Abstract
Dolostone is widely distributed and commonly used as concrete aggregates. A large number of studies have shown that there are significant differences in the expansibility of different dolostones, and the key factors determining the expansibility of alkali carbonate rocks have not been clarified. In this paper, rocks were selected from five different geological ages: Jixianian, Cambrian, Ordovician, Devonian, and Triassic ages. The ordering degree and the content of MgCO3 of dolomites in rocks of different geological ages were determined by X-ray diffraction (XRD). The degree of dedolomitization reaction in rocks cured in 80 °C, 1 mol/L NaOH solution was determined by quantitative X-ray diffraction (QXRD). The morphology of dolomites in rocks was determined by a polarizing microscope. The products of the dedolomitization reaction were determined by field emission electron microscopy (FESEM-EDS). According to the test results, the following conclusions are drawn. There is a good positive correlation between ordering degree and the molar fraction of MgCO3 of dolomites. When the MgCO3 mole fraction of dolomites varies from 47.17% to 49.60%, the higher the MgCO3 mole fraction, the greater the ordering degree of dolomite. By analyzing the degree of the dedolomitization reaction of different dolostone powders cured at 80 °C in 1 mol/L NaOH solution, it is found that the older the geological age of dolostone, the slower the dedolomitization reaction rate and the lower the degree of dedolomitization reaction. The lower the ordering degree of dolomite crystal in the same geological age, the faster the rate of dedolomitization reaction and the higher the degree of dedolomitization reaction.
1. Introduction
Carbonate rocks are widely distributed throughout China, accounting for about one-fifth of the total surface sedimentary rock distribution [1]. It is often used as aggregate in engineering construction, but due to its alkali dolomite reaction (ADR) activity, it has caused serious damage to engineering construction all over the world, resulting in serious hidden dangers to safety and huge repair costs [2,3]. Therefore, it is of great significance to further study the structure of carbonate rocks. ADR is also known as a dedolomitization reaction. Dedolomitization refers to the calcification of dolomite, a common process by which dolomite is converted to calcite by alkali, which may cause rocks to swell [4]. There are two main types of carbonate rocks: dolostone and limestone [5]. The main mineral of dolostone is dolomite, in addition to a small amount of calcite, quartz, feldspar, and clay minerals. The main mineral of limestone is calcite. The structural characteristics of dolostone mainly include mineral content, grain size, and crystallinity. The molar ratio of Ca2+ and Mg2+ in ideal dolomite crystals is the same, but Mg2+ is often replaced by Ca, Fe, and Mn plasma in nature, which does not conform to the structure of ideal dolomite crystals [6]. The ordering degree is a common method to characterize the crystallinity of dolomite crystals. So far, the intensity ratio of I (015)/I (110) obtained by XRD analysis is the most commonly used way to express the ordering degree of dolomite [7,8,9,10]. Previous studies on the crystallographic characteristics of dolomite have been carried out in detail, but they tend to be pure crystallographic studies, and rarely relate the crystallographic characteristics of different dolomites with their dedolomitization reaction [5,9,11]. Gao [12] analyzed a large amount of data on the ordering degree of dolomite and concluded that the ordering degree of penecontemporaneous dolomite is the worst, and that of deep burial dolomite is the best. Zhong [13] analyzed the Triassic dolomite through XRD, analyzed various factors affecting the ordering degree of dolomite and pointed out that the ordering degree of dolomite formed in the burial environment is the best, while the ordering degree of penecontemporaneous dolomite is the worst. Hadley [14] used XRD to study the relationship between ADR activity of aggregates and crystallinity of dolomite crystals, and the results showed that aggregates with and without ADR activity had poor crystallinity of dolomite, so he pointed out that crystallinity would not affect ADR activity of aggregates. In addition, Deng [15] analyzed the ordering degree of dolomite crystals in different rocks and found that the ordering degree will have a certain impact on ADR activity. Gillott [16,17] and Swenson [17,18] studied dolomite structure, and they found that dolomite grain size had a certain influence on ADR reaction rate. Niu [19] studied dolomite with different grain sizes and found that the larger the grain size of dolomite in dolostone, the higher the degree of dedolomitization reaction. Feng [20] analyzed the ordering degree of dolomite at different burial depths, and the results showed that the ordering degree of dolomite crystals increased with burial depth, while the dedolomitization gradually decreased.
In this study, XRD and polarizing microscope are used to determine the microstructure of dolostones of different geological ages, and the influence of factors such as the ordering degree of dolomite crystal on the degree of dedolomitization reaction was discussed, which provided data support for exploring the ACR expansion law of dolostones. Identifying the types of rocks that are likely to cause damage can lead to the more efficient and rational use of resources.
2. Materials and Methods
2.1. Raw Materials
Rocks
By consulting the 1:50,000 scale geological map of China and referring to the regional geological literature, the dolostones of different geological ages are determined. Twelve rock samples from five geological ages were selected for this experiment, as shown in Figure 1. Among them, the rocks WMS-6 and WMS-8 from Tianjin belong to Jixianian age, the rocks BFL-7 and BFL-12 from Linqu county in Shandong Province belong to Cambrian age, the rocks ZC from Taiyuan belong to Ordovician age, the rocks JF, DH-1 and DH-2 from Baoding belong to Ordovician age, the rocks SFP-1, SFP-2 and SFP-3 from Dushan County in Guizhou Province belong to Devonian age and the rocks DJY from Yibin City in Sichuan Province belong to Triassic age.

Figure 1.
The appearance of dolostones.
The chemical analysis method of rock is carried out according to the standard GB/T 176-2017(CIS, 2017). Table 1 shows the chemical composition of rocks. It can be seen from Table 1 that the MgO content in rocks WMS-6, WMS-8, DH-1, DH-2, SFP-1, SFP-2, SFP-3, and JF is relatively similar, about 20%, the MgO content in rocks DJY and BFL-12 is about 15%, and the MgO content in rocks ZC and BFL-7 is only about 5%. The contents of SiO2 are below 10.0% except for rock JF. The content of Fe2O3 and Al2O3 in rock DJY is slightly more than 1%.

Table 1.
Chemical composition of rocks.
