Crack-Resistant Amino Resin Flame-Retardant Coatings Using Waterborne Polyurethane as a Co-Binder Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Synthesis of Melamine-Urea-Formaldehyde (MUF) Resin
2.2.2. Preparation of Waterborne Intumescent Flame-Retardant (WIFR) Coatings
2.3. Characterization
2.3.1. Transparency Test
2.3.2. Cracking Resistance Test
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Cone Calorimetry (CONE) Test
2.3.5. Char Formation and Morphology
3. Results and Discussion
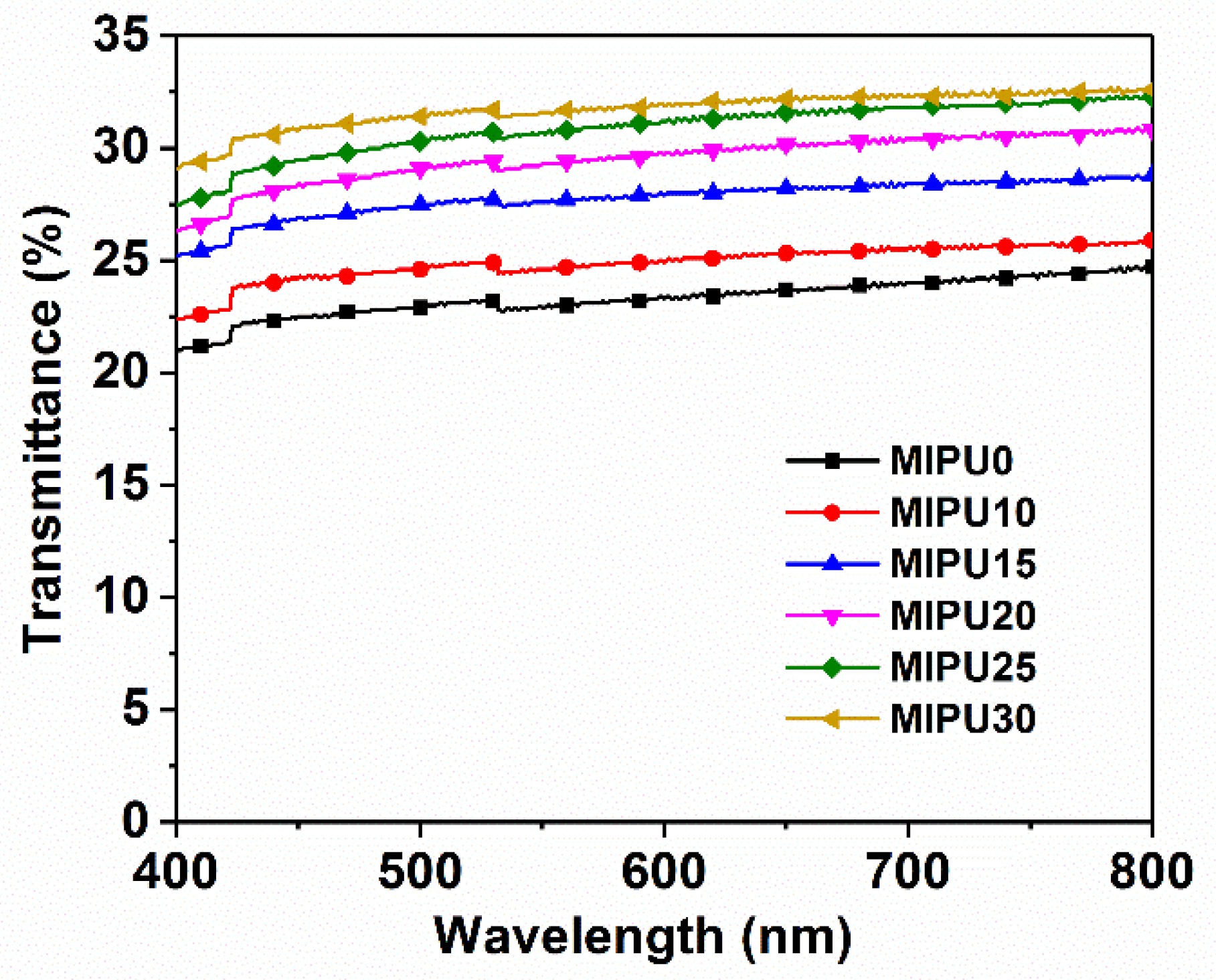
3.1. Optical Transparency of the Coatings
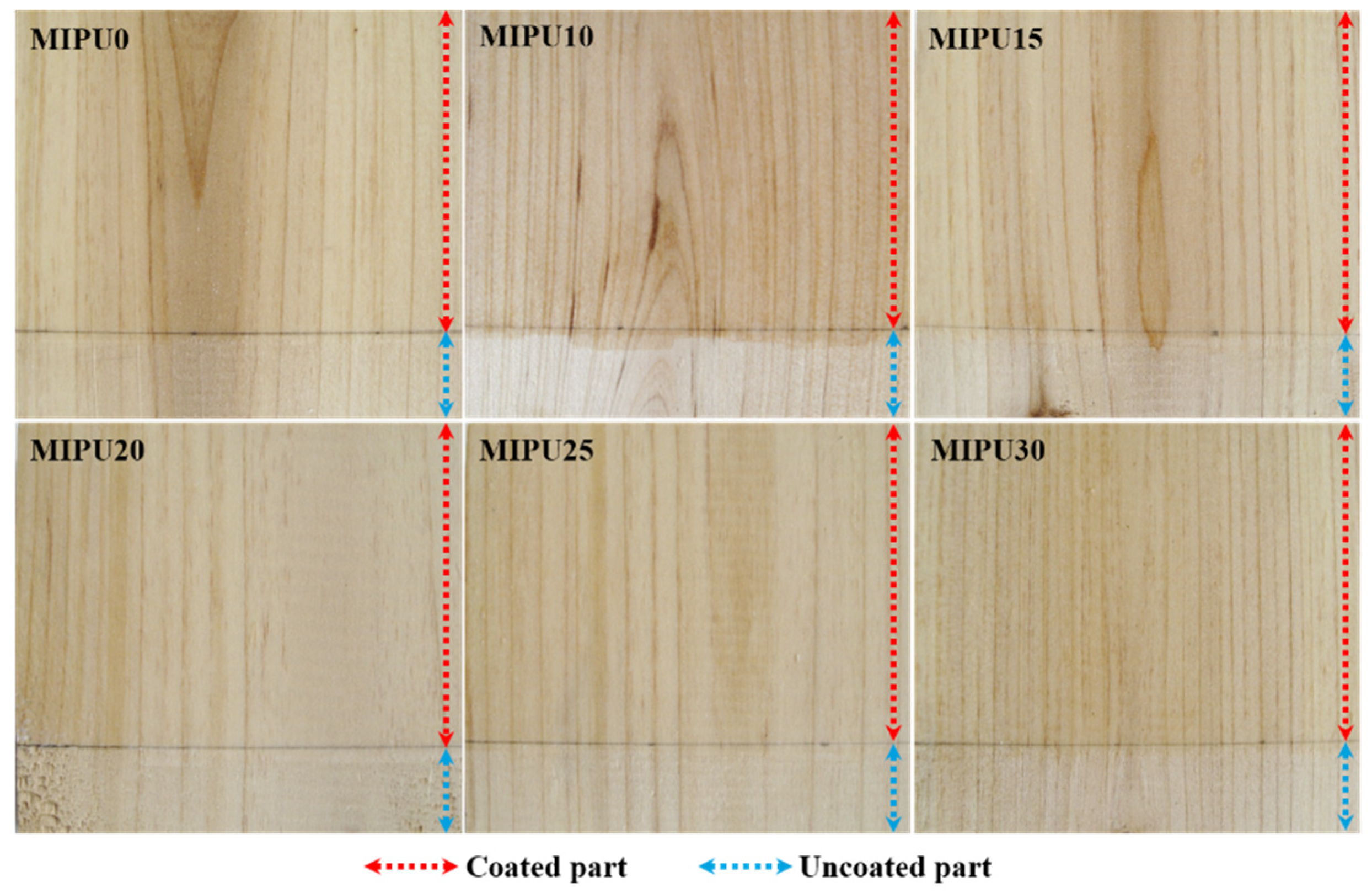
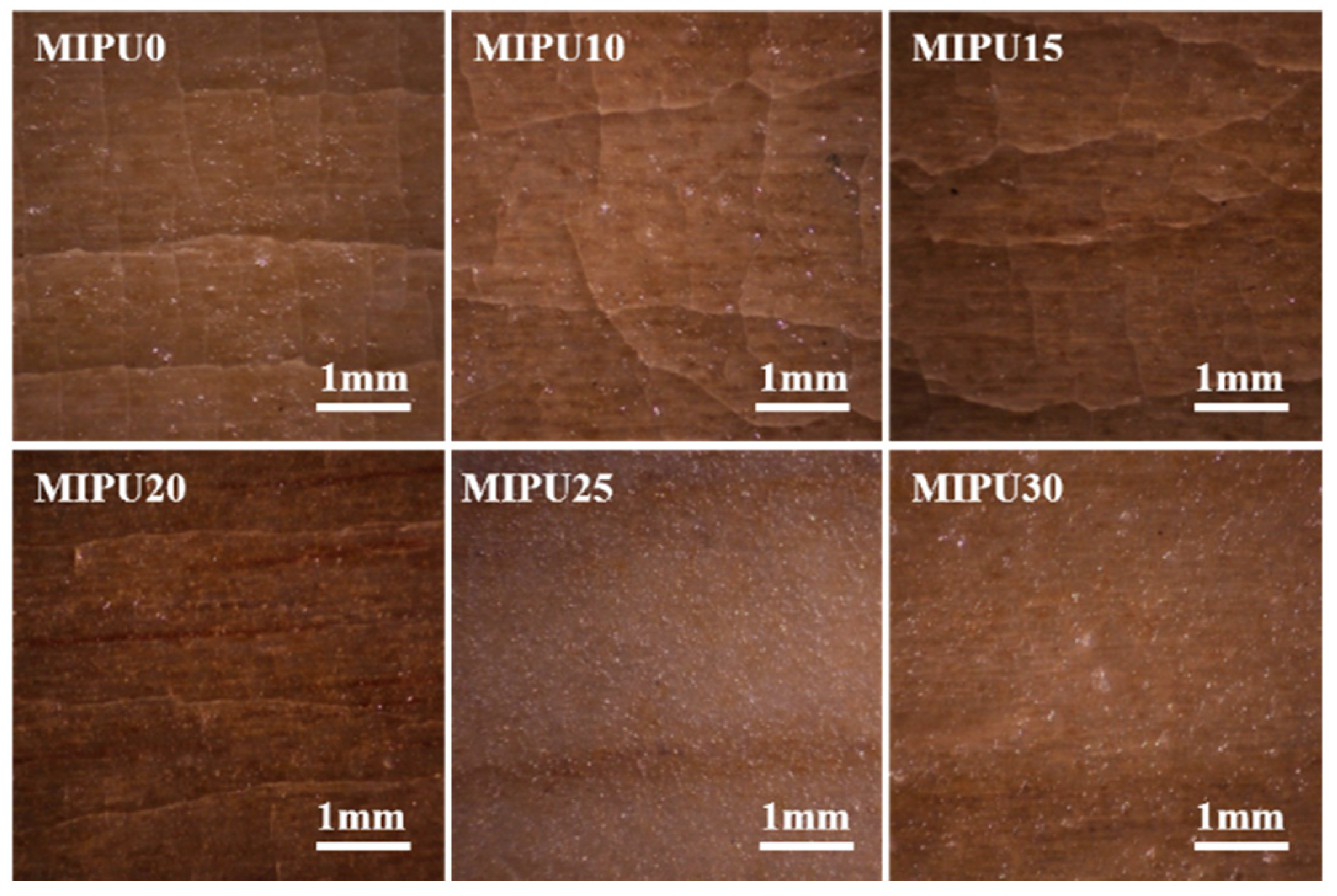
3.2. Crack Resistance of the Coatings
3.3. Thermal Stability of Coatings
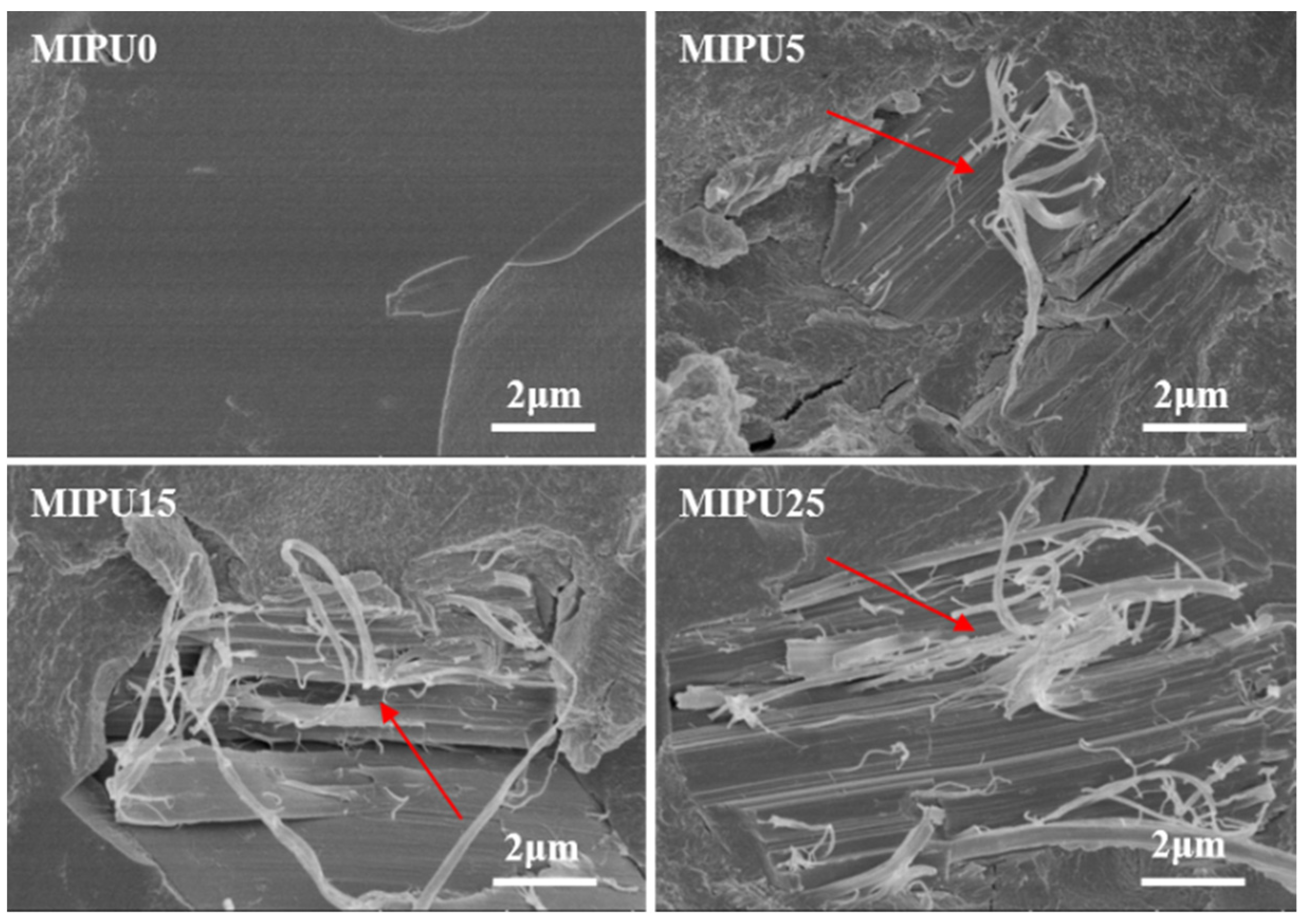
3.4. Morphology of the Expanded Char
3.5. Flame-Retardant Properties of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Guo, J.; Sun, J.; Gu, X.; Feng, W.; Liu, W.; Li, H.; Zhang, S. The preparation of a bisphenol A epoxy resin based ammonium polyphosphate ester and its effect on the char formation of fire resistant transparent coating. Prog. Org. Coat. 2019, 129, 349–356. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, S.; Zhang, Z.; Mai, C.; Xie, Y.; Wang, Q. Fire retardancy of an aqueous, intumescent, and translucent wood varnish based on guanylurea phosphate and melamine-urea-formaldehyde resin. Prog. Org. Coat. 2018, 121, 64–72. