Controlling the Morphology of Barrel-Shaped Nanostructures Grown via CuZn Electro-Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Materials Characterization
2.3. Photocatalysis Tests
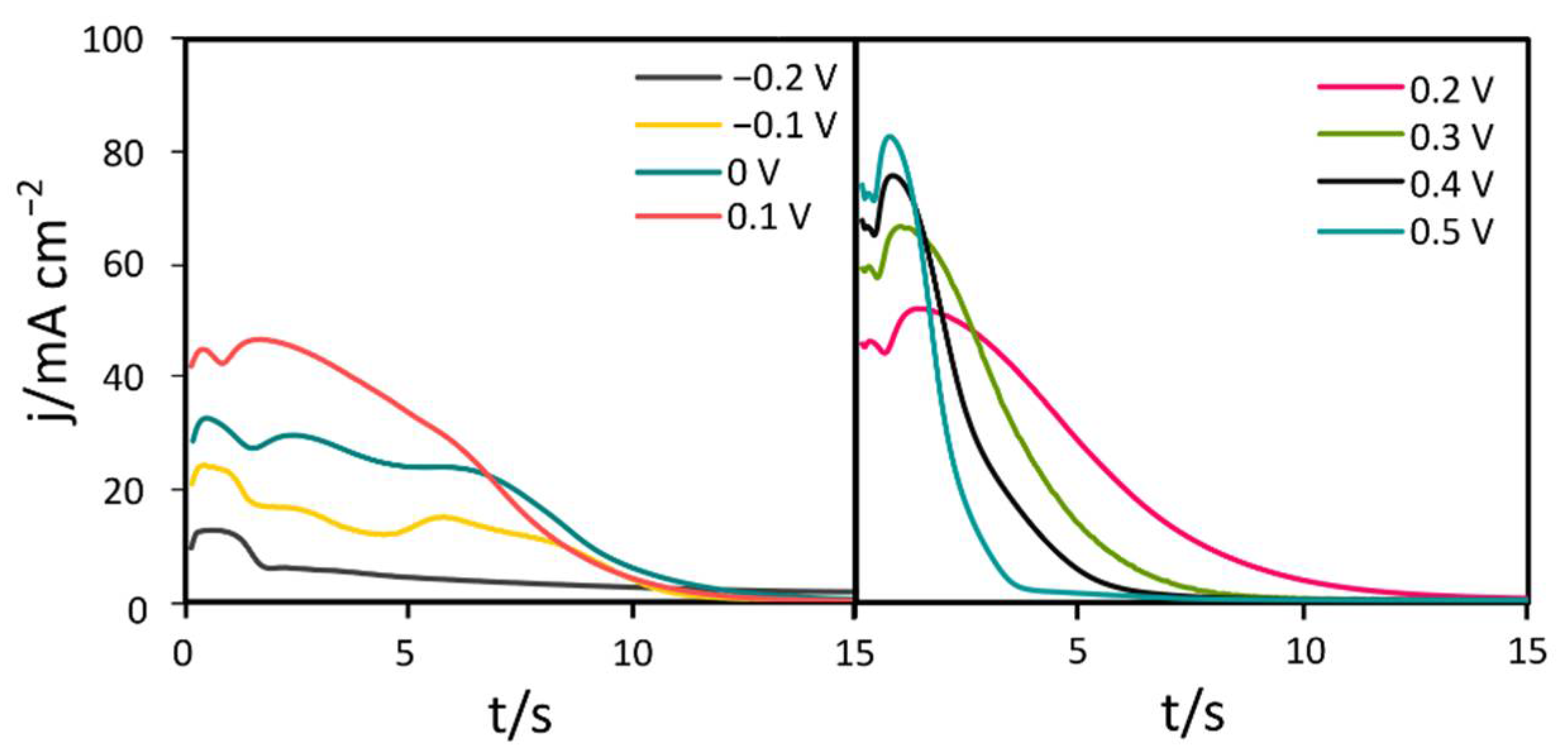
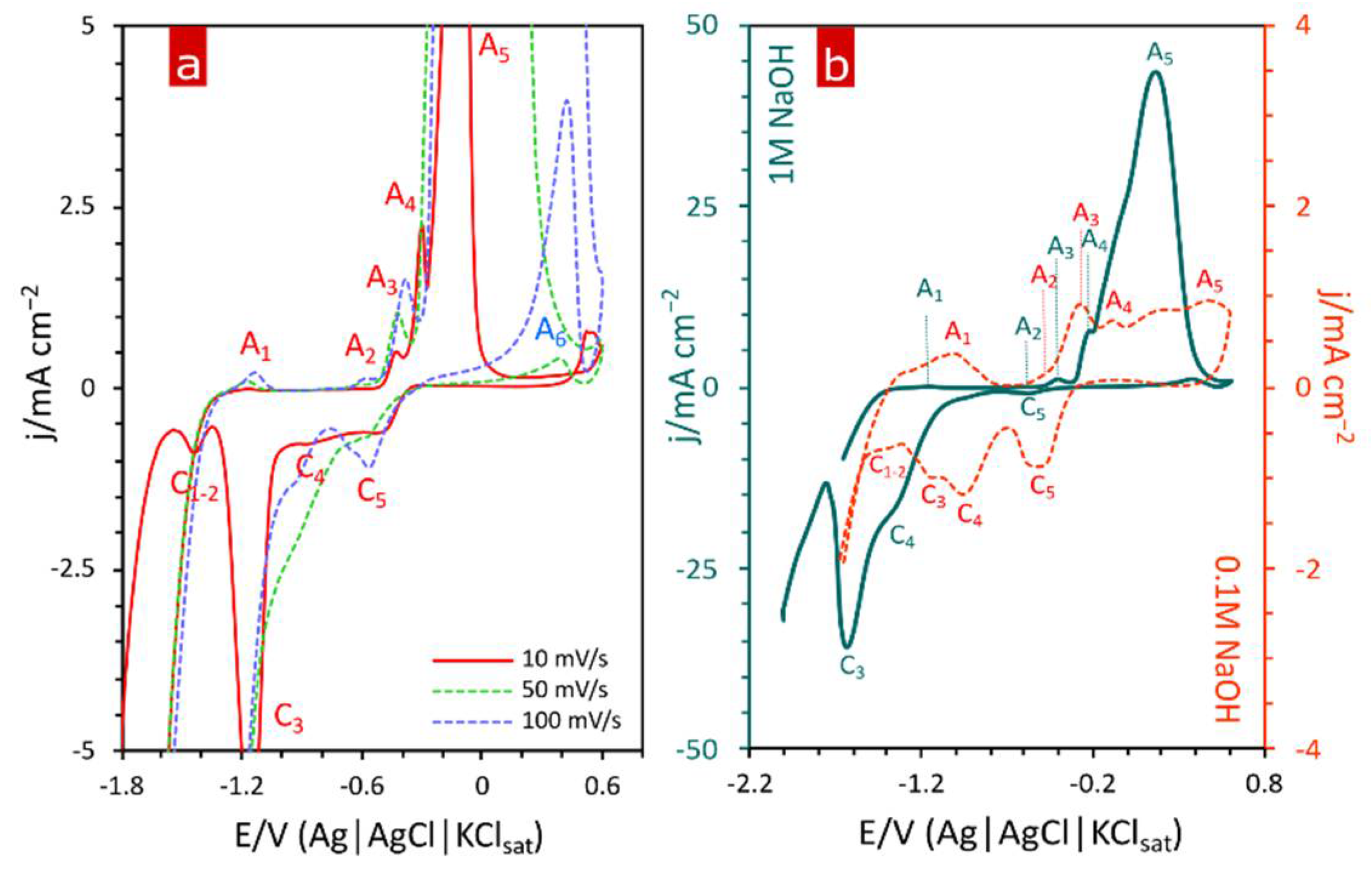
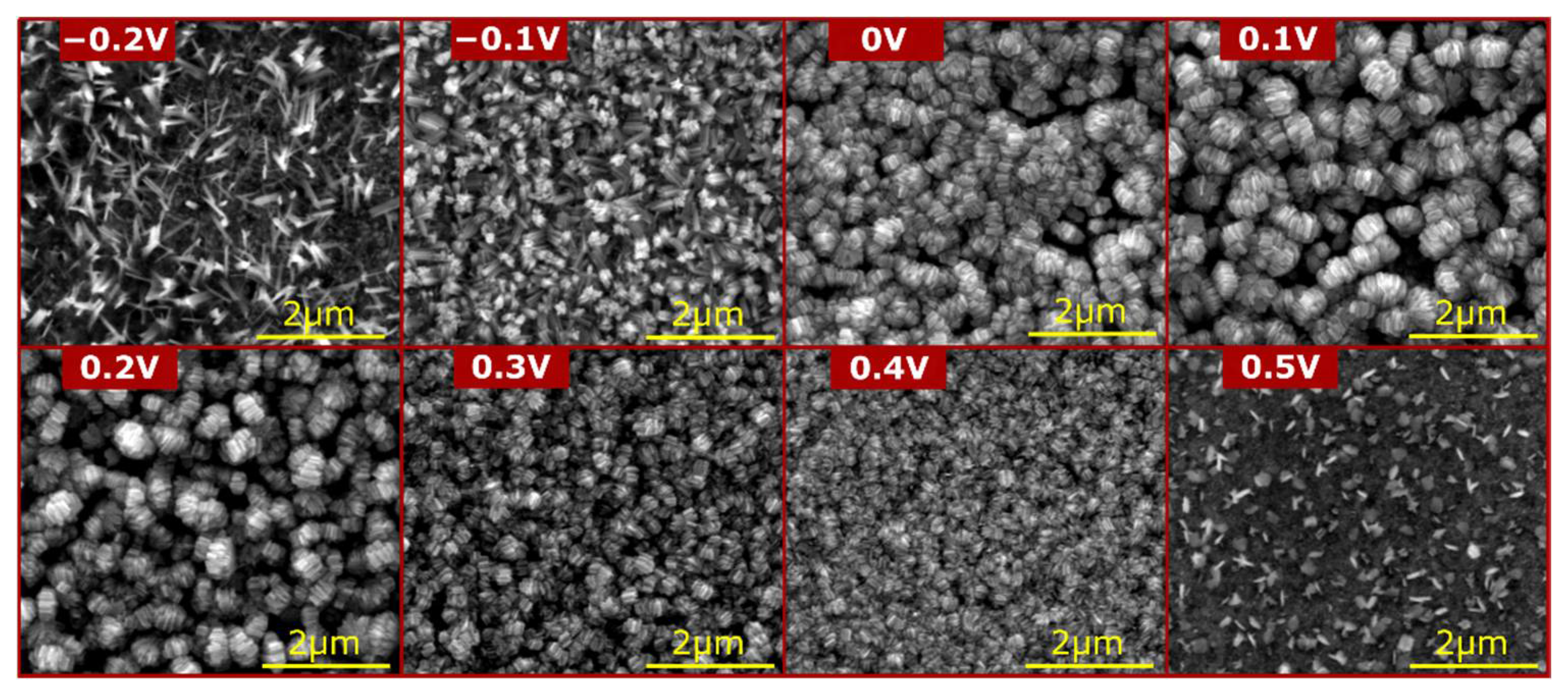
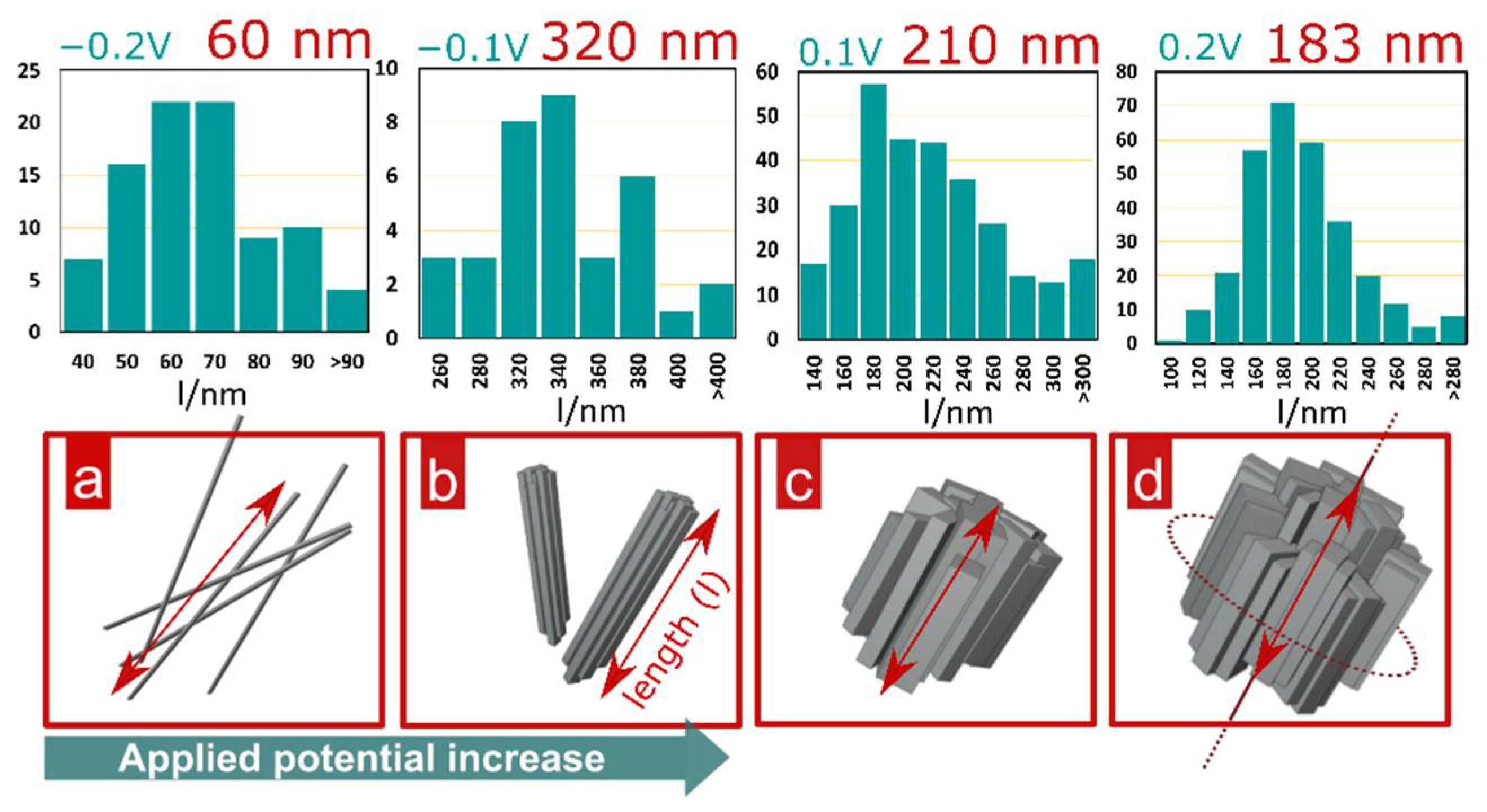
3. Results and Discussion
3.1. Materials Characterization
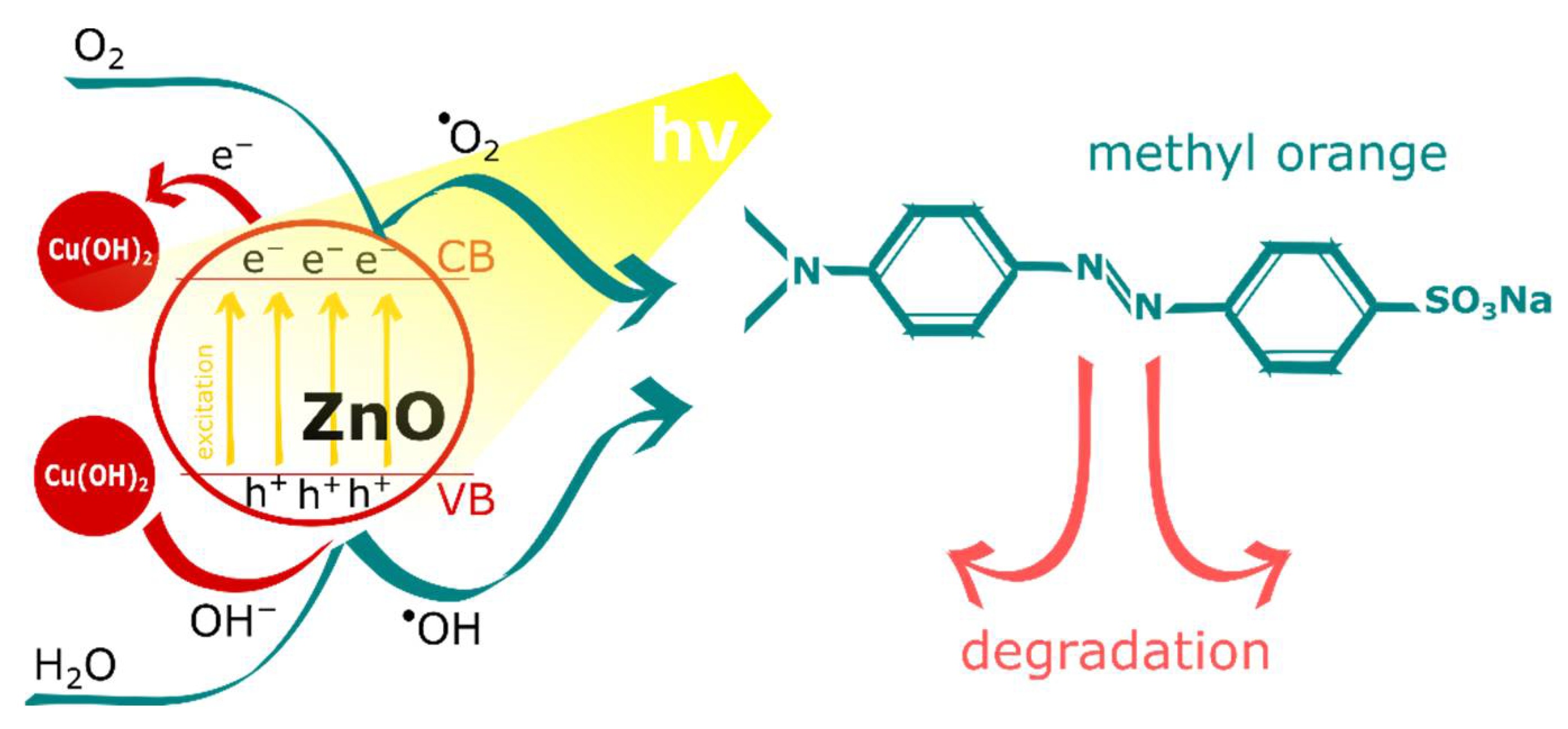
3.2. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 8, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, F.; Zhou, J.; Cai, G.; Cai, L.; Hu, Y.; Wang, D.; Liu, Y.; Guo, L.; Shen, S. N doping to ZnO nanorods for photoelectrochemical water splitting under visible light: Engineered impurity distribution and terraced band structure. Sci. Rep. 2015, 5, 12925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, S.; Jiang, T.; Li, Y.; Zhao, Q.; Li, Y.; Fang, W.; Gong, M. Highly efficient visible-light driven mesoporous graphitic carbon nitride/ZnO nanocomposite photocatalysts. Appl. Catal. B Environ. 2017, 200, 601–610. [Google Scholar] [CrossRef]
- Kumar, E.T.D.; Easwaramoorthi, S.; Rao, J.R. Fluorinated reduced graphene oxide encapsulated ZnO hollow sphere composite as an efficient photocatalyst with increased charge-carrier mobility. Langmuir 2019, 35, 8681–8691. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Tang, Y.; Hu, Q.; Wan, P.; Dai, L.; Yang, X.J. In-situ grown nanoporous Zn-Cu catalysts on brass foils for enhanced electrochemical reduction of carbon dioxide. Appl. Surf. Sci. 2018, 445, 281–286. [Google Scholar] [CrossRef]
