3 Inflammasome Activation via G-Protein–Coupled Receptor CXCR2. J. Immunol. 2017, 199, 1660–1671. [Google Scholar] [CrossRef]
- Paniri, A.; Akhavan-Niaki, H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: Role of lncRNAs in cytokine storm modulation. Life Sci. 2020, 257, 118114. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.-R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Kapasa, E.R.; Giannoudis, P.V.; Jia, X.; Hatton, P.V.; Yang, X.B. The Effect of RANKL/OPG Balance on Reducing Implant Complications. J. Funct. Biomater. 2017, 8, 42. [Google Scholar] [CrossRef]
- Akyol, S.; Akgun, M.Y.; Yetmez, M.; Hanci, M.; Oktar, F.N.; Ben-Nissan, B. Comparative Analysis of NF-κB in the MyD88-Mediated Pathway After Implantation of Titanium Alloy and Stainless Steel and the Role of Regulatory T Cells. World Neurosurg. 2020, 144, e138–e148. [Google Scholar] [CrossRef]
- Sun, Z.; Zeng, J.; Wang, W.; Jia, X.; Wu, Q.; Yu, D.; Mao, Y. Magnoflorine Suppresses MAPK and NF-κB Signaling to Prevent Inflammatory Osteolysis Induced by Titanium Particles In Vivo and Osteoclastogenesis via RANKL In Vitro. Front. Pharmacol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N. Diagnosis of Metal Hypersensitivity in Orthopedics. Oper. Tech. Orthop. 2017, 27, 168–177. [Google Scholar] [CrossRef]
- Chaturvedi, T. Allergy related to dental implant and its clinical significance. Clin. Cosmet. Investig. Dent. 2013, 5, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.W.; Schalock, P.C. Metal Hypersensitivity Reactions to Orthopedic Implants. Dermatol. Ther. 2016, 7, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wawrzynski, J.; Gil, J.A.; Goodman, A.D.; Waryasz, G.R. Hypersensitivity to Orthopedic Implants: A Review of the Literature. Rheumatol. Ther. 2017, 4, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.A.; Crist, B.D. Nickel allergy to orthopaedic implants: A review and case series. J. Clin. Orthop. Trauma 2020, 11, S596–S603. [Google Scholar] [CrossRef] [PubMed]
- Adala, R.; Chakravarthy, M.; Srinivas, V.; Pai, S. Orthopaedic surgery in a patient with metal sensitivity. J. Cutan. Aesthetic Surg. 2011, 4, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef]
- Engelhart, S.; Segal, R.J. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis 2017, 99, 245–249. [Google Scholar]
- Hallab, N.J. A review of the biologic effects of spine implant debris: Fact from fiction. SAS J. 2009, 3, 143–160. [Google Scholar] [CrossRef]
- Hallab, N.J.; Caicedo, M.; McAllister, K.; Skipor, A.; Amstutz, H.; Jacobs, J. Asymptomatic prospective and retrospective cohorts with metal-on-metal hip arthroplasty indicate acquired lymphocyte reactivity varies with metal ion levels on a group basis. J. Orthop. Res. 2012, 31, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Hsueh-Chun, W.; Wang, H.-C.; Wu, M.-C.; Wu, S.-W.; Yeh, M.-L. Evaluation of osseous integration of titanium orthopedic screws with novel SLA treatment in porcine model. PLoS ONE 2017, 12, e0188364. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, Y.; Li, B.; Wang, X.; Li, H.; Liu, S.; Liang, C.; Wang, H. Biological and Mechanical Effects of Micro-Nanostructured Titanium Surface on an Osteoblastic Cell Line In vitro and Osteointegration In vivo. Appl. Biochem. Biotechnol. 2017, 183, 280–292. [Google Scholar] [CrossRef]
- Ota, T.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Shinmura, K.; Yokogawa, N.; Shirai, T.; Murakami, H.; et al. A comparison of bone conductivity on titanium screws inserted into the vertebra using different surface processing. J. Exp. Orthop. 2020, 7, 29. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef]
- Katsuura, Y.; Wright-Chisem, J.; Wright-Chisem, A.; Virk, S.; McAnany, S. The Importance of Surface Technology in Spinal Fusion. HSS J. Musculoskelet. J. Hosp. Spéc. Surg. 2020, 16, 113–116. [Google Scholar] [CrossRef]
- Li, Q.; Shen, A.; Wang, Z. Enhanced osteogenic differentiation of BMSCs and M2-phenotype polarization of macrophages on a titanium surface modified with graphene oxide for potential implant applications. RSC Adv. 2020, 10, 16537–16550. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jansen, J.A.; Yang, F.; Beucken, J.J.V.D. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C 2018, 95, 143–151. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2017, 20, 82–91. [Google Scholar] [CrossRef]
