Tribological Performance of High-Entropy Coatings (HECs): A Review
Abstract
:1. Introduction
2. High-Entropy Alloys (HEAs) and Coatings (HECs)
- High-entropy metallic coatings, including the transition metal-based HECs (contain elements like Al, Co, Cr, Cu, Fe, Mn, Ni, Ti, and V) and the refractory HECs (contain elements with high melting temperature, such as Cr, Hf, Mo, Nb, Ta, Ti, V, W, and Zr,) [34]. The refractory HECs are designed to prevent the substrate materials from high-temperature oxidation, abrasion, wear and corrosion [38,88].
- High-entropy ceramic coatings, e.g., HEAs mixed with oxygen, boron, and other anions. The components are mainly composed of strong nitride/carbides/oxides-forming elements such as Al, Ti, Cr, Si, Nb, Zr, etc. The incorporated N, C, and O in HECs are not the component because the coatings are the mixture of the constituent binary nitrides/carbides/oxides which are still in the state of solid solution with high-entropy effect. These high-entropy ceramic coatings are reported to possess outstanding surface properties, such as high hardness, thermal stability, corrosion resistance, and low diffusivity, which have great potentials in hard protective coatings and diffusion barriers [38]. Particularly, reactive magnetron sputtered high entropy (HE) nitrides have received global attention as a new type of protective coating with excellent mechanical properties. Since the nitrogen content of the films has a strong influence on the structure and mechanical performance of the HE nitrides, several studies reported the HE nitride films and coatings deposited by re-active sputtering at varying nitrogen flow ratios (RN). Only one broad peak was identified in these XRD patterns when depositing with a N2 flow of 0 SCCM (standard-state cubic centimetre per minute), indicating that the coatings had an amorphous structure. HE nitride coatings with a basic FCC structure could be produced as the N2 flow increased. The effects of non-nitride-forming element(s) on the microstructures and mechanical properties on HE nitride films/coatings are still not clear, which needs further studies and is helpful to understand the strengthening mechanism of the HE nitride films and coatings and develop the new HE nitride systems with higher hardness.
- High-entropy composite coatings, in which the HEAs could act as the matrix/binder for hard ceramics and the reinforcements in lightweight alloys, such as Al and Mg alloys. Hard ceramic reinforcements (e.g., TiN, NbC, TiC, and TiB2) [89,90,91] with high melting temperature (Tm) and hardness, excellent wear resistance, and chemical stability, as well as the good metallurgical bonding with HEAs matrix have been synthesized into the high-entropy composite coatings to enhance the surface performances further. To date, some progress has been achieved on high-entropy composite coatings with outstanding properties, such as the TiN/CoCrFeNiTi, NbC/AlCoCrFeNi coatings fabricated by laser cladding and the TiC–TiB2/CoCrCuFeNi coatings synthesized by the in situ plasma transferred arc cladding [38]. Besides hard ceramic reinforcements, the hard Ni60 powders were also adopted as the reinforcements and incorporated into the AlCoCrFeNiTi coating through plasma spraying, by which the hardness and wear resistance at elevated temperature of the sprayed coatings were clearly enhanced [38,66]. Therefore, the surface modification and coatings development with controlled microstructure and properties are the subject of intensive research [92,93,94].
3. Fabrication Routes for HEAs
3.1. HEAs in the Form of Targets and Fibres
3.2. HEAs as a Feedstock
4. Fabrication Routes of High-Entropy Coatings (HECs)
5. Tribological Performance of High-Entropy Coatings (HECs)
5.1. Vapor Deposition and Related Methods
5.2. Thermal Spraying
5.3. Cladding
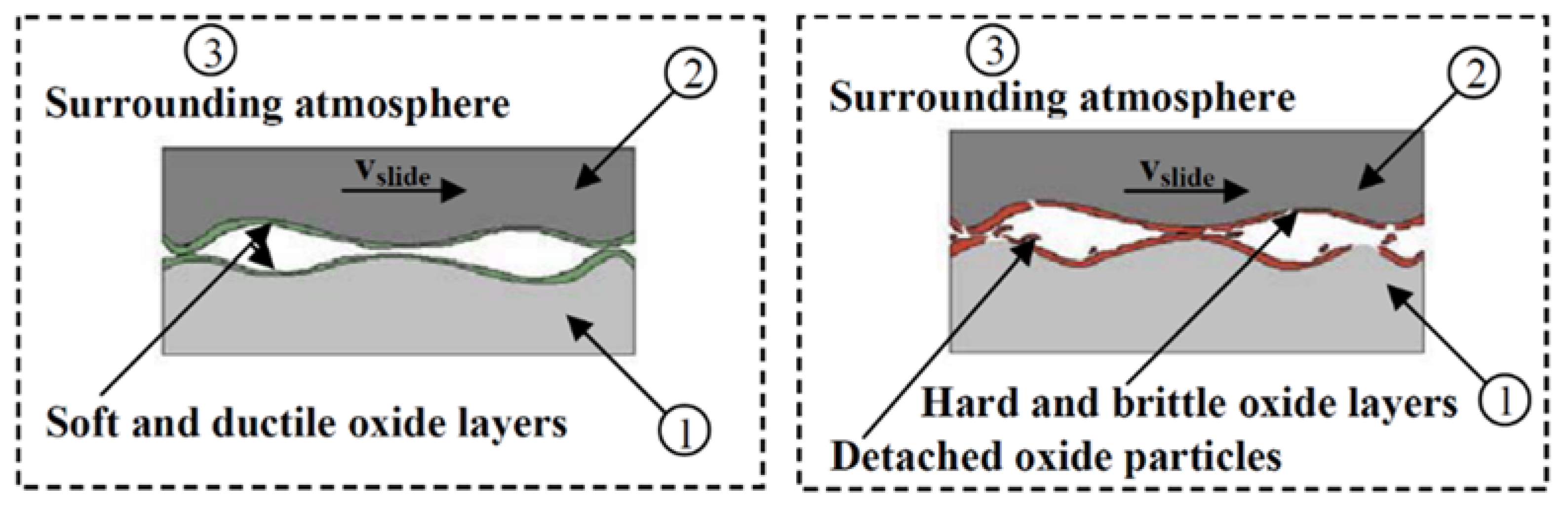
6. Role of Surface (In Situ) Oxides and Interfacial Processes at Tribological Interface
7. Influence of Phase Transition on the Tribological Properties
8. Summary
- The most common fabrication methods for HEAs in the form of rods or ingots (targets) are vacuum arc melting, mechanical alloying (MA) after spark plasma sintering (SPS), and hot drawing for HEA fibre and wire. In contrast, HEA is also employed as a feedstock in thermal spraying and cladding processes. Four synthesis techniques have been reported to prepare HEA feedstocks: mixing, arc melting followed by mechanical milling, mechanical alloying, and gas atomization.
- The authors analysed numerous coating methods for HECs deposition and summarized the articles in which the researchers developed the coating using various methods such as magnetron sputtering, laser cladding, and thermal spraying. There are several advantages to employing magnetron sputtering, including the ability to combine elements for HEAs directly from single or multicomponent targets. Thermal spray and cladding, on the other hand, has the disadvantage of requiring powder and can still result in the typical tortuous microstructure as well as oxidation and phase decomposition (intermetallic phase) challenges. One process can produce thick coatings (i.e., CrMnFeCoNi HEA) with acceptable mechanical properties, while the other can produce thin films (HE carbides and nitrides) with excellent mechanical properties. Thus, the coating technique used is influenced by the final microstructure and required properties.
- The high-entropy coatings developed by vapor deposition-based methods are mainly composed of HE nitride and carbide coatings. The elements such as Zr, Nv, V, Ti, Hf, etc., possess high affinity with nitrogen, which can easily form nitrides using magnetron sputtering. Aside of the elements, the deposition parameters including N2 flow and bias voltage, also have significant influence on the phase formation and final microstructure of the HE nitride coatings. Furthermore, HE nitride coatings form nano-sized structures, resulting in considerable improvements in physical and mechanical properties such as exceptional wear and oxidation resistance and thermal stability. Moreover, the compressive residual stress generated by magnetron sputtering (with manipulation of bias) for the HECs can also reduce the crack formation and contribute to the improved wear resistance.
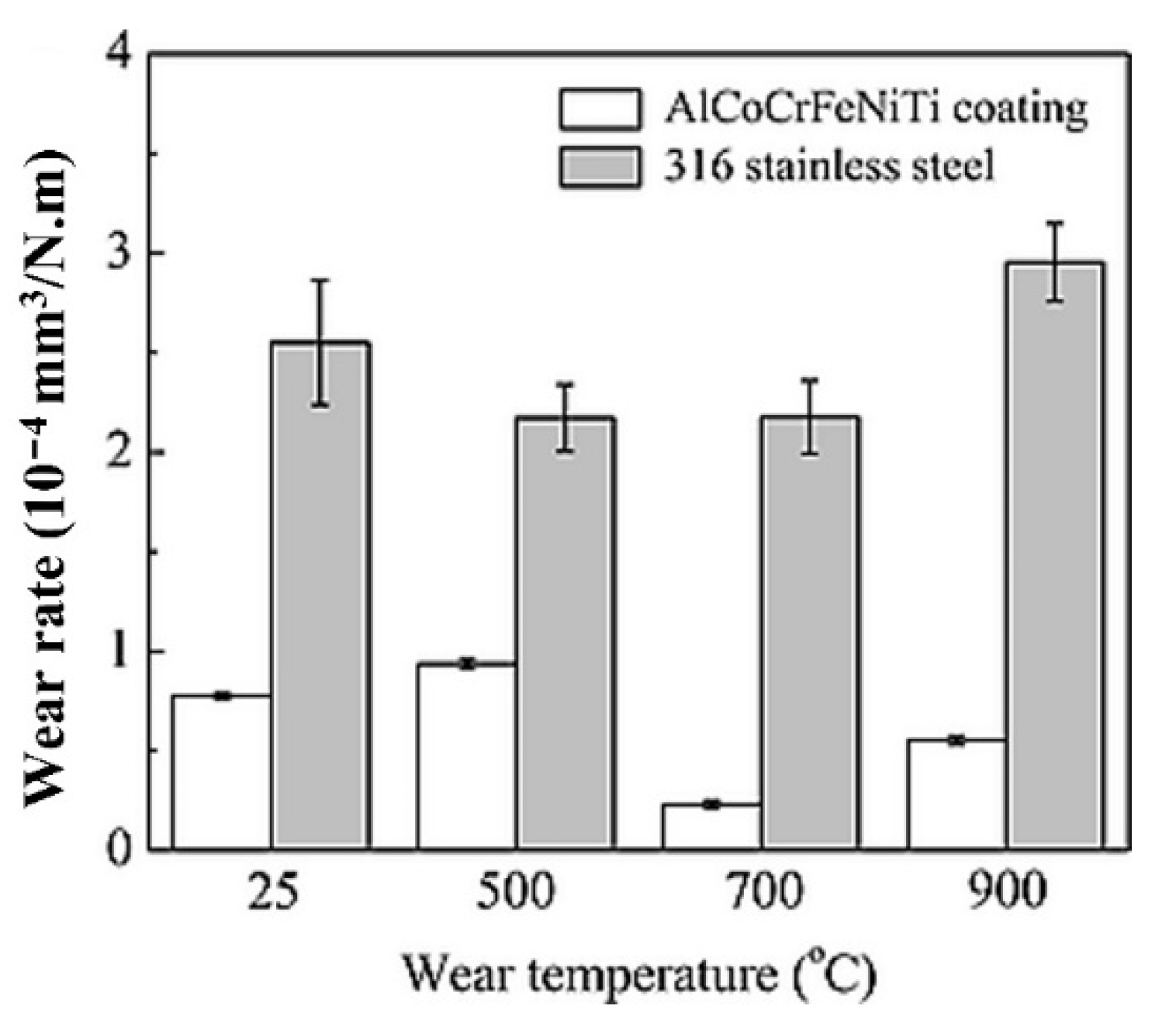
- The HECs developed using thermal spray methods have shown good microstructural stability with excellent mechanical properties. However, the majority of research has been carried on plasma (APS) and HVOF sprayed HECs, and there is relatively limited information available regarding the tribological behaviour of cold sprayed coatings. Currently, the thermal spray process can only generate high entropy metallic and ceramic coatings through the APS process. Because of temperature limitations in the HVOF and cold spray procedures, they cannot be used to develop High-entropy ceramic coatings. Furthermore, the maximum hardness of ~790 HV for HVOF sprayed Al0.6TiCrFeCoNi coating was reported [56]. Plasma-sprayed HECs, on the other hand, outperformed both HVOF and cold-sprayed HECs. So far, the plasma sprayed Al0.2TiCrFeCo1.5Ni1.5 [77] with the addition of 5% Ag has shown the lowest wear rate of 8.9 × 10−6 mm3/N.m at high temperatures. However, due to the limited open-source literature and lack of high temperature wear data, the commercial potential for thermal sprayed HECs in tribological applications must be evaluated further.
- The cladding process is advantageous for developing HECs with low coating dilution and strong metallurgical bonding between substrate and coatings. According to the literature, cladded coatings formed the FCC or BCC matrix as well as small intermetallic phases. Surprisingly, the presence of solid-solution(s) and intermetallic compound(s) in HECs improved tribological behaviour. The ductile solid-solution matrix can help protect the surface against brittle fracture in the combined structures of solid solution(s) and intermetallic compound(s), while the hard intermetallic compound phase can effectively resist abrasive wear and protect the surface against severe plastic deformation. The combined effects of solid-solution(s) and intermetallic compound(s) can significantly improve the wear resistance of HEFs and HECs. It is worth mentioning that the excellent wear resistance of HEFs and HECs cannot be linked to a single factor, but rather to a combination of factors. The investigation of innovative wear-resistant mechanisms for cladded HECs is important, as it is expected to drive the development of new types of wear-resistant HECs.
- According to the literature, HECs have a high hardness due to the formation of hard phases such as BCC, B2, laves, and ordered phases. Many authors have also reported nitride HECs with extremely high hardness greater than ~40 GPa and discussed the effect of N2 concentrations on the mechanical properties of nitride HECs. Whereas cladded and thermally sprayed HECs have been reported to have a maximum hardness of ~10 GPa.
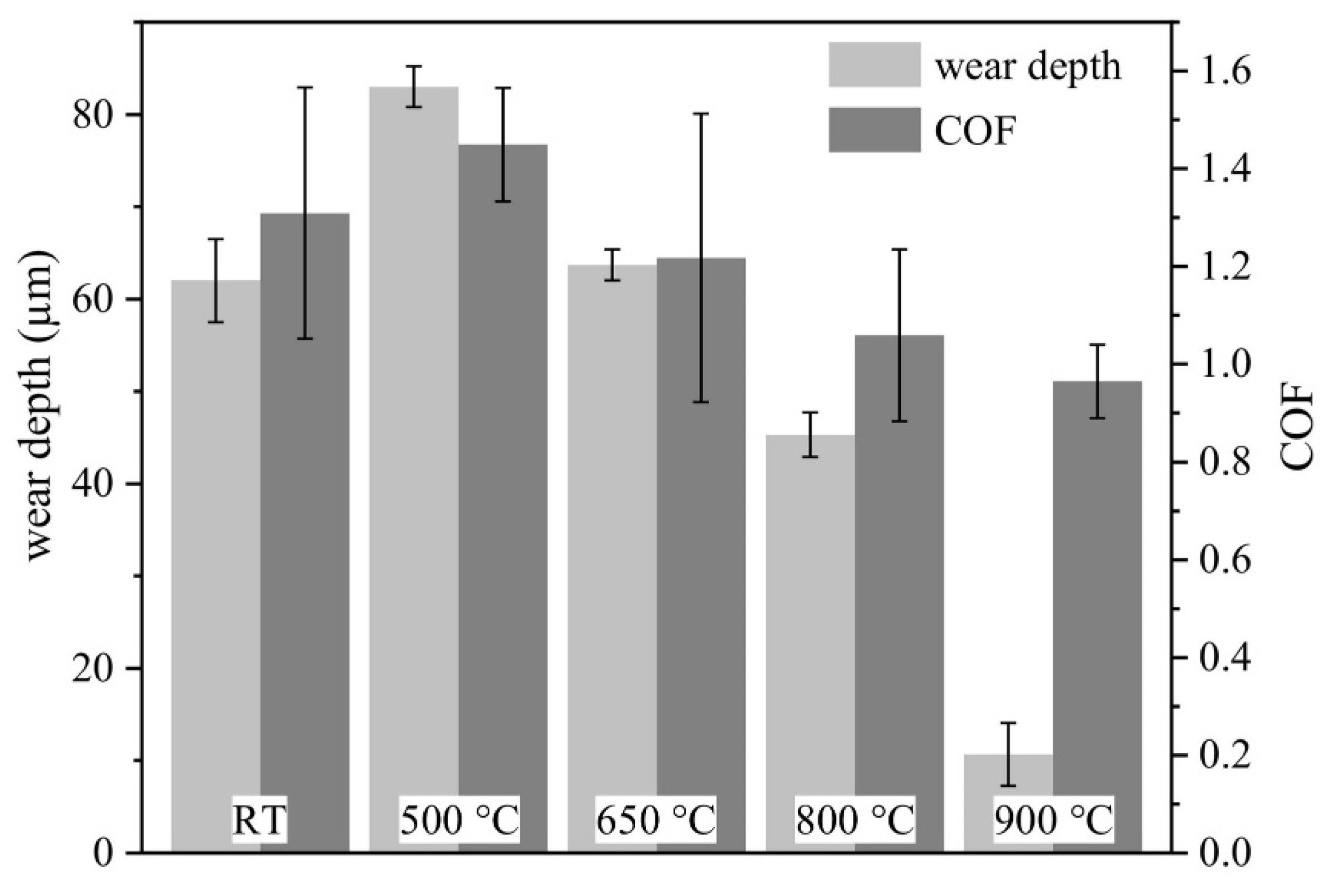
- The authors reviewed the results of tribological testing at room temperature as well as at high temperatures. The authors found that most of the HECs possess a higher wear resistance from room temperature to 750 °C. However, the wear mechanism was abrasive at room temperature to 500 °C, and after that, the dominant mechanism was oxidation.
- The tribological properties of coatings/films can be determined by their mechanical properties. Higher hardness, in particular, is more resistant to plastic deformation under certain applied loads. According to Leyland [189], the tribological properties of coatings could be evaluated using the values H/E and H3/E2 (H and E represent hardness and elastic modulus, respectively), which depicted the long elastic strain to failure and the contact yield pressure in a rigid ball on the elastic/plastic plate contact condition, respectively. As a result, increased hardness can contribute to the attractive wear resistance of HEFs and HECs.