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-protective and flame-retardant coatings-A state-of-the-art review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Gu, J.-W.; Zhang, G.-C.; Dong, S.-l.; Zhang, Q.-Y.; Kong, J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Moretto, H.H.; Schulze, M.; Wagner, G. Silicones. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Song, W.; Wu, M.; He, Y.; Wu, Y.; Qu, W. The Evolution of Intumescent Char in Flame-Retardant Coatings Based on Amino Resin. Coatings 2021, 11, 709. [Google Scholar] [CrossRef]
- Hong, L.; Hu, X.; Rao, W.; Zhang, X. Flame retardancy and crack resistance of transparent intumescent fire-resistive coatings. J. Appl. Polym. Sci. 2015, 132, 42423. [Google Scholar] [CrossRef]
- Christensen, G. Analysis of functional groups in amino resins. Prog. Org. Coat. 1980, 8, 211–239. [Google Scholar] [CrossRef]
- Thouless, M. Cracking and delamination of coatings. J. Vac. Sci. Technol. A Vac. Surf. Film. 1991, 9, 2510–2515. [Google Scholar] [CrossRef]
- Maciulaitis, R.; Grigonis, M.; Malaiskiene, J. The impact of the aging of intumescent fire protective coatings on fire resistance. Fire Saf. J. 2018, 98, 15–23. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, N.; Wang, X. Effects of polyhydric alcohols as etherifying agent on the performance of varnish films. J. Hebei Univ. Nat. Sci. Ed. 2014, 34, 262. [Google Scholar]
