Relevant Aspects of Titanium and Zirconia Dental Implants for Their Fatigue and Osseointegration Behaviors
Abstract
:1. Introduction
2. Materials and Methods
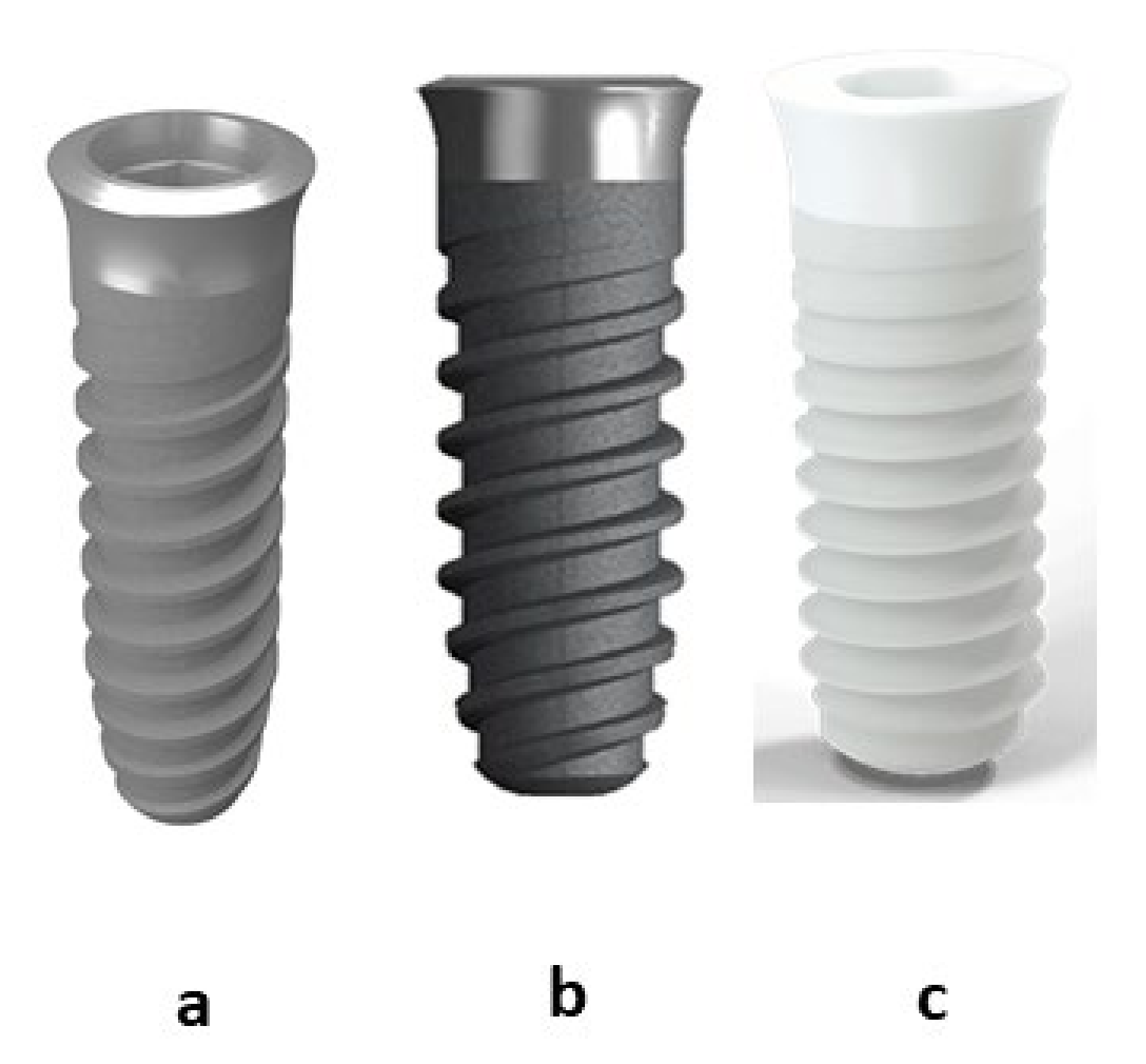
2.1. Dental Implants
- Commercially pure titanium cp-Ti (grade 4) (Klockner Dental Implants, Escaldes Engordany, Andorra) smooth with passivated treatment (citric acid 20% for 15 s) (Ti).
