Histological Evaluation of Restylane Lyft Used as a Scaffold for Dental Pulp Regeneration in Non-Infected Immature Teeth in Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation and Allocation into Study Groups
2.2. Surgical Procedure
2.2.1. Animals’ Anesthesia
Blood Clot Group (BC) (n = 24 Roots)
Restylane Lyft Group (BC + HA) (n = 24 Roots)
Negative Control Group (NC) (n = 10 Roots)
Positive Control Group (PC) (n = 11 Roots)
Animal Postoperative Care
Animal Euthanization and Jaw Resection
2.3. Micro-Computed Tomographic Scanning (µ-CT)
2.4. Histological Preparation
2.5. Histological Evaluation
2.6. Statistical Analysis
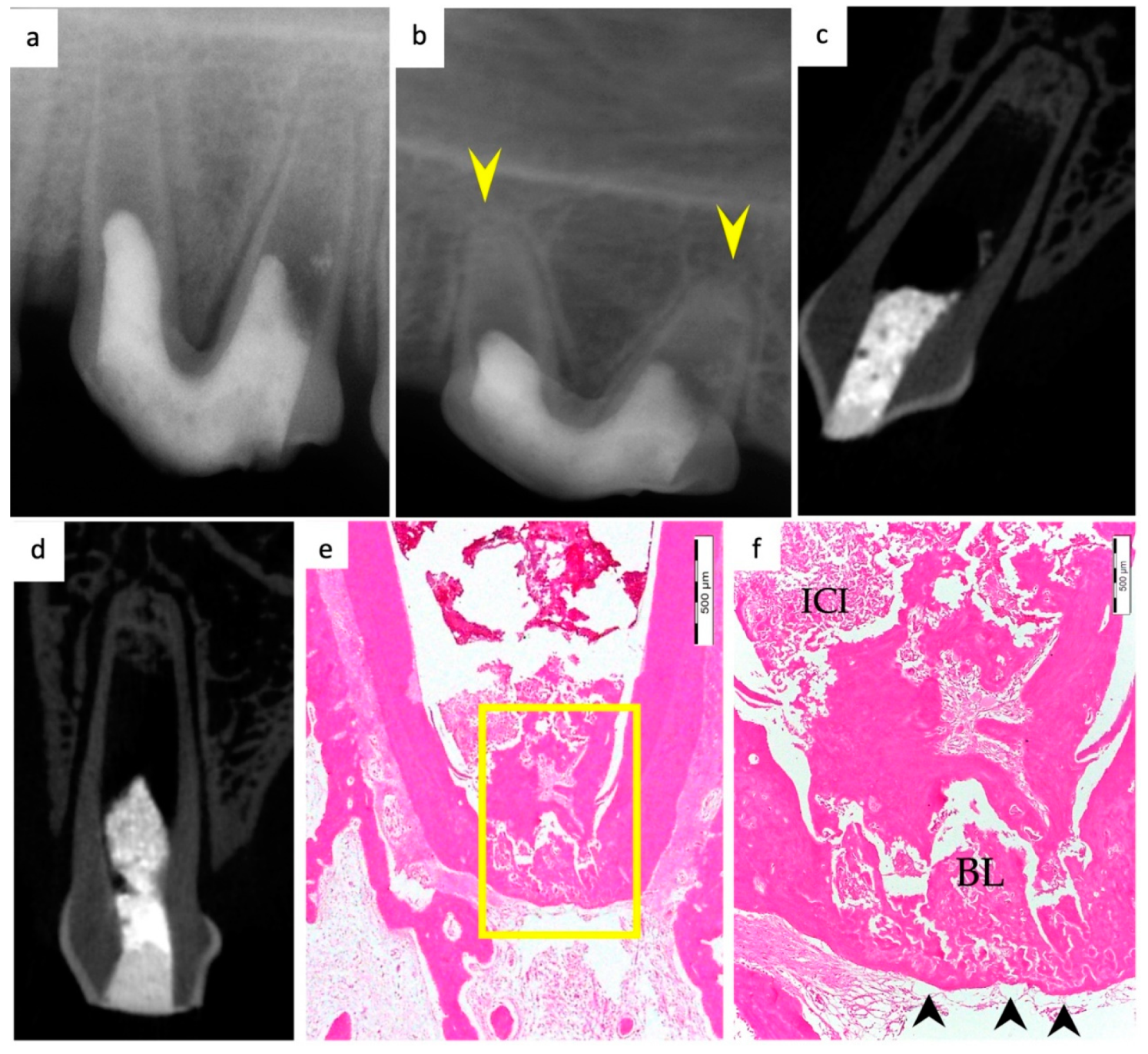
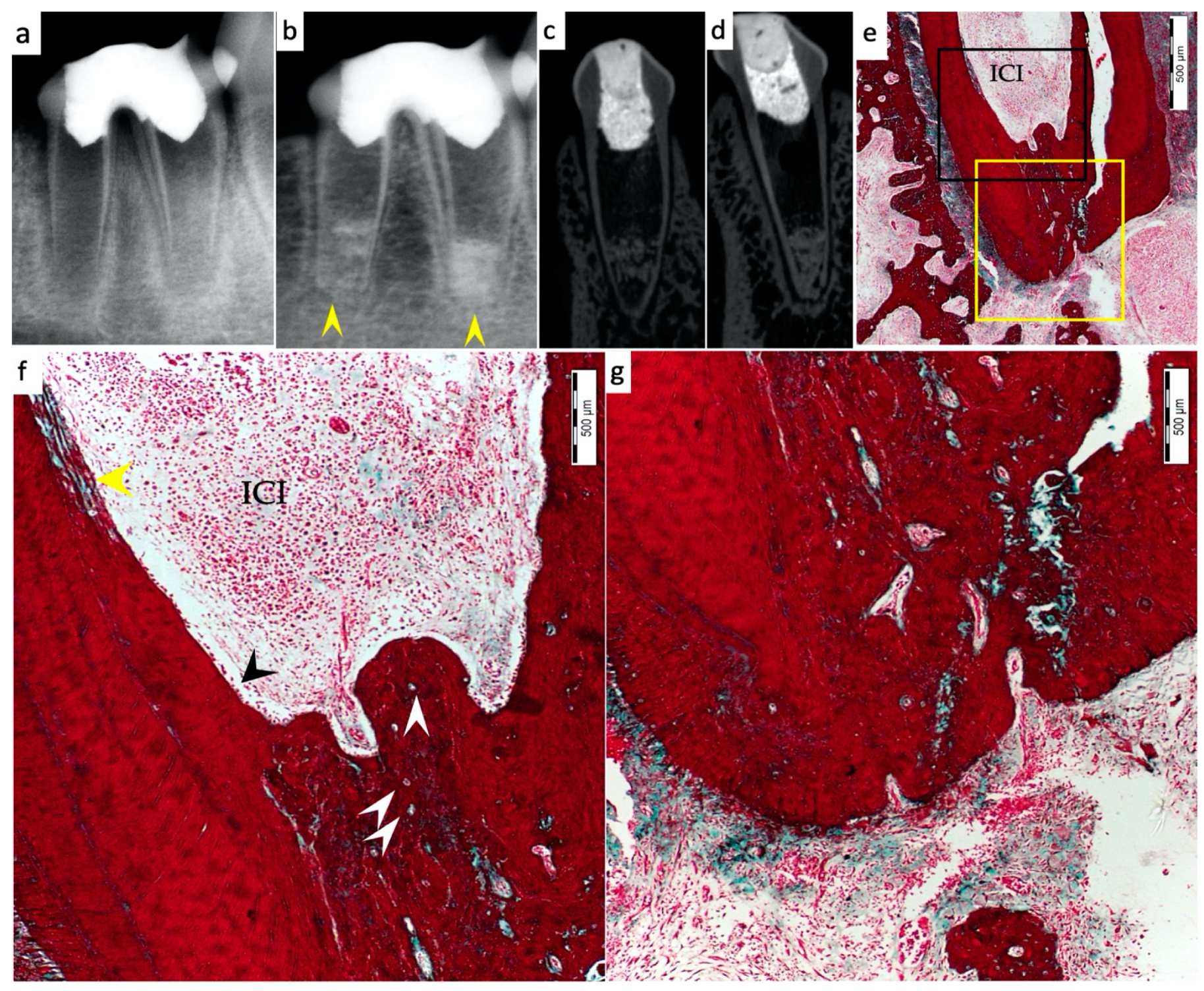