- Ren, D.; Gao, J.; Pan, L.; Wang, Z.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Gratzel, M. Atomic layer deposition of ZnO on CuO enables selective and efficient electroreduction of carbon dioxide to liquid fuels. Angew. Chem. Int. Ed. 2019, 58, 15036–15040. [Google Scholar] [CrossRef]
- Sua, X.; Sund, Y.; Jinb, L.; Zhanga, L.; Yangb, Y.; Kernsb, P.; Liua, B.; Lid, S.; He, J. Hierarchically porous Cu/Zn bimetallic catalysts for highly selective CO2 electroreduction to liquid C2 products. Appl. Catal. B Environ. 2020, 269, 118800. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Saad, M.A.S.; Al-Marri, M.J. Electrocatalytic conversion of CO2 over in-situ grown Cu microstructures on Cu and Zn foils. J. CO2 Util. 2021, 53, 101749. [Google Scholar] [CrossRef]
- Payraa, S.; Challagullaa, S.; Bobdeb, Y.; Chakrabortya, C.; Ghoshb, B.; Roy, S. Probing the photo- and electro-catalytic degradation mechanism of methylene blue dye over ZIF-derived ZnO. J. Hazard. Mat. 2019, 373, 377–388. [Google Scholar] [CrossRef]
- Mudike, R.; Sabbanahalli, C.; Sriramoju, J.B.; Bheemaraju, A.; Halligudra, G.; Muniyappa, M.; Narayanaswamy, M.P.; Kumar CS, A.; Shivaramu, P.D.; Rangappa, D. Copper zinc tin sulfide and multi-walled carbon nanotubes nanocomposite for visible-light-driven photocatalytic applications. Mat. Res. Bull. 2022, 146, 111606. [Google Scholar] [CrossRef]