- Commercially pure titanium cp-Ti (grade 4) sand blasted with alumina (350 μm to 500 μm) projected at 2.5 bars and 200 mm of distance (implant-gun). After the sand blasted treatment, the samples were passivated (citric acid 20% for 15 s) (Ti-R)
- Zirconia-2.5 Y-TZP. (ZrO2)
2.2. Roughness
2.3. Contact Angle and Surface Free Energy
2.4. Residual Stresses
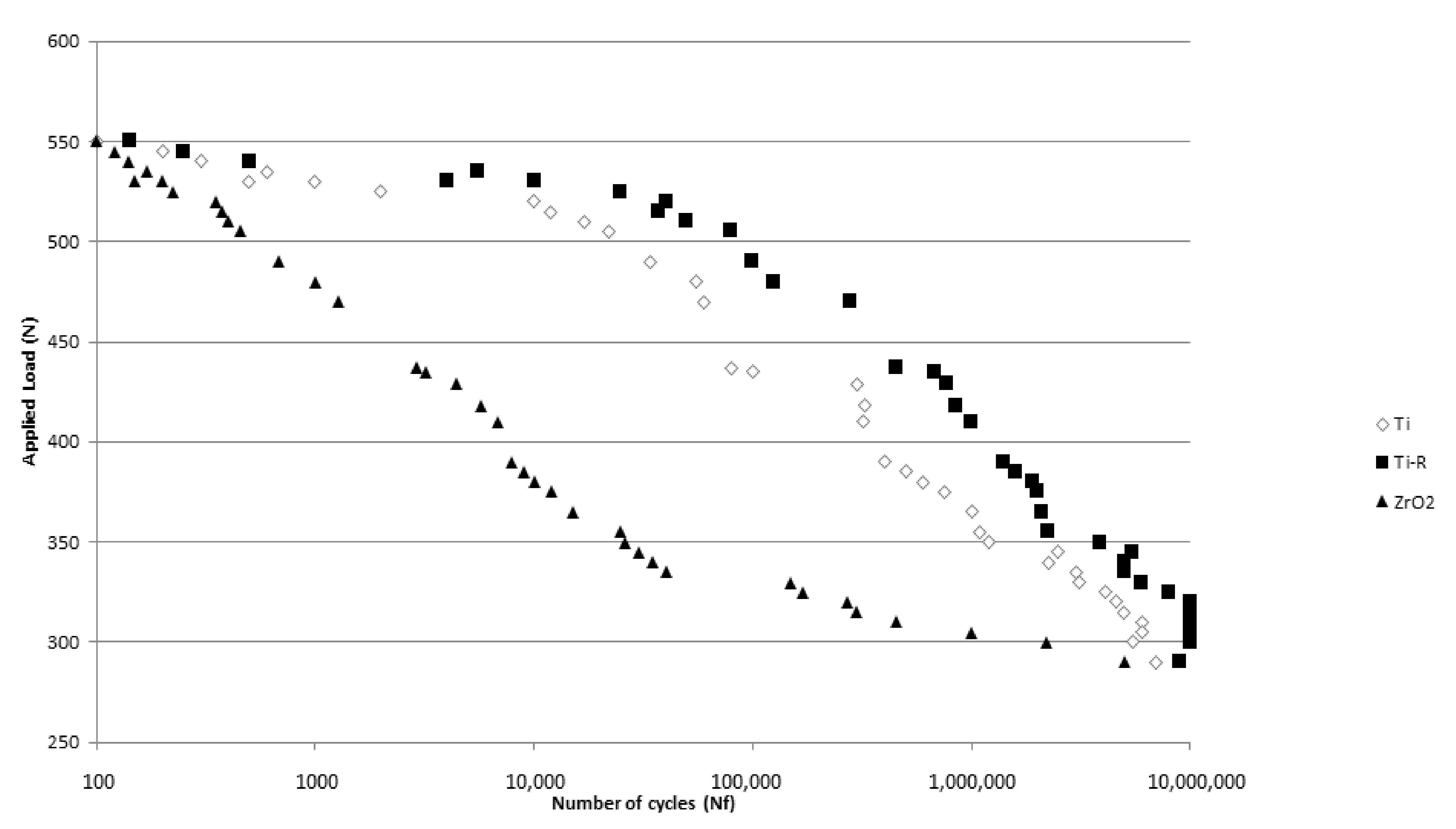
2.5. Fatigue Behavior
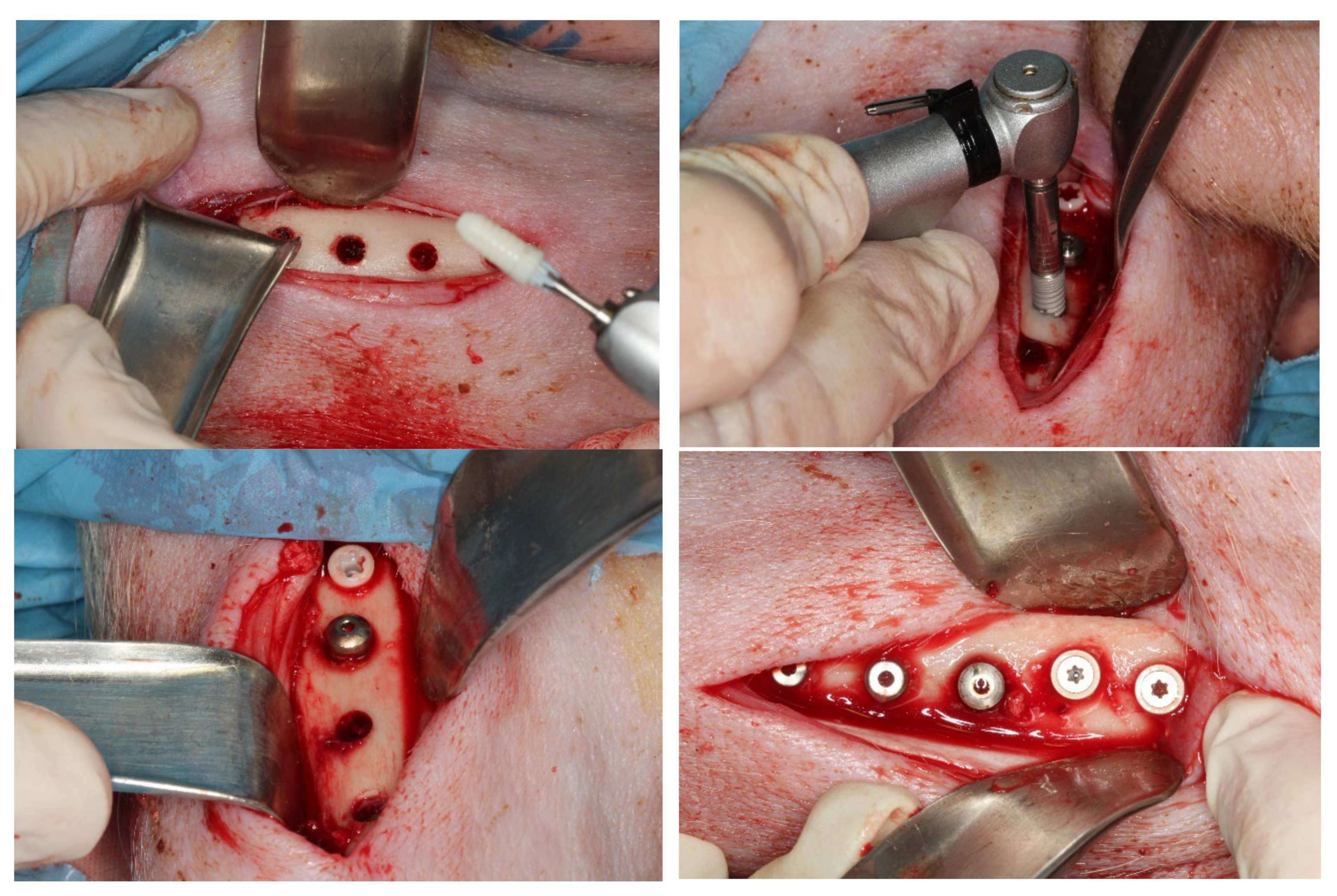
2.6. In Vivo Study
2.7. Surgical Intervention
2.8. Histomorphometric/Histological Analysis
2.9. Specimen Preparation
- −
- Fixation: Bone blocks were placed in 10% formalin for 2 weeks.