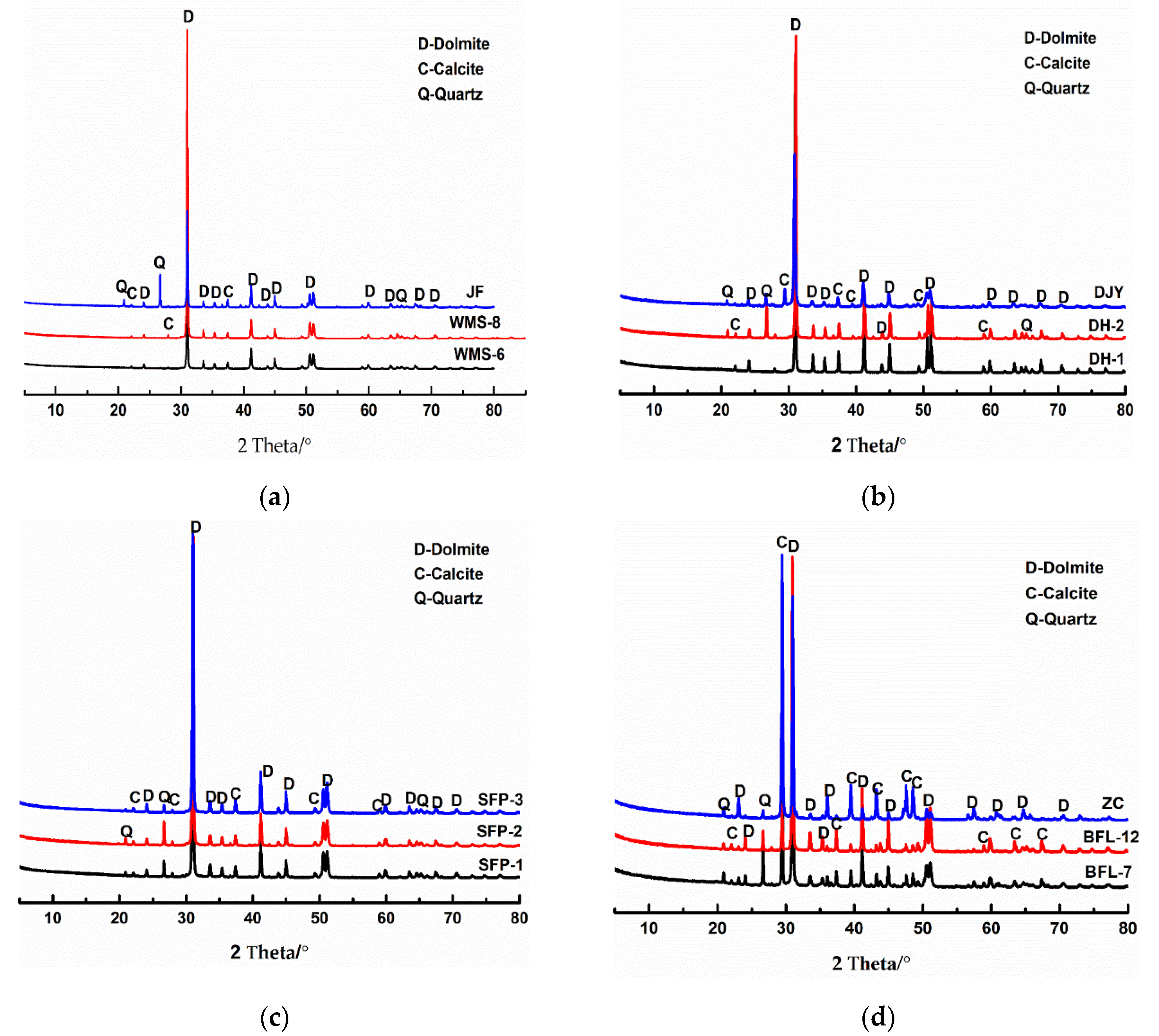
Figure 2 shows the minerals of the studied dolostones determined by XRD (the scanning speed is 10°/min, the scanning angle is 5~80°). From Figure 2, it can be seen that the main minerals in the dolostones WMS-6, WMS-8, BFL-12, DH-1, DH-2, JF, SFP-1, SFP-2, and DJY are dolomite and a small amount of quartz, while the main minerals in the dolomitic limestone ZC and BFL-7 are dolomite, calcite, and quartz.
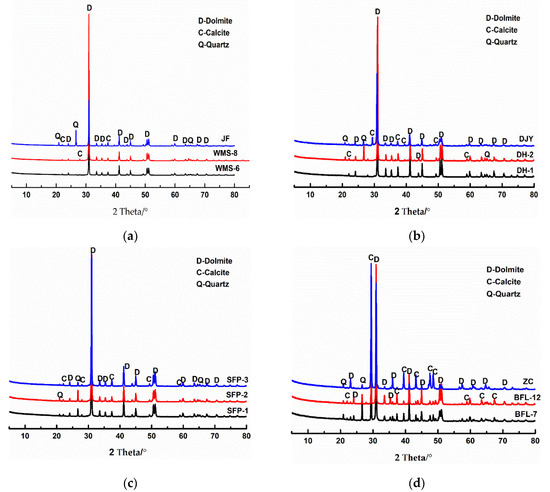
Figure 2.
XRD patterns of rocks: (a–c) dolomite; (d) dolomitic limestone.
2.2. Methods
2.2.1. Ordering Degree of Dolomite
The rocks are ground to about 60 μm to avoid damage to crystallinity caused by excessive grinding, dried and then XRD (the scanning angle is 25~40°, the scanning speed is 2°/min) was used to measure the ordering degree of dolomites. XRD data of rocks were analyzed through the Jade software. The integral intensity value I (015) and I (110) of the diffraction peak of the crystal plane of dolomite (015) and (110), respectively, were read [21,22], and ordering degree of dolomites was calculated according to Formula (1):
The d (104) value of the diffraction peak of dolomite (104) crystal plane on the measurement spectrum was read, and the mole fraction of MgCO3 and CaCO3, respectively, was calculated by using Formulas (2) and (3) [23,24]:
Mole fraction of MgCO3 (%) = −333.33 d (104) + 1011.99
Mole fraction of CaCO3 (%) = 100% − Mole fraction of MgCO3 (%)
2.2.2. Thin Section Petrography
The dolomite grain sizes in rocks of different geological ages were examined by thin slices of different rocks for optical microscope observation. About a hundred pictures were taken of each rock. A polarizing optical microscope (Optiphot-II Pol reflecting light apparatus, 25–400×, Nikon, Tokyo, Japan) with transmitted light was used. The preparation of thin sections was performed according to the section “Thin section specimen preparation” [25].
2.2.3. Dedolomitization Reaction
The dolomite powder was cured in the condition of 80 °C, 1 mol/L NaOH solution, and taken out regularly. After drying, ZnO was used as the internal standard for quantitative analysis by XRD, and then Jade was used to fitting and analyze the XRD pattern to obtain the peak area ratio of ZnO and dolomite, and the content of brucite was calculated by internal standard method. The degree of dedolomitization reaction in rocks is calculated by the ratio of the reduced amount of dolomite to the content of original dolomite, and the scanning speed is 1°/min, the scanning angle is 30~33°.
2.2.4. Microstructure of Dedolomitization Reaction Products
Firstly, 5~10 mm rock particles were taken out after curing in 80 °C, 1 mol/L NaOH solution for 7 days. Then, rock particles were solidified in resin and polished by vibration polishing machine. After rough grinding, fine grinding, polishing, and drying for 24 h, the samples were observed by FE-SEM.