- As previously stated, HECs have been mostly formed of single solid solution FCC, BCC, and FCC + BCC, with the formation of some minor oxides and an intermetallic phase. The authors attempted to correlate the mechanical and tribological properties of HECs with major phase groups (FCC, BCC, and FCC + BCC). HECs with sole BCC phase showed higher hardness and wear properties relative to FCC due to their limited slip systems but were also brittle. Despite having lower wear properties than the BCC group, FCC phase coatings have remained a viable category for some applications, particularly those requiring some ductility. Furthermore, FCC + BCC groups exhibited properties that were intermediate between BCC and FCC groups and may be an interesting group to investigate further with an optimistic future of HECs.
9. Future Scope of High-Entropy Coatings (HECs) for Various Applications
- There is very limited literature available on how to manufacture HEA targets and rods using various manufacturing methods. Hence, it provides a chance to investigate alternate processes in order to get a competitive edge over effective fabrication. According to Kasar et al. [192], one possibility is to use an additive manufacturing technology, such as laser power bed diffusion, to synthesize HEA targets with lubricating elements. These developed HEAs targets/rods with lubricious elements such as Mo, Cu, Nb, Zr, and Ta can be used in the magnetron sputtering method to develop HEFs. However, in order to set the matrix of HEAs compared to conventional alloys, the feasibility of the process and cost factor must be analysed.
- There is very little information available that can reveal the correlation between the powder morphology and the final microstructure and properties of HECs. Therefore, advanced research needs to be carried out on the various HECs by manipulating the powder particle size and morphologies to reveal the relationships between powder particles and microstructure and properties.
- Based on the literature, nitride HE coatings can provide higher mechanical and thermal properties. Also, many authors reported nitride coatings to possess the same properties as superalloys. As suggested by W. Li et al. [33], the effects of the non-nitride-forming element(s) on the microstructures and mechanical properties of HE nitride coatings are still not clear. Therefore, needs further research to understand the strengthening mechanism of the HE nitride coatings and develop the new HE nitride film systems with excellent hardness.
- Based on the design criteria, such as high hardness, the mixture of solid-solutions and intermetallic compounds, the compressive residual stress, and the inclusion of some lubricious elements, new types of HEAs and HECs with the superior wear resistance should be designed, fabricated, and applied.
- This review paper summarizes the different HECs formed by various coating methods. As pointed highlighted by Meghwal et al. [82], there is limited information provided in the literature related to testing conditions and standard practices. Hence, the authors strongly recommend to all the researchers to follow the standard methods for microstructure and thermal characterization [199,200,201,202], tribological testing and mechanical testing procedures [115].
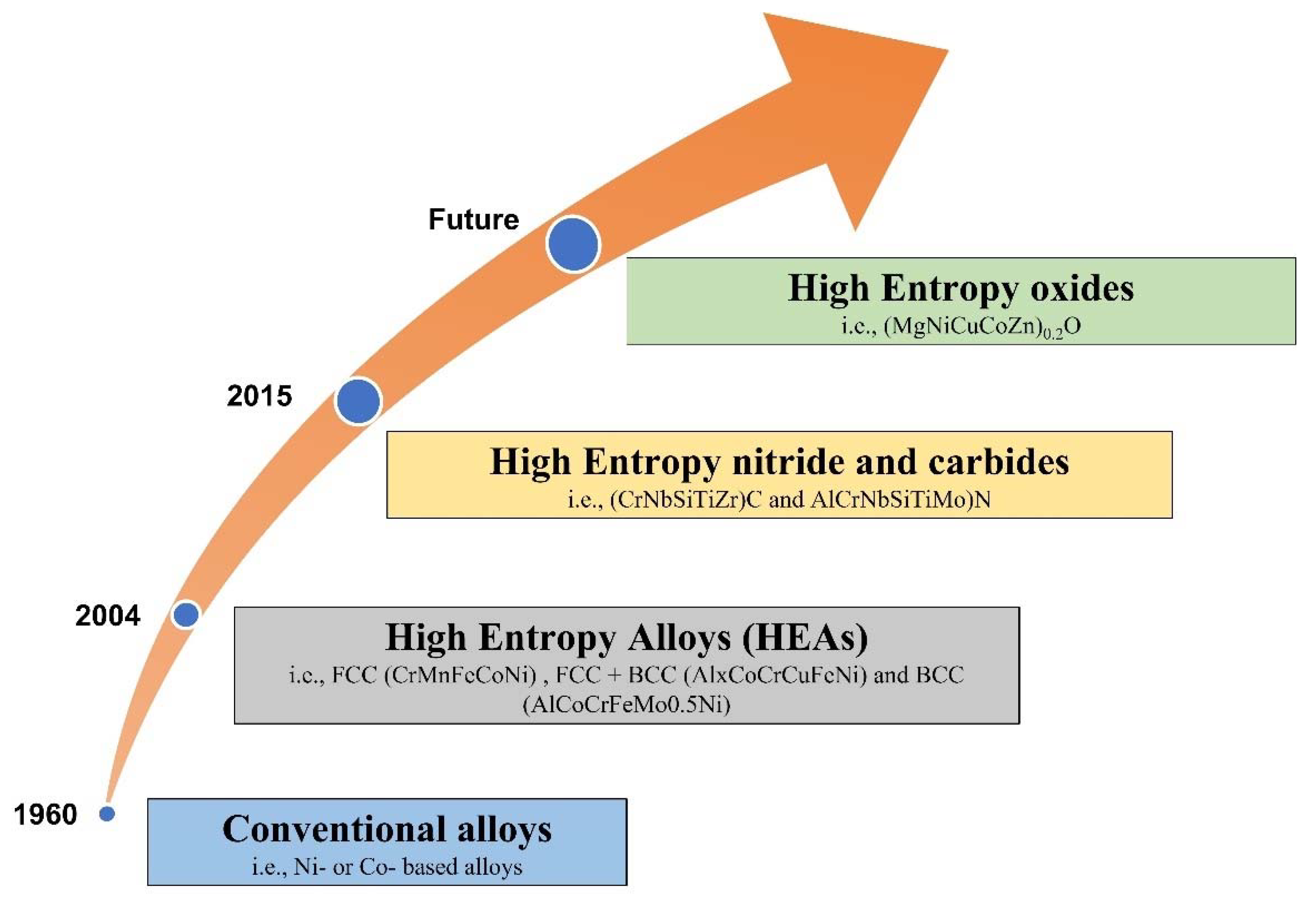
- Currently, more efforts are addressed towards developing HE coatings with improved wear resistance to meet the requirements of industry. In the last decade, the HECs gained global attention due to their combination of properties and novelty. However, there is very limited information available on the efficient means of producing HE carbides and nitrides with higher coating thicknesses. Since the HE carbides and nitrides exhibit excellent mechanical properties, many researchers are optimistic about the future of these HE carbides and nitrides. Figure 32 demonstrates the future trend (in other words evolution) of HEAs. High entropy oxides are also gaining attention of many researchers as a potential solution to these high temperature tribological issues. High-entropy oxides (HEOs) are complex oxides with a single-phase crystal structure and five or more distinct metal cations in the same amount. There have been relatively fewer studies on HEO to date; the first HEO, (MgNiCuCoZn)0.2O in a rock salt structure, was reported in 2015 by Rost et al. [203]. According to the authors, the ability to stabilize all these binary compounds into a single rock salt phase is a direct result of the value of the configurational entropy, which is exceptionally large in the case of a multicomponent (generally up to 5 components) mixture and compensates for the unfavourable correspondent enthalpic contribution. However, for this new class of oxide systems to find the optimum composition regime for obtaining desired functional properties, a combination of experiments and theoretical calculations is needed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Czichos, H.; Woydt, M. Introduction to Tribology and Tribological Parameters. In ASM Handbook of Friction, Lubrication and Wear Technology; ASM International: Almere, The Netherlands, 2017; Volume 18. [Google Scholar]
- Hernandez, S. High Temperature Wear Processes; Luleå Tekniska Universitet: Luleå, Sweden, 2014. [Google Scholar]
- Zum Gahr, K.H. Microstructure and Wear of Materials; Tribology Series 10; Elsevier: New York, NY, USA, 1987; p. 132. [Google Scholar]
- Becker, E.P. Trends in Tribological Materials and Engine Technology. Tribol. Int. 2004, 37, 569–575. [Google Scholar] [CrossRef]
- Botto, D.; Lavella, M. High Temperature Tribological Study of Cobalt-Based Coatings Reinforced with Different Percentages of Alumina. Wear 2014, 318, 89–97. [Google Scholar] [CrossRef]
- Blau, P.J. Elevated-Temperature Tribology of Metallic Materials. Tribol. Int. 2010, 43, 1203–1208. [Google Scholar] [CrossRef]
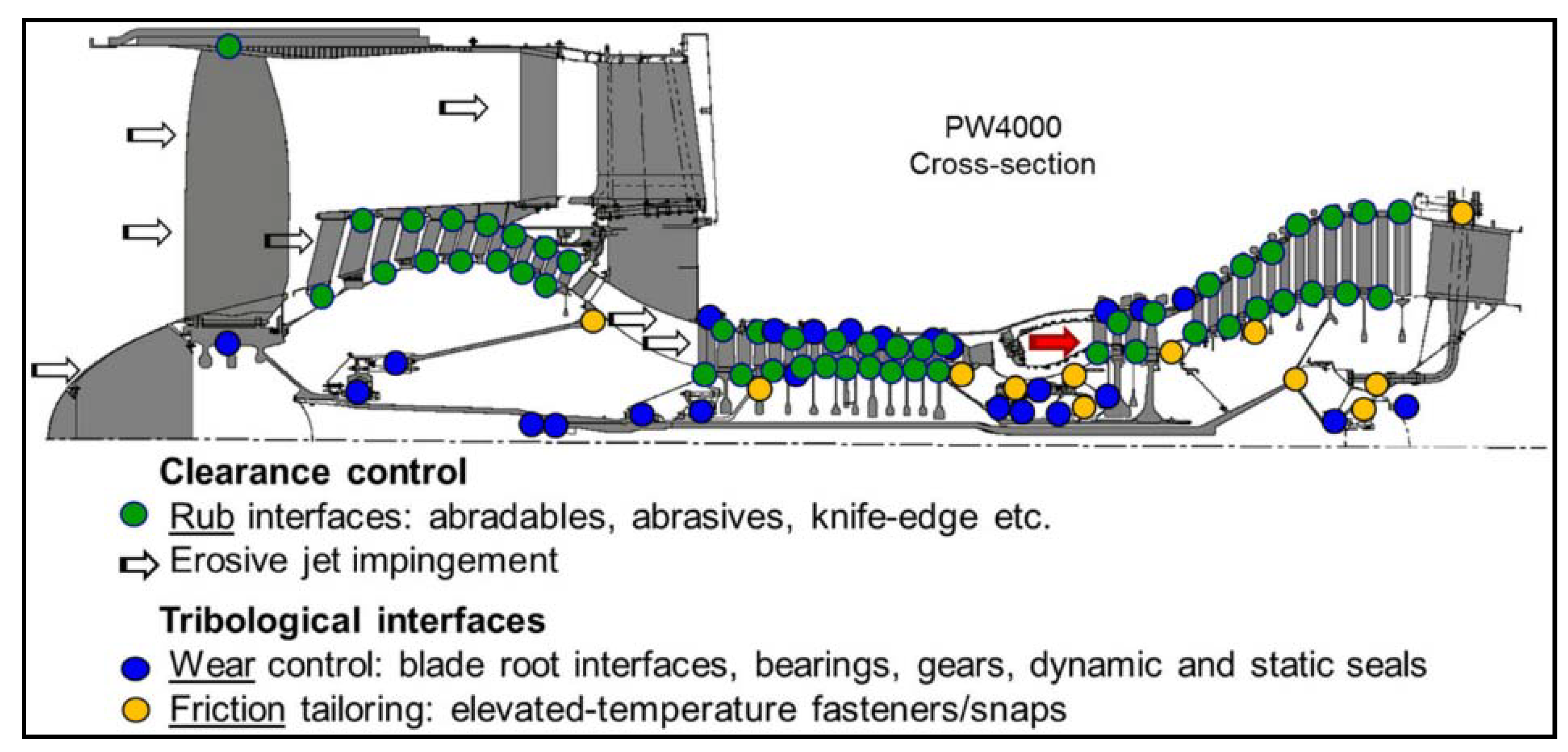
- Agrawal, G.L. Foil Air/Gas Bearing Technology—An Overview. In Proceedings of the ASME 1997 International Gas Turbine and Aeroengine Congress and Exhibition, Orlando, FL, USA, 2–5 June 1997; Volume 78682, p. V001T04A006. [Google Scholar]
- Harris, T.A.; Barnsby, R.M. Tribological Performance Prediction of Aircraft Gas Turbine Mainshaft Ball Bearings. Tribol. Trans. 1998, 41, 60–68. [Google Scholar] [CrossRef]
- Stoyanov, P.; Harrington, K.M.; Frye, A. Insights into the Tribological Characteristic of Cu-Based Coatings under Extreme Contact Conditions. JOM 2020, 72, 2191–2197. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C. Lubricious Oxide Coatings for Extreme Temperature Applications: A Review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Chupp, R.E.; Hendricks, R.C.; Steinetz, B.M. Gas Turbine Engines: Seals. In Encyclopedia of Aerospace Engineering; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Aouadi, S.; Broitman, E.; Figueroa, C.A.; Greczynski, G.; Zapien, J.A.; Stüber, M. ICMCTF 2018—Preface. Surf. Coat. Technol. 2019, 357, 1014. [Google Scholar] [CrossRef]
- Sun, W.; Tan, A.W.-Y.; King, D.J.Y.; Khun, N.W.; Bhowmik, A.; Marinescu, I.; Liu, E. Tribological Behavior of Cold Sprayed Inconel 718 Coatings at Room and Elevated Temperatures. Surf. Coat. Technol. 2020, 385, 125386. [Google Scholar] [CrossRef]
- Majcherczak, D.; Dufrenoy, P.; Berthier, Y. Tribological, Thermal and Mechanical Coupling Aspects of the Dry Sliding Contact. Tribol. Int. 2007, 40, 834–843. [Google Scholar] [CrossRef]
- Wen, S.; Huang, P. Principles of Tribology; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118062892. [Google Scholar]
- Nilsson, M. Tribology in Metal Working. Ph.D. Thesis, Uppsala University, Department of Engineering Sciences, Uppsala, Sweden, 2012. [Google Scholar]
- Alvi, S.A. Synthesis and Characterization of High Entropy Alloy and Coating. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2019. [Google Scholar]
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured High-entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed Pleasures: Multimetallic Cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The Influences of Temperature and Microstructure on the Tensile Properties of a CoCrFeMnNi High-Entropy Alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in Multi-Component AlCoCrCuFeNi High-Entropy Alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue Behavior of Al0.5CoCrCuFeNi High Entropy Alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish Diffusion in Co–Cr–Fe–Mn–Ni High-Entropy Alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Soni, V.; Senkov, O.N.; Gwalani, B.; Miracle, D.B.; Banerjee, R. Microstructural Design for Improving Ductility of an Initially Brittle Refractory High Entropy Alloy. Sci. Rep. 2018, 8, 8816. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A Fracture-Resistant High-Entropy Alloy for Cryogenic Applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alloys, E.; Sheikh, S. Thesis for the Degree of Licentiate of Technology; Kopieringen Mittuniversitetet: Sundsvall, Sweden, 2016. [Google Scholar]
- Porter, D.A.; Easterling, K.E.; Sherif, M. Phase Transformations in Metals and Alloys (Revised Reprint); CRC Press: Boca Raton, FL, USA, 2009; ISBN 1439883572. [Google Scholar]
- Li, W.; Liu, P.; Liaw, P.K. Microstructures and Properties of High-Entropy Alloy Films and Coatings: A Review. Mater. Res. Lett. 2018, 6, 199–229. [Google Scholar] [CrossRef] [Green Version]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Swalin, R.A. Thermodynamics of Solids; John Wiley Sons: New York, NY, USA, 1972; p. 178. [Google Scholar]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and development of high entropy alloys for structural applications. Entropy 2014, 16, 494. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin, Germany, 2016. [Google Scholar]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- Sheikh, S. Alloy Design and Optimization of Mechanical Properties of High-Entropy. Ph.D. Thesis, Chalmers Tekniska Hogskola, Gothenburg, Sweden, 2016. [Google Scholar]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, Y.; Liu, B.; Guo, W.; Xu, L. Microstructure and Wear Behavior of FeCoCrNiMo0.2 High Entropy Coatings Prepared by Air Plasma Spray and the High Velocity Oxy-Fuel Spray Processes. Coatings 2017, 7, 151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure Characterization of Al x CoCrCuFeNi High-Entropy Alloy System with Multiprincipal Elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative Effects of Enthalpy and Entropy on the Phase Stability of Equiatomic High-Entropy Alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Wall, M.T.; Pantawane, M.V.; Joshi, S.; Gantz, F.; Ley, N.A.; Mayer, R.; Spires, A.; Young, M.L.; Dahotre, N. Laser-Coated CoFeNiCrAlTi High Entropy Alloy onto a H13 Steel Die Head. Surf. Coat. Technol. 2020, 387, 125473. [Google Scholar] [CrossRef]
- Chen, S.-T.; Tang, W.-Y.; Kuo, Y.-F.; Chen, S.-Y.; Tsau, C.-H.; Shun, T.-T.; Yeh, J.-W. Microstructure and Properties of Age-Hardenable AlxCrFe1.5MnNi0.5 Alloys. Mater. Sci. Eng. A 2010, 527, 5818–5825. [Google Scholar] [CrossRef]
- Liu, C.M.; Wang, H.M.; Zhang, S.Q.; Tang, H.B.; Zhang, A.L. Microstructure and Oxidation Behavior of New Refractory High Entropy Alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Nong, Z.S.; Lei, Y.N.; Zhu, J.C. Wear and Oxidation Resistances of AlCrFeNiTi-Based High Entropy Alloys. Intermetallics 2018, 101, 144–151. [Google Scholar] [CrossRef]
- Huang, P.; Yeh, J.; Shun, T.; Chen, S. Multi-principal-element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Shen, J.; Vilar, R. Thermal Stability and Oxidation Resistance of Laser Clad TiVCrAlSi High Entropy Alloy Coatings on Ti-6Al-4V Alloy. Surf. Coat. Technol. 2011, 206, 1389–1395. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and Electrochemical Properties of High Entropy Alloys—a Comparison with Type-304 Stainless Steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Chiang, W.-C.; Wu, J.-K. Corrosion Behavior of FeCoNiCrCux High-Entropy Alloys in 3.5% Sodium Chloride Solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Shi, P.; Yu, Y.; Xiong, N.; Liu, M.; Qiao, Z.; Yi, G.; Yao, Q.; Zhao, G.; Xie, E.; Wang, Q. Microstructure and Tribological Behavior of a Novel Atmospheric Plasma Sprayed AlCoCrFeNi High Entropy Alloy Matrix Self-Lubricating Composite Coatings. Tribol. Int. 2020, 151, 106470. [Google Scholar] [CrossRef]
- Qiu, X. Microstructure, Hardness and Corrosion Resistance of Al2CoCrCuFeNiTix High-Entropy Alloy Coatings Prepared by Rapid Solidification. J. Alloys Compd. 2018, 735, 359–364. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Microstructure and Wear Resistance of AlCoCrFeNiTi High-Entropy Alloy Coatings Produced by HVOF. Coatings 2017, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Öte, M.; Königstein, T.; Tan, Z.; He, D. Wear Behavior of HVOF-Sprayed Al0.6TiCrFeCoNi High Entropy Alloy Coatings at Different Temperatures. Surf. Coat. Technol. 2019, 358, 215–222. [Google Scholar] [CrossRef]
- Hsu, W.; Yang, Y.; Chen, C.; Yeh, J. Thermal Sprayed High-Entropy NiCo0.6Fe0.2Cr1.5SiAlTi0.2 Coating with Improved Mechanical Properties and Oxidation Resistance. Intermetallics 2017, 89, 105–110. [Google Scholar] [CrossRef]
- Jin, G.; Cai, Z.; Guan, Y.; Cui, X.; Liu, Z.; Li, Y. Tribological Performance of High-Entropy Coatings (HECs); Institute of Surface/Interface Science and Technology, Key Laboratory of College of Material Science and Chemical Engineering: Harbin, China, 2018; ISBN 8604518258. [Google Scholar]
- Huang, C.; Zhang, Y.; Vilar, R.; Shen, J. Dry Sliding Wear Behavior of Laser Clad TiVCrAlSi High Entropy Alloy Coatings on Ti-6Al-4V Substrate. Mater. Des. 2012, 41, 338–343. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.; Jiao, H. Microstructure and Properties of 6FeNiCoSiCrAlTi High-Entropy Alloy Coating Prepared by Laser Cladding. Appl. Surf. Sci. 2011, 257, 2259–2263. [Google Scholar] [CrossRef]
- Srivastava, M.; Jadhav, M.; Chakradhar, R.P.S.; Muniprakash, M.; Singh, S. Surface & Coatings Technology Synthesis and Properties of High Velocity Oxy-Fuel Sprayed FeCoCrNi2Al High Entropy Alloy Coating. Surf. Coat. Technol. 2019, 378, 124950. [Google Scholar] [CrossRef]
- Geant, V.; Voiculescu, I.; Mitrica, D.; Tudor, A.; Bobzin, K. Processing of AlCoCrFeNiTi High Entropy Alloy by Atmospheric Plasma Spraying. IOP Conf. Ser. Mater. Sci. Eng. 2017, 181, 012015. [Google Scholar] [CrossRef]