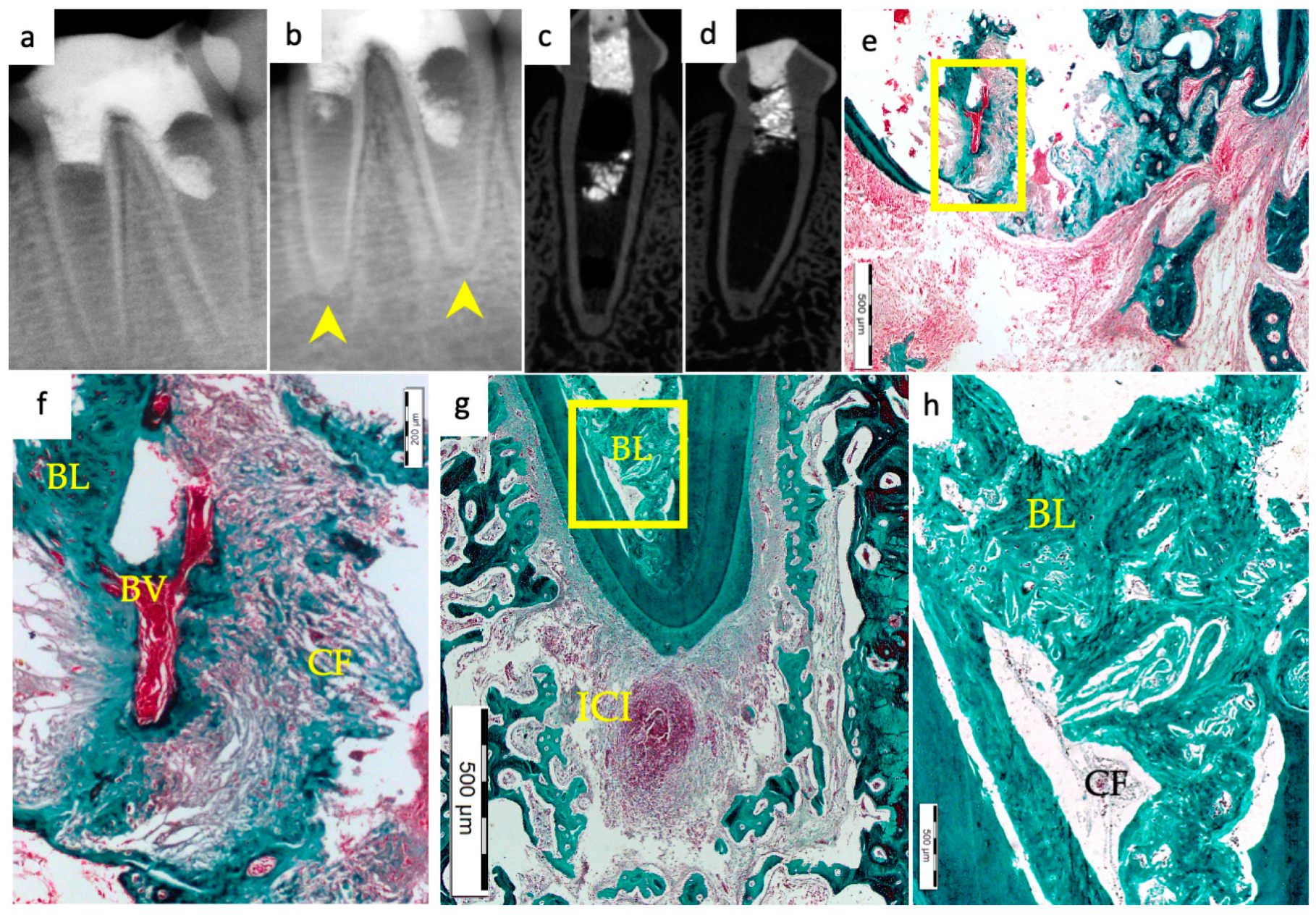
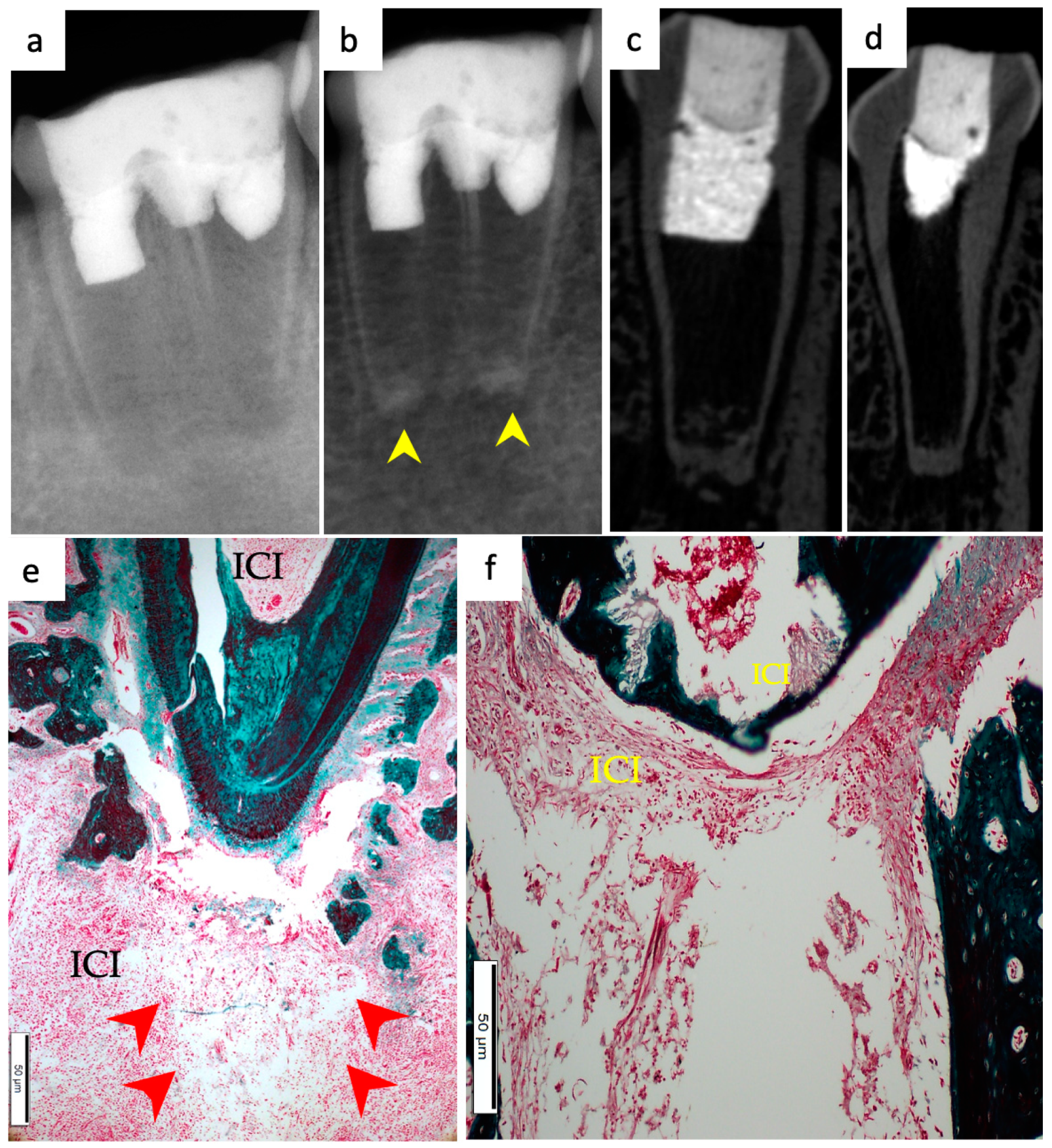
3. Results
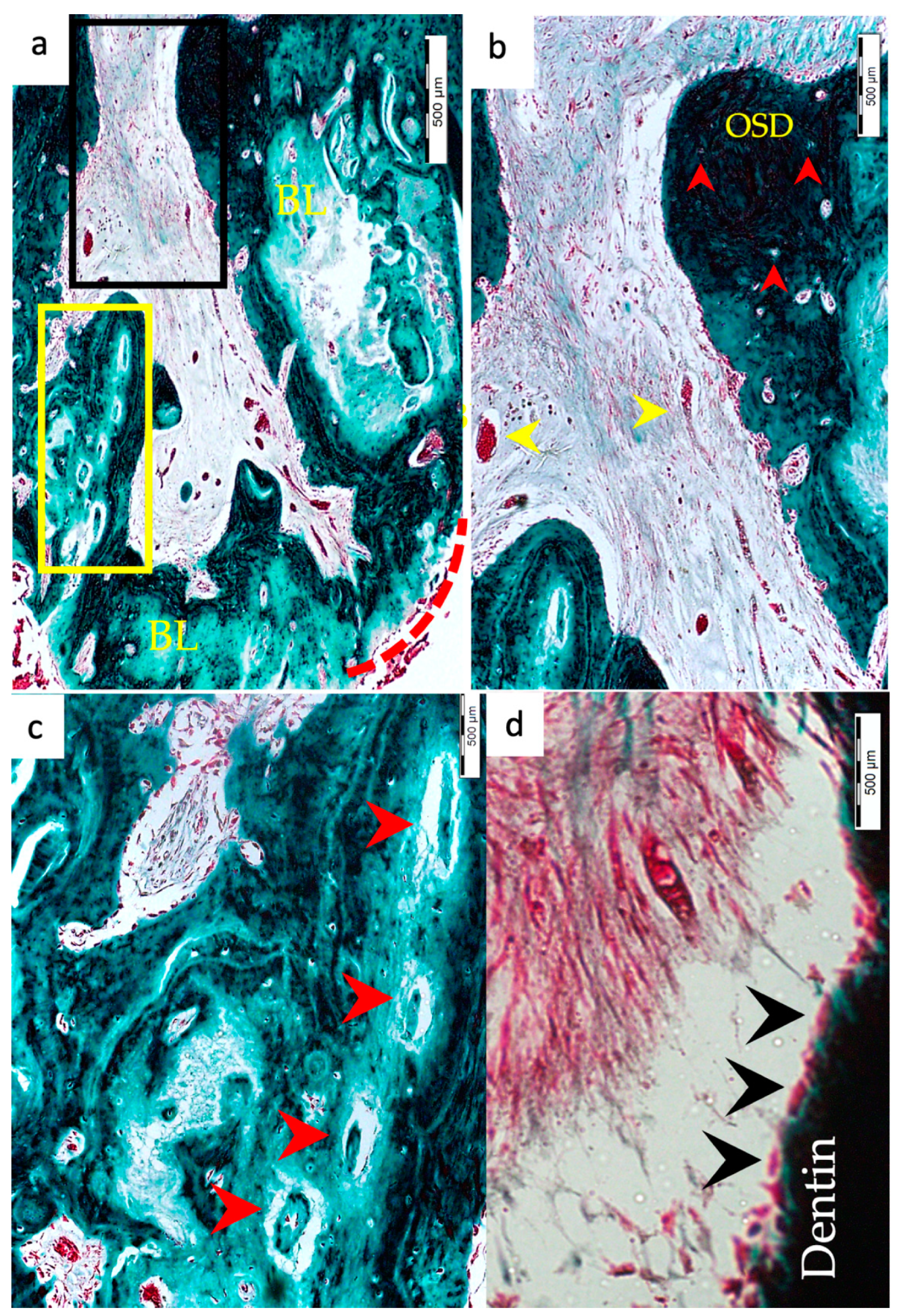
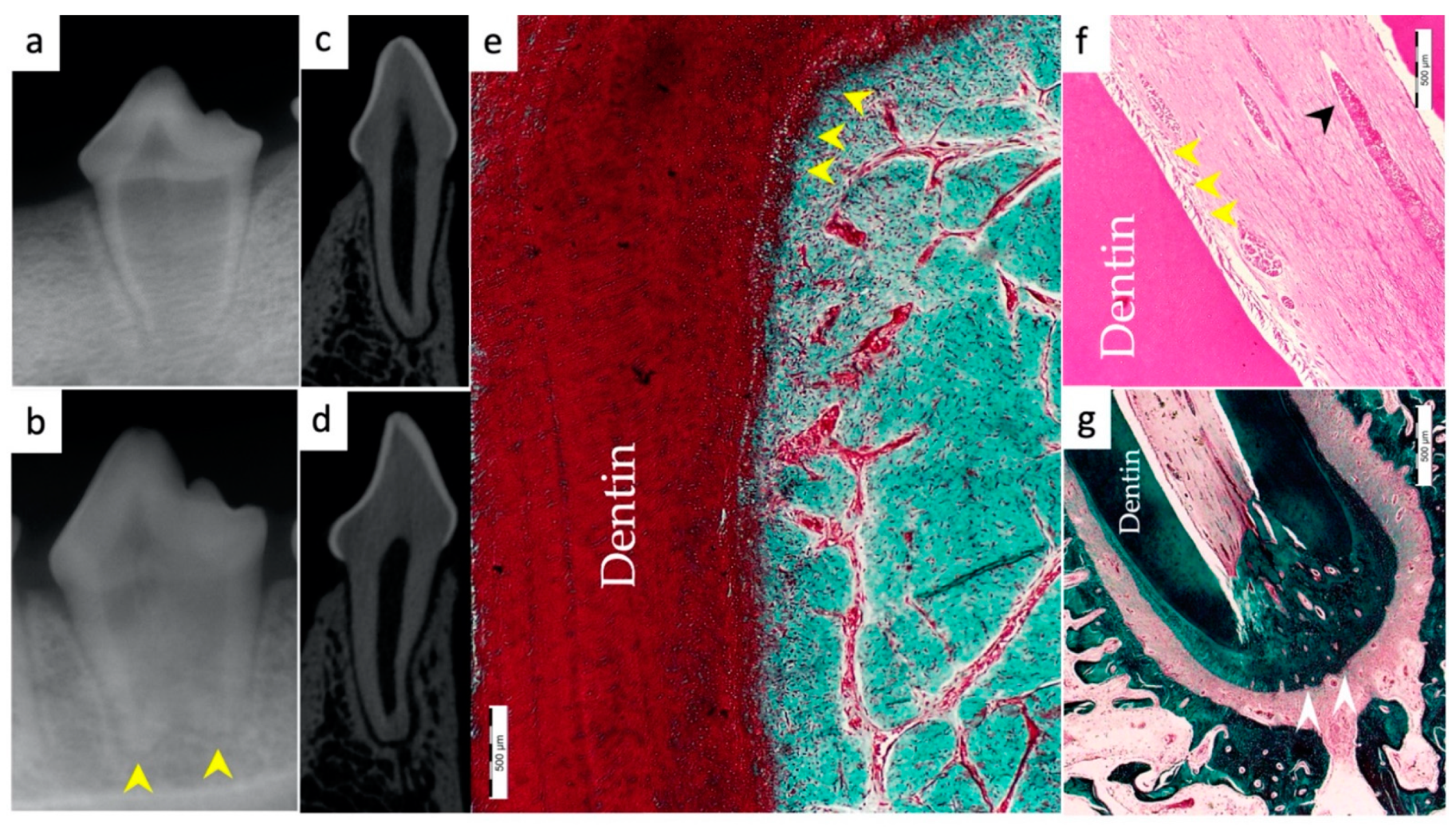
3.1. Hard Tissue Deposition
3.2. Type of Hard Tissue Formed
3.3. Vascularization and Formation of Vascularized Soft Connective Tissue
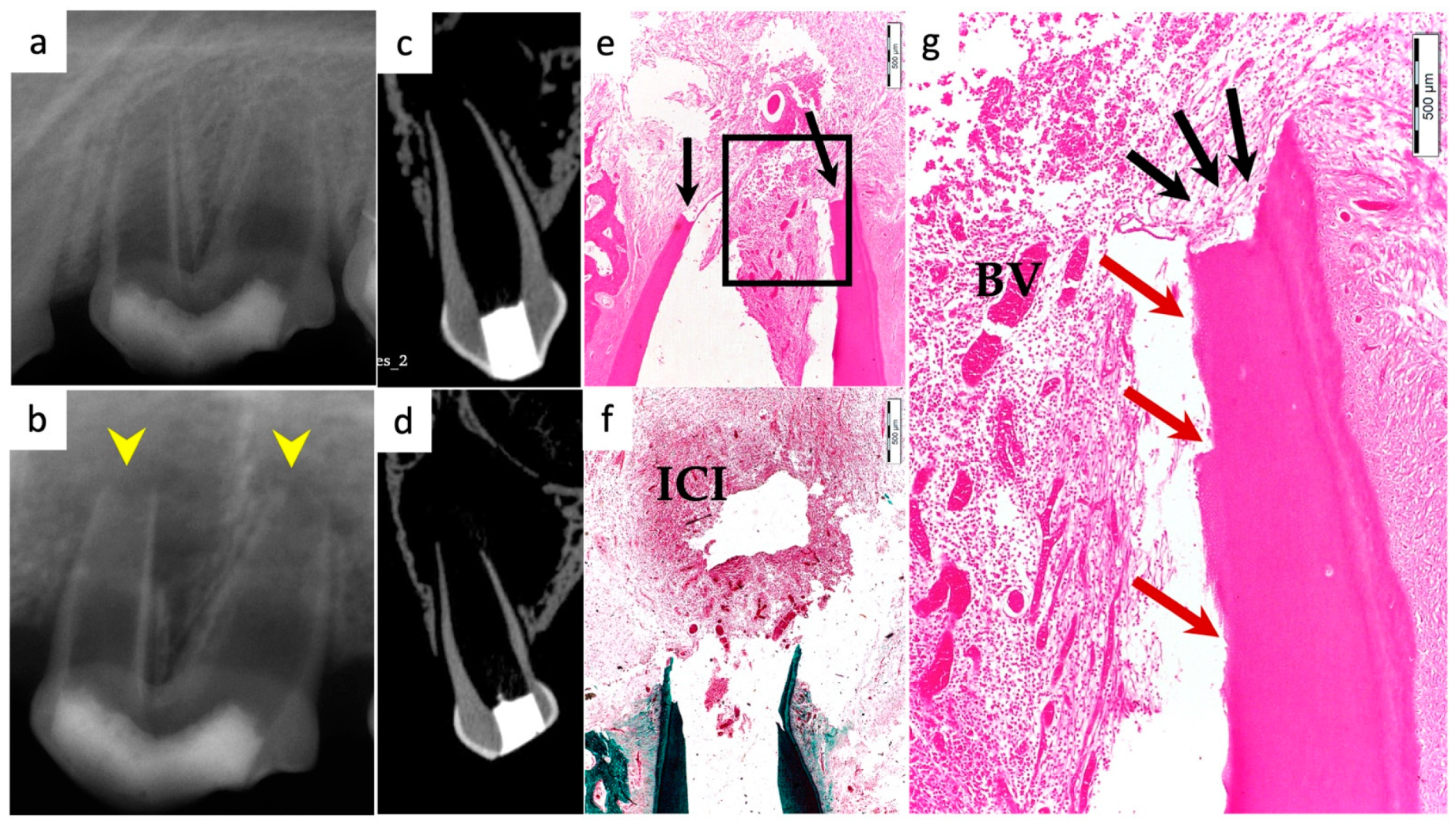
3.4. Degree of Inflammation
3.5. Apical Closure
3.6. Positive and Negative Control Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nygaard-Ostby, B.; Hjortdal, O. Tissue formation in the root canal following pulp removal. Scand. J. Dent. Res. 1971, 79, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, S.I.