- Caneva, M.; Salata, L.A.; De Souza, S.S.; Bressan, E.; Botticelli, D.; Lang, N.P. Hard tissue formation adjacent to implants of various size and configuration immediately placed into extraction sockets: An experimental study in dogs. Clin. Oral Implant. Res. 2010, 21, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Khadija, G.; Saleem, A.; Akhtar, Z.; Naqvi, Z.; Gull, M.; Masood, M.; Mukhtar, S.; Batool, M.; Saleem, N.; Rasheed, T.; et al. Short term exposure to titanium, aluminum and vanadium (Ti 6Al 4V) alloy powder drastically affects behavior and antioxidant metabolites in vital organs of male albino mice. Toxicol. Rep. 2018, 5, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Doe, Y.; Ida, H.; Seiryu, M.; Deguchi, T.; Takeshita, N.; Sasaki, S.; Sasaki, S.; Irie, D.; Tsuru, K.; Ishikawa, K.; et al. Titanium surface treatment by calcium modification with acid-etching promotes osteogenic activity and stability of dental implants. Materialia 2020, 12, 100801. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Milheiro, A.; Nozaki, K.; Kleverlaan, C.J.; Muris, J.; Miura, H.; Feilzer, A.J. In vitro cytotoxicity of metallic ions released from dental alloys. Odontology 2016, 104, 136–142. [Google Scholar] [CrossRef]
- Brayda-Bruno, M.; Fini, M.; Pierini, G.; Giavaresi, G.; Rocca, M.; Giardino, R. Evaluation of Systemic Metal Diffusion after Spinal Pedicular Fixation with Titanium Alloy and Stainless Steel System: A 36-month Experimental Study in Sheep. Int. J. Artif. Organs 2001, 24, 41–49. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Choi, Y.-S.; Hwang, Y.-H.; Cho, H.-W.; Lee, D.-G. Biocompatibility and Biological Corrosion Resistance of Ti–39Nb–6Zr+0.45Al Implant Alloy. J. Funct. Biomater. 2020, 12, 2. [Google Scholar] [CrossRef]
- Maya, S.; Prakash, T.; Das Madhu, K.; Goli, D. Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed. Pharmacother. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Kretzer, J.P.; Mueller, U.; Streit, M.R.; Kiefer, H.; Sonntag, R.; Streicher, R.M.; Reinders, J. Ion release in ceramic bearings for total hip replacement: Results from an in vitro and an in vivo study. Int. Orthop. 2017, 42, 65–70. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Hamidi, M.F.F.A.; Harun, W.S.W.; Samykano, M.; Ghani, S.A.C.; Ghazalli, Z.; Ahmad, F.; Sulong, A.B. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Brewer, J.M.; Grant, M.H. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J. Immunotoxicol. 2011, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Posada, O.M.; Tate, R.J.; Grant, M.H. Toxicity of cobalt-chromium nanoparticles released from a resurfacing hip implant and cobalt ions on primary human lymphocytes in vitro. J. Appl. Toxicol. 2015, 35, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Hedbrant, A.; Eklund, D.; Andersson, L.; Bryngelsson, I.-L.; Persson, A.; Westberg, H.; Särndahl, E. Effects on white blood cell counts and the NLRP3 inflammasome due to dust and cobalt exposure in the hard metal industry. Biomarkers 2021, 27, 60–70. [Google Scholar] [CrossRef]
- Klasson, M.; Lindberg, M.; Westberg, H.; Bryngelsson, I.-L.; Tuerxun, K.; Persson, A.; Särndahl, E. Dermal exposure to cobalt studied in vitro in keratinocytes – effects of cobalt exposure on inflammasome activated cytokines, and mRNA response. Biomarkers 2021, 26, 674–684. [Google Scholar] [CrossRef]
- Wegienka, G.; Johnson, C.C.; Zoratti, E.; Havstad, S. Racial differences in allergic sensitization: Recent findings and future directions. Curr. Allergy Asthma Rep. 2013, 13, 255–261. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Ryhänen, J.; Niemi, E.; Serlo, W.; Niemelä, E.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of Nickel-Titanium Shape Memory Metal and Its Corrosion Behavior in Human Cell Cultures. J. Biomed. Mater. Res. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Haider, W.; Munroe, N.; Tek, V.; Gill, P.K.S.; Tang, Y.; McGoron, A.J. Cytotoxicity of Metal Ions Released from Nitinol Alloys on Endothelial Cells. J. Mater. Eng. Perform. 2011, 20, 816–818. [Google Scholar] [CrossRef]