- Li, Z. Designing and Understanding Novel High-Entropy Alloys towards Superior Properties; Talk Presented at Talk at Universität Kassel; Institut für Werkstofftechnik: Kassel, Germany, 2018. [Google Scholar]
- Tian, L. Microstructure and Wear Behavior of Atmospheric Plasma-Sprayed AlCoCrFeNiTi High-Entropy Alloy Coating. J. Mater. Eng. Perform. 2016, 25, 5513–5521. [Google Scholar] [CrossRef]
- Behaviors, C. Microstructure, Mechanical and Corrosion Behaviors of CoCrFeNiAl0.3 High Entropy Alloy (HEA) Films. Coatings 2017, 7, 156. [Google Scholar] [CrossRef]
- Tian, L.; Feng, Z.; Xiong, W. Microstructure, Microhardness, and Wear Resistance of AlCoCrFeNiTi/Ni60 Coating by Plasma Spraying. Coatings 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.K.; Tan, H.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and Wear Behavior of FeCoNiCrMn High Entropy Alloy Coating Deposited by Plasma Spraying. Surf. Coat. Technol. 2020, 385, 125430. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, S.-K.; Chen, T.-J.; Chu, P.-C.; Yeh, J.-W.; Lin, S.-J. Electrical, Magnetic, and Hall Properties of AlxCoCrFeNi High-Entropy Alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.; Cheng, Y.; Liaw, P.K. High-Entropy Alloys with High Saturation Magnetization, Electrical Resistivity, and Malleability. Sci. Rep. 2013, 3, 1455. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Liu, Y.; Zhao, X.; Cheng, J.; Li, H. Electromagnetic Wave Absorption Properties of Mechanically Alloyed FeCoNiCrAl High Entropy Alloy Powders. Adv. Powder Technol. 2016, 27, 1128–1133. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Li, K.; Zhao, G.-L.; Guo, S.M. Electromagnetic Interference Shielding Effectiveness of High Entropy AlCoCrFeNi Alloy Powder Laden Composites. J. Alloys Compd. 2018, 734, 220–228. [Google Scholar] [CrossRef]
- Berndt, C.C.; Hasan, F.; Tietz, U.; Schmitz, K.-P. A Review of Hydroxyapatite Coatings Manufactured by Thermal Spray. In Advances in Calcium Phosphate Biomaterials; Springer: Berlin, Germany, 2014; pp. 267–329. [Google Scholar]
- Alagarsamy, K.; Fortier, A.; Komarasamy, M.; Kumar, N.; Mohammad, A.; Banerjee, S.; Han, H.-C.; Mishra, R.S. Mechanical Properties of High Entropy Alloy Al 0.1 Cocrfeni for Peripheral Vascular Stent Application. Cardiovasc. Eng. Technol. 2016, 7, 448–454. [Google Scholar] [CrossRef]
- Popescu, G.; Ghiban, B.; Popescu, C.A.; Rosu, L.; Trusca, R.; Carcea, I.; Soare, V.; Dumitrescu, D.; Constantin, I.; Olaru, M.T. New TiZrNbTaFe High Entropy Alloy Used for Medical Applications. IOP Conf. Ser. Mater. Sci. Eng. 2018, 400, 22049. [Google Scholar] [CrossRef] [Green Version]
- Vladescu, A.; Titorencu, I.; Dekhtyar, Y.; Jinga, V.; Pruna, V.; Balaceanu, M.; Dinu, M.; Pana, I.; Vendina, V.; Braic, M. In Vitro Biocompatibility of Si Alloyed Multi-Principal Element Carbide Coatings. PLoS ONE 2016, 11, e0161151. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Yu, Y.; Zhang, Z.; Zhu, S.; Lou, X.; Wang, Z. Study of High Temperature Friction and Wear Performance of (CoCrFeMnNi) 85Ti15 High-Entropy Alloy Coating Prepared by Plasma Cladding. Surf. Coat. Technol. 2020, 384, 125337. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Yan, C.; Zhang, X.; Xiong, D. Microstructure and Tribological Properties of Plasma-Sprayed Al0.2Co1.5CrFeNi1.5Ti-Ag Composite Coating from 25 to 750 °C. J. Mater. Eng. Perform. 2020, 29, 1640–1649. [Google Scholar] [CrossRef]
- Yin, S.; Lupoi, R.; Li, W.; Xu, Y.; Song, B.; Yan, X.; Kuang, M. Cold Sprayed FeCoNiCrMn High Entropy Alloy (HEA) Coating: Microstructure and Tribological Property. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar] [CrossRef]
- Lo, W.-L.; Hsu, S.-Y.; Lin, Y.-C.; Tsai, S.-Y.; Lai, Y.-T.; Duh, J.-G. Improvement of High Entropy Alloy Nitride Coatings (AlCrNbSiTiMo) N on Mechanical and High Temperature Tribological Properties by Tuning Substrate Bias. Surf. Coat. Technol. 2020, 401, 126247. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Y.; Xu, J.; Zhu, C.; Dai, P.; Xue, S. Tribological Properties of Nano/Ultrafine-Grained FeCoCrNiMnAlx High-Entropy Alloys over a Wide Range of Temperatures. J. Alloys Compd. 2020, 817, 153305. [Google Scholar] [CrossRef]
- Patel, P.; Alidokht, S.A.; Sharifi, N.; Roy, A.; Harrington, K.; Stoyanov, P.; Chromik, R.R.; Moreau, C. Microstructural and Tribological Behavior of Thermal Spray CrMnFeCoNi High Entropy Alloy Coatings. J. Therm. Spray Technol. 2022, 31, 1285–1301. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal Spray High-Entropy Alloy Coatings: A Review. J. Thermal Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Canter, N. High-Entropy Alloys. Tribol. Lubr. Technol. 2015, 71, 14. [Google Scholar]
- Chikumba, S.; Rao, V.V. High Entropy Alloys: Development and Applications. In Proceedings of the 7th International Conference on Latest Trends in Engineering & Technology (ICLTET’2015), Irene, Pretoria, South Africa, 26–27 November 2015; pp. 1–5. [Google Scholar]
- Clayton, P. Tribological Behavior of a Titanium-Nickel Alloy. Wear 1993, 162, 202–210. [Google Scholar] [CrossRef]
- Patel, P.; Jamnapara, N.I.; Zala, A.; Kahar, S.D. Investigation of Hot-Dip Aluminized Ti6Al4V Alloy Processed by Different Thermal Treatments in an Oxidizing Atmosphere. Surf. Coat. Technol. 2020, 385, 125323. [Google Scholar] [CrossRef]
- Parekh, T.; Patel, P.; Sasmal, C.S.; Jamnapara, N.I. Effect of Plasma Processed Ti-Al Coating on Oxidation and Tensile Behavior of Ti6Al4V Alloy. Surf. Coat. Technol. 2020, 394, 125704. [Google Scholar] [CrossRef]
- Lee, C.; Song, G.; Gao, M.C.; Feng, R.; Chen, P.; Brechtl, J.; Chen, Y.; An, K.; Guo, W.; Poplawsky, J.D. Lattice Distortion in a Strong and Ductile Refractory High-Entropy Alloy. Acta Mater. 2018, 160, 158–172. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, X.; Liu, Q. Microstructure and Properties of In-Situ TiN Reinforced Laser Cladding CoCr2FeNiTix High-Entropy Alloy Composite Coatings. Surf. Coat. Technol. 2018, 344, 353–358. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Liu, B.; Yi, D.; Yang, X.; Zhang, W.; Chen, G.; Liu, Y.; Bai, P. Influence of NbC Particles on Microstructure and Mechanical Properties of AlCoCrFeNi High-Entropy Alloy Coatings Prepared by Laser Cladding. J. Alloys Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, D.; Liang, X.; Chen, Y. Evolution of Microstructure and Mechanical Properties of in Situ Synthesized TiC–TiB2/CoCrCuFeNi High Entropy Alloy Coatings. Surf. Coat. Technol. 2015, 281, 109–116. [Google Scholar] [CrossRef]
- Jamnapara, N.I.; Mukherjee, S. Coatings for High Temperature Applications. In High Temperature Corrosion; World Scientific: Singapore, 2016; pp. 161–200. [Google Scholar]
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [Green Version]
- Sha, C. Microstructure, Mechanical Properties, Scratch Responses and Wear Behaviours of Transition Metal Nitride and High Entropy Alloy Coatings. Ph.D. Thesis, School of Materials Science and Engineering, UNSW Sydney, Kensington, Australia, 2020. [Google Scholar]
- Braic, M.; Braic, V.; Vladescu, A.; Zoita, C.N.; Balaceanu, M. Solid Solution or Amorphous Phase Formation in TiZr-Based Ternary to Quinternary Multi-Principal-Element Films. Prog. Nat. Sci. Mater. Int. 2014, 24, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.-J.; Tsai, M.-H.; Yeh, J.-W. Machining Performance of Sputter-Deposited (Al0.34Cr0.22Nb0.11Si0.11Ti0.22)50N50 High-Entropy Nitride Coatings. Coatings 2015, 5, 312–325. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Duan, H.; Zhang, H.; Ma, J. Slurry Erosion Resistance of Laser Clad NiCoCrFeAl3 High-Entropy Alloy Coatings. Tribol. Trans. 2015, 58, 1119–1123. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; He, Y.; Li, M.; Guo, S. Formation of Core–Shell Structure in High Entropy Alloy Coating by Laser Cladding. Appl. Surf. Sci. 2016, 363, 543–547. [Google Scholar] [CrossRef]
- Meng, C.; Song, Z.; Wang, G.; Zhuang, W.; Wu, C.; Wang, X. Microstructure and Properties of CoCrFeNiCu High-Entropy Alloy Coating Prepared by Induction Cladding. Mater. Lett. 2022, 314, 131896. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Zhang, P.; Liu, M.; Li, G.-R.; Ye, J.-Q.; Liu, P.; Tong, Y.-X. Electrochemical Preparation and Magnetic Study of Bi–Fe–Co–Ni–Mn High Entropy Alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Anupam, A.; Kumar, S.; Chavan, N.M.; Murty, B.S.; Kottada, R.S. First Report on Cold-Sprayed AlCoCrFeNi High-Entropy Alloy and Its Isothermal Oxidation. J. Mater. Res. 2019, 34, 796–806. [Google Scholar] [CrossRef]
- Lehtonen, J.; Koivuluoto, H.; Ge, Y.; Juselius, A.; Hannula, S.-P. Cold Gas Spraying of a High-Entropy CrFeNiMn Equiatomic Alloy. Coatings 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Li, W.; Song, B.; Yan, X.; Kuang, M.; Xu, Y.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn High Entropy Alloy (HEA) Coating via Cold Spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Hushchyk, D.V.; Yurkova, A.I.; Cherniavsky, V.V.; Bilyk, I.I.; Nakonechnyy, S.O. Nanostructured AlNiCoFeCrTi High-Entropy Coating Performed by Cold Spray. Appl. Nanosci. 2020, 10, 4879–4890. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A Brief Review of High-Entropy Films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. 6-synthesis and processing. In High-Entropy Alloy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–117. [Google Scholar]
- Senkov, O.N.; Woodward, C.F. Microstructure and Properties of a Refractory NbCrMo0.5Ta0.5TiZr Alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Ma, S.G.; Qiao, J.W.; Wang, Z.H.; Yang, H.J.; Zhang, Y. Microstructural Features and Tensile Behaviors of the Al0.5CrCuFeNi2 High-Entropy Alloys by Cold Rolling and Subsequent Annealing. Mater. Des. 2015, 88, 1057–1062. [Google Scholar] [CrossRef]
- Khan, N.A. Entropy Alloy (HEA) and High Entropy Ceramic (HEC) Thin Films. Ph.D. Thesis, The University of Sydney, Sydney, Australia, 2021. [Google Scholar]
- Ramanathan, A.; Krishnan, P.K.; Muraliraja, R. A Review on the Production of Metal Matrix Composites through Stir Casting–Furnace Design, Properties, Challenges, and Research Opportunities. J. Manuf. Process. 2019, 42, 213–245. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Feng, T.; Zhang, Y.; Sha, G.; Lewandowski, J.J.; Liaw, P.K.; Zhang, Y. High-Entropy Al0.3CoCrFeNi Alloy Fibers with High Tensile Strength and Ductility at Ambient and Cryogenic Temperatures. Acta Mater. 2017, 123, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Beijing, T.; Xing, Q. High Entropy Alloys-Manufacturing Routes. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Kohrt, C.; Lampke, T. Processing of AlCoCrFeNiTi High Entropy Alloy by Atmospheric Plasma Spraying. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 181, p. 12015. [Google Scholar]
- Ang, A.S.; Berndt, C.C.; Sesso, M.L.; Anupam, A.; Kottada, R.S.; Murty, B.S. Plasma-Sprayed High Entropy Alloys: Microstructure and Properties of AlCoCrFeNi and MnCoCrFeNi. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 791–800. [Google Scholar] [CrossRef]
- Wang, L.M.; Chen, C.C.; Yeh, J.W.; Ke, S.T. The Microstructure and Strengthening Mechanism of Thermal Spray Coating NixCo0.6Fe0.2CrySizAlTi0.2 High-Entropy Alloys. Mater. Chem. Phys. 2011, 126, 880–885. [Google Scholar] [CrossRef]
- Hsu, W.L.; Murakami, H.; Yeh, J.W.; Yeh, A.C.; Shimoda, K. On the Study of Thermal-Sprayed Ni0.2Co0.6Fe0.2CrSi0.2AlTi0.2 HEA Overlay Coating. Surf. Coat. Technol. 2017, 316, 71–74. [Google Scholar] [CrossRef]
- Jadhav, M.; Singh, S.; Srivastava, M.; Kumar, G.S.V. An Investigation on High Entropy Alloy for Bond Coat Application in Thermal Barrier Coating System. J. Alloys Compd. 2019, 783, 662–673. [Google Scholar] [CrossRef]
- Cai, Z.; Jin, G.; Cui, X.; Liu, Z.; Zheng, W.; Li, Y.; Wang, L. Synthesis and Microstructure Characterization of Ni-Cr-Co-Ti-V-Al High Entropy Alloy Coating on Ti-6Al-4V Substrate by Laser Surface Alloying. Mater. Charact. 2016, 120, 229–233. [Google Scholar] [CrossRef]
- He, B.; Zhang, N.; Lin, D.; Zhang, Y.; Dong, F.; Li, D. The Phase Evolution and Property of FeCoCrNiAlTix High-Entropy Alloying Coatings on Q253 via Laser Cladding. Coatings 2017, 7, 157. [Google Scholar] [CrossRef]
- Tian, L.; Fu, M.; Xiong, W. Microstructural Evolution of AlCoCrFeNiSi High-Entropy Alloy Powder during Mechanical Alloying and Its Coating Performance. Materials 2018, 11, 320. [Google Scholar] [CrossRef] [Green Version]
- Löbel, M.; Lindner, T.; Clauß, S.; Pippig, R.; Dietrich, D.; Lampke, T. Microstructure and Wear Behavior of the High-Velocity-Oxygen-Fuel Sprayed and Spark Plasma Sintered High-Entropy Alloy AlCrFeCoNi. Adv. Eng. Mater. 2021, 23, 2001253. [Google Scholar] [CrossRef]
- Mu, Y.K.; Jia, Y.D.; Xu, L.; Jia, Y.F.; Tan, X.H.; Yi, J.; Wang, G.; Liaw, P.K. Nano Oxides Reinforced High-Entropy Alloy Coatings Synthesized by Atmospheric Plasma Spraying. Mater. Res. Lett. 2019, 7, 312–319. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Chen, J.-H.; Stadler, S.; Chen, S.-H. Properties of Atomized AlCoCrFeNi High-Entropy Alloy Powders and Their Phase-Adjustable Coatings Prepared via Plasma Spray Process. Appl. Surf. Sci. 2019, 478, 478–486. [Google Scholar] [CrossRef]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.; Meng, G. Microstructure of Laser Re-Melted AlCoCrCuFeNi High Entropy Alloy Coatings Produced by Plasma Spraying. Entropy 2013, 15, 2833–2845. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Qi, W.; Xie, L.; Yang, X.; Li, J.; Zhang, Y. Microstructure and Corrosion Behavior of (CoCrFeNi) 95Nb5 High-Entropy Alloy Coating Fabricated by Plasma Spraying. Materials 2019, 12, 694. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.J.; Lin, X.; Wang, Y.Y.; Xie, C.S.; Yue, T.M. Microstructure and Corrosion Resistance of Cu0· 9NiAlCoCrFe High Entropy Alloy Coating on AZ91D Magnesium Alloys by Laser Cladding. Mater. Res. Innov. 2014, 18, S2-1008–S2-1011. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. High-Temperature Wear Behaviour of AlCoCrFeNiTi0.5 Coatings Produced by HVOF. Surf. Coat. Technol. 2020, 403, 126379. [Google Scholar] [CrossRef]
