Optimizing the Thermomechanical Process of Nickel-Based ODS Superalloys by an Efficient Method
Abstract
:1. Introduction
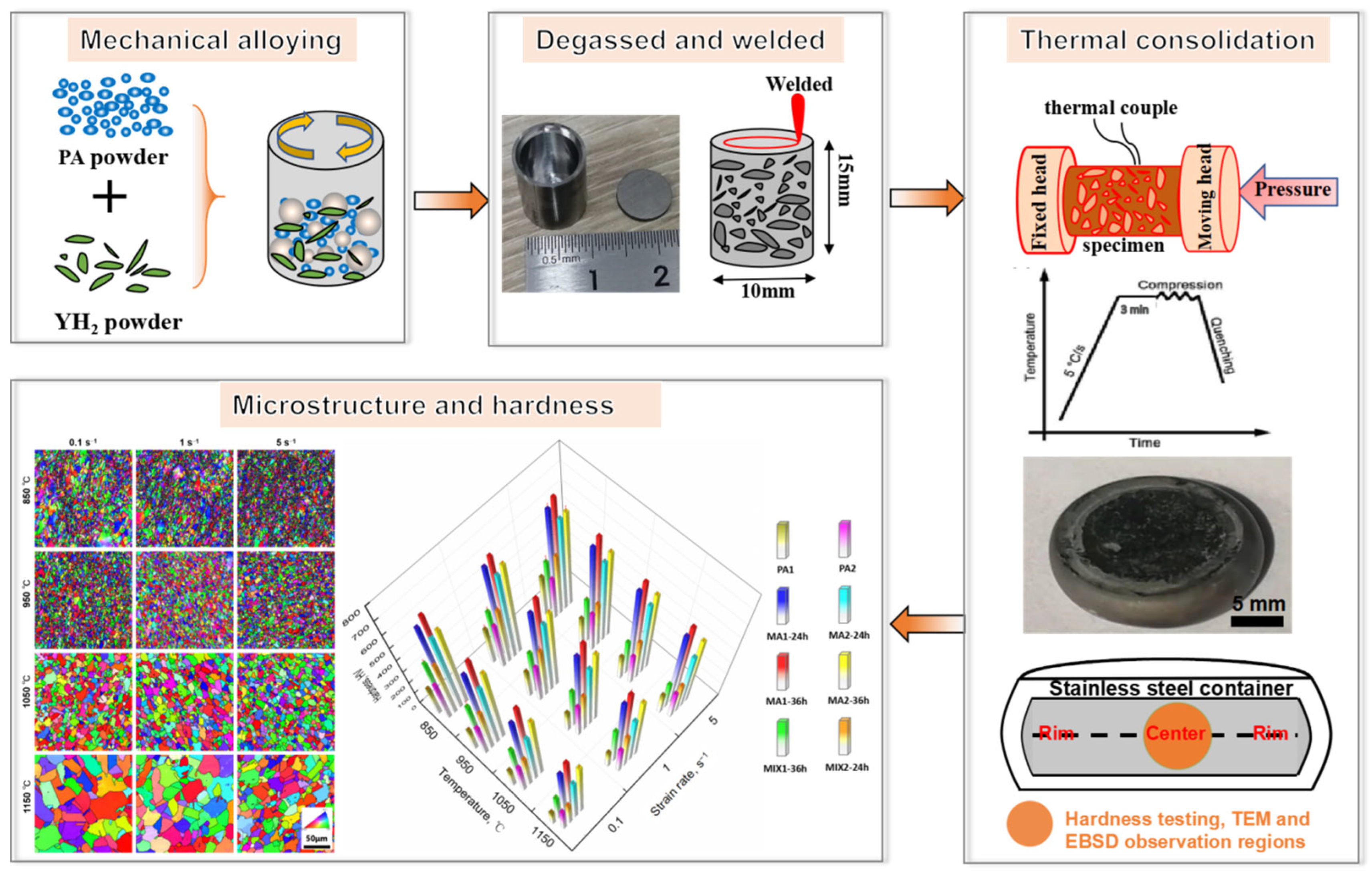
2. Materials and Experiment Methods
3. Results and Discussion
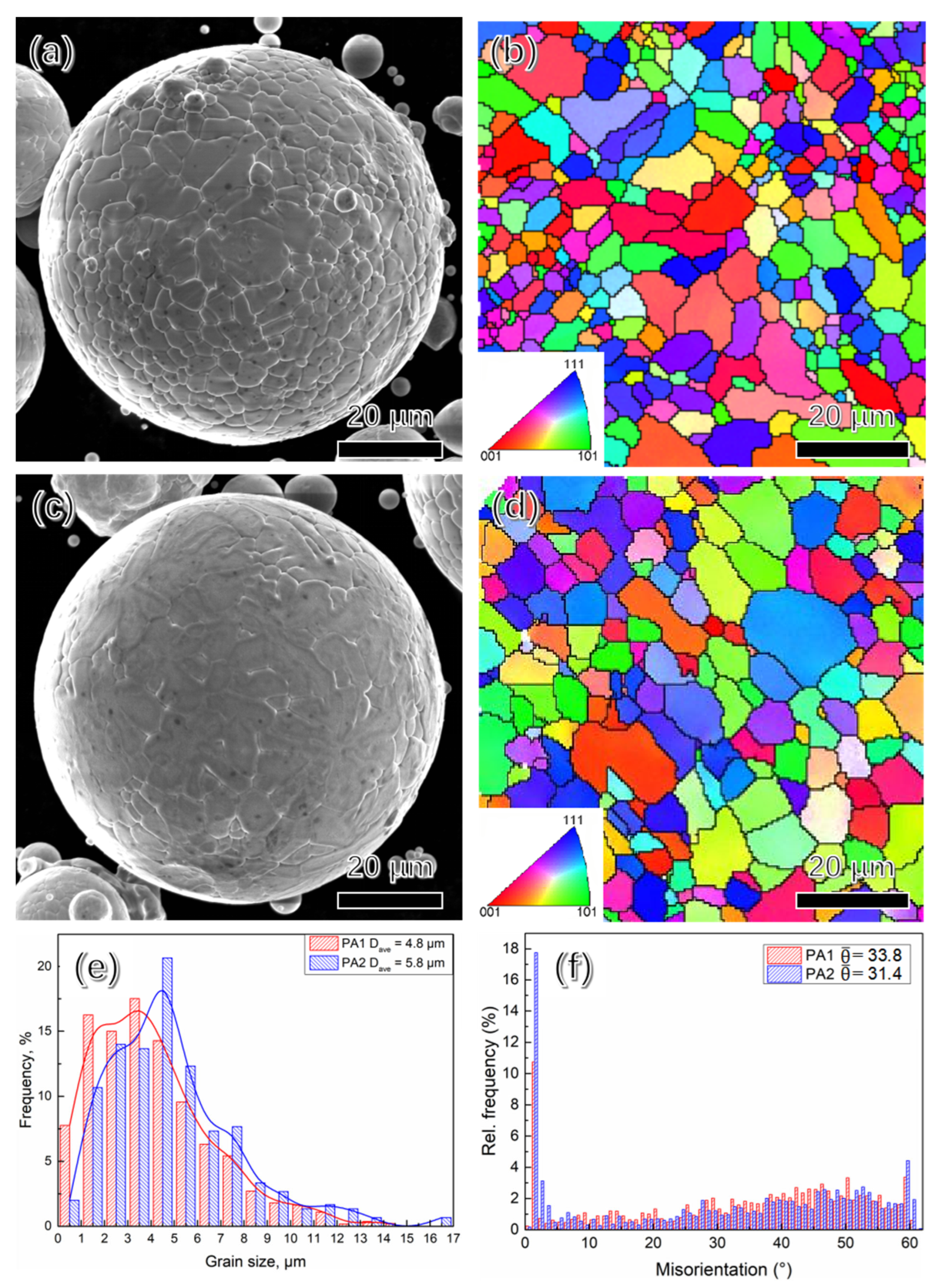
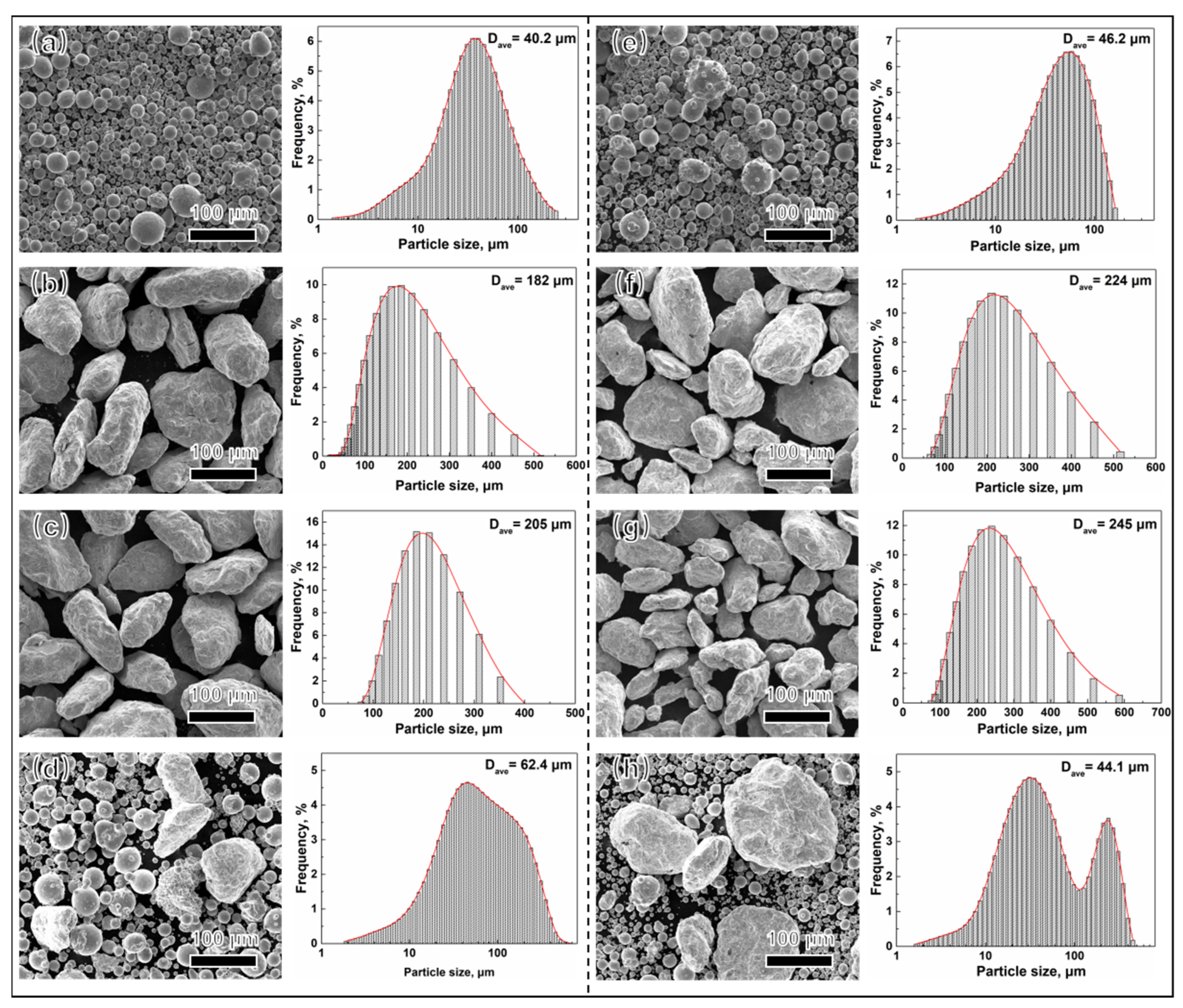
3.1. Morphologies of Powder Analysis