- −
- Dehydration: With alcohols at different concentrations under constant agitation:
- 70% alcohol for 3 days
- 80% alcohol for 3 days
- 96% alcohol for 3 days
- 99.8% alcohol for 3 days
- Technovit 7200® + BPO: alcohol (30:70) for three days.
- Technovit 7200® + BPO: alcohol (50:50) for three days.
- Technovit 7200® + BPO: alcohol (70:30) for three days.
- Technovit 7200® + BPO (100) for three days.
- Technovit 7200® + BPO (100) for three days under vacuum.
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.I.; Han, J.H.; Lee, I.S.; Lee, K.H.; Shin, M.C.; Choi, B.B. New titanium alloys for biomaterials: A study of mechanical and corrosion properties and cytotoxicity. Bio-Med Mater. Eng. 1997, 7, 253–263. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Niespodziana, K.; Jurczyk, K.; Jurczyk, M. The synthesis of titanium alloys for biomedical applications. Rev. Adv. Mater. Sci. 2008, 18, 236–240. [Google Scholar]
- Uporabo, B. A review of the surface modifications of titanium alloys for biomedical applications. Mater. Tehnol. 2017, 51, 181–193. [Google Scholar]
- Nicula, R.; Lüthen, F.; Stir, M.; Nebe, B.; Burkel, E. Spark plasma sintering synthesis of porous nanocrystalline titanium alloys for biomedical applications. Biomol. Eng. 2007, 24, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Bannon, B.P.; Mild, E.E. Titanium Alloys for Biomaterial Application: An Overview. In Titanium Alloys in Surgical Implants. ASTM-STP796; Luckey, H.A., Kubli, F., Eds.; ASTM: Philadelphia, PA, USA, 1981; pp. 7–14. [Google Scholar]
- Lemons, J.E. Application of Materials in Medicine and Dentistry. Dental Implants. In Biomaterials Science: An Introduction to Materials in Medicine; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 308–318. [Google Scholar]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.A.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F.J. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Climent, M.; Lázaro, P.; Rios, J.V.; Lluch, S.; Marqués, M.; Guillem-Martí, J.; Gil, F.J. Influence of acid-etching after grit-blasted on osseointegration of titanium dental implants: In vitro and in vivo studies. J. Mater. Sci. Mater. Med. 2013, 24, 2047–2055. [Google Scholar] [CrossRef]
- Gil, F.J.; Rodríguez, D.; Planell, J.A.; Cortada, M.; Giner, L.; Costa, S. Galvanic corrosion behaviour of Titanium implants coupled to dental alloys. J. Mater. Sci. Mater. Med. 2000, 11, 287–293. [Google Scholar]
- Gil, F.J.; Sánchez, L.A.; Espias, A.; Planell, J.A. In vitro corrosion behaviour and metallic ion release of different prosthodontic alloys. Int. Dent. J. 1999, 49, 347–351. [Google Scholar] [CrossRef]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent. Mater. 2007, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure tianium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef]
- Rodrigues, D.; Valderrama, P.; Wilson, T.; Palmer, K.; Thomas, A.; Sridhar, S.; Sadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [Green Version]
- Variola, F.; Lauria, A.; Nanci, A.; Rosei, F. Influence of Treatment Conditions on the Chemical Oxidative Activity of H2SO4/H2O2Mixtures for Modulating the Topography of Titanium. Adv. Eng. Mater. 2009, 11, B227–B234. [Google Scholar] [CrossRef]
- Variola, F.; Francis-Zalzal, S.; Leduc, A.; Barbeau, J.; Nanci, A. Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int. J. Nanomed. 2014, 9, 2319. [Google Scholar] [CrossRef] [Green Version]