- Cao, F.; Munroe, P.; Zhou, Z.; Xie, Z. Microstructure and Mechanical Properties of a Multilayered CoCrNi/Ti Coating with Varying Crystal Structure. Surf. Coat. Technol. 2018, 350, 596–602. [Google Scholar] [CrossRef]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and Tribological Properties of TiTaHfNbZr High Entropy Alloy Coatings Deposited on Ti6Al4V Substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Corrosion Behavior of (Ti–Al–Cr–Si–V) XNy Coatings on Mild Steels Derived from RF Magnetron Sputtering. Surf. Coat. Technol. 2008, 203, 558–561. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, Structure, and Properties of an AlCrMoNbZr High-Entropy Alloy Coating for Accident-Tolerant Fuel Cladding. Surf. Coat. Technol. 2018, 347, 13–19. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.R.; Braic, M. Nanostructured Multi-Element (TiZrNbHfTa) N and (TiZrNbHfTa) C Hard Coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of Nitrogen Content on Structure and Mechanical Properties of Multi-Element (AlCrNbSiTiV) N Coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Ren, B.; Shen, Z.; Liu, Z. Structure and Mechanical Properties of Multi-Element (AlCrMnMoNiZr) Nx Coatings by Reactive Magnetron Sputtering. J. Alloys Compd. 2013, 560, 171–176. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Deng, M.-J.; Chang, Z.-C.; Kuo, B.-H.; Chen, E.-C.; Chang, S.-Y.; Shieu, F.-S. Oxidation Resistance and Characterization of (AlCrMoTaTi)-Six-N Coating Deposited via Magnetron Sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Johansson, K.; Riekehr, L.; Fritze, S.; Lewin, E. Multicomponent Hf-Nb-Ti-V-Zr Nitride Coatings by Reactive Magnetron Sputter Deposition. Surf. Coat. Technol. 2018, 349, 529–539. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Yakushchenko, I.V.; Bondar, O.V.; Beresnev, V.M.; Oyoshi, K.; Ivasishin, O.M.; Amekura, H.; Takeda, Y.; Opielak, M.; Kozak, C. Irradiation Resistance, Microstructure and Mechanical Properties of Nanostructured (TiZrHfVNbTa)N Coatings. J. Alloys Compd. 2016, 679, 155–163. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D. Structure and Properties of Nanostructured (Ti-Hf-Zr-V-Nb) N Coatings. J. Nanomater. 2013, 201, 5. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Yakushchenko, I.V.; Bagdasaryan, A.A.; Bondar, O.V.; Krause-Rehberg, R.; Abadias, G.; Chartier, P.; Oyoshi, K.; Takeda, Y.; Beresnev, V.M. Microstructure, Physical and Chemical Properties of Nanostructured (Ti–Hf–Zr–V–Nb) N Coatings under Different Deposition Conditions. Mater. Chem. Phys. 2014, 147, 1079–1091. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M.; Smyrnova, K.V.; Kravchenko, Y.O.; Zukowski, P.V.; Bondarenko, G.G. The Influence of Nitrogen Pressure on the Fabrication of the Two-Phase Superhard Nanocomposite (TiZrNbAlYCr) N Coatings. Mater. Lett. 2018, 211, 316–318. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.K.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and Tribological Properties of Plasma Sprayed FeCoNiCrSiAlx High Entropy Alloy Coatings. Wear 2020, 448–449, 203209. [Google Scholar] [CrossRef]
- Qiu, X.W.; Zhang, Y.P.; Liu, C.G. Effect of Ti Content on Structure and Properties of Al2CrFeNiCoCuTix High-Entropy Alloy Coatings. J. Alloys Compd. 2014, 585, 282–286. [Google Scholar] [CrossRef]
- Juan, Y.F.; Li, J.; Jiang, Y.Q.; Jia, W.L.; Lu, Z.J. Modified Criterions for Phase Prediction in the Multi-Component Laser-Clad Coatings and Investigations into Microstructural Evolution/Wear Resistance of FeCrCoNiAlMox Laser-Clad Coatings. Appl. Surf. Sci. 2019, 465, 700–714. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q. MoFeCrTiWAlNb Refractory High-Entropy Alloy Coating Fabricated by Rectangular-Spot Laser Cladding. Intermetallics 2018, 102, 78–87. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Juan, Y.F.; Lu, Z.J.; Jia, W.L. Evolution in Microstructure and Corrosion Behavior of AlCoCrxFeNi High-Entropy Alloy Coatings Fabricated by Laser Cladding. J. Alloys Compd. 2019, 775, 1–14. [Google Scholar] [CrossRef]
- Shu, F.Y.; Wu, L.; Zhao, H.Y.; Sui, S.H.; Zhou, L.; Zhang, J.; He, W.X.; He, P.; Xu, B.S. Microstructure and High-Temperature Wear Mechanism of Laser Cladded CoCrBFeNiSi High-Entropy Alloy Amorphous Coating. Mater. Lett. 2018, 211, 235–238. [Google Scholar] [CrossRef]
- Shu, F.; Zhang, B.; Liu, T.; Sui, S.; Liu, Y.; He, P.; Liu, B.; Xu, B. Effects of Laser Power on Microstructure and Properties of Laser Cladded CoCrBFeNiSi High-Entropy Alloy Amorphous Coatings. Surf. Coat. Technol. 2019, 358, 667–675. [Google Scholar] [CrossRef]
- Ni, C.; Shi, Y.; Liu, J.; Huang, G. Characterization of Al0.5FeCu0.7NiCoCr High-Entropy Alloy Coating on Aluminum Alloy by Laser Cladding. Opt. Laser Technol. 2018, 105, 257–263. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.Z. Synthesis and Characterization of FeCoNiCrCu High-Entropy Alloy Coating by Laser Cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; Cui, X.; Jin, G.; Li, Y.; Liu, Z.; Dong, M. Design and Microstructure Characterization of FeCoNiAlCu High-Entropy Alloy Coating by Plasma Cladding: In Comparison with Thermodynamic Calculation. Surf. Coat. Technol. 2017, 330, 163–169. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, Y.; Li, J.; Liu, Y.; Liu, Y. Microstructure and Mechanical Properties of FeCoCrNiNbX High-Entropy Alloy Coatings. Phys. B Condens. Matter 2018, 550, 112–116. [Google Scholar] [CrossRef]
- Lu, J.; Wang, B.; Qiu, X.; Peng, Z.; Ma, M. Microstructure Evolution and Properties of CrCuFexNiTi High-Entropy Alloy Coating by Plasma Cladding on Q235. Surf. Coat. Technol. 2017, 328, 313–318. [Google Scholar] [CrossRef]
- Küçük, Y. Effect of Counterbody on the Dry Sliding Wear Performance of Plasma Sprayed Calcia-Stabilized Zirconia Coating. Int. J. Refract. Met. Hard Mater. 2020, 92, 105284. [Google Scholar] [CrossRef]
- Alvi, S.; Akhtar, F. High Temperature Tribology of CuMoTaWV High Entropy Alloy. Wear 2019, 426–427, 412–419. [Google Scholar] [CrossRef]
- Alvi, S.; Jarzabek, D.M.; Kohan, M.G.; Hedman, D.; Jenczyk, P.; Natile, M.M.; Vomiero, A.; Akhtar, F. Synthesis and Mechanical Characterization of a CuMoTaWV High-Entropy Film by Magnetron Sputtering. ACS Appl. Mater. Interfaces 2020, 12, 21070–21079. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, Z.; Hong, Y.; Lu, B.; Liu, J.; Li, Y.; Pu, J. Improved Tribological Behavior of Plasma-Nitrided AlCrTiV and AlCrTiVSi High-Entropy Alloy Films. Tribol. Int. 2021, 163, 107195. [Google Scholar] [CrossRef]
- Lai, C.-H.; Cheng, K.-H.; Lin, S.-J.; Yeh, J.-W. Mechanical and Tribological Properties of Multi-Element (AlCrTaTiZr) N Coatings. Surf. Coat. Technol. 2008, 202, 3732–3738. [Google Scholar] [CrossRef]
- Si, Y.; Wang, G.; Wen, M.; Tong, Y.; Wang, W.; Li, Y.; Yan, L.; Yu, W.; Zhang, S.; Ren, P. Corrosion and Friction Resistance of TiVCrZrWNx High Entropy Ceramics Coatings Prepared by Magnetron Sputtering. Ceram. Int. 2022, 48, 9342–9352. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Xia, Y. Synthesis and Characterization of Super-Hard AlCrTiVZr High-Entropy Alloy Nitride Films Deposited by HiPIMS. Appl. Surf. Sci. 2020, 523, 146529. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Yu, X.; Wang, L.; Huang, W.; Lu, Z. Insight into the Structure and Tribological and Corrosion Performance of High Entropy (CrNbSiTiZr) C Films: First-Principles and Experimental Study. Surf. Coat. Technol. 2021, 421, 127468. [Google Scholar] [CrossRef]
- Jin, G.; Cai, Z.; Guan, Y.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Zhang, D. High Temperature Wear Performance of Laser-Cladded FeNiCoAlCu High-Entropy Alloy Coating. Appl. Surf. Sci. 2018, 445, 113–122. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.Z.; Vilar, R. Microstructure Characterization of Laser Clad TiVCrAlSi High Entropy Alloy Coating on Ti-6Al-4V Substrate. Adv. Mater. Res. 2011, 154, 621–625. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Yi, J.Z.; Zhang, C.H. Synthesis and Characterization of FeCoCrAlCu High-Entropy Alloy Coating by Laser Surface Alloying. Surf. Coat. Technol. 2015, 262, 64–69. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Jin, G. Microstructure and Wear Resistance of Laser Cladded Ni-Cr-Co-Ti-V High-Entropy Alloy Coating after Laser Remelting Processing. Opt. Laser Technol. 2018, 99, 276–281. [Google Scholar] [CrossRef]
- Ye, Y.X.; Liu, C.Z.; Wang, H.; Nieh, T.G. Friction and Wear Behavior of a Single-Phase Equiatomic TiZrHfNb High-Entropy Alloy Studied Using a Nanoscratch Technique. Acta Mater. 2018, 147, 78–89. [Google Scholar] [CrossRef]
- Minghong, S.; Li, Z.; Junwei, Z.; Na, L.; Taizeng, L.; Ning, W. Effects of Annealing on the Microstructure and Wear Resistance of AlCoCrFeNiTi0.5 High-Entropy Alloy Coating Prepared by Laser Cladding. Rare Met. Mater. Eng. 2017, 46, 1237–1240. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Chen, L.; Lin, X.; Huang, H.; Tang, S.; Du, F. Wear and Corrosion Resistance of Al0.5CoCrCuFeNi High-Entropy Alloy Coating Deposited on AZ91D Magnesium Alloy by Laser Cladding. Entropy 2018, 20, 915. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Wu, W.; Cao, Z.; Deng, D.; Li, T. Microstructure Evolution and Wear Behavior of the Laser Cladded CoFeNi2V0.5Nb0.75 and CoFeNi2V0.5Nb High-Entropy Alloy Coatings. J. Therm. Spray Technol. 2016, 25, 806–814. [Google Scholar] [CrossRef]
- Huo, W.; Shi, H.; Ren, X.; Zhang, J. Microstructure and Wear Behavior of CoCrFeMnNbNi High-Entropy Alloy Coating by TIG Cladding. Adv. Mater. Sci. Eng. 2015, 2015, 647351. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shi, Y.; Olugbade, E. Microstructure, Mechanical, and Corrosion Resistance Properties of Al0.8CrFeCoNiCux High-Entropy Alloy Coatings on Aluminum by Laser Cladding. Mater. Res. Express 2020, 7, 026504. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Manladan, S.M.; Geng, K.; Hu, S. Wear Resistance of FeCoCrNiMnAlx High-Entropy Alloy Coatings at High Temperature. Appl. Surf. Sci. 2020, 512, 145736. [Google Scholar] [CrossRef]
- Liu, H.; Gao, W.; Liu, J.; Du, X.; Li, X.; Yang, H. Microstructure and Properties of CoCrFeNiTi High-Entropy Alloy Coating Fabricated by Laser Cladding. J. Mater. Eng. Perform. 2020, 29, 7170–7178. [Google Scholar] [CrossRef]
- Erdoğan, A.; Gök, M.S.; Zeytin, S. Analysis of the High-Temperature Dry Sliding Behavior of CoCrFeNiTi0.5Alx High-Entropy Alloys. Friction 2020, 8, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Eakins, E.; Jayaseelan, D.D.; Lee, W.E. Toward Oxidation-Resistant ZrB2-SiC Ultra High Temperature Ceramics. Metall. Mater. Trans. A 2011, 42, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.B.; Fridrici, V.; Kapsa, P. A Systematic Approach for the Selection of Tribological Coatings. Wear 2011, 271, 2132–2143. [Google Scholar] [CrossRef]
- Blau, P.J.; DeVore, C.E. Sliding Behavior of Alumina/Nickel and Alumina/Nickel Aluminide Couples at Room and Elevated Temperature. J. Tribol. 1988, 110, 646–652. [Google Scholar] [CrossRef]
- Quinn, T.F.J. Oxidational Wear Modelling: Part II. The General Theory of Oxidational Wear. Wear 1994, 175, 199–208. [Google Scholar] [CrossRef]
- Bhushan, B. Introduction to Tribology; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1118403223. [Google Scholar]
- Quinn, T.F.J. Oxidational Wear. Wear 1971, 18, 413–419. [Google Scholar] [CrossRef]
- Stott, F.H.; Glascott, J.; Wood, G.C. Models for the Generation of Oxides during Sliding Wear. Proc. R. Soc. Lond. A. Math. Phys. Sci. 1985, 402, 167–186. [Google Scholar]
- Hardell, J. High Temperature Tribology of High Strength Boron Steel and Tool Steels; Luleå University of Technolog: Luleå, Sweden, 2007. [Google Scholar]
- Blau, P.J.; Brummett, T.M.; Pint, B.A. Effects of Prior Surface Damage on High-Temperature Oxidation of Fe-, Ni-, and Co-Based Alloys. Wear 2009, 267, 380–386. [Google Scholar] [CrossRef]
- Stott, F.H. The Role of Oxidation in the Wear of Alloys. Tribol. Int. 1998, 31, 61–71. [Google Scholar] [CrossRef]
- Stott, F.H. High-Temperature Sliding Wear of Metals. Tribol. Int. 2002, 35, 489–495. [Google Scholar] [CrossRef]
- Chromik, R.R.; Alidokht, S.A.; Shockley, J.M.; Zhang, Y. Tribological Coatings Prepared by Cold Spray. In Cold-Spray Coatings: Recent Trends and Future Perspectives; Cavaliere, P., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 321–348. ISBN 978-3-319-67183-3. [Google Scholar]
- Mozgovoy, S.; Hardell, J.; Deng, L.; Oldenburg, M.; Prakash, B. Effect of Temperature on Friction and Wear of Prehardened Tool Steel during Sliding against 22MnB5 Steel. Tribol. Surf. Interfaces 2014, 8, 65–73. [Google Scholar] [CrossRef]
- Geiger, M.; Merklein, M.; Lechler, J. Determination of Tribological Conditions within Hot Stamping. Prod. Eng. 2008, 2, 269. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the Significance of the H/E Ratio in Wear Control: A Nanocomposite Coating Approach to Optimised Tribological Behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Berns, H.; Koch, S. Influence of Abrasive Particles on Wear Mechanism and Wear Resistance in Sliding Abrasion Tests at Elevated Temperatures. Wear 1999, 233, 424–430. [Google Scholar] [CrossRef]
- Soemantri, S.; McGee, A.C.; Finnie, I. Some Aspects of Abrasive Wear at Elevated Temperatures. Wear 1985, 104, 77–91. [Google Scholar] [CrossRef]
- Scalaro, K.; Menezes, P.L. Tribological Properties of High-Entropy Alloys under Dry Conditions for a Wide Temperature Range—A Review. Materials 2021, 14, 5814. [Google Scholar]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The Sliding Wear Behaviour of CoCrFeMnNi and AlxCoCrFeNi High Entropy Alloys at Elevated Temperatures. Wear 2019, 428, 32–44. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive Wear Behavior of AlxCoCrCuFeNi High-Entropy Alloys as a Function of Aluminum Content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of Vanadium Addition on the Microstructure and Properties of AlCoCrFeNi High Entropy Alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Chen, M.-R.; Lin, S.-J.; Yeh, J.-W.; Chuang, M.-H.; Chen, S.-K.; Huang, Y.-S. Effect of Vanadium Addition on the Microstructure, Hardness, and Wear Resistance of Al0.5CoCrCuFeNi High-Entropy Alloy. Metall. Mater. Trans. A 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Influence of Titanium on Microstructure, Phase Formation and Wear Behaviour of AlCoCrFeNiTix High-Entropy Alloy. Entropy 2018, 20, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM E1920-03; Guide for Metallographic Preparation of Thermal Sprayed Coatings. ASTM West Conshohocken: West Conshohocken, PA, USA, 2014.