- Chabri, S.; Dhara, A.; Show, B.; Adak, D.; Sinha, A.; Mukherjee, N. Mesoporous CuO–ZnO p–n heterojunction based nanocomposites with high specific surface area for enhanced photocatalysis and electrochemical sensing. Catal. Sci. Technol. 2016, 6, 3238–3252. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Behjati, S.; Sheibani, S.; Herritsch, J.; Gottfried, J.M. Photodegradation of dyes in batch and continuous reactors by Cu2O-CuO nano-photocatalyst on Cu foils prepared by chemical-thermal oxidation. Mater. Res. Bull. 2020, 130, 110920. [Google Scholar] [CrossRef]
- Ozdemir, E.T.; Kartal, U.; Dikici, T.; Erol, M.; Yurddaskal, M. A comparative study on structural, morphological and photocatalytic properties of anodically grown ZnO nanowires under varying parameters. J. Mater. Sci. Mater. Electron. 2021, 32, 27398–27408. [Google Scholar] [CrossRef]
- Brudzisz, A.; Giziński, D.; Wierzbicka, E.; Karczewski, K.; Tiringer, U.; Taheri, P.; Stępniowski, W.J. Pom-pom-like nanowire clusters prepared by potentiostatic oxidation of copper in NH4HCO3 solution. Surf. Coat. Technol. 2021, 425, 127674. [Google Scholar] [CrossRef]
- Giziński, D.; Brudzisz, A.; Santos, J.S.; Trivinho-Strixino, F.; Stępniowski, W.J.; Czujko, T. Nanostructured anodic copper oxides as catalysts in electrochemical and photoelectrochemical reactions. Catalysts 2020, 10, 1338. [Google Scholar] [CrossRef]