3. Results
3.1. Analysis of Morphology and Distribution Characteristics of Dolomites
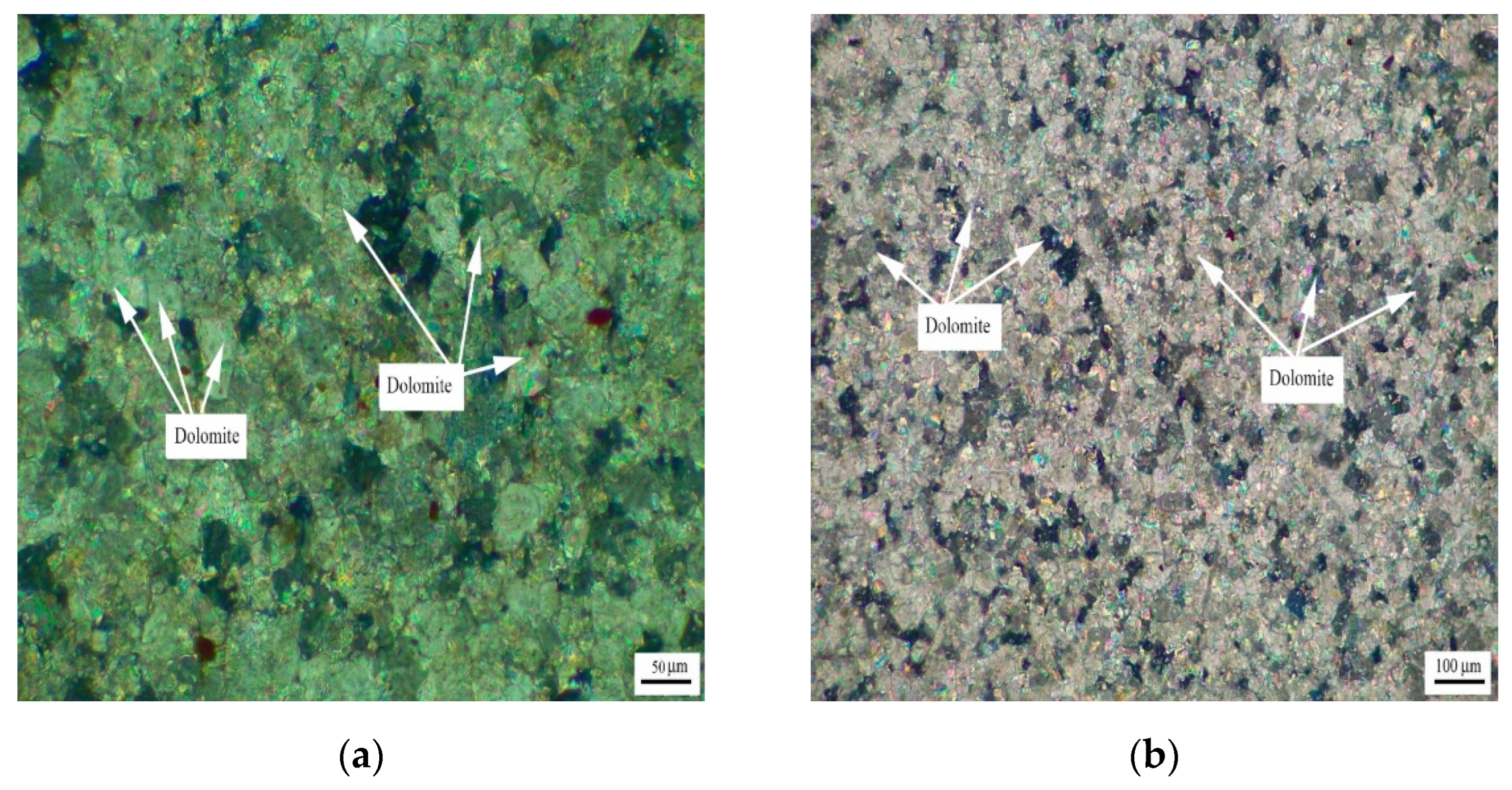
Figure 3 shows the distribution of dolomite grains of Jixianian rocks WMS-6 and WMS-8. It can be seen that the dolomite grains are mosaic distribution, which is mainly caused by the existence of a large number of dolomite grains with different grain sizes in the rock. The dolomite crystals are dominated by anhedral crystals.
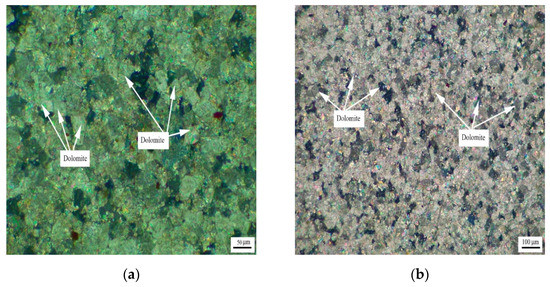
Figure 3.
Distribution of dolomites in Jixianian rocks (a) WMS-6 and (b) WMS-8.
Figure 4 shows the distribution of dolomite grains of Cambrian rocks BFL-7 and BFL-12, and the dolomite grains of rock BFL-7 are dispersed in the calcite matrix. The dolomite grains in BFL-12 are mainly distributed in a mosaic state, and a few dolomite grains are dispersed in the calcite matrix. The dolomite grains in BFL-7 and BFL-12 are mainly anhedral with a little subhedral.
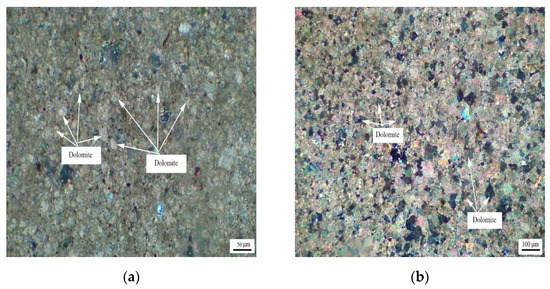
Figure 4.
Distribution of dolomites in Cambrian rocks (a) BFL-7 and (b) BFL-12.
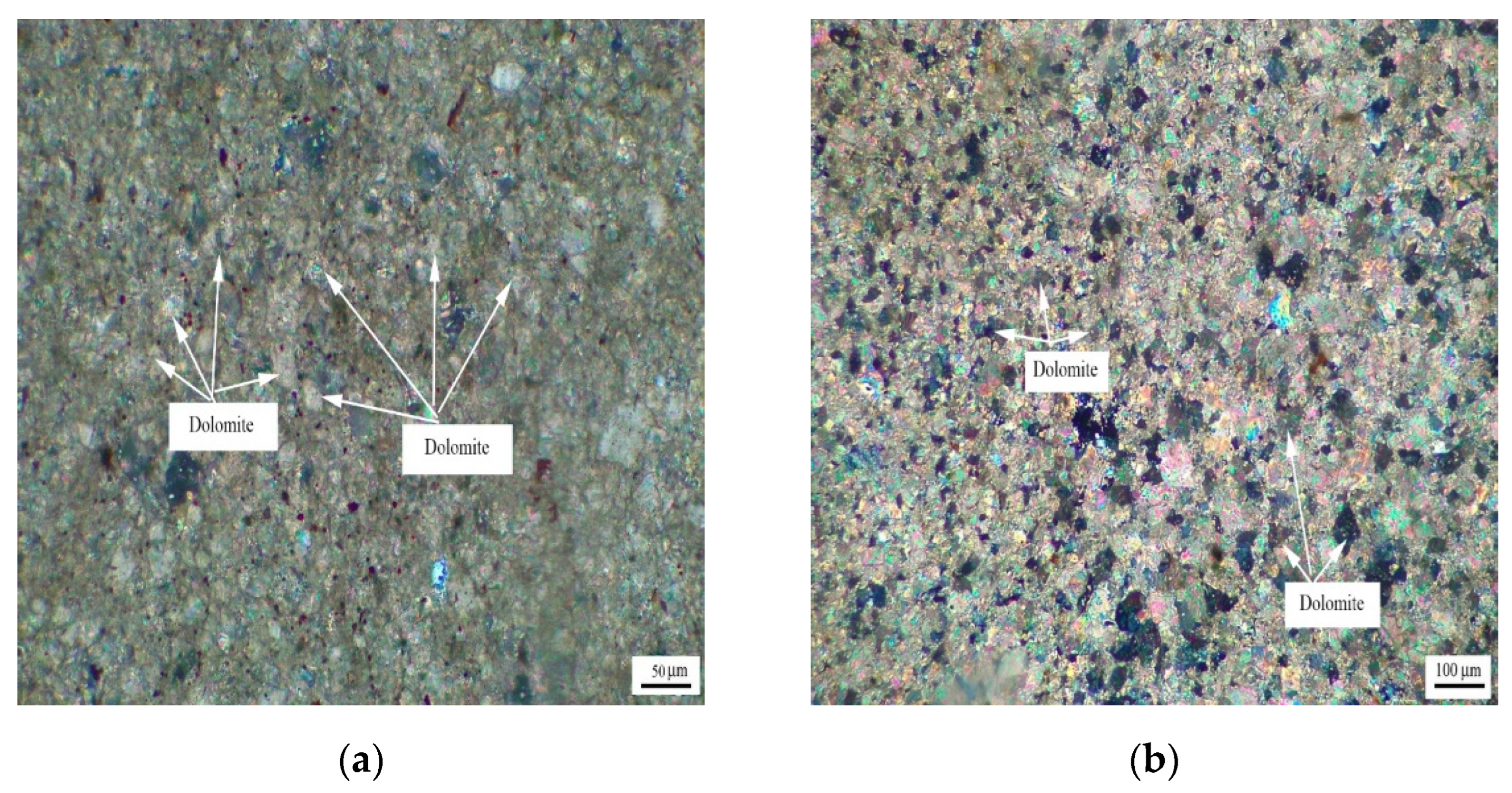
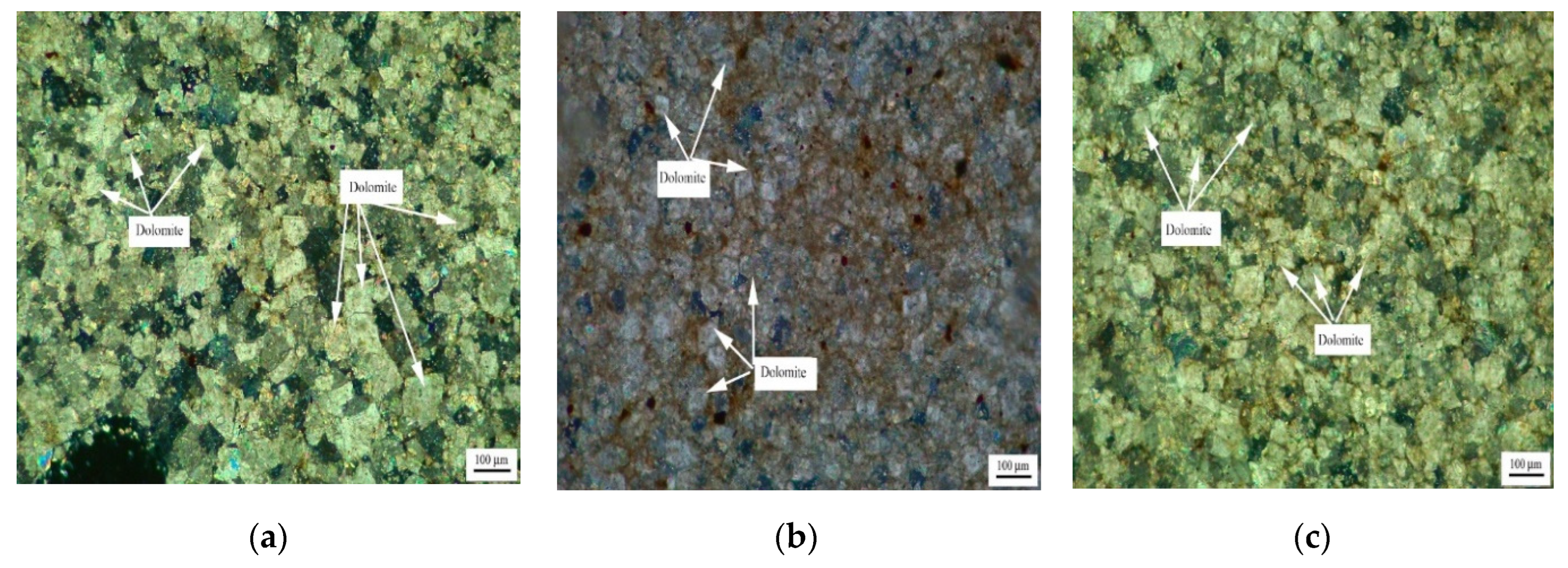