- Cruz, N.; Gil, J.; Punset, M.; Manero, J.M.; Tondela, J.P.; Verdeguer, P.; Aparicio, C.; Rúperez, E. Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy DentalMeshes. Coatings 2022, 12, 154. [Google Scholar] [CrossRef]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Camposilvan, E.; Leone, R.; Gremillarda, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Mohan, P.; Yuan, B.; Patterson, T.; Desai, V.H.; Sohn, Y.H. Degradation of yttria-stabilized zirconia thermal barrier coatings by vanadium pentoxide, phosphorous pentoxide, and sodium sulfate. J. Am. Ceram. Soc. 2007, 90, 3601–3607. [Google Scholar] [CrossRef]
- Lin, J.-D.; Duh, J.-G. Fracture toughness and hardness of ceria- and yttria-doped tetragonal zirconia ceramics. Mat. Chem. Phys. 2002, 78, 253–261. [Google Scholar] [CrossRef]
- Sevilla, P.; Sandino, C.; Arciniegas, M.; Martínez-Gomis, J.; Peraire, M.; Gil, F.J. Evaluating mechanical properties and degradation of YTZP dental implants. Mater. Sci. Eng. C 2010, 30, 14–19. [Google Scholar] [CrossRef]
- Wenz, H.J.; Bartsch, J.; Wolfart, S.; Kern, M. Osseointegration and clinical success of zirconia dental implants: A systematic review. Int. J. Prosthodont. 2008, 21, 27–36. [Google Scholar] [PubMed]
- Yılmaz, E.; Feyza, N.; Gökçe, A.; Fındık, F. Production and Characterization of a Bone-Like Porous Ti/Ti-Hydroxyapatite Functionally Graded Material. J. Mater. Eng. Perform. 2020, 29, 6455–6467. [Google Scholar] [CrossRef]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Gulsoy, O. Assessment of Ti–16Nb–xZr alloys produced via PIM for implant applications. J. Therm. Anal. Calorim. 2018, 134, 7–14. [Google Scholar] [CrossRef]
- Pereira, G.K.R.; Guilardi, L.F.; Dapieve, K.S.; Kleverlaan, C.J.; Rippe, M.; Valandro, L.F. Mechanical reliability, fatigue strength and survival analysis of new polycrystalline translucent zirconia ceramics for monolithic restorations. J. Mech. Beh. Biomed. Mater. 2018, 85, 57–65. [Google Scholar] [CrossRef]
- Gil, J.; Delgado-García-Menocal, J.A.; Velasco-Ortega, E.; Bosch, B.; Delgado, L.; Pérez-Antoñanzas, R.; Fernández-Fairén, M. Comparison of zirconia degradation in dental implants and femoral balls: An X-ray diffraction and nanoindentation study. Int. J. Implant. Dent. 2021, 18, 103. [Google Scholar] [CrossRef]
- Gottlow, J.; Barkarmo, S.; Sennerby, L. An experimental comparison of two different clinically used implant designs and surfaces. Clin. Implant Dent. Relat. Res. 2012, 14, e204–e212. [Google Scholar] [CrossRef]
- Scarano, A.; Degidi, M.; Iezzi, G.; Petrone, G.; Piattelli, A. Correlation between implant stability quotient and bone-implant contact: A retrospective histological and histomorphometrical study of seven titanium implants retrieved from humans. Clin. Implant Dent. Relat. Res. 2006, 8, 218–222. [Google Scholar] [CrossRef]
- Karl, M.; Irastorza-Landa, A. Does implant design affect primary stability in extraction sites? Quintessence Int. 2017, 48, 219–224. [Google Scholar]
- Irinakis, T.; Wiebe, C. Initial torque stability of a new bone condensing dental implant. A cohort study of 140 consecutively placed implants. J. Oral Implantol. 2009, 35, 277–282. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; Sennerby, L.; Meredith, N. Influence of implant taper on the primary and secondary stability of osseointegrated titanium implants. Clin. Oral Implants. Res. 2004, 15, 474–480. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.T.; Lee, J.-Y.; Yi, Y.-J. A randomized controlled clinical trial of two types of tapered implants on immediate loading in the posterior maxilla and mandible. Int. J. Oral Maxillofac. Implant. 2013, 28, 1602–1611. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of Implant Thread Geometry on Secondary Stability, Bone Density, and Bone-to-Implant Contact: A Biomechanical and Histological Analysis. Implant. Dent. 2015, 24, 384–391. [Google Scholar] [CrossRef]