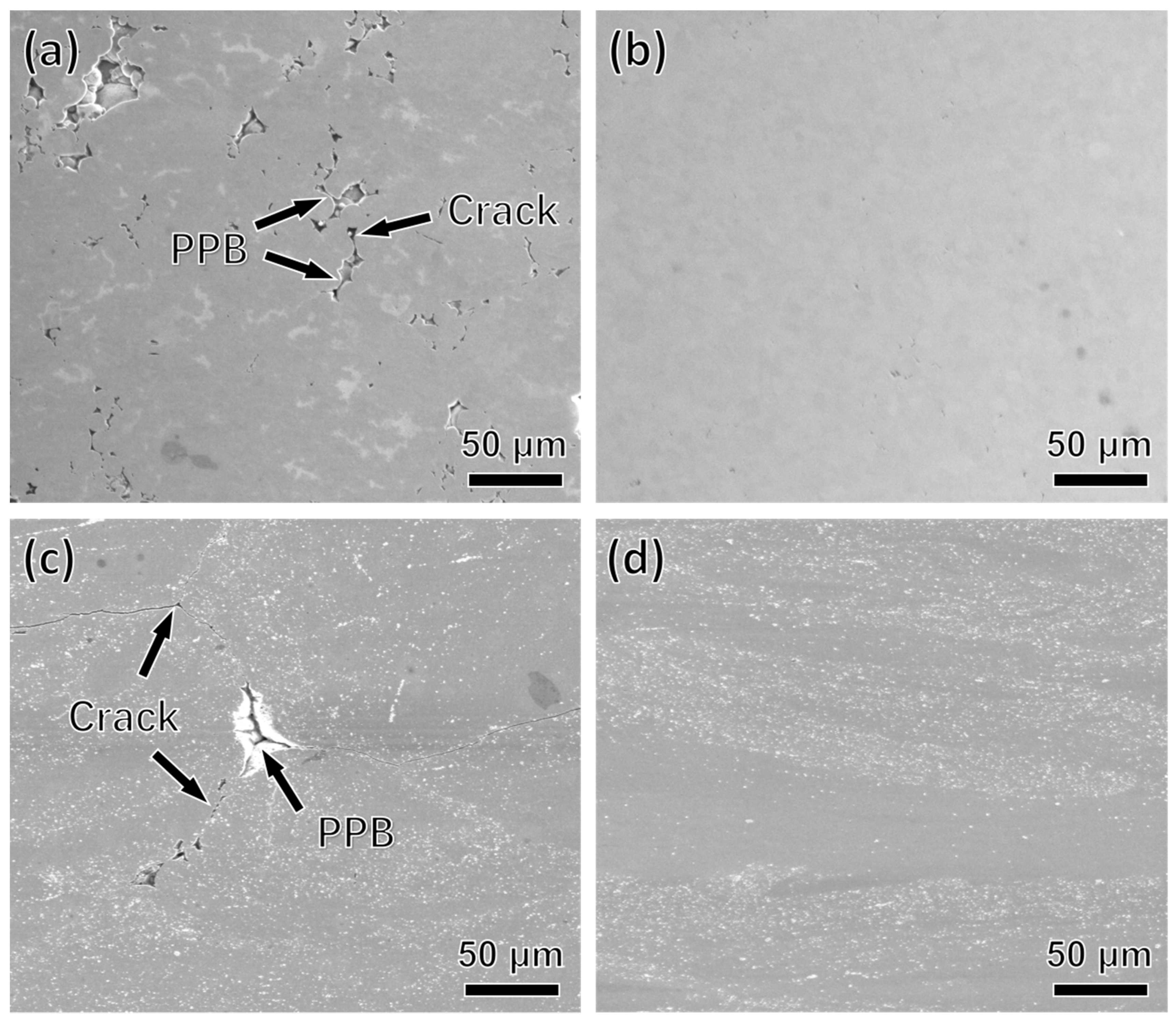
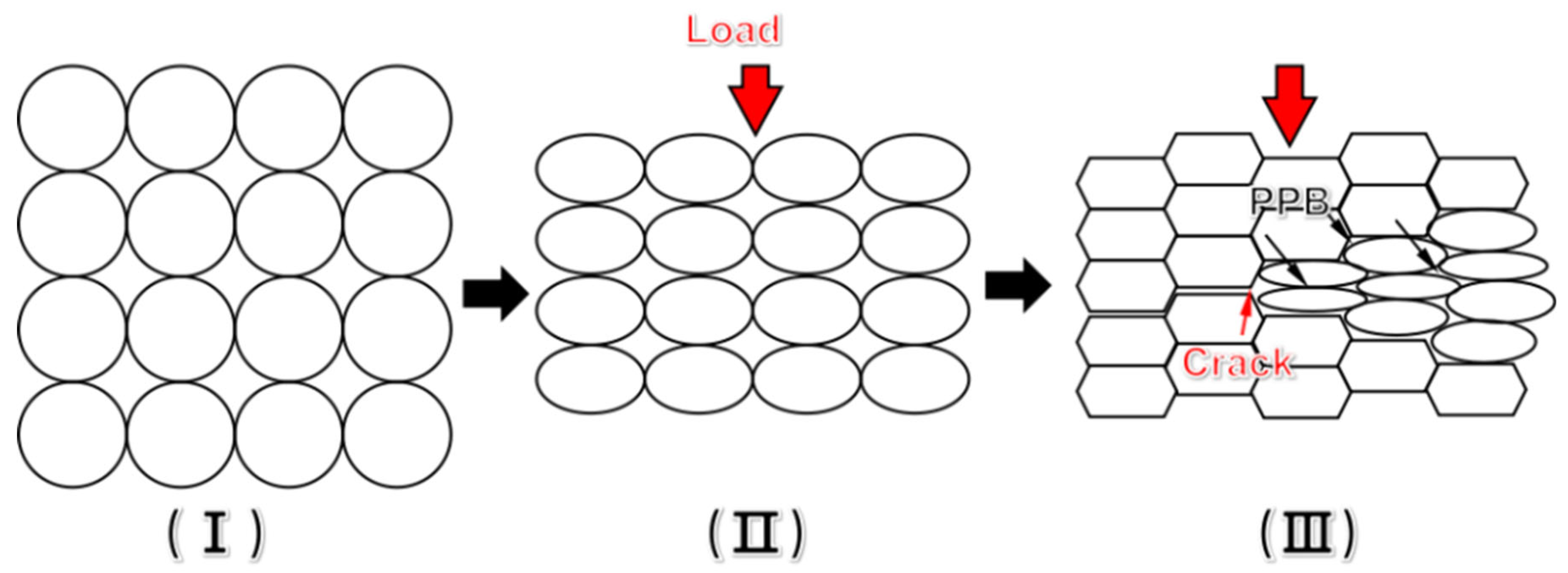
3.2. Macroscopic Cracking Analysis
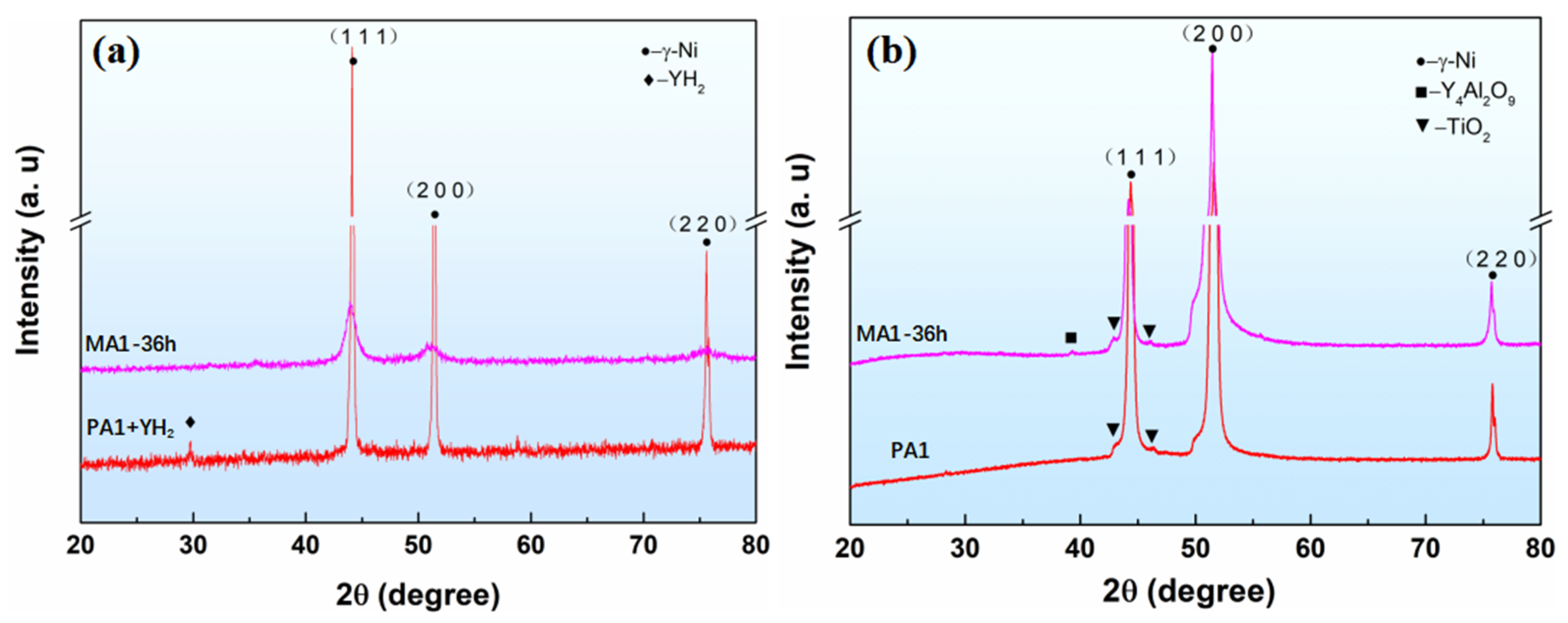
3.3. XRD of the Powder and Alloys Analysis
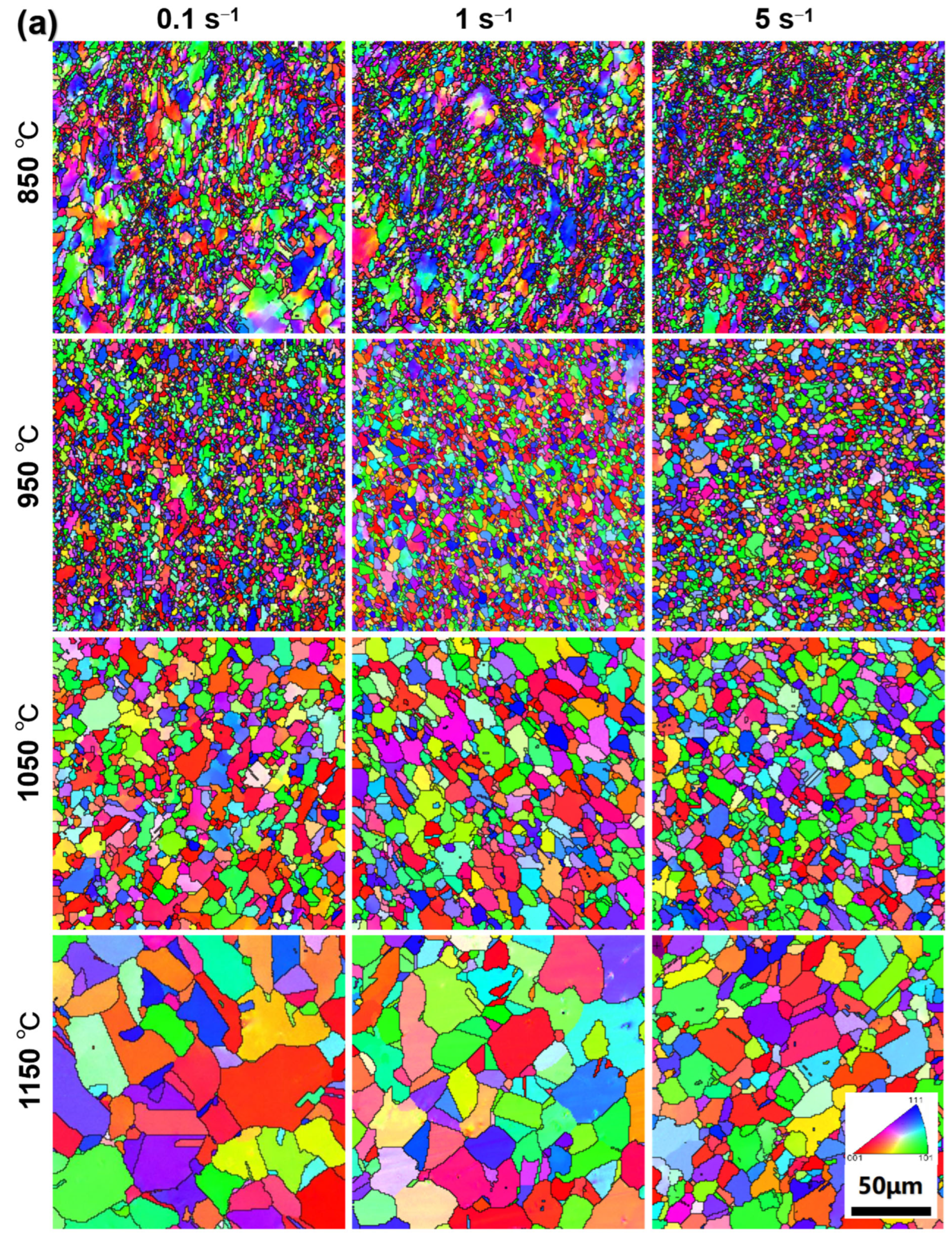
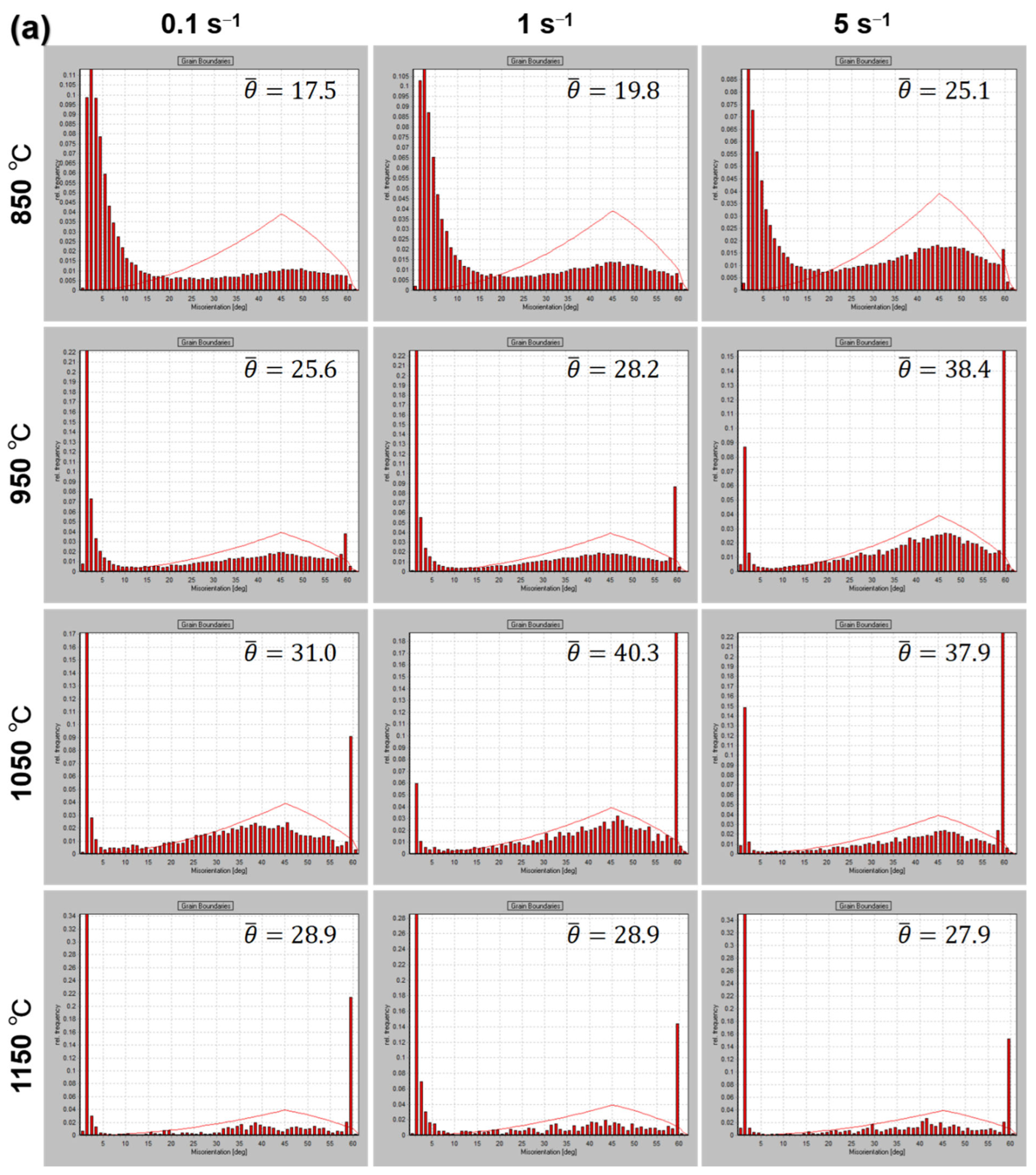
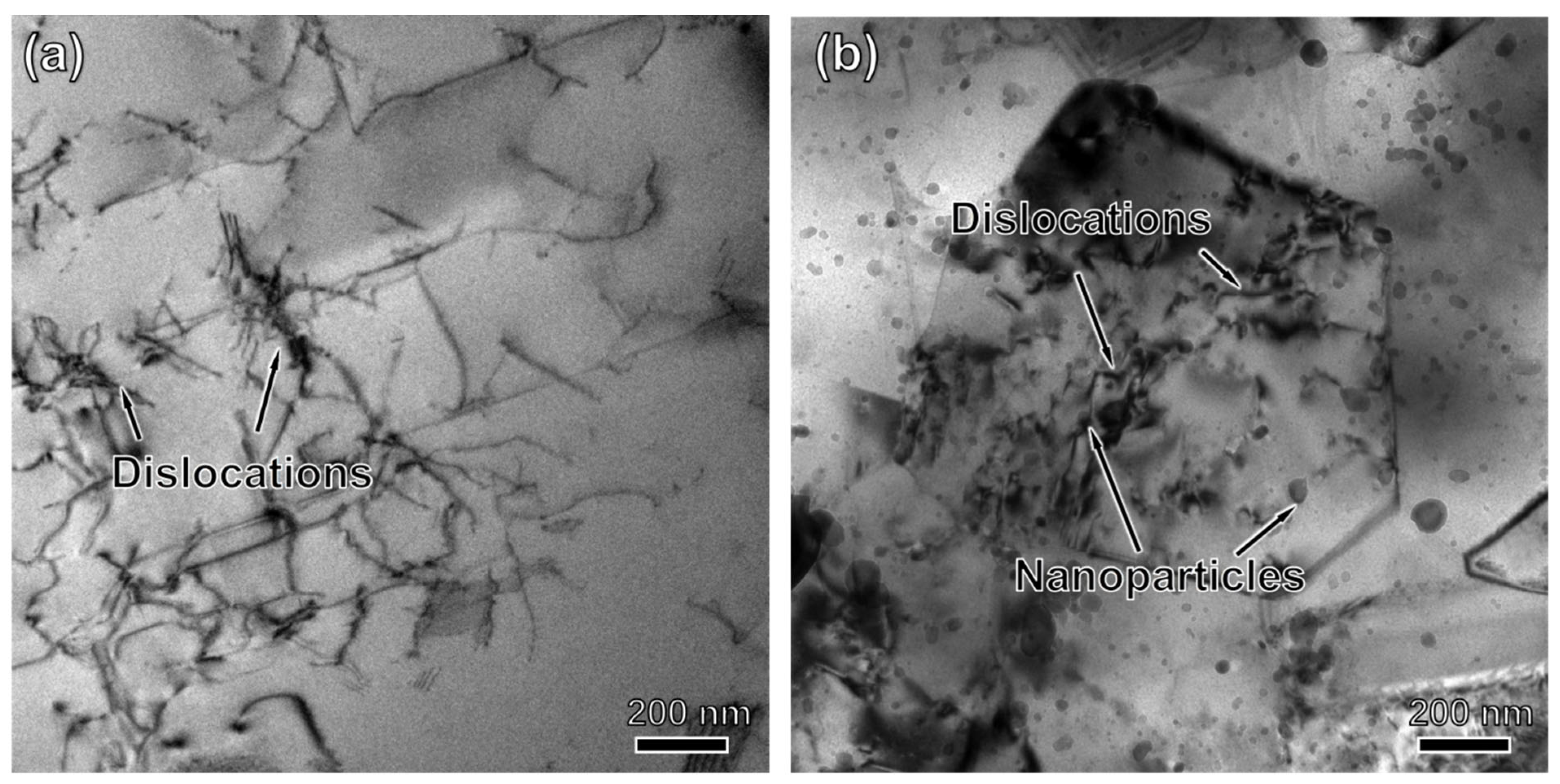