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.K.; Lim, G.S.; Singh, M.; Fial, A.V. Quantitative Assessment of Root Development after Regenerative Endodontic Therapy: A Systematic Review and Meta-Analysis. J. Endod. 2020, 46, 1856–1866.e1852. [Google Scholar] [CrossRef]
- Hashemi-Beni, B.; Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Khademi, A.A. Tissue engineering: Dentin—pulp complex regeneration approaches (A review). Tissue Cell 2017, 49, 552–564. [Google Scholar] [CrossRef]
- Raghavendra, S.; Gathani, K. Scaffolds in regenerative endodontics: A review. Dent. Res. J. 2016, 13, 379–386. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. J. Endod. 2013, 39, S30–S43. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Murray, P.E. Platelet-Rich Plasma and Platelet-Rich Fibrin Can Induce Apical Closure More Frequently Than Blood-Clot Revascularization for the Regeneration of Immature Permanent Teeth: A Meta-Analysis of Clinical Efficacy. Front. Bioeng. Biotechnol. 2018, 6, 139. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, R.; Trope, M. Revitalization procedures in two traumatized incisors with different biological outcomes. J. Endod. 2012, 38, 411–414. [Google Scholar] [CrossRef]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Bohl, K.S.; Shon, J.; Rutherford, B.; Mooney, D.J. Role of synthetic extracellular matrix in development of engineered dental pulp. J. Biomater. Sci. Polym. Ed. 1998, 9, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.T.