Figure 5 shows the distribution of dolomite grains of Ordovician rocks ZC, JF, DH-1, and DH-2. It can be seen from Figure 5a that dolomite grains of rock ZC are dispersed in the calcite matrix. Dolomite grains have a high degree of idiomorphic crystal, and dolomite crystals are mainly euhedral crystals and subhedral crystals. The crystals support each other in a network shape. It can be seen from Figure 5b that the dolomite crystals of rock JF are dominated by anhedral crystals. It can be seen from Figure 5c,d that dolomite crystals of rocks DH-1 and DH-2 are mosaic distribution. The dolomite crystals in rock DH-1 are mainly euhedral crystals and subhedral crystals, while the dolomite crystals in DH-2 are anhedral crystals.

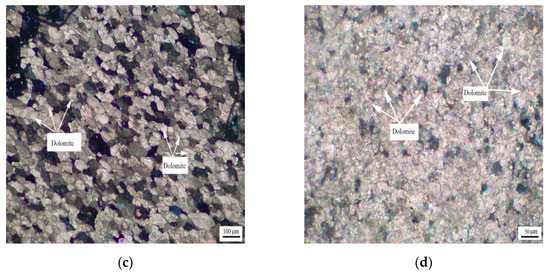
Figure 5.
Distribution of dolomites in Ordovician rocks (a) ZC, (b) JF, (c) DH-1, and (d) DH-2.
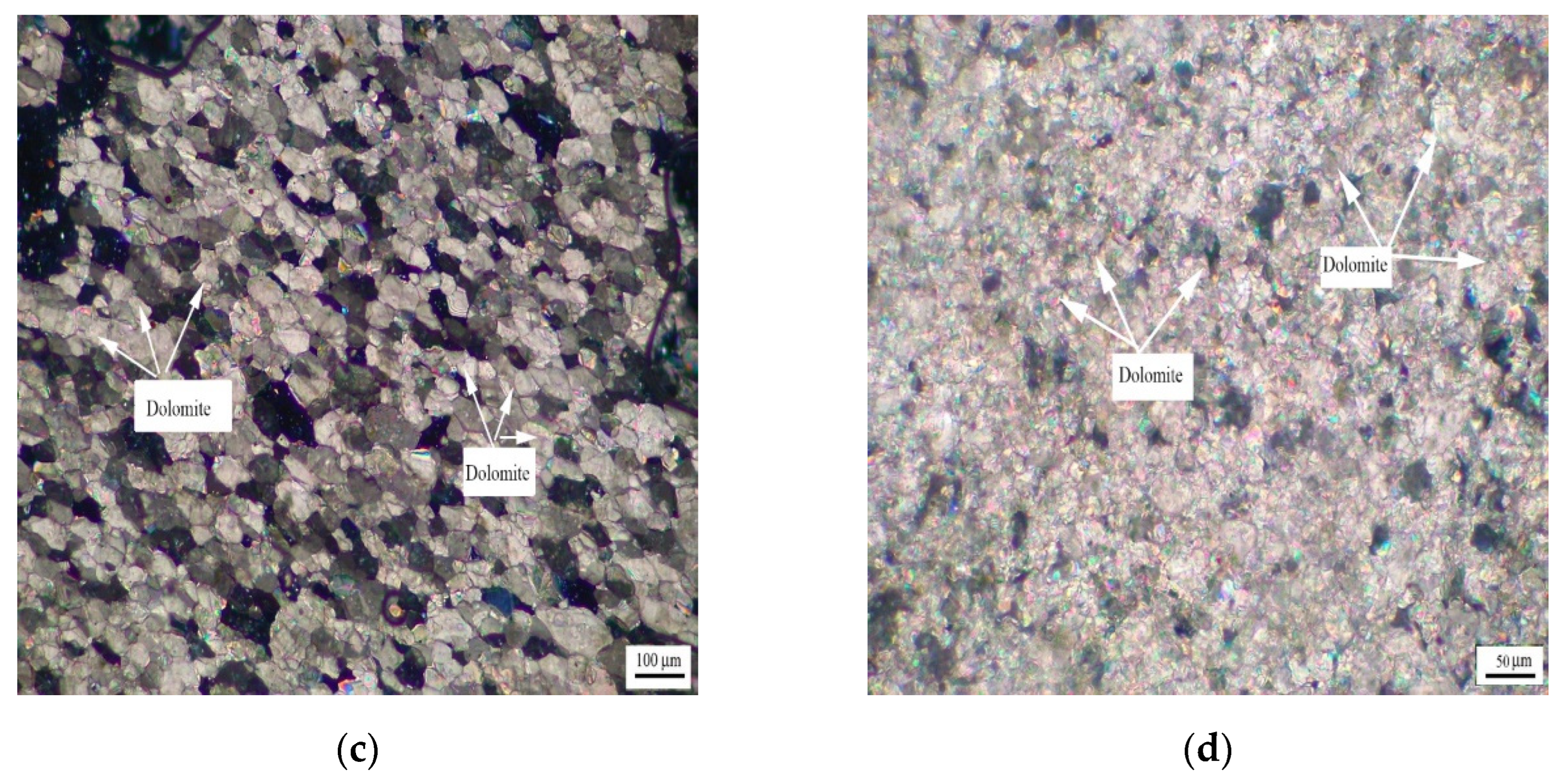
Figure 6 shows the distribution of dolomite grains of Devonian rocks SFP-1, SFP-2, and SFP-3. It can be seen from Figure 6a–c that the dolomite grains in rocks SFP-1, SFP-2, and SFP-3 are mosaic distribution. The dolomite crystals in rocks SFP-1, SFP-2 and SFP-3 are mainly euhedral crystals with a small number of subhedral crystals.