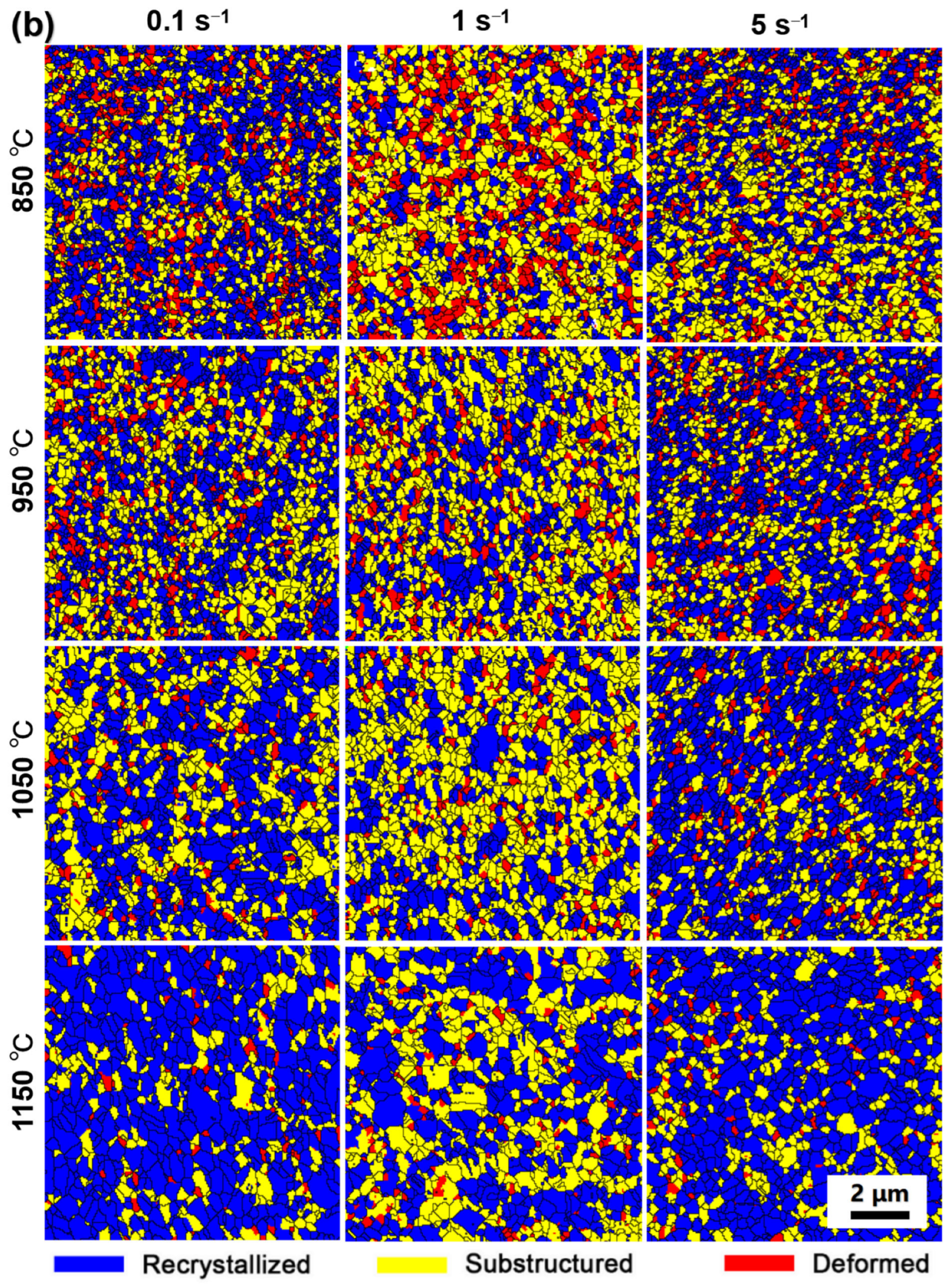
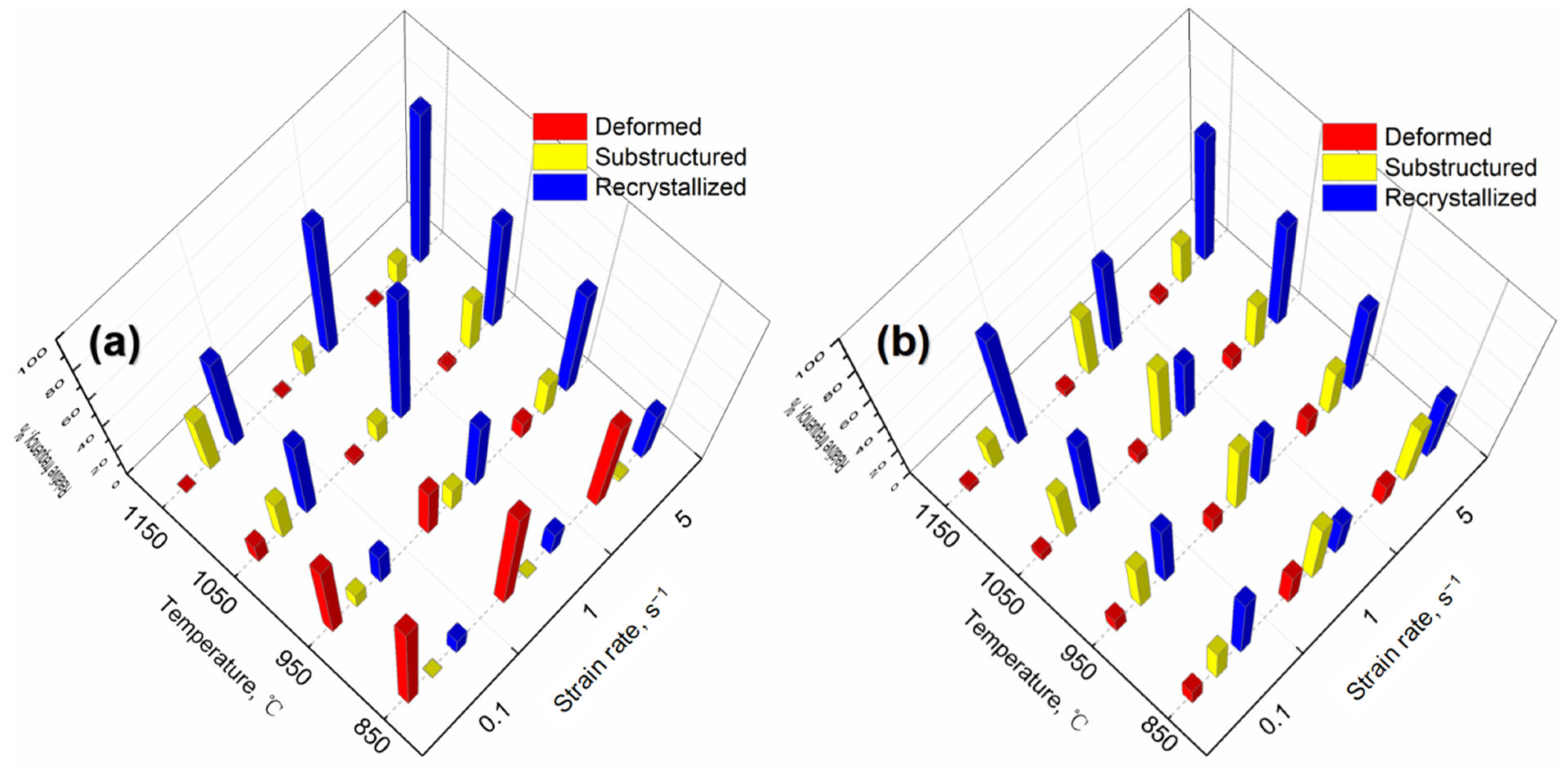
3.4. Microstructure Characterization
3.5. The Variation of Hardness
4. Discussion
4.1. Microstructure Evolution during Consolidation
4.2. Strengthening Mechanism of Nickel-Based ODS Superalloys
5. Conclusions
- (1)
- The PPB of nickel-based superalloys is difficult to be broken and eliminated at low temperature, which facilitates the crack nucleation and propagation during thermal consolidation. With increasing of temperature, the thermal activation of the material increases, the PPB is broken and eliminated quickly at elevated temperature and high stress.
- (2)
- The grain size is sensitive to the consolidation temperature, the average grain size increases with the increase of consolidation temperature. The average grain size of samples consolidation at strain rate 5 s−1 decreases and more uniform than low strain rate, since higher strain rate inhabits DRV and limits the time of boundary migration.
- (3)
- The hardness of nickel-based superalloys decreases with the increase of the consolidation temperature, strain rate, and the hardness increases after ball-milled for longer time. In addition, the hardness of nickel-based ODS superalloys is significantly higher than that of nickel-based superalloys without ODS due to the grain boundary strengthening and nano-oxides strengthening.