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Prescott, R.S.; Alsanea, R.; Fayad, M.I.; Johnson, B.R.; Wenckus, C.S.; Hao, J.; John, A.S.; George, A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J. Endod. 2008, 34, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.R.; Lee, D.H.; Chung, P.H.; Yang, H.C. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e94–e100. [Google Scholar] [CrossRef]
- Cavalcanti, B.N.; Zeitlin, B.D.; Nor, J.E. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent. Mater. 2013, 29, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for Tissue Engineering In Dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Inuyama, Y.; Kitamura, C.; Nishihara, T.; Morotomi, T.; Nagayoshi, M.; Tabata, Y.; Matsuo, K.; Chen, K.K.; Terashita, M. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 120–128. [Google Scholar] [CrossRef]
- Coimbra, P.; Alves, P.; Valente, T.A.M.; Santos, R.; Correia, I.J.; Ferreira, P. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: Synthesis and characterization. Int. J. Biol. Macromol. 2011, 49, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Yeh, Y.Y.; Lung, J.; Yang, Y.C.; Yuan, K. Mineralization Effect of Hyaluronan on Dental Pulp Cells via CD44. J. Endod. 2016, 42, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration (FDA). Medical Device Definition. 2015. Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/ucm051512.htm (accessed on 17 February 2019).