- Lan, T.H.; Du, J.K.; Pan, C.Y.; Lee, H.E.; Chung, W.H. Biomechanical analysis of alveolar bone stress around implants with different thread designs and pitches in the mandibular molar area. Clin. Oral. Investig. 2012, 16, 363–369. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Namgung, C.; Lee, J.-H.; Lim, Y.-J. The influence of thread geometry on implant osseointegration under immediate loading: A literature review. J. Adv. Prosthodont. 2014, 6, 547–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, T.G., Jr.; Miller, R.J.; Trushkowsky, R.; Dard, M. Tapered Implants in Dentistry: Revitalizing Concepts with Technology: A Review. Adv. Dent. Res. 2016, 28, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.; Calvo-Guirado, J.L.; Lazić, Z.; Gómez-Moreno, G.; Ćalasan, D.; Guardia, J.; Čolic, S.; Aguilar-Salvatierra, A.; Gačić, B.; Delgado-Ruiz, R.; et al. Evaluation of primary stability of self-tapping and non-self-tapping dental implants. A 12-week clinical study. Clin. Implant. Dent. Relat. Res. 2013, 15, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Aparicio, C.; Rodriguez, D.; Gil, F.J. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 2011, 31, 320–324. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2014, 18, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Albrektsson, T.; Branemark, P.I.; Hansson, H.A.; Lindstrom, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Sasaki, K.; Nishida, N.; Kokubo, T.; Nakamura, T. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Monsalve-Guil, L.; Jiménez-Guerra, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pequeroles, M.; Perez, R.A.; Gil, F.J. Importance of the roughness and residual stresses of dental implants on fatigue and osseointegration behavior. In vivo study in rabbits. J. Oral Implantol. 2016, 42, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the Titanium Implant Surface Treatment on the Surface Roughness and Chemical Composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef] [Green Version]
- Nkenke, E.L.B.; Weinzierl, K.; Thams, U.; Neugebauer, J.; Steveling, H.; Radespiel-Troger, M.; Neukam, F.W. Bone contact, growth, and density around immediately loaded implants in the mandible of mini pigs. Clin. Oral Implant. Res. 2003, 14, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; Pfingstner, G.; Busenlechner, D.; Dobsak, T.; Reich, K.; Heimel, P.; Gruber, R. Osteocyte lacunar density and area in newly formed bone of the augmented sinus. Clin. Oral Implant. Res. 2012, 24, 285–289. [Google Scholar] [CrossRef]
- Schlegel, K.A.; Donath, K.; Rupprecht, S.; Falk, S.; Zimmermann, R.; Felszeghy, E.; Wiltfang, J. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials 2004, 25, 5387–5393. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on Titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Review: Zirconia: Established facts and perspectives for a Biomedical in dental implantology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88B, 519–529. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V.; Atieh, M.; Ma, S.; Duncan, W. Ceramic implants (Y-TZP). Are they a viable alternative to titanium implants for the support of overdentures? A randomized clinical trial. Clin. Oral Implant. Res. 2014, 25, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Johansson, C.B.; Wennerberg, A.; Albrektsson, T. Quantitative and qualitative investigations of surface enlarged titanium and titanium alloys implants. Clin. Oral Implant. Res. 1998, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpswon, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation and protein synthesis of human osteoblast-like vcells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Manero, J.M.; Gil, F.J.; Padros, E.; Planell, J.A. Applications of environmental scanning electron microscopy (ESEM) in biomaterials field. Microsc. Res. Tech. 2003, 61, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Kasemo, B.; Gold, J. Implant Surfaces and Interface Processes. Adv. Dent. Res. 1999, 13, 8–20. [Google Scholar] [CrossRef]