- Chen, X.; Afreen, S.; Yu, X.; Dong, C.; Kong, Q. Modified melamine-formaldehyde resins improve tensile strength along with antifouling and flame retardancy in impregnation of cellulose paper. RSC Adv. 2019, 9, 36788–36795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, K.; Han, J.; Yu, C.; Fu, S. Graft interpenetrating polymer networks of urethane-modified bismaleimide and epoxy (I): Mechanical behavior and morphology. Polymer 2001, 42, 2491–2500. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Palraj, S.; Maruthan, K.; Selvaraj, M. Preparation and characterization of heat-resistant interpenetrating polymer network (IPN). Prog. Org. Coat. 2007, 59, 21–27. [Google Scholar] [CrossRef]
- Trakulsujaritchok, T.; Hourston, D.J. Damping characteristics and mechanical properties of silica filled PUR/PEMA simultaneous interpenetrating polymer networks. Eur. Polym. J. 2006, 42, 2968–2976. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chou, N.K.; Wu, S.H.; Hsu, S.H.; Whu, S.W.; Ho, G.H.; Tsai, C.L.; Wang, S.S.; Chu, S.H.; Hsieh, K.H. Physical properties of water-borne polyurethane blended with chitosan. J. Appl. Polym. Sci. 2007, 101, 2683–2689. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Mengistie, D.A.; Chen, Y.; Wang, L.; Loghin, C.; Nierstrasz, V. Electrically conductive highly elastic polyamide/lycra fabric treated with PEDOT:PSS and polyurethane. J. Mater. Sci. 2019, 54, 9591–9602. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Zhang, Y.; Wang, C.; Sun, Y.; Yuan, Z.; Pan, Y.; Xie, H.; Cheng, R. Thermal, mechanical, and morphological properties of soybean oil-based polyurethane/epoxy resin interpenetrating polymer networks (IPNs). J. Therm. Anal. Calorim. 2014, 117, 773–781. [Google Scholar] [CrossRef]
- Cheng, H.T.; Lee, Y.S.; Liu, H.C.; Lee, W.J. The effect of component addition order on the properties of epoxy resin/polyurethane resin interpenetrating polymer network structure. J. Appl. Polym. Sci. 2021, 138, 49833. [Google Scholar] [CrossRef]
- Kausar, A. Interpenetrating polymer network and nanocomposite IPN of polyurethane/epoxy: A review on fundamentals and advancements. Polym.-Plast. Technol. Mater. 2019, 58, 691–706. [Google Scholar] [CrossRef]
- Kumar, H.; Somashekar, R.; Mahesh, S. Microcrystalline parameters of polyurethane/polymethyl methacrylate semi interpenetrating polymer networks by WAXS. Mater. Sci. Eng. A 2007, 447, 58–64. [Google Scholar] [CrossRef]
- Roşu, D.; Ciobanu, C.; Caşcaval, C. Polyhydroxyacrylate–polyurethane semi-interpenetrating polymer networks. Eur. Polym. J. 2001, 37, 587–595. [Google Scholar] [CrossRef]