- Lu, P.; Zhou, W.; Li, Y.; Wang, J.C.; Wu, P. Abnormal room temperature ferromagnetism in CuO/ZnO nanocomposites via hydrothermal method. Appl. Surf. Sci. 2017, 399, 396–402. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Zhang, L.; Zhang, L.; Li, J.; Yang, X.; Peng, F.; Sun, Y. Structural tailored ZnO@Cu2O heterostructure-decorated mesh with dual functionalities for oil/water separation and photodegradation. J. Alloys Compd. 2022, 896, 162763. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y. Facile synthesis and photocatalytic activity of ZnO-CuO nanocomposite. Superlattice. Microst. 2010, 47, 615–623. [Google Scholar] [CrossRef]
- Li, C.J.; Cao, X.; Li, W.H.; Zhang, B.W.; Xiao, L.Q. Co-synthesis of CuO-ZnO nanoflowers by low voltage liquid plasma discharge with brass electrode. J. Alloys Compd. 2019, 773, 762–769. [Google Scholar] [CrossRef]
- Sukhov, I.A.; Shafeev, G.A.; Voronov, V.V.; Sygletou, M.; Stratakis, E.; Fotakis, C. Generation of nanoparticles of bronze and brass by laser ablation in liquid. Appl. Surf. Sci. 2014, 302, 79–82. [Google Scholar] [CrossRef]
- Nami, M.; Sheibani, S.; Rashchi, F. Photocatalytic performance of coupled semiconductor ZnO–CuO nanocomposite coating prepared by a facile brass anodization process. Mater. Sci. Semicond. Process. 2021, 135, 106083. [Google Scholar] [CrossRef]