- Hsu, C.-Y.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. Effect of Iron Content on Wear Behavior of AlCoCrFexMo0.5Ni High-Entropy Alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Miller, R.A. Thermal Barrier Coatings for Aircraft Engines: History and Directions. J. Therm. Spray Technol. 1997, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.A.; Kuczmarski, M.A. Burner Rig for Small Particle Erosion Testing of Thermal Barrier Coatings. J. Test. Eval. 2014, 42, 648–658. [Google Scholar] [CrossRef]
- Slifka, A.J.; Filla, B.J.; Phelps, J.M.; Bancke, G.; Berndt, C.C. Thermal Conductivity of a Zirconia Thermal Barrier Coating. J. Therm. Spray Technol. 1998, 7, 43–46. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]


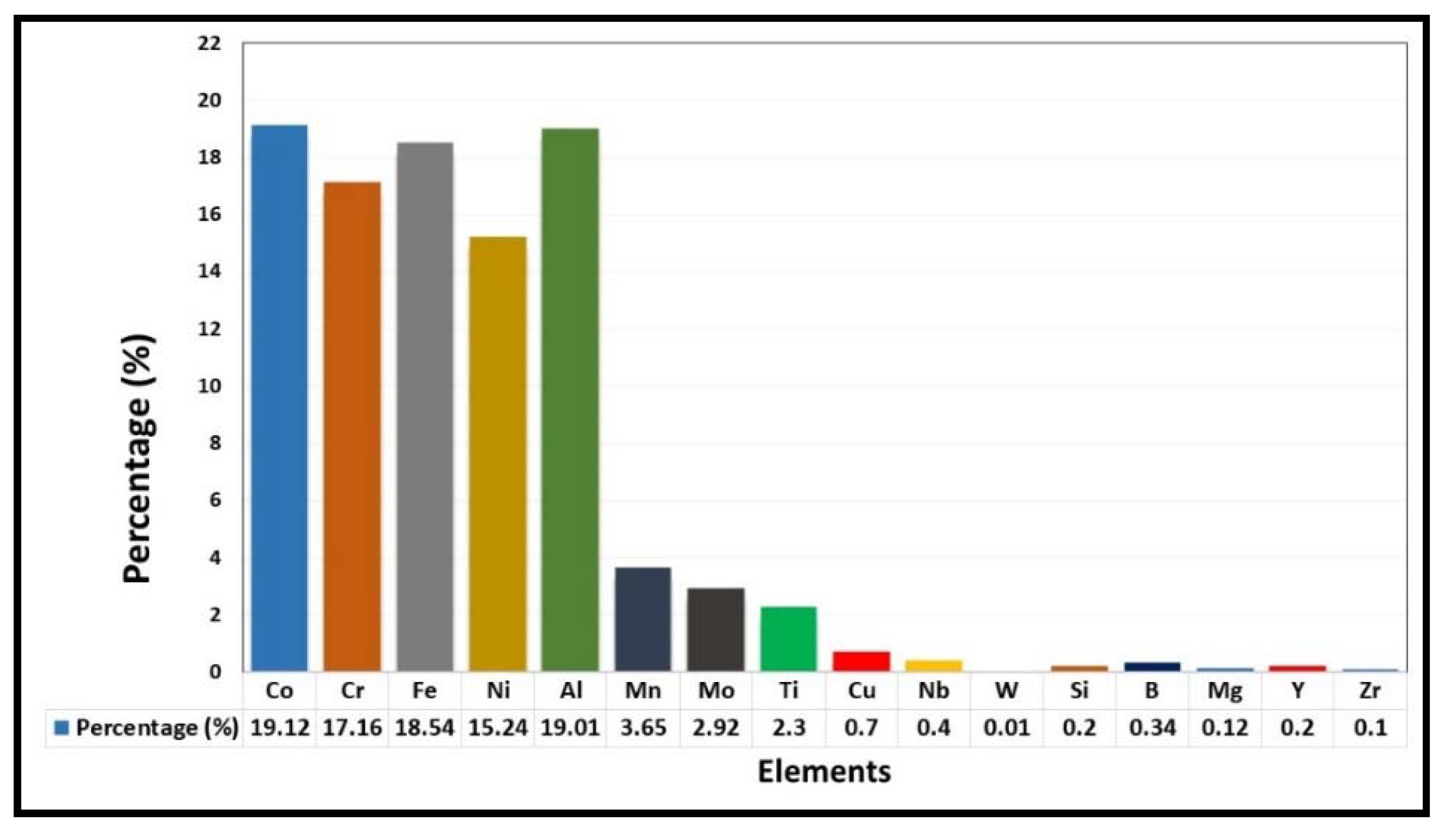

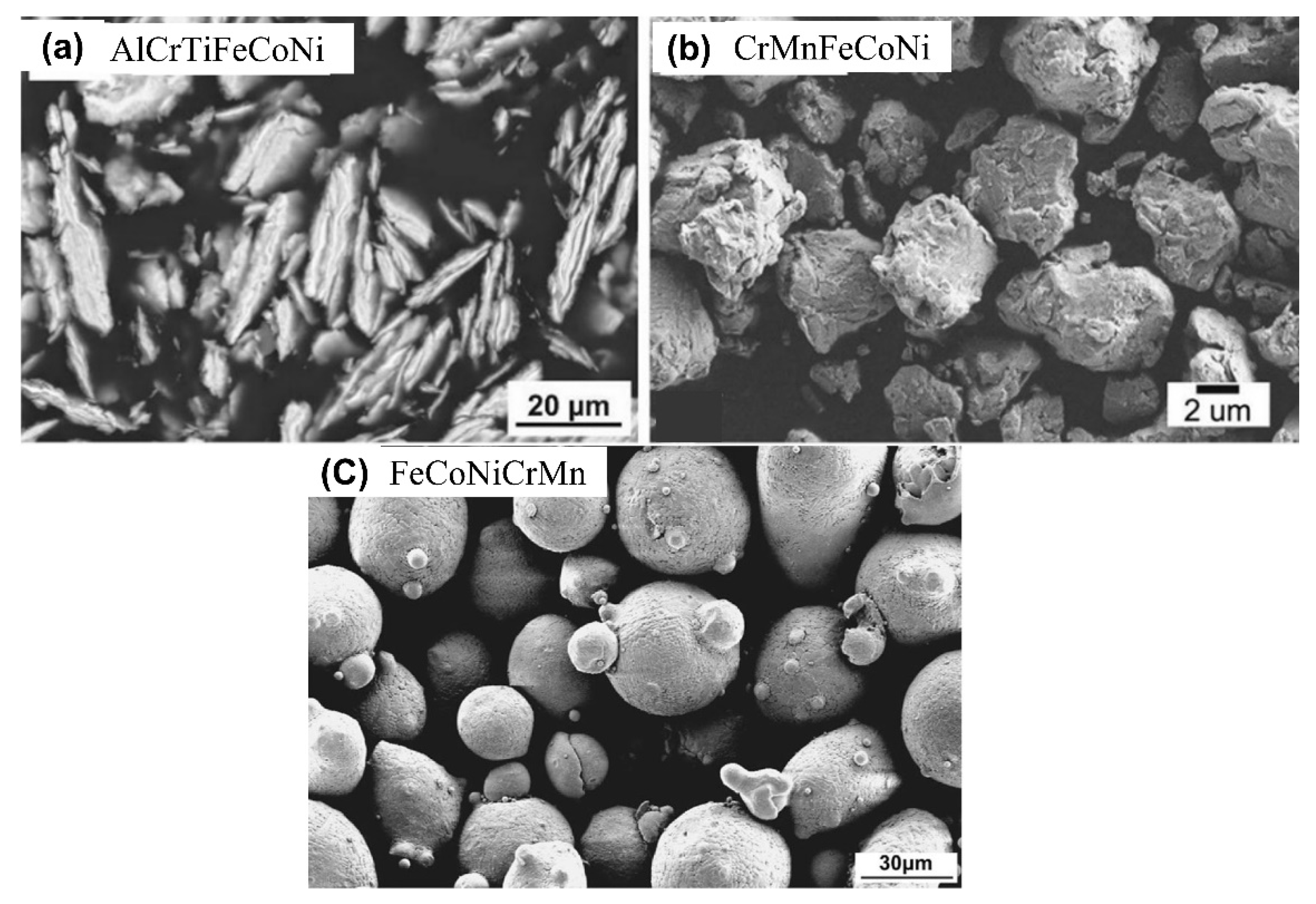
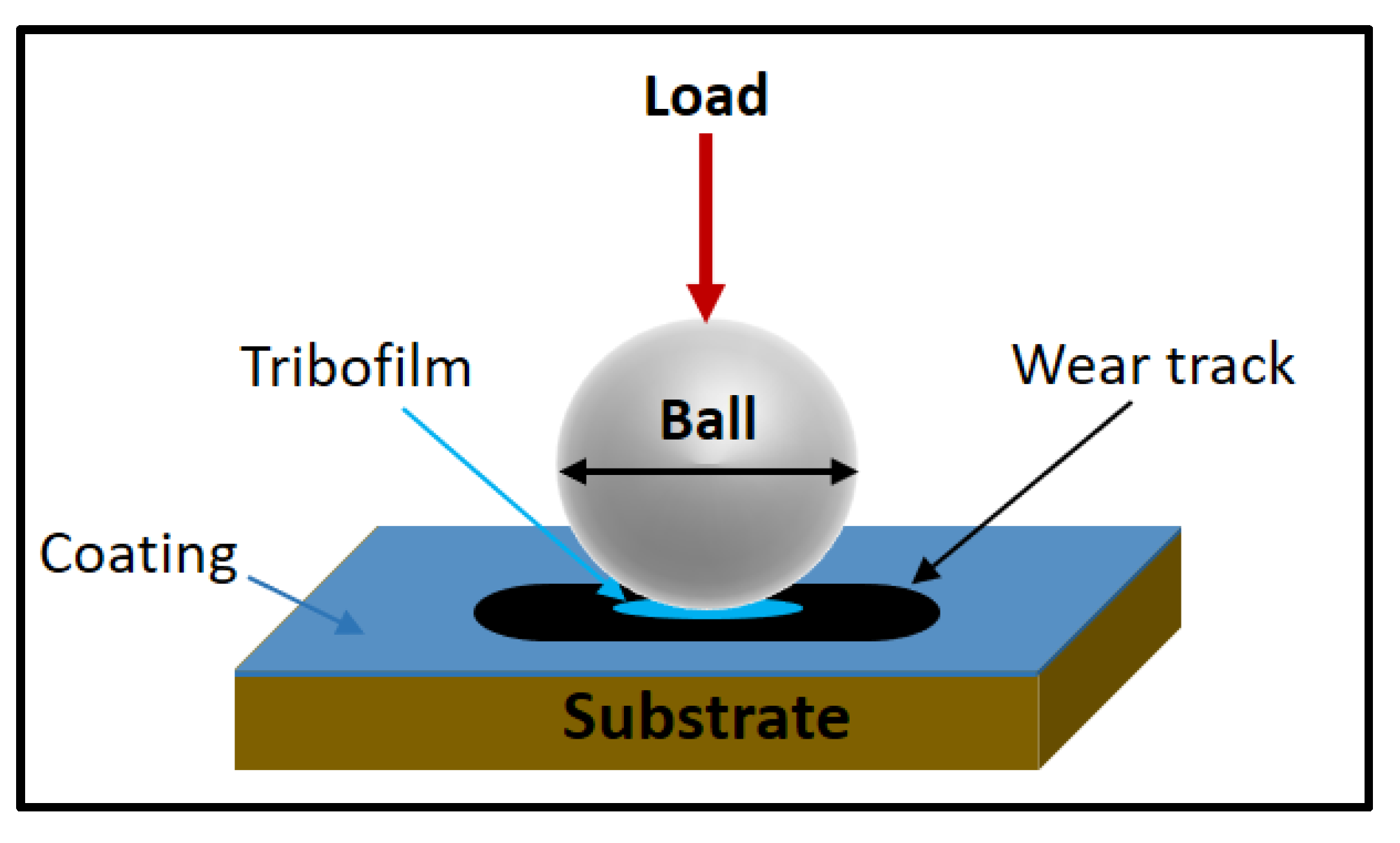
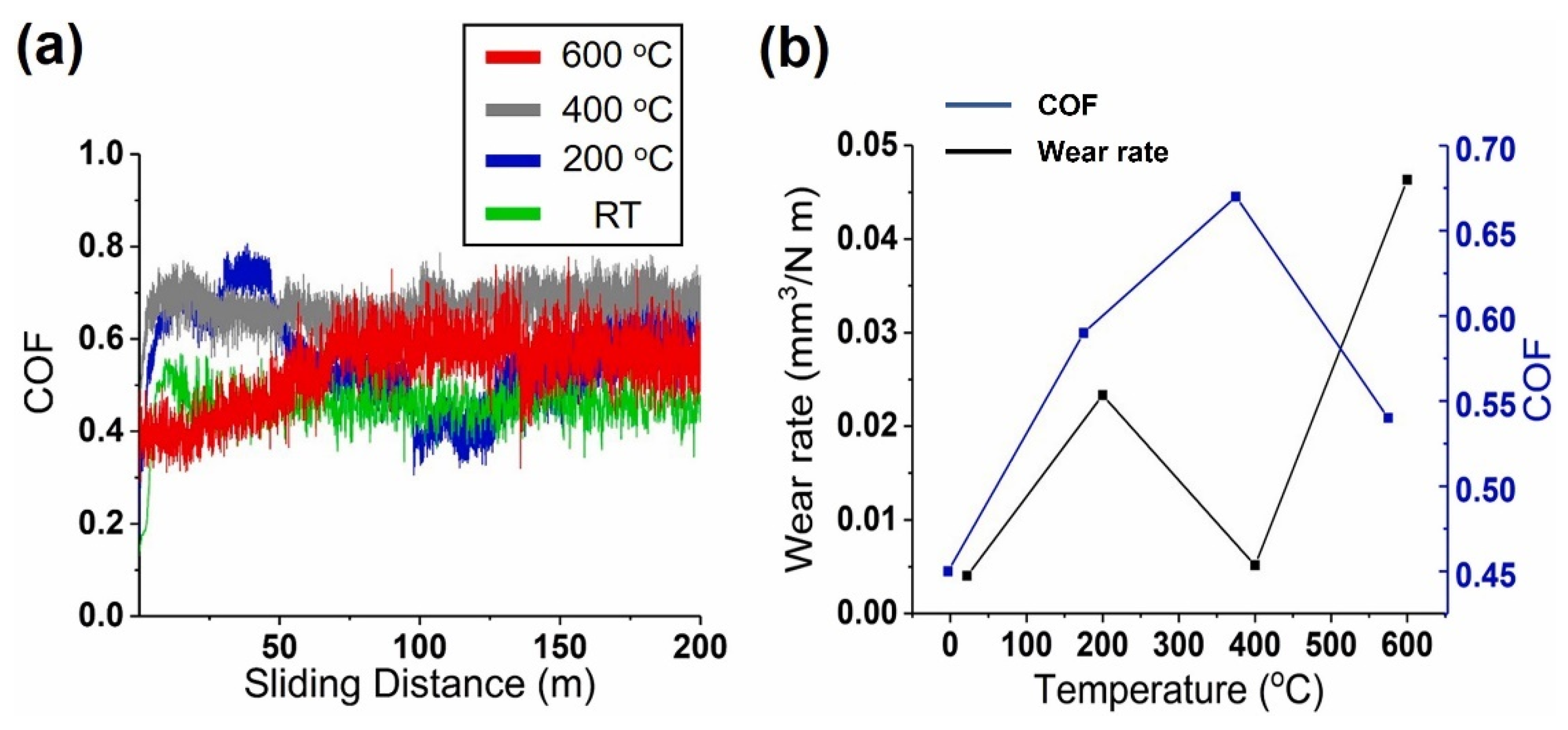
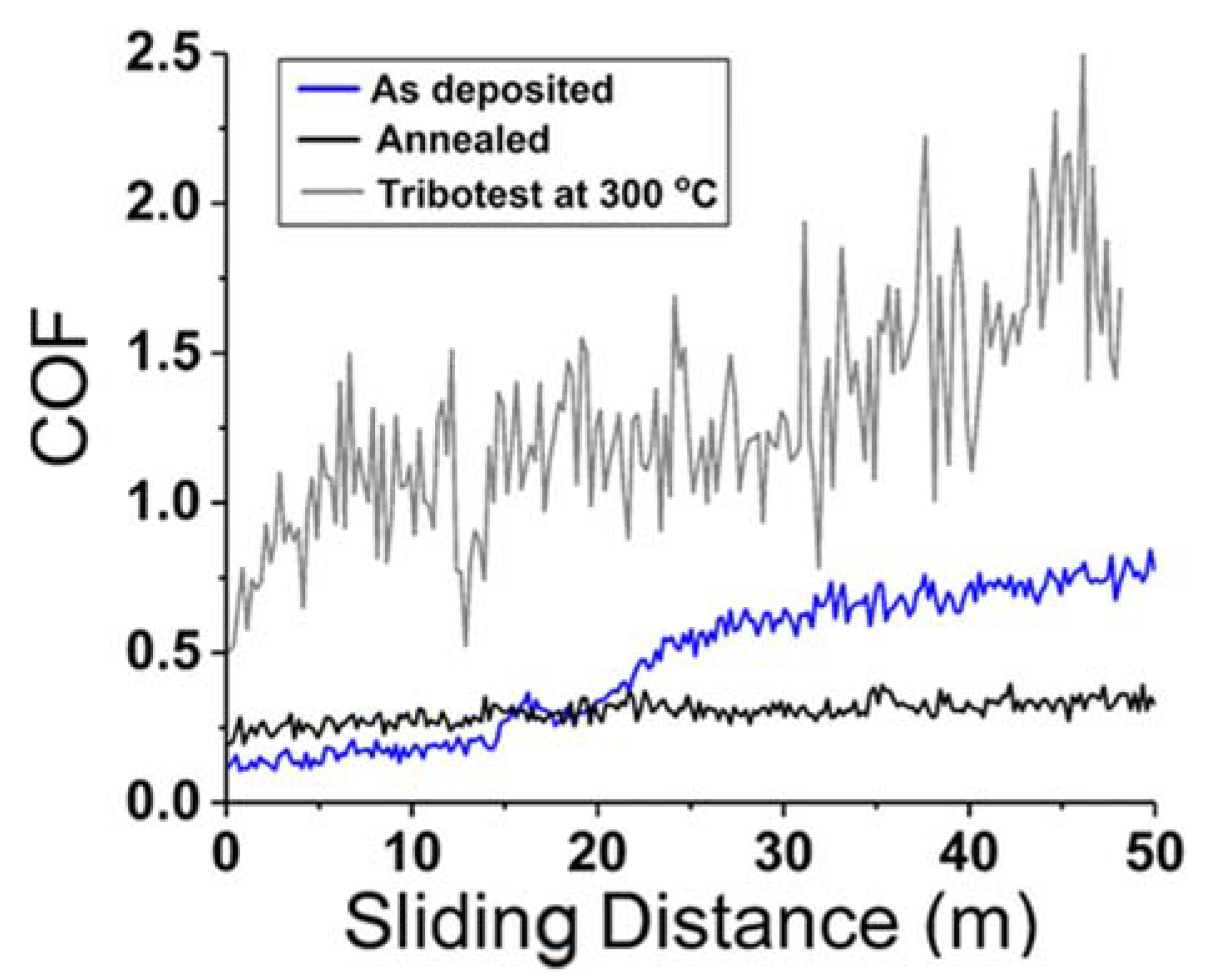
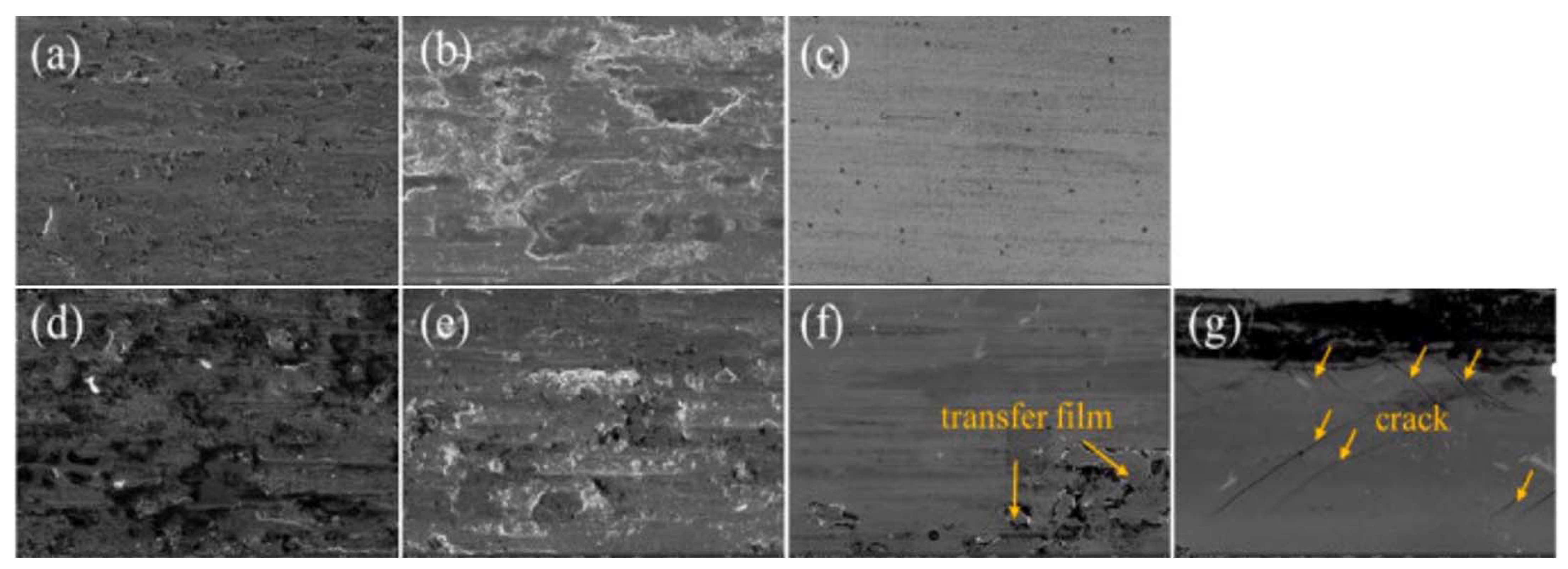
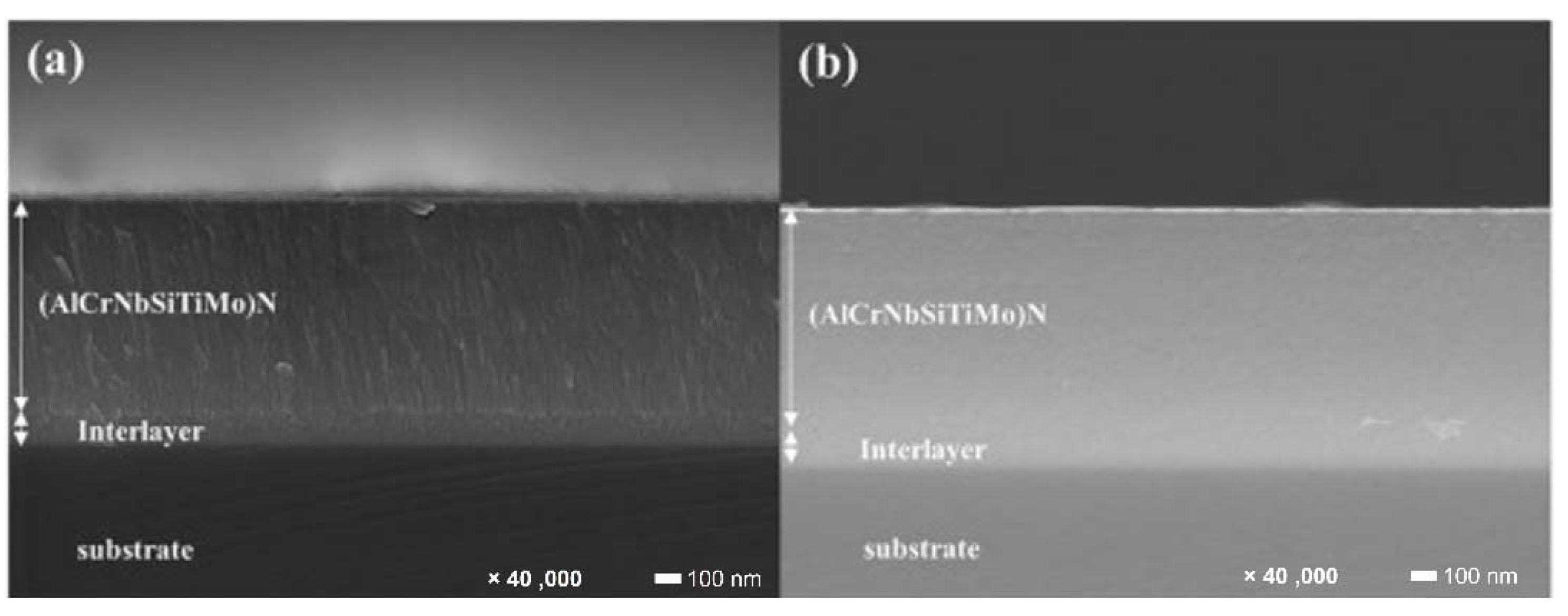
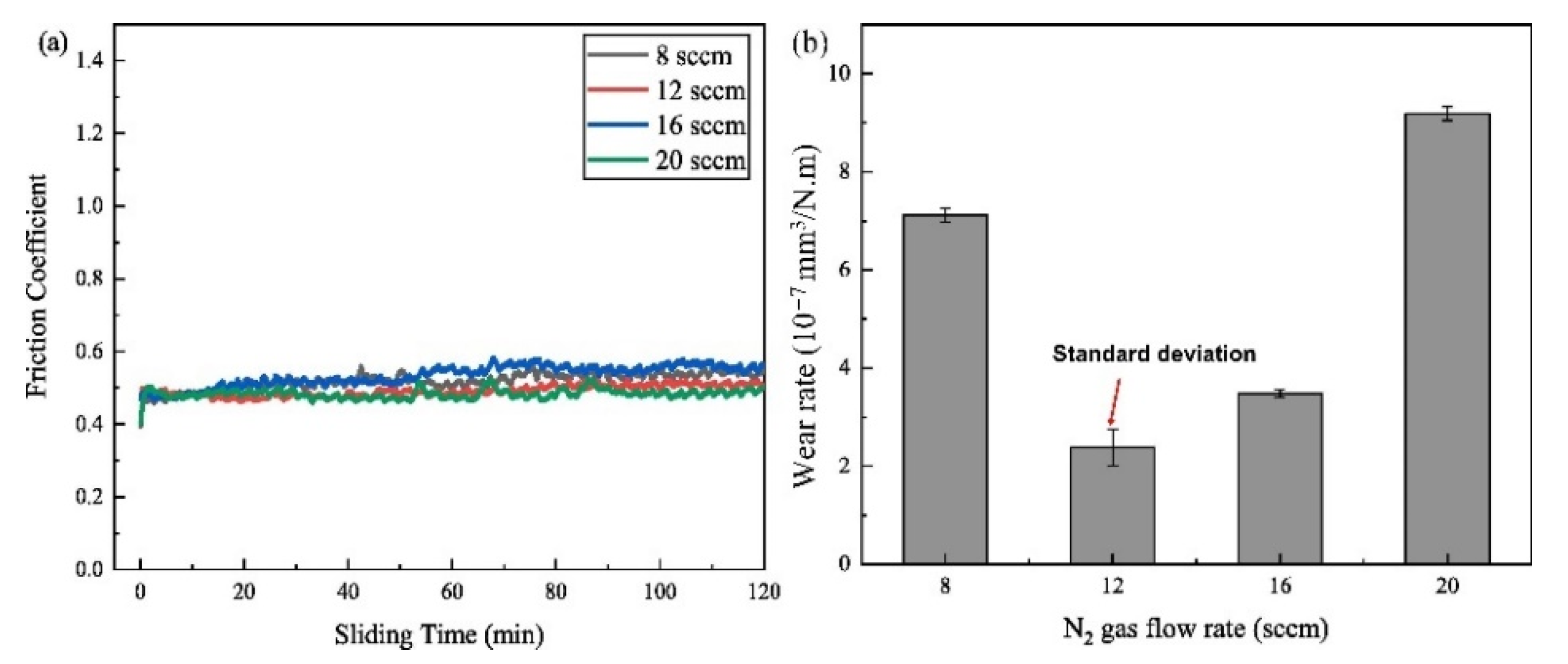

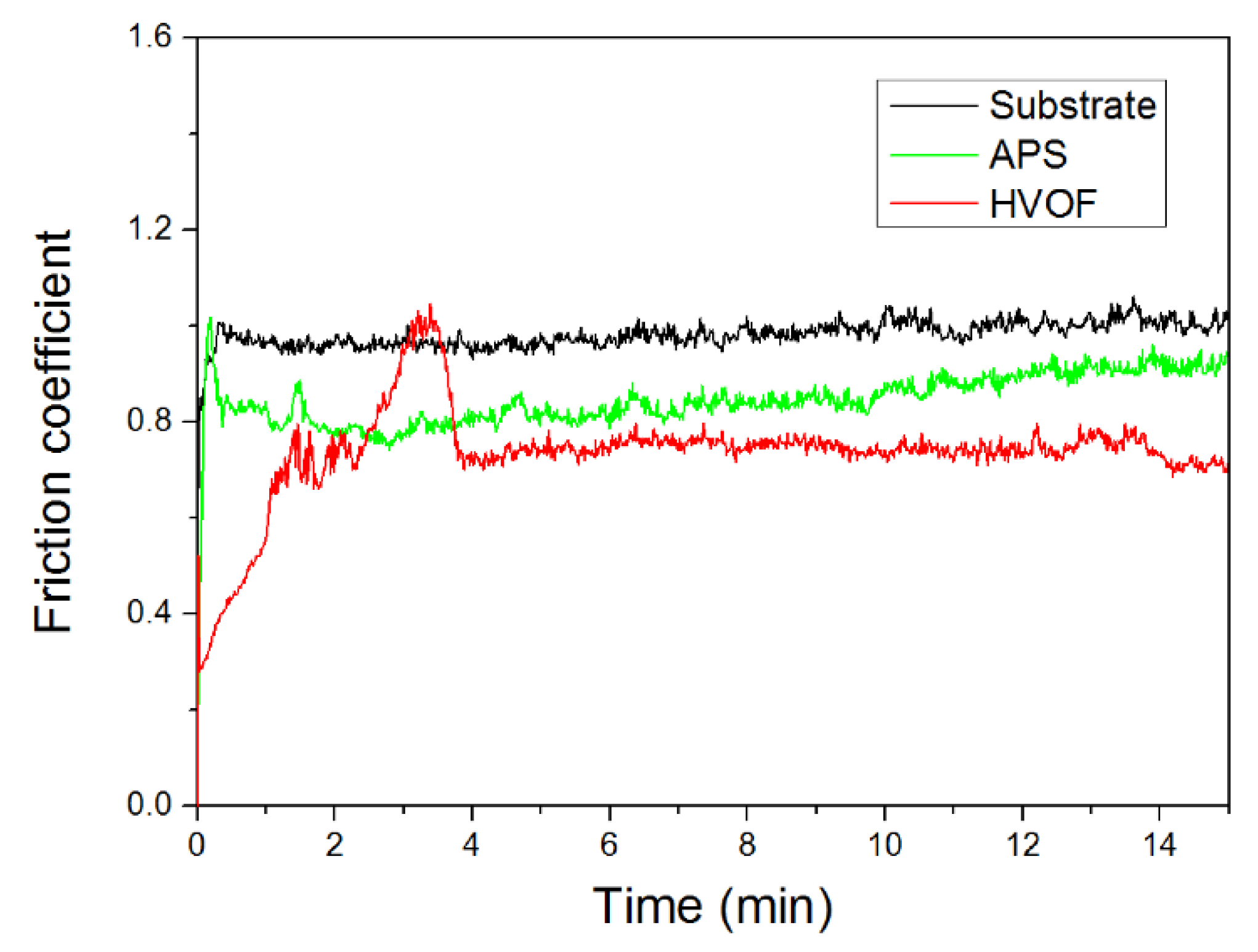
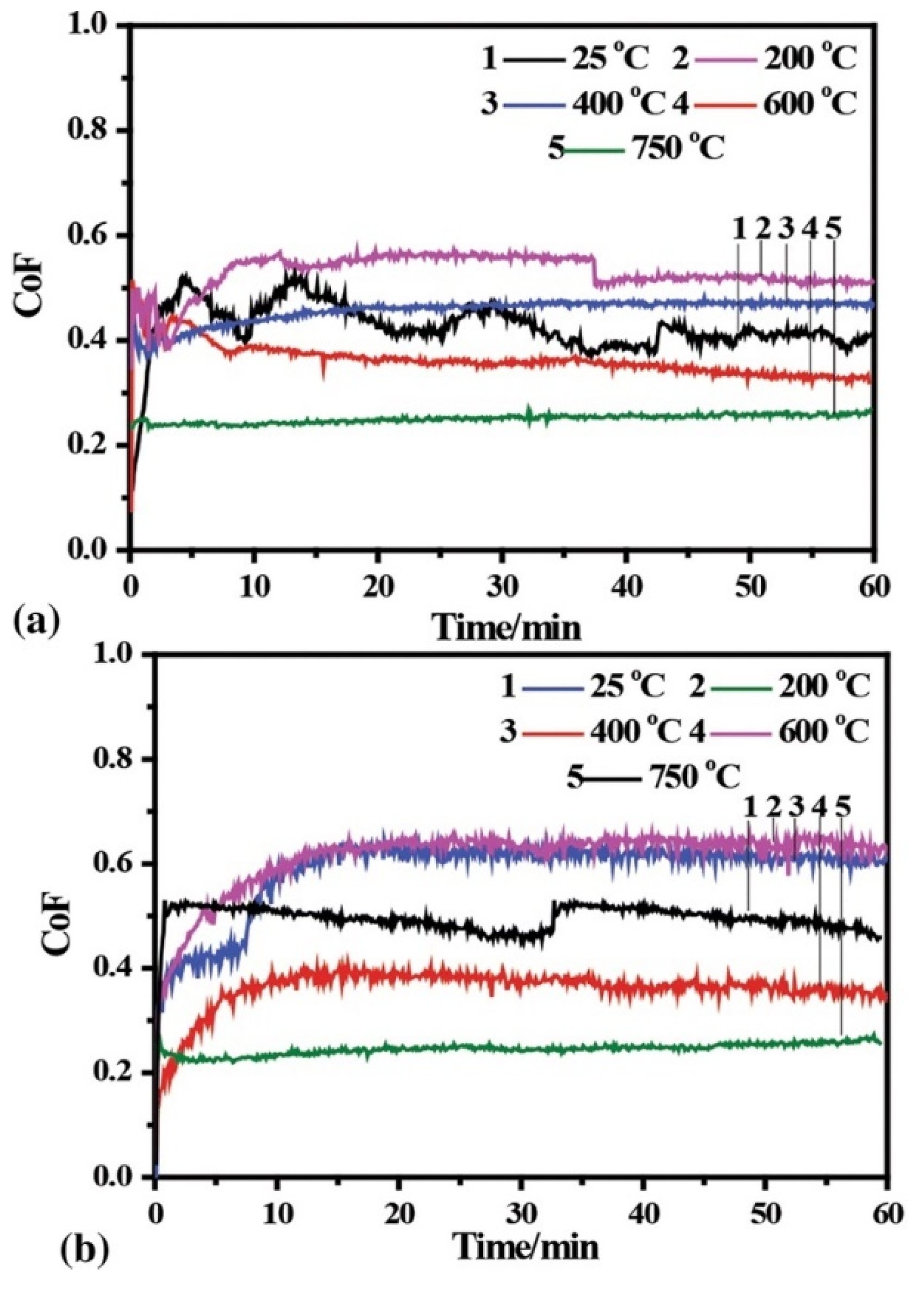
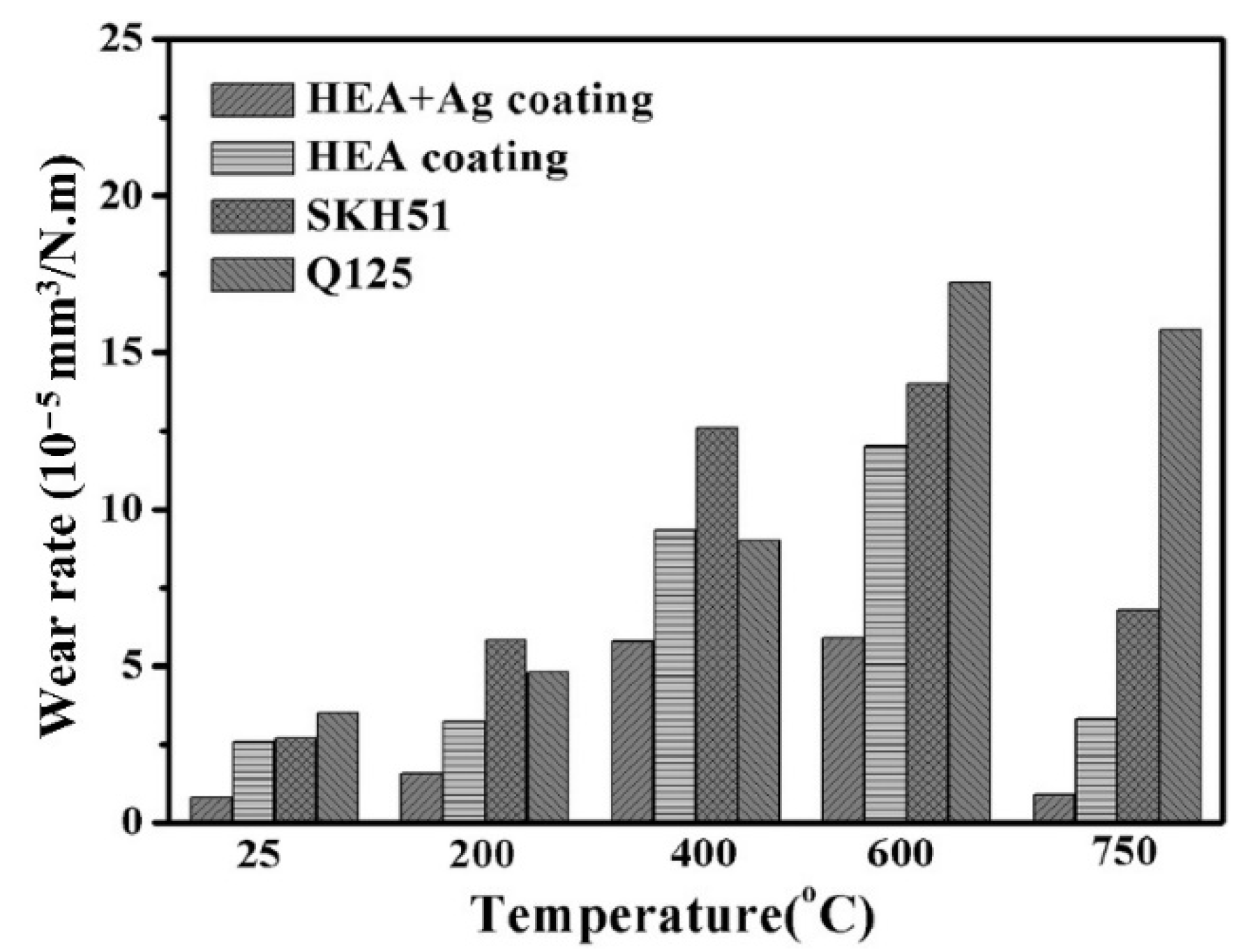
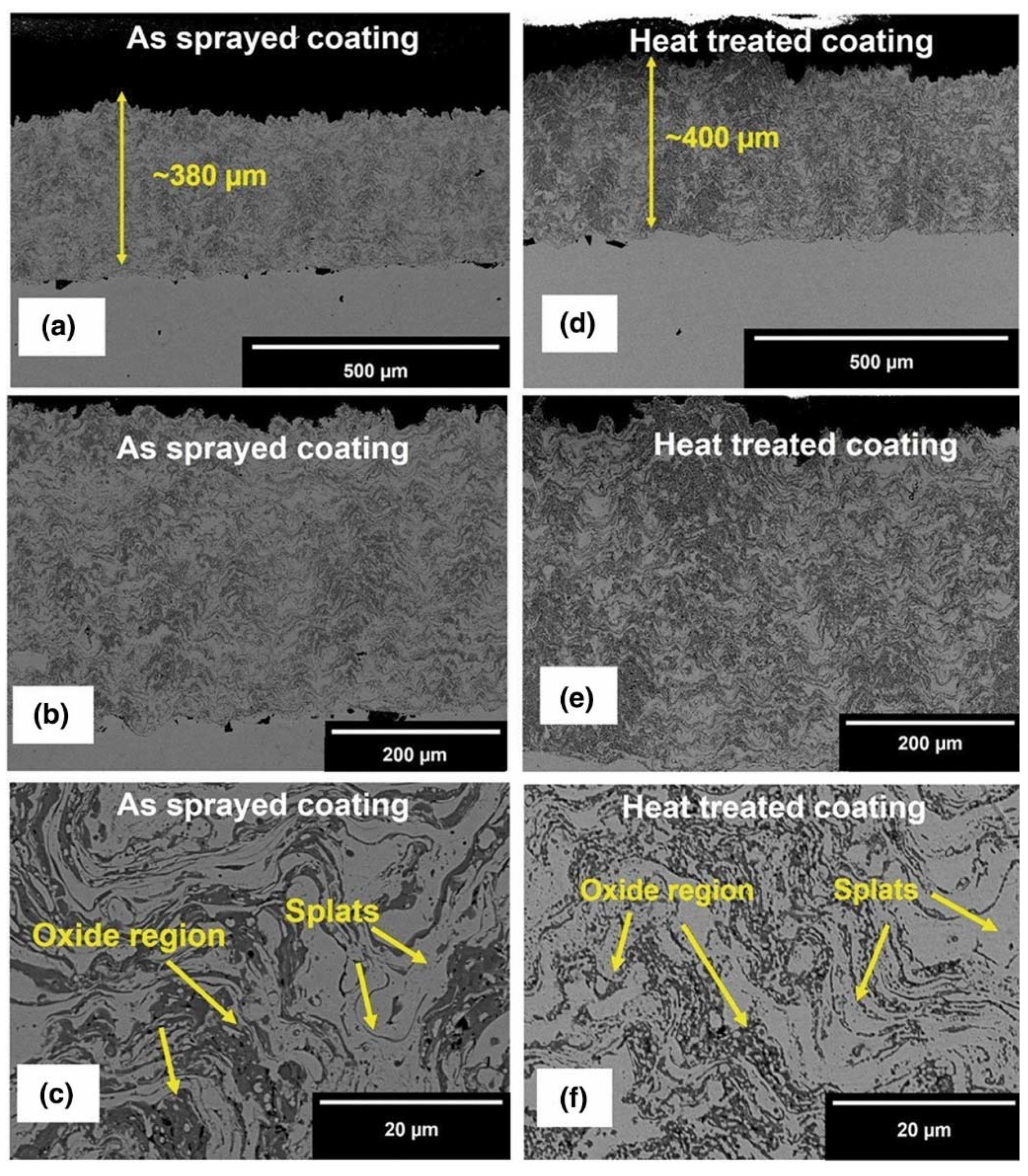

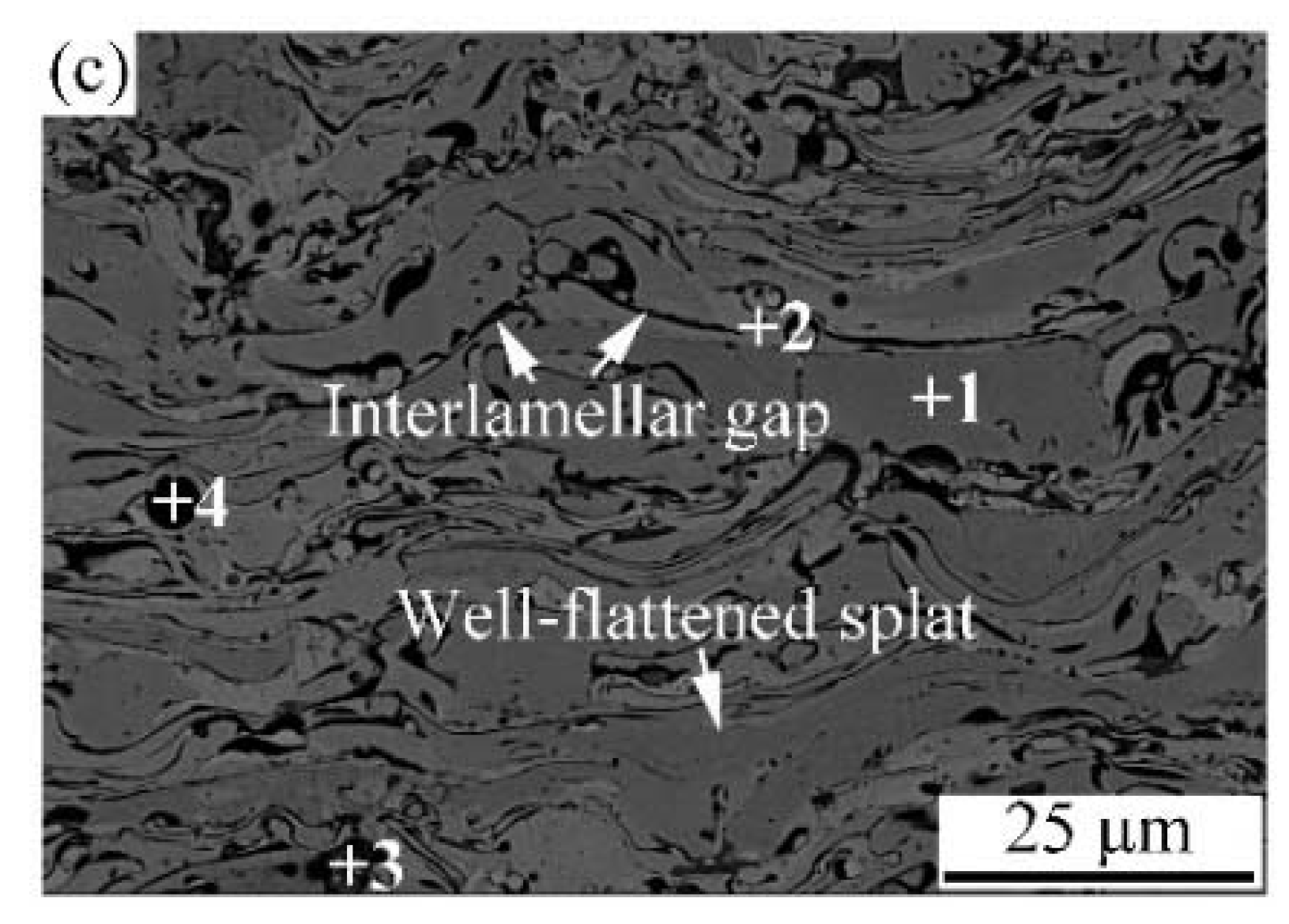


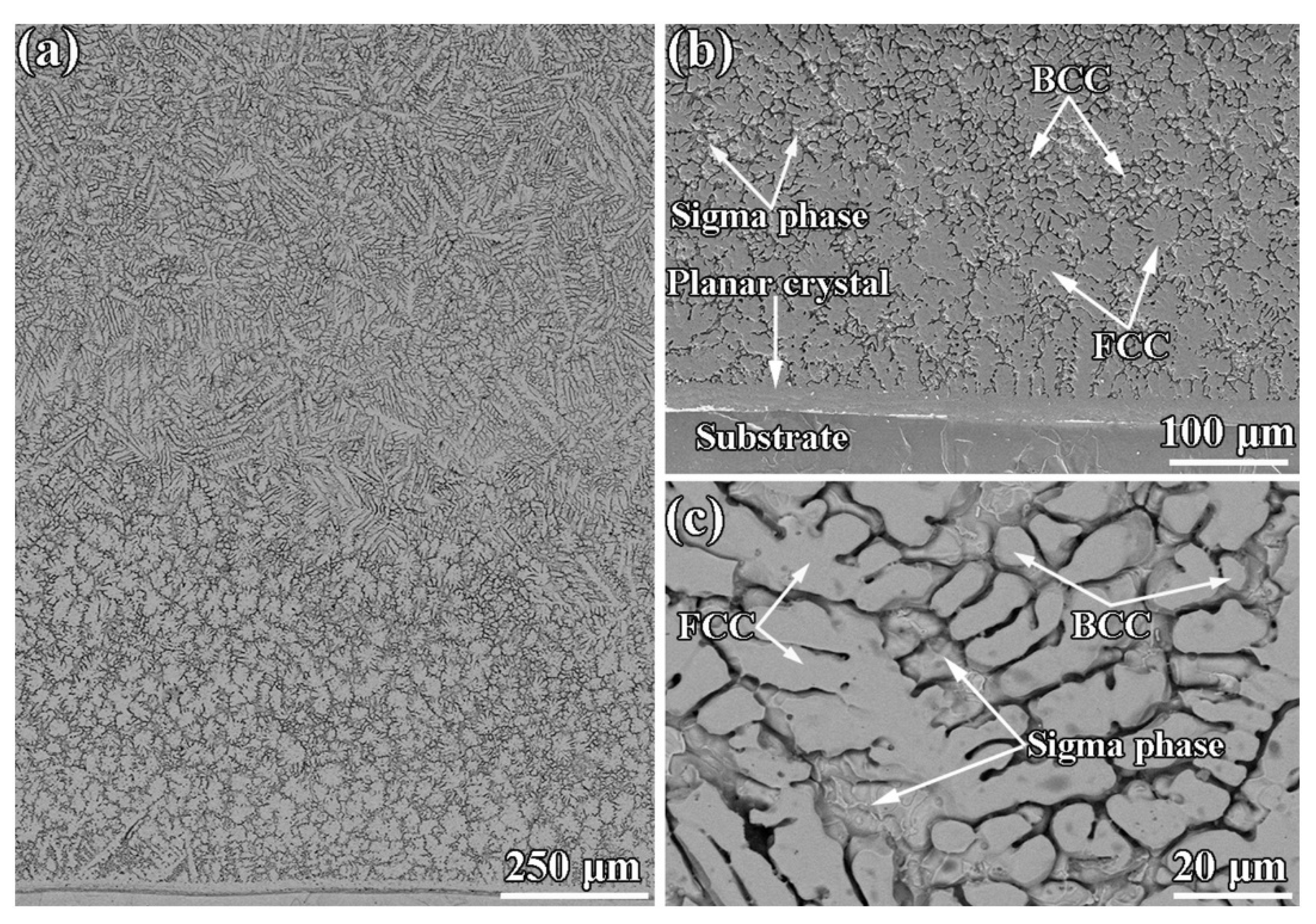
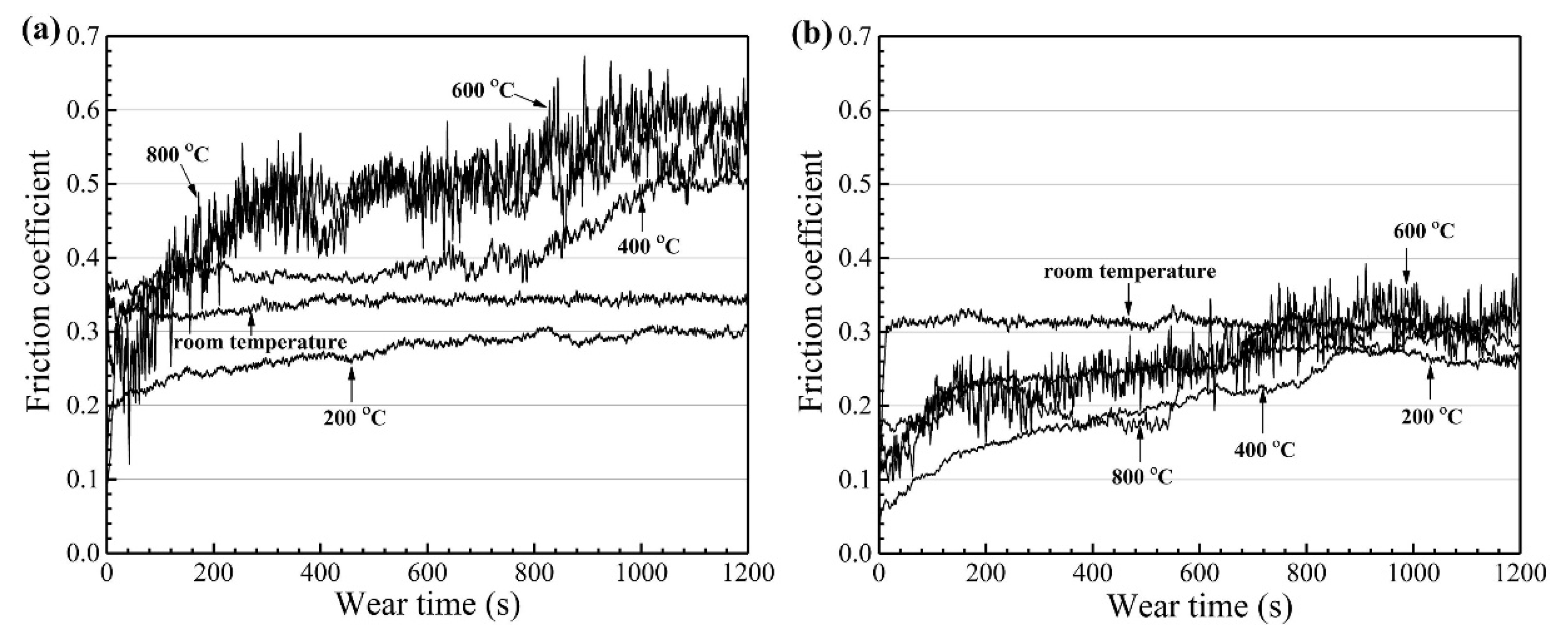
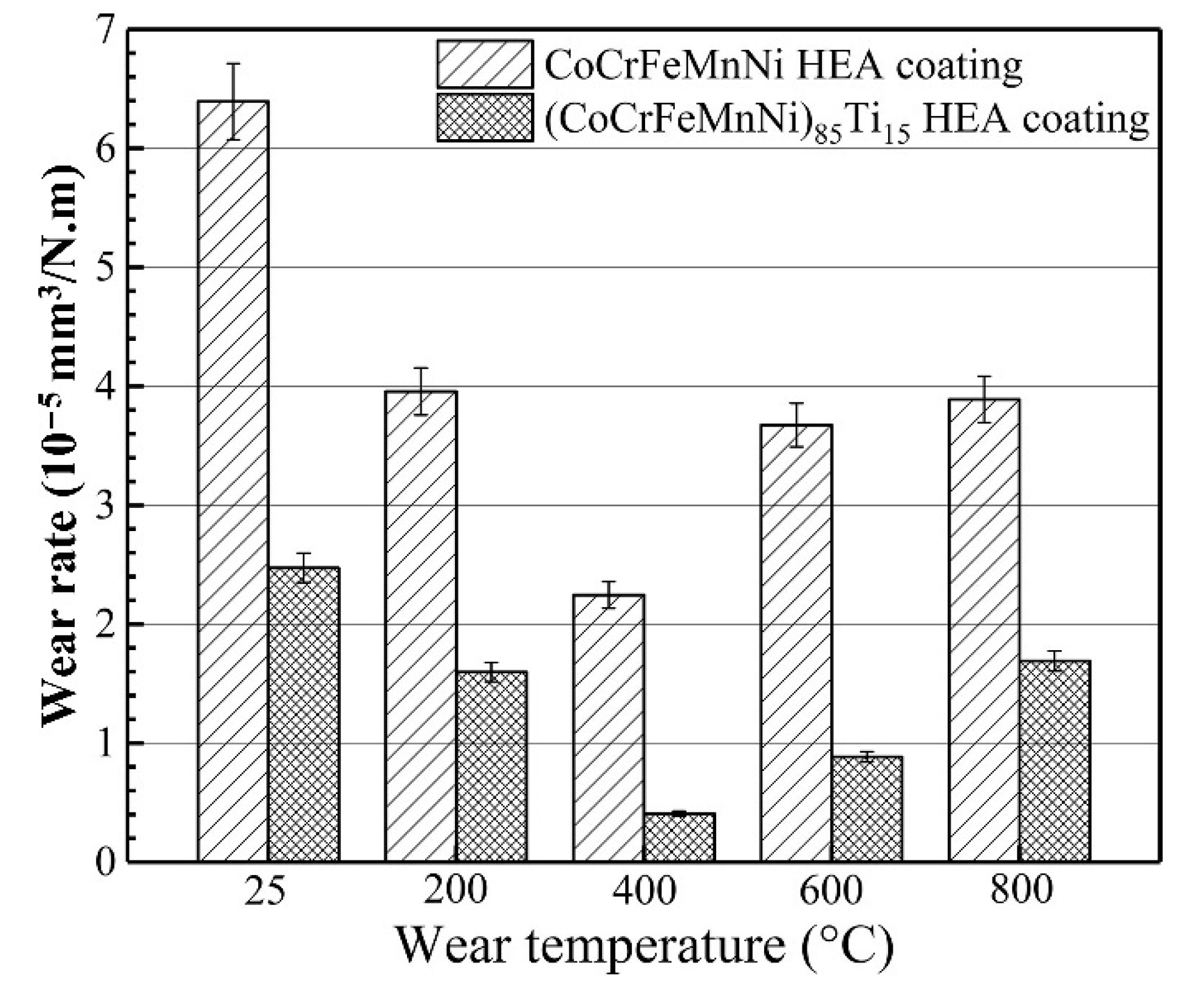

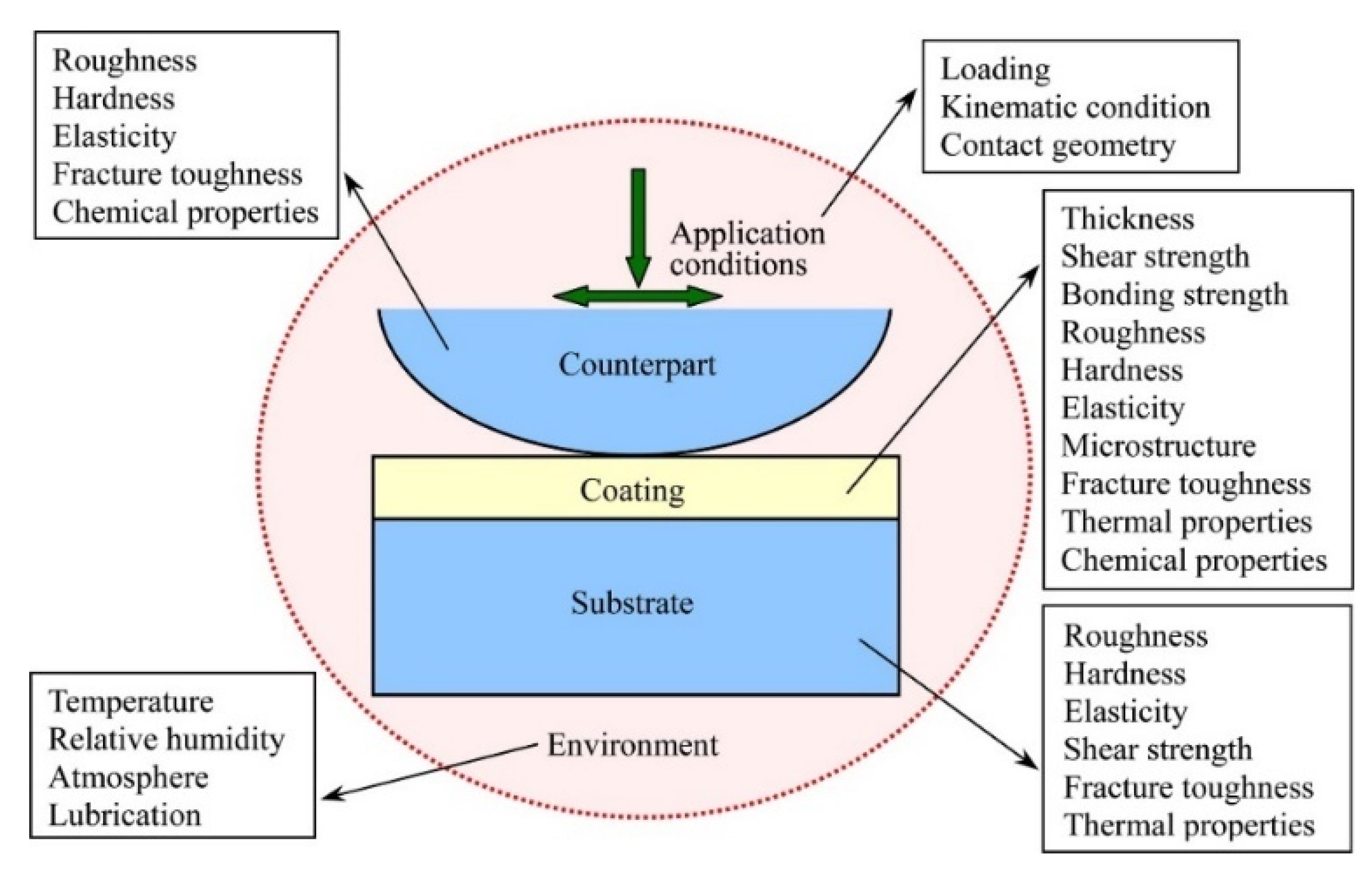


| Powder Preparation Methods | Discussion | Particle Size and Geometry | Phases Observed | Refs. |
|---|---|---|---|---|
| Blending | Mixing of the powders without promoting any bonding between the particles (Not recommended) | 75–80 µm with irregular shape | BCC + FCC | [66,114] |
| Arc Melting + Mechanical milling | First, intermix the desired alloy by arc melting and then crush the ingot into smaller particles with ball milling (partially recommended due to powder flowability) | 30–45 µm with irregular shape | Ordered BCC and FCC | [49,57,116,117] |
| Mechanical alloying | High-speed rotation and high-energy impacts for cold welding and fracturing, resulting in ‘mixing’ at an atomic scale (recommended) | Wet Milling: 20–35 µm with irregular shape | BCC+ FCC | [64,102,115,118,119,120] |
| Dry Milling: 20–70 µm with flaky structure | ||||
| Gas atomization | The liquid alloy passes through a nozzle under high pressure (inert gas) and then the fragmentation of liquid streams into spherical droplets | 30–220 µm with spherical particles | BCC + B2 | [55,104,114,121,122,123,124,125,126,127] |