- Vlad, S.; Vlad, A.; Oprea, S. Interpenetrating polymer networks based on polyurethane and polysiloxane. Eur. Polym. J. 2002, 38, 829–835. [Google Scholar] [CrossRef]
- Wang, G.Y.; Hu, C.P. Simultaneous interpenetrating networks consisting of polyurethane and graft vinyl ester resin: Effect of different radical initiators. J. Appl. Polym. Sci. 2002, 84, 1629–1636. [Google Scholar] [CrossRef]
- Liao, H.; Li, H.; Liu, Y.; Wang, Q. A flame-retardant composite foam: Foaming polyurethane in melamine formaldehyde foam skeleton. Polym. Int. 2019, 68, 410–417. [Google Scholar] [CrossRef]
- Jin, F.-L.; Feng, L.; Shi, Q.-B.; Park, S.-J. Physico-mechanical and fire properties of polyurethane/melamine-formaldehyde interpenetrating polymer network foams. Macromol. Res. 2016, 24, 773–776. [Google Scholar] [CrossRef]
- Wu, M.; Song, W.; Wu, Y.; Qu, W. Preparation and characterization of the flame retardant decorated plywood based on the intumescent flame retardant adhesive. Materials 2020, 13, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 5660-1; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone calorimeter Method). International Organization for Standardization: Geneve, Switzerland, 2002.
- Guo, X.; Liu, H.; Zhang, J.; Huang, J. Effects of polyoxymethylene as a polymeric nucleating agent on the isothermal crystallization and visible transmittance of poly (lactic acid). Ind. Eng. Chem. Res. 2014, 53, 16754–16762. [Google Scholar] [CrossRef]