- AlHowaish, N.A.; AlSudani, D.I.; AlMuraikhi, N.A. Evaluation of a hyaluronic acid hydrogel (Restylane Lyft) as a scaffold for dental pulp regeneration in a regenerative endodontic organotype model. Odontology 2022. [Google Scholar] [CrossRef] [PubMed]
- Chrepa, V.; Austah, O.; Diogenes, A. Evaluation of a Commercially Available Hyaluronic Acid Hydrogel (Restylane) as Injectable Scaffold for Dental Pulp Regeneration: An In Vitro Evaluation. J. Endod. 2017, 43, 257–262. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. The National Academies Collection: Reports funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US) National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Scouten, C.W.; O’Connor, R.; Cunningham, M. Perfusion Fixation of Research Animals. Microsc. Today 2006, 14, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.D.; Chen, X.; Bao, Z.F.; Chen, M.; Ding, Z.J.; Zhong, M. Histologic comparison between platelet-rich plasma and blood clot in regenerative endodontic treatment: An animal study. J. Endod. 2014, 40, 1388–1393. [Google Scholar] [CrossRef]
- Londero, C.d.L.D.; Pagliarin, C.M.L.; Felippe, M.C.S.; Felippe, W.T.; Danesi, C.C.; Barletta, F.B. Histologic Analysis of the Influence of a Gelatin-based Scaffold in the Repair of Immature Dog Teeth Subjected to Regenerative Endodontic Treatment. J. Endod. 2015, 41, 1619–1625. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D.; Schmalz, G. Scaffolds for dental pulp tissue engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Rathee, K.; Kaur, A.; Miglani, N. Pulp Regeneration in an Immature Maxillary Central Incisor Using Hyaluronic Acid Hydrogel. Contemp. Clin. Dent. 2021, 12, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.R.; Diogenes, A. Direct Effect of Intracanal Medicaments on Survival of Stem Cells of the Apical Papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef]
- Verma, P.; Nosrat, A.; Kim, J.R.; Price, J.B.; Wang, P.; Bair, E.; Xu, H.H.; Fouad, A.F. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J. Dent. Res. 2017, 96, 100–106. [Google Scholar] [CrossRef]
- Fouad, A.F.; Nosrat, A. Pulp regeneration in previously infected root canal space. Endod. Top. 2013, 28, 24–37. [Google Scholar] [CrossRef]
- Althumairy, R.I.; Teixeira, F.B.; Diogenes, A. Effect of Dentin Conditioning with Intracanal Medicaments on Survival of Stem Cells of Apical Papilla. J. Endod. 2014, 40, 521–525. [Google Scholar] [CrossRef]
- AlSaeed, T.; Nosrat, A.; Melo, M.A.; Wang, P.; Romberg, E.; Xu, H.; Fouad, A.F. Antibacterial Efficacy and Discoloration Potential of Endodontic Topical Antibiotics. J. Endod. 2018, 44, 1110–1114. [Google Scholar] [CrossRef]
- Alenazy, M.S.; Al-Nazhan, S.; Mosadomi, H.A. Histologic, Radiographic, and Micro-Computed Tomography Evaluation of Experimentally Enlarged Root Apices in Dog Teeth with Apical Periodontitis after Regenerative Treatment. Curr. Ther. Res. Clin. Exp. 2021, 94, 100620. [Google Scholar] [CrossRef]
- Thibodeau, B.; Teixeira, F.; Yamauchi, M.; Caplan, D.J.; Trope, M. Pulp revascularization of immature dog teeth with apical periodontitis. J. Endod. 2007, 33, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J. Endod. 2010, 36, 56–63. [Google Scholar] [CrossRef] [PubMed]
- West, D.C.; Kumar, S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp. Cell Res. 1989, 183, 179–196. [Google Scholar] [CrossRef]
- Sattar, A.; Kumar, S.; West, D.C. Does hyaluronan have a role in endothelial cell proliferation of the synovium? Semin. Arthritis Rheum. 1992, 22, 37–43. [Google Scholar] [CrossRef]
- Sattar, A.; Rooney, P.; Kumar, S.; Pye, D.; West, D.C.; Scott, I.; Ledger, P. Application of angiogenic oligosaccharides of hyaluronan increases blood vessel numbers in rat skin. J. Investig. Dermatol. 1994, 103, 576–579. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.; Tobias, R.S.; Cassidy, N.; Bégue-Kirn, C.; Ruch, J.V.; Lesot, H. Influence of substrate nature and immobilization of implanted dentin matrix components during induction of reparative dentinogenesis. Connect. Tissue Res. 1995, 32, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.A.; Ulset, A.S.; Bender, J.; Jansen, J.A.; Christensen, B.E.; Leeuwenburgh, S.C. Effects of physical and chemical treatments on the molecular weight and degradation of alginate-hydroxyapatite composites. Macromol. Biosci. 2014, 14, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Erisken, C.; Kalyon, D.M.; Zhou, J.; Kim, S.G.; Mao, J.J. Viscoelastic Properties of Dental Pulp Tissue and Ramifications on Biomaterial Development for Pulp Regeneration. J. Endod. 2015, 41, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.D.; Abueva, C.; Kim, B.; Lee, B.T. Chitosan-hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr. Polym. 2015, 115, 160–169. [Google Scholar] [CrossRef]
- Evanko, S.P.; Johnson, P.Y.; Braun, K.R.; Underhill, C.B.; Dudhia, J.; Wight, T.N. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch. Biochem. Biophys. 2001, 394, 29–38. [Google Scholar] [CrossRef]
- Noble, P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002, 21, 25–29. [Google Scholar] [CrossRef]