- (4)
- Basically, in order to obtain fine-grains, excellent properties and less cracking risk, consolidation temperature of near 1050 °C and strain rate of 5 s−1 are suggested.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verhiest, K.; Almazouzi, A.; Wispelaere, N.D.; Petrov, R.; Claessens, S. Development of oxides dispersion strengthened steels for high temperature nuclear reactor applications. J. Nucl. Mater. 2009, 385, 308–311. [Google Scholar] [CrossRef]
- De Carlan, Y.; Bechade, J.L.; Dubuisson, P.; Seran, J.L.; Billot, P.; Bougault, A.; Cozzikaa, T.; Doriota, S.; Hamona, D.; Henry, J.; et al. CEA developments of new ferritic ODS alloys for nuclear applications. J. Nucl. Mater. 2009, 386–388, 430–432. [Google Scholar] [CrossRef]
- Ukai, S.; Harada, M.; Okada, H.; Inoue, M.; Nomura, S.; Shikakura, S.; Nishidab, T.; Fujiwarab, M.; Asabe, K. Tube manufacturing and mechanical properties of oxide dispersion strengthened ferritic steel. J. Nucl. Mater. 1993, 204, 74–80. [Google Scholar] [CrossRef]
- Benjamin, J.S.; Volin, T.E. The mechanism of mechanical alloying. Metall. Trans. 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- Dong, H.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Effect of hafnium addition on the microstructure and tensile properties of aluminum added high-Cr ODS steels. J. Alloy. Compd. 2017, 702, 538–545. [Google Scholar] [CrossRef]
- Park, J.; Jang, J.; Kim, T.K.; Kim, S.-J.; Ahn, J.-H. Formation of Nanostructures in Ni-22Cr-11Fe-1X (X = Y2O3, TiO2) Alloys by High-Energy Ball-Milling. J. Nanosci. Nanotechnol. 2011, 11, 6213–6218. [Google Scholar] [CrossRef]
- Srolovitz, D.J.; Luton, M.J.; Petkovic-Luton, R.; Barnett, D.M.; Nix, W.D. Diffusionally modified dislocation-particle elastic interactions. Acta Metall. 1984, 32, 1079–1088. [Google Scholar] [CrossRef]
- Arzt, E.; Rösler, J. The kinetics of dislocation climb over hard particles—II. Effects of an attractive particle-dislocation interaction. Acta Metall. 1988, 36, 1053–1060. [Google Scholar] [CrossRef]
- Chinnappan, R. Thermodynamic Stability of Oxide Phases of Fe-Cr Based ODS Steels via Quantum Mechanical Calculations. Procedia Eng. 2014, 86, 788–798. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Byun, T.S.; Lee, J.H.; Min, J.Y.; Kim, S.W.; Park, C.H.; Lee, B.H. Effects of processing condition on the microstructural and tensile properties of 14Cr-based oxide dispersion strengthened alloys. J. Nucl. Mater. 2014, 449, 300–307. [Google Scholar] [CrossRef]
- Kishimoto, H.; Alinger, M.J.; Odette, G.R.; Yamamoto, T. TEM examination of microstructural evolution during processing of 14CrYWTi nanostructured ferritic alloys. J. Nucl. Mater. 2004, 329–333, 369–371. [Google Scholar] [CrossRef]
- Odette, G.R. Recent Progress in Developing and Qualifying Nanostructured Ferritic Alloys for Advanced Fission and Fusion Applications. Jom 2014, 66, 2427–2441. [Google Scholar] [CrossRef]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent developments in irradiation-resistant steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Tan, L.; He, G.; Liu, F.; Li, Y.; Jiang, L. Effects of Temperature and Pressure of Hot Isostatic Pressing on the Grain Structure of Powder Metallurgy Superalloy. Materials 2018, 11, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wang, Z.; Wang, Z.; Zhong, J.; Zhao, L.; Jiang, L.; Zhou, R.; Liu, Y.; Huang, L.; Tan, L.; et al. High-Throughput Method–Accelerated Design of Ni-Based Superalloys. Adv. Funct. Mater. 2022. [Google Scholar] [CrossRef]
- Tan, L.; Li, Y.; He, G.; Liu, F.; Nie, Y.; Jiang, L. Optimized hot workability of a powder metallurgy nickel-base superalloy. Mater. Charact. 2019, 147, 340–352. [Google Scholar] [CrossRef]
- Somani, M.C.; Muraleedharan, K.; Prasad, Y.V.R.K.; Singh, V. Mechanical processing and microstructural control in hot working of hot isostatically pressed P/M IN-100 superalloy. Mater. Sci. Eng. A 1998, 245, 88–99. [Google Scholar] [CrossRef]
- He, G.; Liu, F.; Huang, L.; Jiang, L. Hot deformation behaviors of a new hot isostatically pressed nickel based powder metallurgy superalloy. J. Mater. Res. 2016, 31, 3567–3579. [Google Scholar] [CrossRef]
- He, G.; Liu, F.; Huang, L.; Jiang, L. Analysis of forging cracks during hot compression of powder metallurgy nickel-based superalloy on simulation and experiment. Adv. Eng. Mater. 2016, 18, 1823–1832. [Google Scholar] [CrossRef]
- Tan, L.; Li, Y.; Liu, C.; Yang, C.; Ding, H.; Huang, L.; Liu, F.; Qin, Z.; Jiang, L. The evolution history of superalloy powders during hot consolidation and plastic deformation. Mater. Charact. 2018, 140, 30–38. [Google Scholar] [CrossRef]
- Tang, Q.X.; Ukai, S.; Oono, N.; Hayashi, S.; Leng, B.; Sugino, Y.; Han, W.; Okuda, T. Oxide Particle Refinement in 4.5 mass%Al Ni-Based ODS Superalloys. Mater. Trans. 2012, 53, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.; Park, J.J.; Rhee, C.K. Synthesis and structural properties of Ni-20Cr-Y2O3 nanocomposite alloy prepared by a very high energy mechanical milling. Mater. Chem. Phys. 2012, 137, 129–134. [Google Scholar] [CrossRef]
- McCarley, J.; Tin, S. Utilization of hot deformation to trigger strain induced boundary migration (SIBM) in Ni-base superalloys. Mater. Sci. Eng. A 2018, 720, 189–202. [Google Scholar] [CrossRef]
- Kumar, S.S.S.; Raghu, T.; Bhattacharjee, P.P.; Appa Rao, G.; Borah, U. Evolution of microstructure and microtexture during hot deformation in an advanced P/M nickel base superalloy. Mater. Charact. 2018, 146, 217–236. [Google Scholar] [CrossRef]
- Konkova, T.; Rahimi, S.; Mironov, S.; Baker, T.N. Effect of strain level on the evolution of microstructure in a recently developed AD730 nickel based superalloy during hot forging. Mater. Charact. 2018, 139, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Marthinsen, K.; Zhao, Q.; Logé, R.E. The double-edge effect of second-phase particles on the recrystallization behaviour and associated mechanical properties of metallic materials. Prog. Mater. Sci. 2018, 92, 284–359. [Google Scholar] [CrossRef]