| Coating Methods | Classification of Coating Methods | Process Description | Characteristics of Coatings | Refs. |
|---|---|---|---|---|
| Vapor deposition and related methods | Magnetron Sputtering | The surface of the target material is eroded by high-energy ions within the confined gaseous plasma, and the liberated atoms travel through the vacuum environment and deposit onto a substrate to form a thin film [38]. Limited for thin-films coatings. | Columnar or epitaxial structure with FCC and/or amorphous solid solution, the thickness can be achieved from 1 µm to 5µ m with a hardness of 7.9 to 10 GPa for high entropy (HE) metallic coatings and (>10 GPa) for HE nitride coatings. | [129,130,131,132,133,134,135,136,137] |
| Vacuum arc deposition | Deposition of thin film by using the heat energy of arc to evaporate the target materials onto the surface of the substrate [38]. | Columnar structure with FCC and BCC solid solution, the thickness can be achieved from 1 µm to 10 µm with hardness ranging from 300 to 750 HV, mostly for HE metallic coatings. | [138,139,140,141] | |
| Thermal Spraying | High-Velocity Oxygen Fuel (HVOF) | By using the combustion heat of the fuel and oxygen, spraying the powder particles on the substrate with high velocity. Known for dense coatings with low porosity, low oxide contents, and high bonding Strength [38]. | Lamellar grains with BCC solid solution and thickness can be achieved from 100 to 500 µm with a hardness of 700–800 HV, mostly for HE metallic coatings. | [66,70,138] |
| Atmospheric Plasma Spraying (APS) | Melting of the powder particles by using plasma heat energy source and then spraying the powder particles on the base material. Known for high strength of interfacial bonding, high deposition efficiency and high oxide contents [38]. | Lamellar structure with BCC and FCC phase, the thickness of the coating can be achieved from 275 to 570 µm with a hardness of 310–850 HV, mostly for HE metallic coatings. | [41,49,64,66,67,77,117,121,142] | |
| Cold Spraying | Solid-state coating deposition technique, no oxidation, phase transformation and residual thermal stress occur. Limited only for low strength materials [38]. | Equiaxed structure with FCC solid solution, the thickness can be achieved from 1 mm to 5 mm with a hardness of 400–550 HV, mostly for HE metallic coatings. | [121,123,142] | |
| Cladding | Laser Cladding | Pre-placed powders and the thin substrate surface layer are simultaneously melted and solidified rapidly under the heat source of laser [38]. | Dendrite or equiaxed grains, BCC or FCC the solid solution with intermetallic compounds and the thickness of 200 µm to 1.5 mm with harness ranging from 345 to 1100 HV, mostly for HE metallic coatings. | [54,60,89,90,99,100,143,144,145,146,147,148,149,150] |