- Ronold, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. Analysing the optimal value for titanium implant roughness in bone attachment using a tensile test. Biomaterials 2003, 24, 4559–4564. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Bosshardt, D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Depprich, R. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahlert, M.; Röhling, S.; Wieland, M.; Sprecher, C.M.; Kniha, H.; Milz, S. Osseointegration of zirconia and titanium dental implants: A histological and histomorphometrical study in the maxilla of pigs. Clin. Oral Implant. Res. 2009, 20, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Weng, D.; Krämer, S.; Biesterfeld, S.; Jahn-Eimermacher, A.; Wagner, W. Osseointegration one-piece zirconia implants compared with a titanium implant of identical design: A histomorphometric study in the dog. Clin. Oral Implant. Res. 2010, 21, 350–356. [Google Scholar] [CrossRef] [PubMed]



| Implants | O | C | N | H | Al | Fe | Ti |
|---|---|---|---|---|---|---|---|
| Ti | 0.35 ± 0.09 | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.12 ± 0.04 | 0.30 ± 0.09 | Balance |
| Ti-R | 0.92 ± 0.13 | 0.08 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.01 | 2.56 ± 0.76 | 0.32 ± 0.08 | Balance |
| Oxides | Percentage | ||||||
| ZrO2(+HfO2) | 95.5 ± 1.5 | ||||||
| Y2O3 | 4.0 ± 0.6 | ||||||
| Al2O3 | 0.5 ± 0.1 | ||||||
| Implant | Ra (μm) | Rz (μm) |
|---|---|---|
| Ti | 0.33 ± 0.18 | 3.10 ± 0.69 |
| Ti-R | 1.98 ± 0.39 * | 9.98 ± 1.34 * |
| ZrO2 | 0.32 ± 0.19 | 3.00 ± 0.34 |
| Sample | CA (°) | SFE (mJ/m2) | |||
|---|---|---|---|---|---|
| WA | DIIO | ϒ | ϒD | ϒP | |
| Ti | 75.66 ± 2.67 | 43.60 ± 1.72 | 38.16 ± 1.16 | 35.60 ± 0.82 | 2.56 ± 0.75 |
| Ti-R | 94.65 ± 2.87 * | 47.67 ± 1.73 * | 31.47 ± 0.90 * | 21.53 ± 0.81 * | 4.94 ± 0.47 * |
| ZrO2 | 70.08 ± 3,57 | 45.10 ± 1.89 | 40.69 ± 0.87 | 37.45 ± 0.98 | 3.24 ± 0.41 |
| Implant | Residual Stress (MPa) |
|---|---|
| Ti | −250.2 ± 8.9 * |
| Ti-R | −440.9 ± 19.3 ** |
| ZrO2 | −190.3 ± 5.2 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragoneses, J.; Valverde, N.L.; Fernandez-Dominguez, M.; Mena-Alvarez, J.; Rodriguez, C.; Gil, J.; Aragoneses, J.M. Relevant Aspects of Titanium and Zirconia Dental Implants for Their Fatigue and Osseointegration Behaviors. Materials 2022, 15, 4036. https://doi.org/10.3390/ma15114036
Aragoneses J, Valverde NL, Fernandez-Dominguez M, Mena-Alvarez J, Rodriguez C, Gil J, Aragoneses JM. Relevant Aspects of Titanium and Zirconia Dental Implants for Their Fatigue and Osseointegration Behaviors. Materials. 2022; 15(11):4036. https://doi.org/10.3390/ma15114036
Chicago/Turabian StyleAragoneses, Javier, Nansi Lopez Valverde, Manuel Fernandez-Dominguez, Jesús Mena-Alvarez, Cinthia Rodriguez, Javier Gil, and Juan Manuel Aragoneses. 2022. "Relevant Aspects of Titanium and Zirconia Dental Implants for Their Fatigue and Osseointegration Behaviors" Materials 15, no. 11: 4036. https://doi.org/10.3390/ma15114036
APA StyleAragoneses, J., Valverde, N. L., Fernandez-Dominguez, M., Mena-Alvarez, J., Rodriguez, C., Gil, J., & Aragoneses, J. M. (2022). Relevant Aspects of Titanium and Zirconia Dental Implants for Their Fatigue and Osseointegration Behaviors. Materials, 15(11), 4036. https://doi.org/10.3390/ma15114036