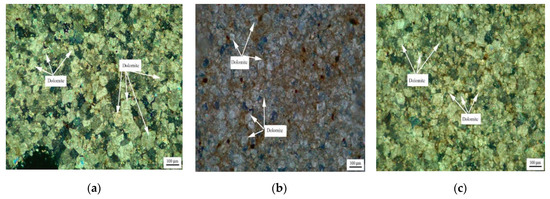
Figure 6.
Distribution of dolomites in Devonian rocks (a) SFP-1, (b) SFP-2, and (c) SFP-3.
Figure 7 shows the distribution of dolomite grains of Triassic rock DJY. It can be seen from Figure 7 that the dolomite grains in DJY are mainly mosaic distribution, and a few dolomite grains are dispersed in the calcite matrix. The dolomite crystals are mainly anhedral crystals.

Figure 7.
Distribution of dolomites in Triassic rock DJY.
3.2. The Ordering Degree of Dolomite
The ordering degree and MgCO3 mole fraction of dolomites were analyzed by XRD to characterize the crystallinity of dolomite crystals. Table 2 shows the analysis of rocks of different geological ages by XRD, and the calculation of MgCO3 mole fraction and ordering degree of corresponding dolomites. It can be seen from Table 3 that the ordering degree and the content of MgCO3 of dolomites are significantly different in rocks of different geological ages.

Table 2.
The MgCO3 mole fraction and ordering degree of dolomite crystals in rocks.

Table 3.
The ordering degree of dolomite and degree of dedolomitization reaction.
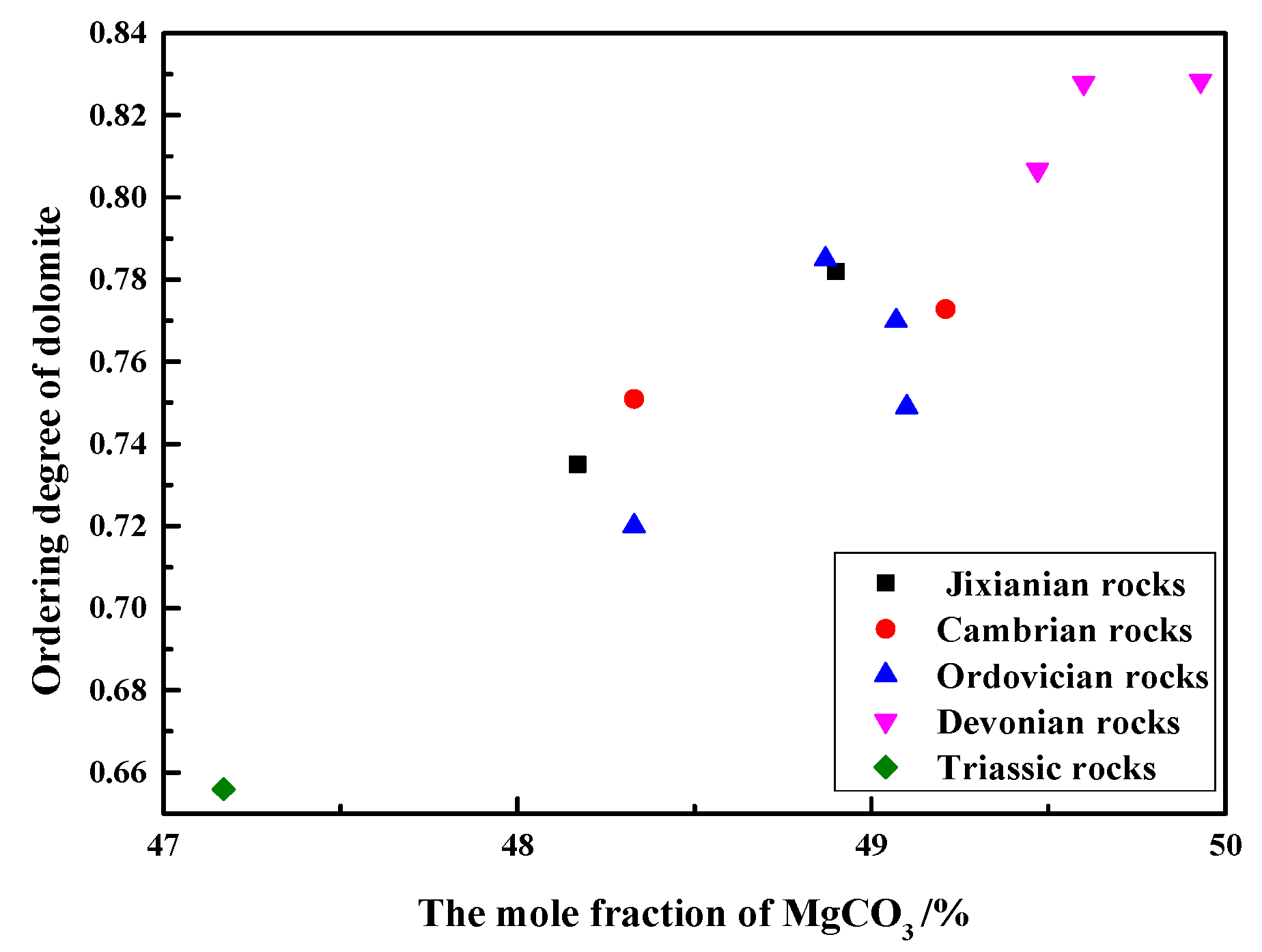
Figure 8 shows the relationship between the ordering degree of dolomites in rocks of different geological ages and the MgCO3 mole fraction of dolomites. As can be seen from Figure 7, the ordering degree of dolomite crystals in Devonian rocks SFP-1, SFP-2, and SFP-3 are the highest, up to 0.83, while the ordering degree of dolomite crystals of Triassic rocks DJY is the lowest, only 0.61. It can also be seen that when the mole fraction of MgCO3 varies from 47.17% to 49.60%, there is an approximately linear relationship between the MgCO3 mole fraction and the ordering degree of dolomite crystals. The ordering degree of dolomites increases with the increase in the MgCO3 mole fraction. When the molar fraction of MgCO3 of dolomite crystals is close to 50%, the order degree is higher. When the content of Mg2+ and Ca2+ in dolomite crystals is close to each other, Mg2+ and Ca2+ are more likely to be regularly distributed. Dolomite is formed in a low-salinity environment with a low ratio of Mg2+ to Ca2+, so the crystallization rate of dolomite is relatively slow, so the ordering degree of dolomite is high.