- He, G.; Tan, L.; Liu, F.; Huang, L.; Huang, Z.; Jiang, L. Revealing the role of strain rate during multi-pass compression in an advanced polycrystalline nickel base superalloy. Mater. Charact. 2017, 128, 123–133. [Google Scholar] [CrossRef]
- Zener, C. Theory of growth of spherical precipitates from solid solution. J. Appl. Phys. 1949, 20, 950–953. [Google Scholar] [CrossRef]
- De Castro, V.; Marquis, E.A.; Lozano-Perez, S.; Pareja, R.; Jenkins, M.L. Stability of nanoscale secondary phases in an oxide dispersion strengthened Fe–12Cr alloy. Acta Mater. 2011, 59, 3927–3936. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Logé, R.E. A review of dynamic recrystallization phenomena in metallic materials. Mater. Des. 2016, 111, 548–574. [Google Scholar] [CrossRef]
- Sakasegawa, H.; Ohtsuka, S.; Ukai, S.; Tanigawa, H.; Fujiwara, M.; Ogiwara, H.; Kohyama, A. Particle size effects in mechanically alloyed 9Cr ODS steel powder. J. Nucl. Mater. 2007, 367–370, 185–190. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Ukai, S.; Sakasegawa, H.; Fujiwara, M.; Kaito, T.; Narita, T. Nano-mesoscopic structural characterization of 9Cr-ODS martensitic steel for improving creep strength. J. Nucl. Mater. 2007, 367–370, 160–165. [Google Scholar] [CrossRef]
- Read, H.G.; Murakami, H. Microstructural influences on the decomposition of an Al-containing ferritic stainless steel. Appl. Surf. Sci. 1996, 94–95, 334–342. [Google Scholar] [CrossRef]
- Ukai, S.; Ohtsuka, S. Low cycle fatigue properties of ODS ferritic–martensitic steels at high temperature. J. Nucl. Mater. 2007, 367–370, 234–238. [Google Scholar] [CrossRef]
- Lee, J.S.; Kimura, A.; Ukai, S.; Fujiwara, M. Effects of hydrogen on the mechanical properties of oxide dispersion strengthening steels. J. Nucl. Mater. 2004, 329–333, 1122–1126. [Google Scholar] [CrossRef]
- Yoshitake, T.; Ohmori, T.; Miyakawa, S. Burst properties of irradiated oxide dispersion strengthened ferritic steel claddings. J. Nucl. Mater. 2002, 307–311, 788–792. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Ukai, S.; Fujiwara, M.; Kaito, T.; Narita, T. Improvement of 9Cr-ODS martensitic steel properties by controlling excess oxygen and titanium contents. J. Nucl. Mater. 2004, 329–333, 372–376. [Google Scholar] [CrossRef]
- Miller, M.K.; Kenik, E.A.; Russell, K.F.; Heatherly, L.; Hoelzer, D.T.; Maziasz, P.J. Atom probe tomography of nanoscale particles in ODS ferritic alloys. Mater. Sci. Eng. A 2003, 353, 140–145. [Google Scholar] [CrossRef]
- Tan, L.; Huang, Z.; Liu, F.; He, G.; Wang, X.; Huang, L.; Zhang, Y.; Jiang, L. Effects of strain amount and strain rate on grain structure of a novel high Co nickel-based polycrystalline superalloy. Mater. Des. 2017, 131, 60–68. [Google Scholar] [CrossRef]
- Wusatowska-Sarnek, A.M.; Miura, H.; Sakai, T. Nucleation and microtexture development under dynamic recrystallization of copper. Mater. Sci. Eng. A 2002, 323, 177–186. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Ukai, S.; Oono, N.; Liu, C. Effects of annealing temperature on nanoscale particles in oxide dispersion strengthened Fe-15Cr alloy powders with Ti and Zr additions. J. Alloy. Compd. 2017, 693, 177–187. [Google Scholar] [CrossRef]
- Yan, P.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Effects of Hf addition on the thermal stability of 16Cr-ODS steels at elevated aging temperatures. J. Alloy. Compd. 2018, 739, 368–379. [Google Scholar] [CrossRef]
- Jorge-Badiola, D.; Iza-Mendia, A.; Gutiérrez, I. Study by EBSD of the development of the substructure in a hot deformed 304 stainless steel. Mater. Sci. Eng. A 2005, 394, 445–454. [Google Scholar] [CrossRef]
- Bui, Q.H.; Dirras, G.; Ramtani, S.; Gubicza, J. On the strengthening behavior of ultrafine-grained nickel processed from nanopowders. Mater. Sci. Eng. A 2010, 527, 3227–3235. [Google Scholar] [CrossRef]
- Rosenberg, J.; Piehler, H. Calculation of the Taylor Factor and Lattice Rotations for Bcc Metals Deforming by Pencil Glide. Metall. Trans. 1971, 2, 257–259. [Google Scholar] [CrossRef]
- Wasilkowska, A.; Bartsch, M.; Messerschmidt, U.; Herzog, R.; Czyrska-Filemonowicz, A. Creep mechanisms of ferritic oxide dispersion strengthened alloys. J. Mater. Process. Technol. 2003, 133, 218–224. [Google Scholar] [CrossRef]
- Xu, H.; Li, W.; Sha, X.; Meng, J.; Kang, C.; Wang, W.; Zang, X.; Wang, Z. Effects of Zr addition on the microstructural stability of 15Cr-ODS steels under elevated-temperature annealing. Fusion Eng. Des. 2019, 138, 231–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, T.; Liu, D.; Du, J.; Wang, Y.; Zhang, C.; Suo, J. 9Cr-ODS steel composite material reinforced by Ta layers. J. Alloy. Compd. 2016, 682, 294–301. [Google Scholar] [CrossRef]








| Al | Cr | Fe | Ti | Y | C | Ni | |
|---|---|---|---|---|---|---|---|
| PA1 | 0.25 | 20.5 | 0.67 | 0.56 | 0 | 0.054 | Bal. |
| PA2 | 0.25 | 21 | 0.85 | 0.57 | 0.68 | 0.059 | Bal. |
| Specimen | Powder | Mill Time/h | Temperature/°C | Strain Rate/s−1 |
|---|---|---|---|---|
| PA1 | PA1 powder | 0 | 850/950/ 1050/1150 | 0.1/1/5 |
| MA1-24h | PA1 powder and 0.6%YH2 powder | 24 | ||
| MA1-36h | PA1 powder and 0.6%YH2 powder | 36 | ||
| MIX1-36h | MA1-36h powder and PA2 powder (ratio 1:2) | 12 (mixed) | ||
| PA2 | PA2 powder | 0 | ||
| MA2-24h | PA2 powder | 24 | ||
| MA2-36h | PA2 powder | 36 | ||
| MIX2-24h | MA2-24h powder and PA2 (ratio 1:2) | 12 (mixed) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Liu, F.; Tan, L.; Tian, Z.; Qin, Z.; Huang, L.; Xiao, X.; Wang, G.; Chen, P.; Liu, B. Optimizing the Thermomechanical Process of Nickel-Based ODS Superalloys by an Efficient Method. Materials 2022, 15, 4087. https://doi.org/10.3390/ma15124087
He W, Liu F, Tan L, Tian Z, Qin Z, Huang L, Xiao X, Wang G, Chen P, Liu B. Optimizing the Thermomechanical Process of Nickel-Based ODS Superalloys by an Efficient Method. Materials. 2022; 15(12):4087. https://doi.org/10.3390/ma15124087
Chicago/Turabian StyleHe, Wuqiang, Feng Liu, Liming Tan, Zhihui Tian, Zijun Qin, Lan Huang, Xiangyou Xiao, Guowei Wang, Pan Chen, and Baogang Liu. 2022. "Optimizing the Thermomechanical Process of Nickel-Based ODS Superalloys by an Efficient Method" Materials 15, no. 12: 4087. https://doi.org/10.3390/ma15124087