- Procaccini, R.; Schreiner, W.H.; Vázquez, M.; Ceré, S. Surface study of films formed on copper and brass at open circuit potential. Appl. Surf. Sci. 2013, 268, 171–178. [Google Scholar] [CrossRef]
- Qiu, P.; Leygraf, C. Initial oxidation of brass induced by humidified air. Appl. Surf. Sci. 2011, 258, 1235–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, R.W.; Breiter, M.W. The anodic dissolution and passivation of zinc in concentrated potassium hydroxide solutions. J. Electrochem. Soc. 1969, 116, 719–729. [Google Scholar] [CrossRef]
- Assaf, F.H.; El-Rehim, S.S.A.; Zaky, A.M. Cyclic voltammetric behaviour of α-brass in alkaline media. Corr. J. 2001, 36, 143–150. [Google Scholar] [CrossRef]
- Pickering, H.W.; Byrne, P.J. Partial currents during anodic dissolution of Cu-Zn alloys at constant potential. J. Electrochem. Soc. 1969, 116, 1492–1496. [Google Scholar] [CrossRef]
- Mika, K.; Socha, R.P.; Nyga, P.; Wiercigroch, E.; Małek, K.; Jarosz, M.; Uchacz, T.; Sulka, G.D.; Zaraska, L. Electrochemical synthesis and characterization of dark nanoporous zinc oxide films. Electrochim. Acta 2019, 305, 349–359. [Google Scholar] [CrossRef]
- Anantharaj, S.; Sugime, H.; Noda, S. Ultrafast growth of a Cu(OH)2−CuO nanoneedle array on Cu foil for methanol oxidation electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 27327–27338. [Google Scholar] [CrossRef]