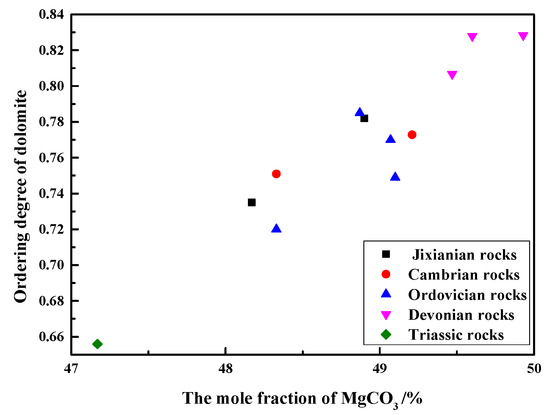
Figure 8.
Relationship between ordering degree and the mole fraction of MgCO3 of dolomites.
3.3. The Degree of Dedolomitization Reaction
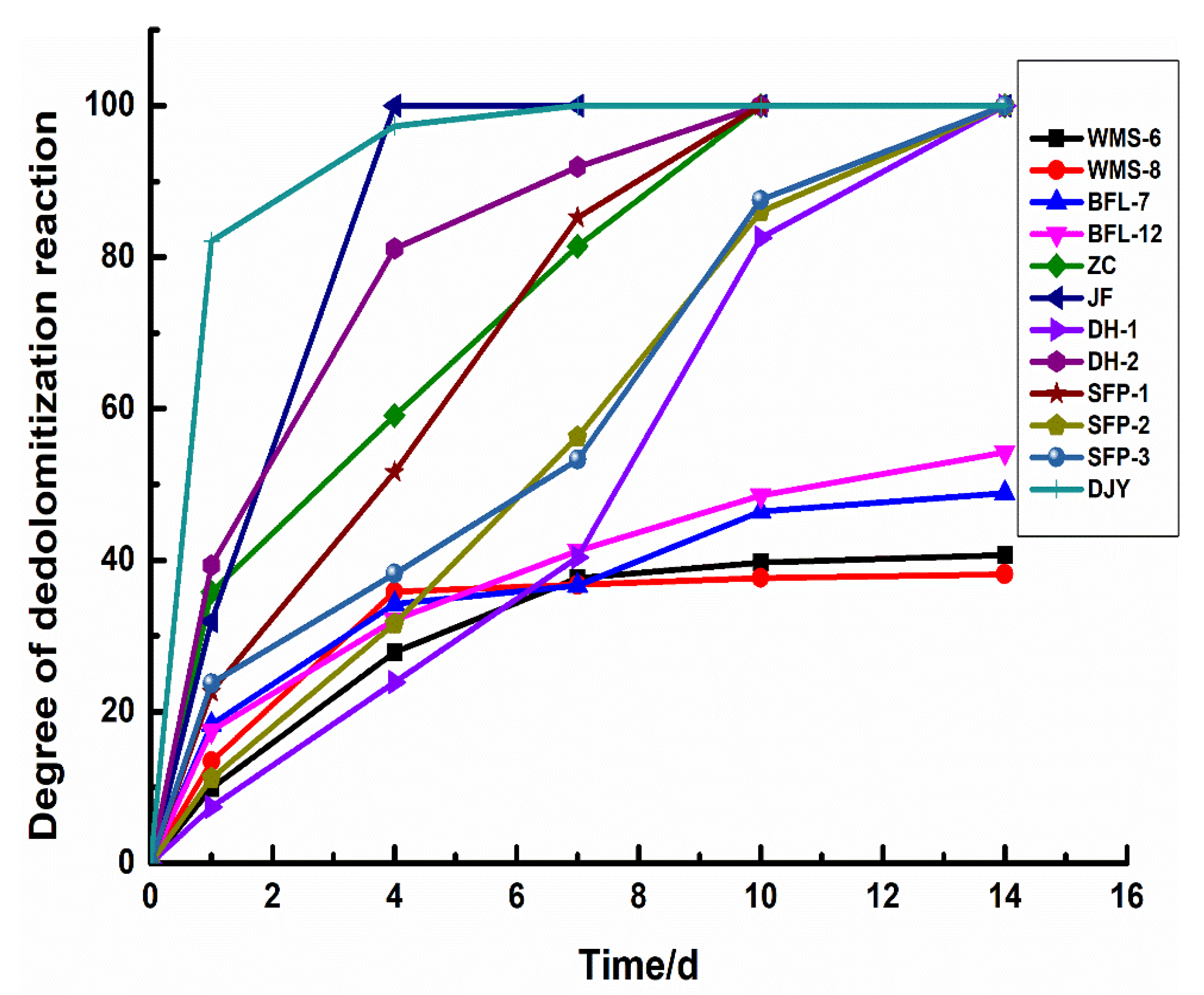
Figure 9 shows the degree of dedolomitization reaction in rocks of different geological ages at different reaction times. It can be seen that there are certain differences in the reaction rates of dolomite in dolostones. The dedolomitization reaction rate of Jixianian rocks WMS-6 and WMS-8 is relatively slow in the early stage. After curing for 4 days, the degree of dedolomitization reaction almost does not change at about 30%. The degree of dedolomitization of the Cambrian rocks BFL-7 and BFL-12 is relatively slow and tends to be stable at about 40%. The dolomite of Ordovician rocks ZC, DH-1, and DH-2 react completely after curing for about 10 days, while the dolomite of Ordovician rocks JF reacted completely at 4 days. The dolomite in Devonian rocks SFP-1, SFP-2, and SFP-3 reacted completely at about 10 days. The dedolomitization reaction rate of Triassic rocks is the highest in the early stage and can be fully reacted at 7 days. The dedolomitization reaction rate of the rocks in the Jixianian and Cambrian ages are the slowest. The dedolomitization reaction rate of Ordovician rocks is slightly faster than that of Devonian rocks and can be fully reacted with the increase in curing time. Therefore, geological age has a certain influence on the dedolomitization reaction in rocks, and the older the geological age of rocks, the lower the degree of dedolomitization reaction and the slower the reaction rate.
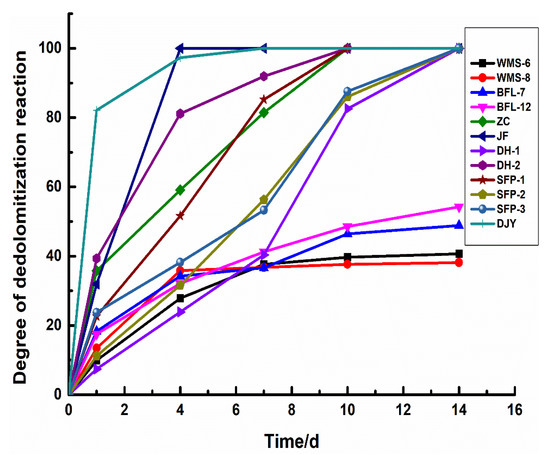
Figure 9.
The degree of dedolomitization reaction in rocks.
3.4. Influence of Ordering Degree of Dolomite on Degree of Dedolomitization Reaction
The degree of dedolomitization reaction of different dolostones is different, and the reason for the difference is not clear. By analyzing the ordering degree of dolomites, the reason for the difference in the degree of dedolomitization reaction is explained.
Table 3 shows the ordering degree of dolomites and the degree of dedolomitization reaction in the dolostones. The ordering degree of dolomite of Jixianian rocks WMS-6 and WMS-8 is 0.74 and 0.78, respectively, and the degree of dedolomitization reaction in WMS-6 is greater than that in WMS-8 at 14 d. The ordering degree of dolomite of Cambrian rocks BFL-7 and BFL-12 is 0.77 and 0.75, respectively. The degree of dedolomitization reaction of dolostone in BFL-7 and BFL-12 is close. The ordering degrees of dolomites of Ordovician rocks ZC, JF, DH-1, and DH-2 are 0.77, 0.72, 0.78, and 0.74, respectively. The dedolomitization rate of dolomites of JF is the fastest, and that of dolomites of DH-1 is the slowest. The ordering degree of dolomites of Devonian rocks SFP-1, SFP-2, and SFP-3 are 0.81, 0.83, and 0.83, respectively. The dedolomitization reaction rate of dolomites in SFP-1 is the fastest, and the dedolomitization reaction rate of dolomites of SFP-2 and SFP-3 are close to each other. The ordering degree of dolomite of Triassic rock DJY is only 0.61, and the dedolomitization reaction rate of rock DJY is the highest. It can be seen that the lower the ordering degree of dolomites in the same geological age, the faster the dedolomitization reaction rate of dolomite, and the higher the degree of dedolomitization reaction.