- Stocks, D.; Sundaram, H.; Michaels, J.; Durrani, M.J.; Wortzman, M.S.; Nelson, D.B. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J. Drugs Dermatol. JDD 2011, 10, 974–980. [Google Scholar] [PubMed]
- Yamauchi, N.; Yamauchi, S.; Nagaoka, H.; Duggan, D.; Zhong, S.; Lee, S.M.; Teixeira, F.B.; Yamauchi, M. Tissue engineering strategies for immature teeth with apical periodontitis. J. Endod. 2011, 37, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.L.; Toole, B.P. Hyaluronate inhibition of cell proliferation. Arthritis Care Res. 1987, 30, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Kutty, J.K.; Cho, E.; Soo Lee, J.; Vyavahare, N.R.; Webb, K. The effect of hyaluronic acid incorporation on fibroblast spreading and proliferation within PEG-diacrylate based semi-interpenetrating networks. Biomaterials 2007, 28, 4928–4938. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, Y.; He, Y.; Yang, C.; Wang, Y.; Shi, X.; Wei, G. Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol. 2010, 29, 107–116. [Google Scholar] [CrossRef]
- Landau, M.; Fagien, S. Science of Hyaluronic Acid Beyond Filling: Fibroblasts and Their Response to the Extracellular Matrix. Plast. Reconstr. Surg. 2015, 136, 188s–195s. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, Y.; Hwang, S.J. Effect of bFGF and fibroblasts combined with hyaluronic acid-based hydrogels on soft tissue augmentation: An experimental study in rats. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 47. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Huang, G.T.; Cheung, G.S.; Dissanayaka, W.L.; Zhu, W. Transplantation of dental pulp stem cells and platelet-rich plasma for pulp regeneration. J. Endod. 2012, 38, 1604–1609. [Google Scholar] [CrossRef]
| (1) Histological hard tissue deposition on radicular dentin walls | Present Absent |
| (2) Type of hard tissues formed within the root canal space | Absent Dentin-like matrix: Presence of odontoblast-like cells with associated odontoblastic processes, predentin, and/or dentin-like calcified areas Bone-like: Presence of a Haversian system with uniformly distributed osteocyte-like cells Cementum-like: Cementum-like mineralized tissue lining and adhering onto the dentin of the root with or without embedded cementocyte-like cells |
| (3) Vascularization | Present Absent |
| (4) Presence or absence of soft connective tissues within root canal space | Present (new vascularized fibrous connective tissue with fibroblasts and collagen fibers) Absent |
| (5) Degree of inflammatory cells infiltrated within pulpal canal space and/or in periapical area | None (0): Absence of inflammatory cells Mild (1): Small number of dispersed inflammatory cells Moderate (2): Focal aggregation of inflammatory cells Severe (3): Intense inflammatory infiltrate and tissue alterations |
| (6) Presence or absence of histological apical closure | Present Absent |
| Parameter | NC (n = 10) | PC (n = 11) | BC (n = 22) | BC + HA (n = 20) | p-Value |
|---|---|---|---|---|---|
| (1) Histological hard tissue deposition on radicular dentin walls | |||||
| Present | 0% (0) | 100% (11) | 86.3% (19) | 80% (16) | 0.44 * |
| Absent | 100% (10) | 0% (0) | 13.4% (3) | 20% (4) | |
| (2) Type of hard tissues formed along the root canal walls and within pulp spaces | |||||
| Absent | 0% (0) | 0% (0) | 0.6 * | ||
| Dentin-like | 22.7% (5) | 20% (4) | |||
| Bone-like | 27.3% (6) | 35% (7) | |||
| Cementum-like | 50% (11) | 45% (9) | |||
| (3) Vascularization | |||||
| Present | 0% (0) | 100% (11) | 54.6% (11) | 85% (17) | 0.04 * |
| Absent | 100% (10) | 0% (0) | 45% (9) | 15% (3) | |
| (4) Presence or absence of vascularized soft connective tissues within root canal space | |||||
| Present | 0% (0) | 100% (11) | 9.1% (2) | 40% (8) | 0.029 * |
| Absent | 100% (10) | 0% (0) | 90.9% (20) | 60% (12) | |
| (5) Inflammation within pulp spaces and/or the periapical area | |||||
| None | 0% (0) | 81.8% (9) | 18.2% (4) | 10% (2) | 0.048 † |
| Mild | 0% (0) | 18.2% (2) | 68.2% (15) | 45% (9) | |
| Moderate | 10% (1) | 0% (0) | 9.1% (2) | 35% (7) | |
| Severe | 90% (9) | 0% (0) | 4.5% (1) | 10% (2) | |
| (6) Apical closure | |||||
| Present | 0% (0) | 100% (11) | 72.7% (16) | 85% (17) | 0.46 * |
| Absent | 100% (10) | 0% (0) | 27.3% (6) | 15% (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlHowaish, N.A.; AlSudani, D.I.; Khounganian, R.; AlMuraikhi, N. Histological Evaluation of Restylane Lyft Used as a Scaffold for Dental Pulp Regeneration in Non-Infected Immature Teeth in Dogs. Materials 2022, 15, 4095. https://doi.org/10.3390/ma15124095
AlHowaish NA, AlSudani DI, Khounganian R, AlMuraikhi N. Histological Evaluation of Restylane Lyft Used as a Scaffold for Dental Pulp Regeneration in Non-Infected Immature Teeth in Dogs. Materials. 2022; 15(12):4095. https://doi.org/10.3390/ma15124095
Chicago/Turabian StyleAlHowaish, Norah A., Dina I. AlSudani, Rita Khounganian, and Nehal AlMuraikhi. 2022. "Histological Evaluation of Restylane Lyft Used as a Scaffold for Dental Pulp Regeneration in Non-Infected Immature Teeth in Dogs" Materials 15, no. 12: 4095. https://doi.org/10.3390/ma15124095