| Plasma Cladding | Melting with higher heat input and bigger blowing force and mixing of the molten powder are abundant to obtain the homogeneous coating [38]. | Columnar or equiaxed grains, BCC or FCC solid solution and the thickness can be produced from (1 mm to 2.5 mm) with harness ranging from 485 to 730 HV, mostly for HE metallic coatings. | [76,151,152,153] |
| High-Entropy Coating (HEC) System | Deposition Method | Counter Ball | Applied Load (N) | Wear Speed (m/s) | Test Temp. (°C) | Sliding Distance (m) | Volume Wear Rate (×10−6 mm3/N.m) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CuMoTaWV | Spark Plasma Sintering | Si3N4 | 5 | 0.1 | RT | 200 | 2.19 | [155] |
| 200 | 4.94 | |||||||
| 400 | 2.46 | |||||||
| 600 | 1.39 | |||||||
| CuMoTaWV | Magnetron sputtering | E52100 steel | 1 | 0.1 | RT | 50 | 6.4 | [156] |
| 300 | 25 | |||||||
| AlCrTiVSi | Magnetron sputtering | GCr15 steel | 1 | 0.0157 | RT | 28 | Unmeasurable | [157] |
| AlCrTiVSi-N | Unmeasurable | |||||||
| AlCrTiVSi | Magnetron sputtering | Al2O3 | 1 | 0.0157 | RT | 28 | Wear out | |
| AlCrTiVSi-N | 21 ± 2.4 | |||||||
| TiTaHfNbZr | Magnetron sputtering | Al2O3 | 1 | 0.01 | RT | 30 | 230 | [130] |
| 2 | 660 | |||||||
| 3 | 630 | |||||||
| AlCrNbSiTiMo) N | Magnetron sputtering | Al2O3 | 3 | 0.032 | 700 | 75 | 1.2 | [79] |
| (CrNbSiTiZr)C | Magnetron sputtering | GCr15 steel | 2 | 0.12 | RT | 216 | 4.2 | [161] |
| (AlCrTiVZr)N | HiPIMS | GCr15 steel | 30 | 0.004 | RT | 29 | 0.23 | [160] |
| AlSiTiCrFeCoNiMo0.5 | APS | Al2O3 | 10 | 0.5 | 200 | 20 | 4.94 | [49] |
| AlSiTiCrFeNiMo0.5 | APS | Al2O3 | 10 | 0.5 | 400 | 20 | 2.46 | |
| AlSiTiCrFeNiMo0.5 | APS | Al2O3 | 10 | 0.5 | 600 | 20 | 1.39 | |
| AlCoCrFeNiSi | APS | Si3N4 | 5 | 0.3 | RT | 540 | 38 ± 8 | [121] |
| AlCoCrFeNiTi | APS | Si3N4 | 5 | 0.3 | 500 | 360 | 93 ± 10 | [64] |
| 700 | 23 ± 10 | |||||||
| 900 | 43 ± 20 | |||||||
| AlSi0.2Ti0.2CrFe0.2Co0.6Ni0.2 | APS | SiC | 10 | 0.5 | RT | 20 | 479 ± 12 | [117] |
| HVOF | SiC | 10 | 0.5 | 20 | 509 ± 17 | |||
| CrFeCoNiMo0.2 | APS | GCr15 steel | 10 | 0.05 | RT | 45 | 3.9 | [41] |
| CrFeCoNiMo0.2 | HVOF | GCr15 steel | 10 | 0.05 | 45 | 480 | ||
| AlTiCrFeCoNi/Ni60 | APS | Si3N4 | 5 | 0.3 | RT | 360 | 55 ± 6 | [66] |
| 500 | 67 ± 5 | |||||||
| Al0.2TiCrFeCo1.5Ni1.5-5 wt.% Ag | APS | Si3N4 | 5 | 0.157 | RT | 566 | 8 | [77] |
| 200 | 19.7 | |||||||
| 400 | 52.6 | |||||||
| 600 | 52.8 | |||||||
| 750 | 4.8 | |||||||