3.5. Microscopic Analysis of Products after Dedolomitization
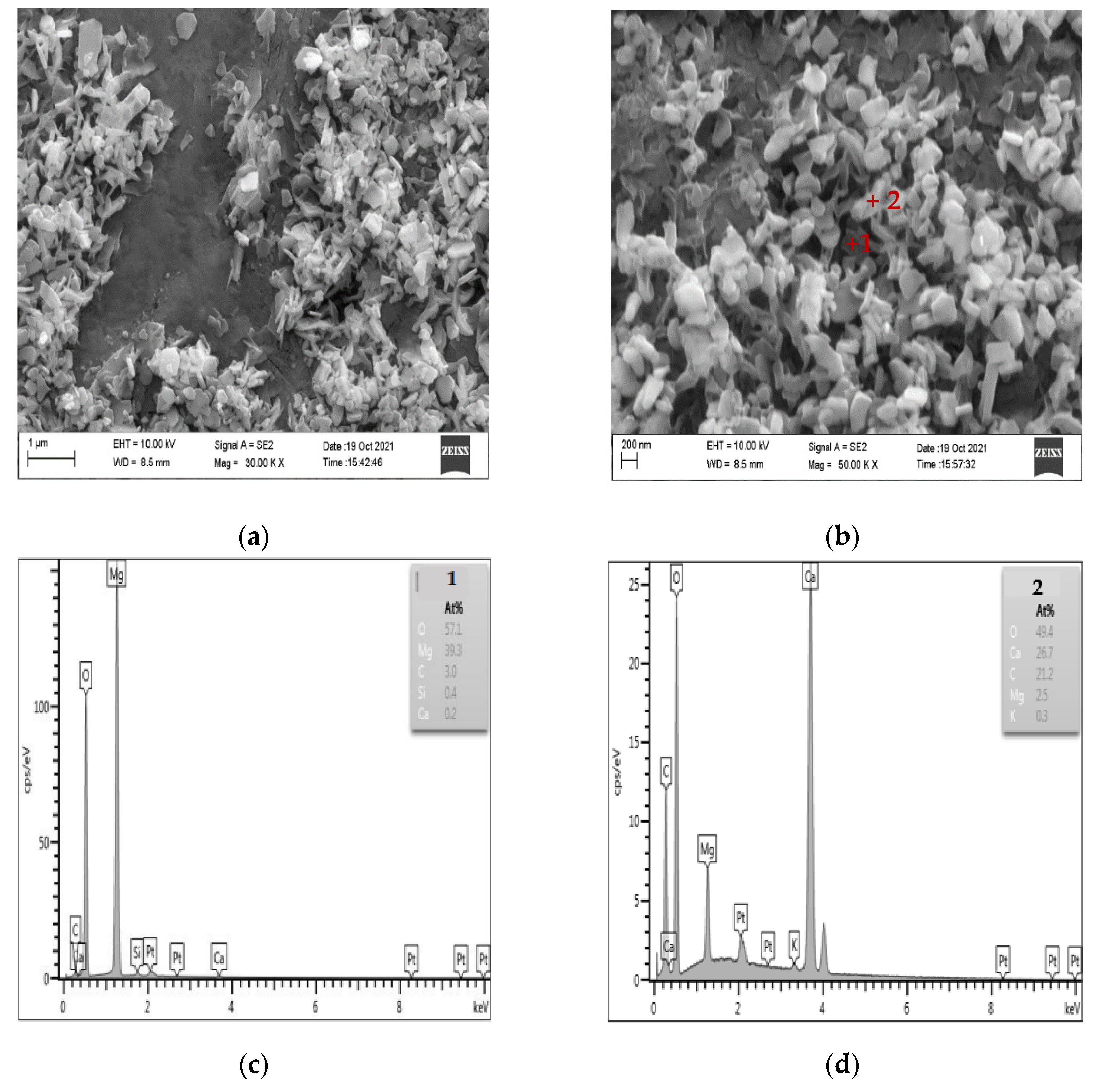
Figure 10 shows the SEM-EDS diagram of the products generated after the dedolomitization reaction. It can be seen from Figure 10a,b that a large number of flaky and granular substances are formed around dolomite after the dedolomitization reaction. The results of Figure 10c,d EDS show that flaky substance (point 1) is mainly composed of Mg and O, which can be judged as brucite after dedolomitization reaction, while granular substance (point 2) is mainly composed of Ca, C and O, which is another product calcite after de dolomitization reaction. The shape of calcite is mostly granular and columnar, and the shape of brucite is mainly flake. These reaction products are distributed and accumulated around dolomite crystals, and it can be seen that there are a large number of pores between brucite and calcite.
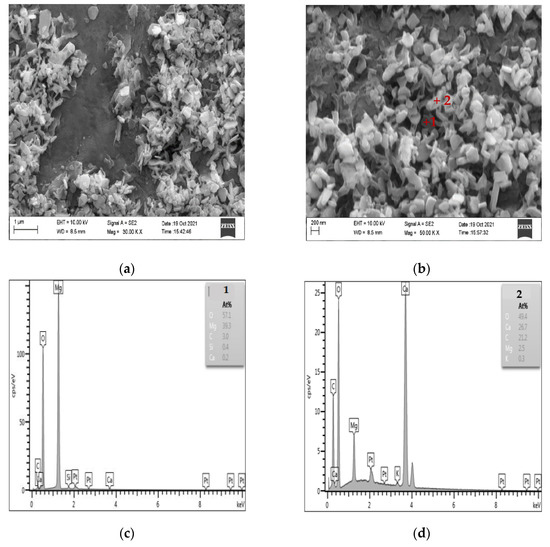
Figure 10.
SEM-EDS images of dedolomitization reaction products. (a,b) The secondary electron image of dedolomitization reaction product (c) The elements composition of point 1 (d) The elements composition of point 2.
4. Discussion
The influence of the morphology of dolomite on the dedolomitization reaction was discussed. The dolomites of rocks WMS-6, WMS-8, Bfl-7, Bfl-12, JF, DH-2, and DJY are mainly anhedral crystals. Among them, the degree of dedolomitization reaction of dolostones WMS-6, WMS-8, BFL-7, and BFL-12 is relatively low, and the degree of dedolomitization reaction is only about 40% at 14 d. The degree of dedolomitization reaction of dolostones DH-2 and DJY is close to 100% at 7 days. The degree of dedolomitization reaction of dolostone ZC, SFP-1, SFP-2, and SFP-3 with a good euhedral degree is close to 100% at about 14 d, and there is a great difference in the degree of dedolomitization reaction at the initial stage of dedolomitization reaction, among which, the degree of dedolomitization reaction of rock ZC reaches 59.1% at 4 d. However, the degree of dedolomitization reaction of rock SFP-2 is only 31.6% in 4 d. There is no correlation between the crystal morphology of dolomites and the degree of dedolomitization reaction, so the crystal morphology of dolomite in dolostones is not the key factor to determine the degree of dedolomitization reaction.
5. Conclusions
Through the study of rocks’ microstructure and dedolomitization reaction in dolostones of different geological ages, the conclusions are as follows.