- Dezfoolian, M.; Rashchi, F.; Nekouei, R.K. Synthesis of copper and zinc oxides nanostructures by brass anodization in alkaline media. Surf. Coat. Technol. 2015, 275, 245–251. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Al-Osta, A.A.; Jadhav, V.V.; Zate, M.K.; Mane, R.S.; Huic, K.N.; Han, S.-H. Electrochemical supercapacitors of anodized-brass-templated NiO nanostructured electrodes. Scr. Mater. 2014, 99, 29–32. [Google Scholar] [CrossRef]
- Eissa, D.S.; El-Hagar, S.S.; Ashour, E.A.; Allam, N.K. Electrochemical nano-patterning of brass for stable and visible light-induced photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 14588–14595. [Google Scholar] [CrossRef]
- Ryczek, K.; Kozieł, M.; Wiercigroch, E.; Małek, K.; Jarosz, M.; Sulka, G.D.; Zaraska, L. Fast fabrication of nanostructured semiconducting oxides by anodic oxidation of brass. Mater. Sci. Semicond. Process. 2020, 113, 105035. [Google Scholar] [CrossRef]
- Singh, S.; Barick, K.C.; Bahadur, D. Shape-controlled hierarchical ZnO architectures: Photocatalytic and antibacterial activities. Cryst. Eng. Comm. 2013, 15, 4631–4639. [Google Scholar] [CrossRef]
- Hong, R.Y.; Li, J.H.; Chen, L.L.; Liu, D.Q.; Li, H.Z.; Zheng, Y.; Ding, J. Synthesis, surface modification and photocatalytic property of ZnO nanoparticles. Powder Technol. 2009, 189, 426–432. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Selvaraj, R.; Kalimuthu, K.R.; Kalimuthu, V. A type-II MoS2/ZnO heterostructure with enhanced photocatalytic activity. Mater. Lett. 2019, 243, 183–186. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Z.; Tao, P.; Jin, B.; Wu, Z.; Huang, Y. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 nanospheres modified porous g-C3N4. Mater. Chem. Phys. 2014, 143, 1462–1468. [Google Scholar] [CrossRef]
- Yang, K.; Yu, Q.; Li, H.; Ren, X.; Pan, J.; Li, X. Design of a higher positive valence band in g-C3N4 photocatalyst for enhanced photocatalytic activity by copper hydroxide deposition. Mater. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Yu, J.; Ran, J. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364–1371. [Google Scholar] [CrossRef]
- Bandara, J.; Klehm, U.; Kiwi, J. Raschig rings-Fe2O3 composite photocatalyst activate in the degradation of 4-chlorophenol and Orange II under daylight irradiation. Appl. Catal. B Environ. 2007, 76, 73–81. [Google Scholar] [CrossRef]

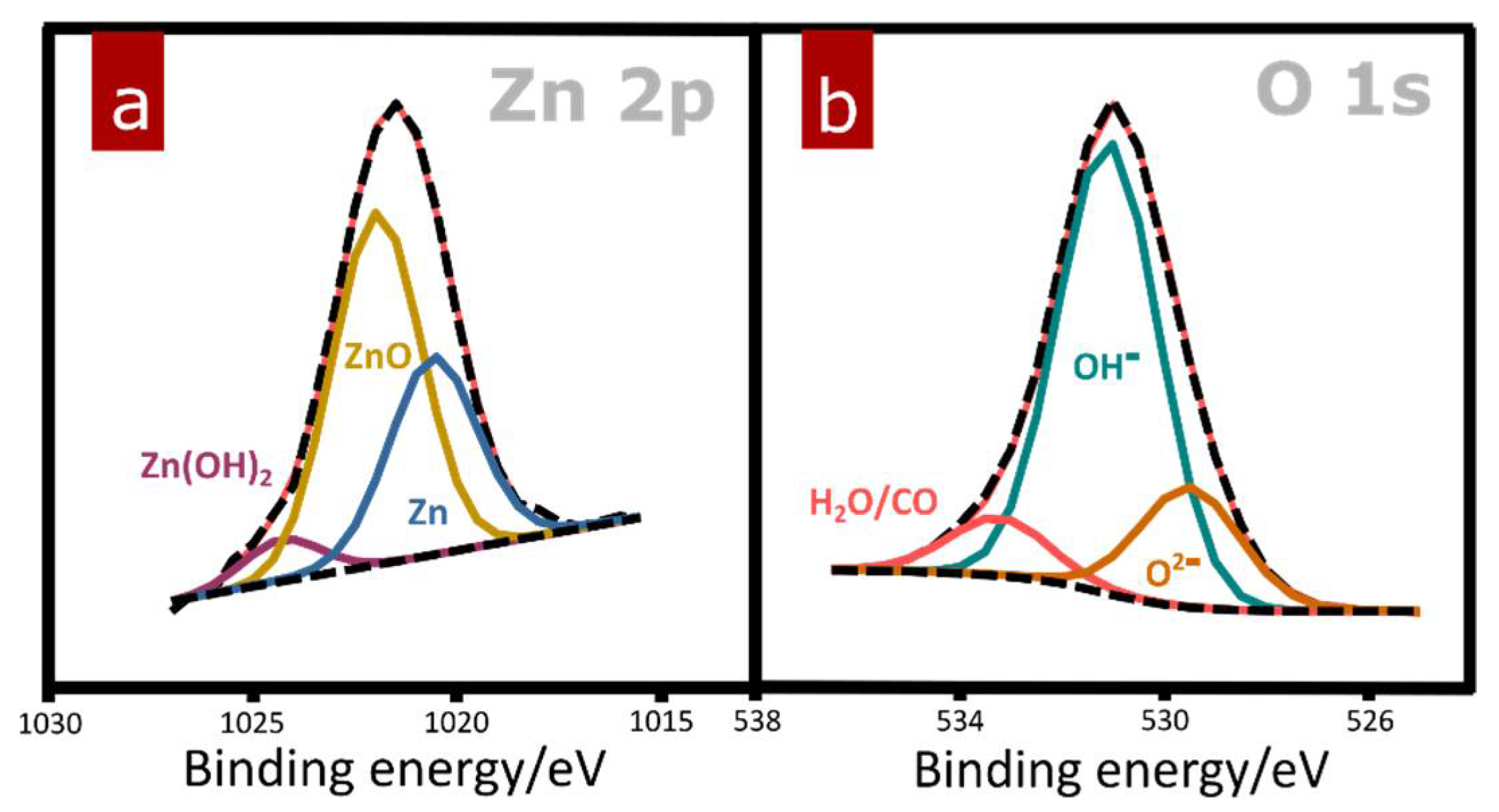

| Substrate | Film Composition after Electro-Oxidation | Morphology | Electrolyte Composition | Voltage | Ref |
|---|---|---|---|---|---|
| CuZn | CuO-ZnO (after annealing) | particles | 1 M oxalic acid | 40 V (25 min) | [32] |
| CuZn (65:35) | CuO-ZnO | flowers, petals cubes | 0.05, 0.1, 0.2 M NaOH and KOH (with NH4Cl, NH4F, NH4SO4) | 6, 12, 24 V (15, 30, 60 min) | [30] |
| CuZn (70:30) | CuO-ZnO; Cu-Zn-O | flowers | 0.1 M, 1 M NaHCO3 | 12 V (5 min) | [33] |