| Al0.2TiCrFeCo1.5Ni1.5 | APS | Si3N4 | 5 | 0.157 | RT | 566 | 25 | [77] |
| 200 | 31 | |||||||
| 400 | 81.7 | |||||||
| 600 | 124.4 | |||||||
| 750 | 48 | |||||||
| Al0.5SiCrFeCoNi | APS | WC-12Co | 20 | 0.04 | RT | 100 | 55 | [142] |
| Al1.0SiCrFeCoNi | 43 | |||||||
| Al1.5SiCrFeCoNi | 30 | |||||||
| CrMnFeCoNi | APS | WC-Co | 20 | --- | RT | 100 | 270 | [67] |
| CrMnFeCoNi | HVOF | Al2O3 | 5 | 0.0314 | RT | 100 | 393 ± 32 | [81] |
| CrMnFeCoNi-annealed | 365 ± 41 | |||||||
| Al0.6TiCrFeCoNi | HVOF | Al2O3 | 5 | 0.10 | RT | 500 | 104.4 | [56] |
| 300 | 275.7 | |||||||
| 500 | 267.4 | |||||||
| CrMnFeCoNi | Cold spray | WC-Co | 5 | 0.1 | RT | 200 | 476 ± 22 | [104] |
| CoCrFeNiTi0.5 | Laser cladding | WC | 10 | ---- | RT | 216 | 41.68 | [174] |
| 250 | 50.92 | |||||||
| 500 | 92.59 | |||||||
| CoCrFeNiTi0.5Al0.5 | Laser cladding | WC | 10 | ---- | RT | 216 | 42.12 | [174] |
| 250 | 45.83 | |||||||
| 500 | 57.87 | |||||||
| CoCrFeNiTi0.5Al | Laser cladding | WC | 10 | ---- | RT | 216 | 2.3 | [174] |
| 250 | 41.87 | |||||||
| 500 | 50.14 | |||||||
| TiVCrAlSi | Laser cladding | GCr15 steel | 10 | ---- | RT- 5Hz | 566 | 25 ± 2 | [59] |
| RT-10Hz | 22 ± 5 | |||||||
| RT-15Hz | 21 ± 5 | |||||||
| Al0.8CrFeCoNiCu0.5 | Laser cladding | GCr15 steel | 98 | 0.209 | RT | 360 | 11.9 | [171] |
| Al0.8CrFeCoNiCu0.75 | 14.1 | |||||||
| Al0.8CrFeCoNiCu | 19.0 | |||||||
| CoCrFeMnNi | Plasma cladding | Si3N4 | 25 | 0.157 | RT | 188 | 64. | [76] |
| 200 | 39.7 | |||||||
| 400 | 22.5 | |||||||
| 600 | 36.0 | |||||||
| 800 | 38.0 | |||||||
| CoCrFeMnNi)85Ti15 | Plasma cladding | Si3N4 | 25 | 0.157 | RT | 188 | 25.0 | [76] |
| 200 | 17.0 | |||||||
| 400 | 4.5 | |||||||
| 600 | 8.2 | |||||||
| 800 | 17.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.; Roy, A.; Sharifi, N.; Stoyanov, P.; Chromik, R.R.; Moreau, C. Tribological Performance of High-Entropy Coatings (HECs): A Review. Materials 2022, 15, 3699. https://doi.org/10.3390/ma15103699
Patel P, Roy A, Sharifi N, Stoyanov P, Chromik RR, Moreau C. Tribological Performance of High-Entropy Coatings (HECs): A Review. Materials. 2022; 15(10):3699. https://doi.org/10.3390/ma15103699
Chicago/Turabian StylePatel, Payank, Amit Roy, Navid Sharifi, Pantcho Stoyanov, Richard R. Chromik, and Christian Moreau. 2022. "Tribological Performance of High-Entropy Coatings (HECs): A Review" Materials 15, no. 10: 3699. https://doi.org/10.3390/ma15103699
APA StylePatel, P., Roy, A., Sharifi, N., Stoyanov, P., Chromik, R. R., & Moreau, C. (2022). Tribological Performance of High-Entropy Coatings (HECs): A Review. Materials, 15(10), 3699. https://doi.org/10.3390/ma15103699