- The ordering degree of dolomite crystals in Devonian rocks is the highest, which is 0.83. The ordering degree of dolomite crystals in Jixianian, Cambrian, and Ordovician rocks is about 0.75. The ordering degree of dolomite crystals in Triassic rocks is the lowest, which is 0.61. The MgCO3 mole fraction of dolomite crystals in the test rocks ranges from 0.4717 to 0.4960. With the increase in the MgCO3 mole fraction, the ordering degree of dolomite increases, and the relationship between the MgCO3 mole fraction and the ordering degree is approximately linear.
- After curing in a 1 mol/L NaOH solution at 80 °C, the dedolomitization reaction rate of dolomite in 0.045–0.080 mm Triassic rock powders is the fastest, and the dolomite completely reacts at 7 days. The dedolomitization rate in the Jixianian rock powder sample is the slowest, and the dedolomitization reaction degree reaches about 40% at 7 days, and then hardly changes. The dedolomitization reaction degree of the dolomite in the Cambrian rock powder sample reaches about 50% at 10 d and then tends to be stable. The dedolomitization reaction rates of powder samples in Ordovician and Devonian rocks are similar, and the dolomites in the rocks can fully react after 10 days and 14 days, respectively. Therefore, it can be inferred that the older the geological age of rocks, the slower the dedolomitization reaction rate, and the lower the degree of dedolomitization reaction.
- The effects of the ordering degree of dolomites on the degree of dedolomitization reaction in different dolostones are analyzed. The lower the ordering degree of dolomite crystals in rocks of the same geological age, the faster the rate of dedolomitization reaction and the higher the degree of dedolomitization reaction.
- The products of the dedolomitization reaction of dolostones were determined by SEM-EDS, and the calcite and brucite were distributed around the dolomite crystals, and there were many tiny pores between the calcite and brucite.
Author Contributions
Conceptualization, M.D.; data curation, Z.M., Z.F., X.L., L.Y., T.Z. and X.H.; writing—original draft preparation, Z.F.; writing—review and editing, M.D. and Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Open Fund Project of the National Laboratory for High-Performance Civil Engineering Materials (2021CEM012) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the assistance from Zhongyang Mao, Tao Zhang, Xiang Liu, and Lei Yi from NJTECH, and the staff from the State Key Laboratory of Materials-Oriented Chemical Engineering.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, J.; Kang, Y.; Liu, D. Experimental research on drilling alkali sensitivity of carbonate gas reservoirs in Sichuan—Chongqing region. Nat. Gas Ind. 2005, 25, 60–61. [Google Scholar]
- Alexander, M.G. Alkali–aggregate reaction. In Developments in the Formulation and Reinforcement of Concrete, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–113. [Google Scholar]
- Hird, K. Petrography and Geochemistry of Some Carboniferous and Precambrian Dolomites. Ph.D. Thesis, University of Durham, Durham, UK, 1985. [Google Scholar]
- Donatelli, J.L. Dedolomitization and Other Diagenesis in the Backreef Setting of the Permian Reef Complex in Dark Canyon, New Mexico. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2016. [Google Scholar]
- Banerjee, A. Estimation of dolomite formation: Dolomite precipitation and dolomitization. J. Geol. Soc. India 2016, 87, 561–572. [Google Scholar] [CrossRef]
- Zhang, J.; Shou, J.; Zhang, T.; Pan, L.; Zhou, J. New Approach on the Study of Dolomite Origin: The crystal structure analysis of dolomite. Acta Sedimentol. Sin. 2014, 32, 550–559. [Google Scholar]
- Zucchini, A.; Comodi, P.; Katerinopoulou, A.; Balic-Zunic, T.; McCammon, C.; Frondini, F. Order–disorder–reorder process in thermally treated dolomite samples: A combined powder and single-crystal X-ray diffraction study. Phys. Chem. Miner. 2012, 39, 319–328. [Google Scholar] [CrossRef]
- McKenzie, J.A. Holocene Dolomitization of Calcium Carbonate Sediments from the Coastal Sabkhas of Abu Dhabi, U.A.E.: A Stable Isotope Study. J. Geol. 1981, 89, 185–198. [Google Scholar] [CrossRef]
- Alonso-Zarza, A.M.; Martin-Perez, A. Dolomite in caves: Recent dolomite formation in oxic, non-sulfate environments. Castanar Cave, Spain. Sediment. Geol. 2008, 205, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Pina, C.M.; Pimentel, C.; Crespo, Á. Dolomite cation order in the geological record. Chem. Geol. 2020, 547, 119667. [Google Scholar] [CrossRef]
- Geske, A.; Goldstein, R.H.; Mavromatis, V.; Richter, D.K.; Buhl, D.; Kluge, T.; John, C.M.; Immenhauser, A. The magnesium isotope (δ26 Mg) signature of dolomites. Geochim. Et Cosmochim. Acta 2015, 149, 131–151. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, S. Controlling factors of dolomite ordering degree. Sci. Technol. Innov. Her. 2018, 15, 7. [Google Scholar]
- Zhong, Q.; Huang, S.J.; Zou, M.L. Controlling factors of order degree of dolomite in carbonate rocks: A case study from Lower Paleozoic in Tahe Oilfield and Triassic in northeastern Sichuan Basin. Lithol. Reserv. 2009, 21, 50–55. [Google Scholar]
- Hadley, D.W. Alkali-reactivity of carbonate rocks-expansion and dedolomitization. Highw. Res. Board 1961, 40, 462–474. [Google Scholar]
- Deng, M.; Qian, G. Ordered index and dedolomitization of dolomite crystals. J. Nanjing Univ. Chem. Technol. 2001, 23, 1–5. [Google Scholar]
- Gillott, J.E.; Duncan, M.A.G.; Swenson, E.G. Alkali-aggregate reaction in nova Scotia IV. Character of the reaction. Cem. Concr. Res. 1973, 3, 521–535. [Google Scholar] [CrossRef] [Green Version]
- Swenson, E.G.; Gillott, J.E. Alkali-carbonate rock reaction. Highw. Res. Rec. 1964, 45, 21–40. [Google Scholar]
- Swenson, E.G. A reactive aggregate undetected by ASTM tests. ASTM Bull. 1957, 226, 48–51. [Google Scholar]
- Niu, L.L.; Deng, M.; Yang, Y.F. Influence of Grain Size of Dolomites on Alkali-Dolomite Reaction and Expandability of Dolostone. Bull. Chin. Ceram. Soc. 2015, 34, 2757–2763. [Google Scholar]
- Feng, S.H.; Hong, L.I.; Jiang, J.J.; Lei, Y.; Niu, Y.Z.; Yang, R.; Liu, Y.J. The Multiple Dolomitizations in Ordovician Majiagou Carbonate Rocks in Liujiang Basin, Qinhuangdao Area, North China. Acta Sedimentol. Sin. 2017, 35, 664–680. [Google Scholar]
- Kaczmarek, S.E.; Sibley, D.F. A Comparison of Nanometer-Scale Growth and Dissolution Features on Natural and Synthetic Dolomite Crystals: Implications for the Origin of Dolomite. J. Sediment. Res. 2007, 77, 424–432. [Google Scholar] [CrossRef]
- Deffeyes, K.S.; Lucia, F.J.; Weylt, P.K. Dolomitization: Observations on the Island of Bonaire, Netherlands Antittes. Science 1964, 143, 678–679. [Google Scholar] [CrossRef]
- Huang, S.J. Experimental research methods of carbonate rocks. Mineral. Petrol. 1990, 10, 114–117. [Google Scholar]
- Zeng, L.; Wan, M.X.; Peng, Y. Dolomite Sequentiality and Its Application to Petroleum Geology. Nat. Gas Explor. M Dev. 2004, 27, 64–66. [Google Scholar]
- French, W.J. Concrete petrography: A review. Q. J. Eng. Geol. 1991, 24, 17–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).