- Bogdanski, D.; Koller, M.; Bram, M.; Stöver, D.; Buchkremer, H.; Choi, J.; Epple, M.; Muhr, G. Schnelle Analyse Der Biokompatibilität Mittels Gradierter Probekörper Am Beispiel Von Ni-NiTi-Ti. Biomed. Eng. Biomed. Tech. 2002, 47, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.J.L.; Dreher, M.L.; Zheng, J.; Chen, L.; Madamba, D.; Miyashiro, K.; Trépanier, C.; Nagaraja, S. Effects of Oxide Layer Composition and Radial Compression on Nickel Release in Nitinol Stents. Shape Mem. Superelast. 2015, 1, 319–327. [Google Scholar] [CrossRef]
- Nagaraja, S.; Sullivan, S.J.; Stafford, P.R.; Lucas, A.D.; Malkin, E. Impact of nitinol stent surface processing on in-vivo nickel release and biological response. Acta Biomater. 2018, 72, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, T.; Rokicki, R. Modification of Nitinol Biomaterial for Medical Applications. World Sci. News 2018, 96, 35–58. [Google Scholar]
- Lou, J.; Gao, Z.; Zhang, J.; He, H.; Wang, X. Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films. Coatings 2021, 11, 1543. [Google Scholar] [CrossRef]
- Pacheco, K.A. Allergy to Surgical Implants. Clin. Rev. Allergy Immunol. 2018, 56, 72–85. [Google Scholar] [CrossRef]
- Voggenreiter, G.; Leiting, S.; Brauer, H.; Leiting, P.; Majetschak, M.; Bardenheuer, M.; Obertacke, U. Immuno-inflammatory tissue reaction to stainless-steel and titanium plates used for internal fixation of long bones. Biomaterials 2002, 24, 247–254. [Google Scholar] [CrossRef]
- Mahendra, G.; Pandit, H.; Kliskey, K.; Murray, D.; Gill, H.S.; Athanasou, N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009, 80, 653–659. [Google Scholar] [CrossRef]
- Akyol, S.; Bozkus, H.; Cinar, S.A.; Hanci, M.M. Which is Better: Stainless Steel or Titanium Alloy? Turk. Neurosurg. 2017, 28, 4. [Google Scholar] [CrossRef]
- Niempoog, S.; Kukreja, S. Neuropathy Caused by Metal Hypersensitivity after Placement of Stainless Steel Plate. Case Rep. Orthop. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Thomas, P.; Von Der Helm, C.; Schopf, C.; Mazoochian, F.; Frommelt, L.; Gollwitzer, H.; Schneider, J.; Flaig, M.; Krenn, V.; Thomas, B.; et al. Patients with Intolerance Reactions to Total Knee Replacement: Combined Assessment of Allergy Diagnostics, Periprosthetic Histology, and Peri-implant Cytokine Expression Pattern. BioMed Res. Int. 2015, 2015, 910156. [Google Scholar] [CrossRef]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Merritt, K.; Brown, S.A. Release of hexavalent chromium from corrosion of stainless steel and cobalt?chromium alloys. J. Biomed. Mater. Res. 1995, 29, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Watchmaker, J.; Collins, R.; Chaney, K. Allergic Contact Dermatitis to Manganese in Metallic Implant. Dermatitis 2015, 26, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yin, Y.; Gong, L.; Liang, Z.; Zhu, C.; Ren, C.; Zheng, N.; Zhang, Q.; Liu, H.; Liu, W.; et al. Manganese nanodepot augments host immune response against coronavirus. Nano Res. 2020, 14, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, M.S.; Desai, R.; McAllister, K.; Reddy, A.; Jacobs, J.J.; Hallab, N.J. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. J. Orthop. Res. 2008, 27, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.I.P.; Gomes, A.M.; Rodrigues, M.F.; Pinto, T.S.; Fernandes, C.J.D.C.; Bezerra, F.J.; Zambuzzi, W.F. Cobalt-chromium-enriched medium ameliorates shear-stressed endothelial cell performance. J. Trace Elements Med. Biol. 2019, 54, 163–171. [Google Scholar] [CrossRef]
- Anderson, J.A.; Lamichhane, S.; Mani, G. Macrophage responses to 316L stainless steel and cobalt chromium alloys with different surface topographies. J. Biomed. Mater. Res. Part A 2016, 104, 2658–2672. [Google Scholar] [CrossRef]
- Safranski, D.; Dupont, K.; Gall, K. Pseudoelastic NiTiNOL in Orthopaedic Applications. Shape Mem Superelast. 2020, 6, 332–341. [Google Scholar] [CrossRef]
- Gholampour, S.; Gholampour, H. Correlation of a new hydrodynamic index with other effective indexes in Chiari I malformation patients with different associations. Sci. Rep. 2020, 10, 15907. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