| CuZn | CuO-ZnO | spherical structure | 1 M NaOH | 30–60 V (10 min) | [34] |
| CuZn (65:35) | CuO-ZnO | flowers | 0.1 M NaOH + 0.025 M NH4Cl | 12 V (15–45 min) | [22] |
| CuZn (63:37) | Cu(OH)2-ZnO | barrels | 1 M NaOH | (−0.2)–0.5 V (10 min) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giziński, D.; Mojsilović, K.; Brudzisz, A.; Tiringer, U.; Vasilić, R.; Taheri, P.; Stępniowski, W.J. Controlling the Morphology of Barrel-Shaped Nanostructures Grown via CuZn Electro-Oxidation. Materials 2022, 15, 3961. https://doi.org/10.3390/ma15113961
Giziński D, Mojsilović K, Brudzisz A, Tiringer U, Vasilić R, Taheri P, Stępniowski WJ. Controlling the Morphology of Barrel-Shaped Nanostructures Grown via CuZn Electro-Oxidation. Materials. 2022; 15(11):3961. https://doi.org/10.3390/ma15113961
Chicago/Turabian StyleGiziński, Damian, Kristina Mojsilović, Anna Brudzisz, Urša Tiringer, Rastko Vasilić, Peyman Taheri, and Wojciech J. Stępniowski. 2022. "Controlling the Morphology of Barrel-Shaped Nanostructures Grown via CuZn Electro-Oxidation" Materials 15, no. 11: 3961. https://doi.org/10.3390/ma15113961
APA StyleGiziński, D., Mojsilović, K., Brudzisz, A., Tiringer, U., Vasilić, R., Taheri, P., & Stępniowski, W. J. (2022). Controlling the Morphology of Barrel-Shaped Nanostructures Grown via CuZn Electro-Oxidation. Materials, 15(11), 3961. https://doi.org/10.3390/ma15113961