- Jang, B.; Uhlmann, D.R.; Sande, J.V. Ductile—“brittle transition in polymers. J. Appl. Polym. Sci. 1984, 29, 3409–3420. [Google Scholar] [CrossRef]
- Yari, H.; Mohseni, M.; Messori, M. Toughened acrylic/melamine thermosetting clear coats using POSS molecules: Mechanical and morphological studies. Polymer 2015, 63, 19–29. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, S.Y.; Jang, S.G.; Shin, G.Y.; Yoo, J.H. Fusion zone microstructural evolution of Al-10% Si coated hot stamping steel during laser welding. ISIJ Int. 2019, 59, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-T.; Cheng, S.-X.; Zhuo, R.-X. Poly (vinyl alcohol)/poly (N-isopropylacrylamide) semi-interpenetrating polymer network hydrogels with rapid response to temperature changes. Colloid Polym. Sci. 2003, 281, 580–583. [Google Scholar] [CrossRef]
- Moussa, K.; Decker, C. Semi—Interpenetrating polymer networks synthesis by photocrosslinking of acrylic monomers in a polymer matrix. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2633–2642. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Influence of nano-LDHs on char formation and fire-resistant properties of flame-retardant coating. Prog. Org. Coat. 2005, 53, 29–37. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Chen, S.; Zhou, Y. Synthesis and fire properties of rigid polyurethane foams made from a polyol derived from melamine and cardanol. Polym. Degrad. Stab. 2014, 110, 27–34. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and nitrogen-containing polyols: Synergistic effect on the thermal property and flame retardancy of rigid polyurethane foam composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Chen, S.; Li, X.; Ma, H. Synthesis and characterization of water borne intumescent fire retardant varnish based on phosphate resin acid cold cured amino resin. Prog. Org. Coat. 2012, 74, 608–614. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Martinka, J.; Martinka, F.; Rantuch, P.; Hrušovský, I.; Blinová, L.; Balog, K. Calorific value and fire risk of selected fast-growing wood species. J. Therm. Anal. Calorim. 2018, 131, 899–906. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Qin, D.; Sun, J.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. Intumescent flame retardant behavior of triazine group and ammonium polyphosphate in waterborne polyurethane. Polym. Degrad. Stab. 2021, 183, 109439. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, J. Regulating effects of nitrogenous bases on the char structure and flame retardancy of polypropylene/intumescent flame retardant composites. ACS Sustain. Chem. Eng. 2017, 5, 2375–2383. [Google Scholar] [CrossRef]
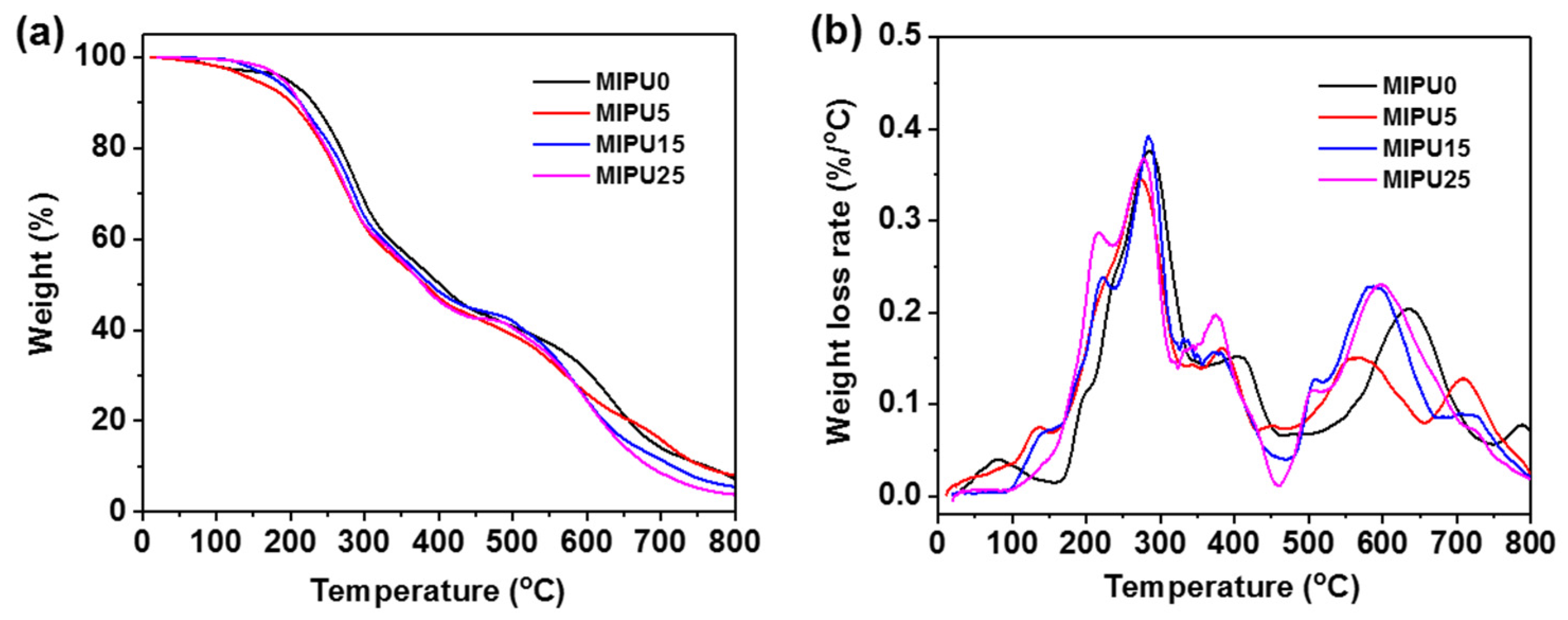

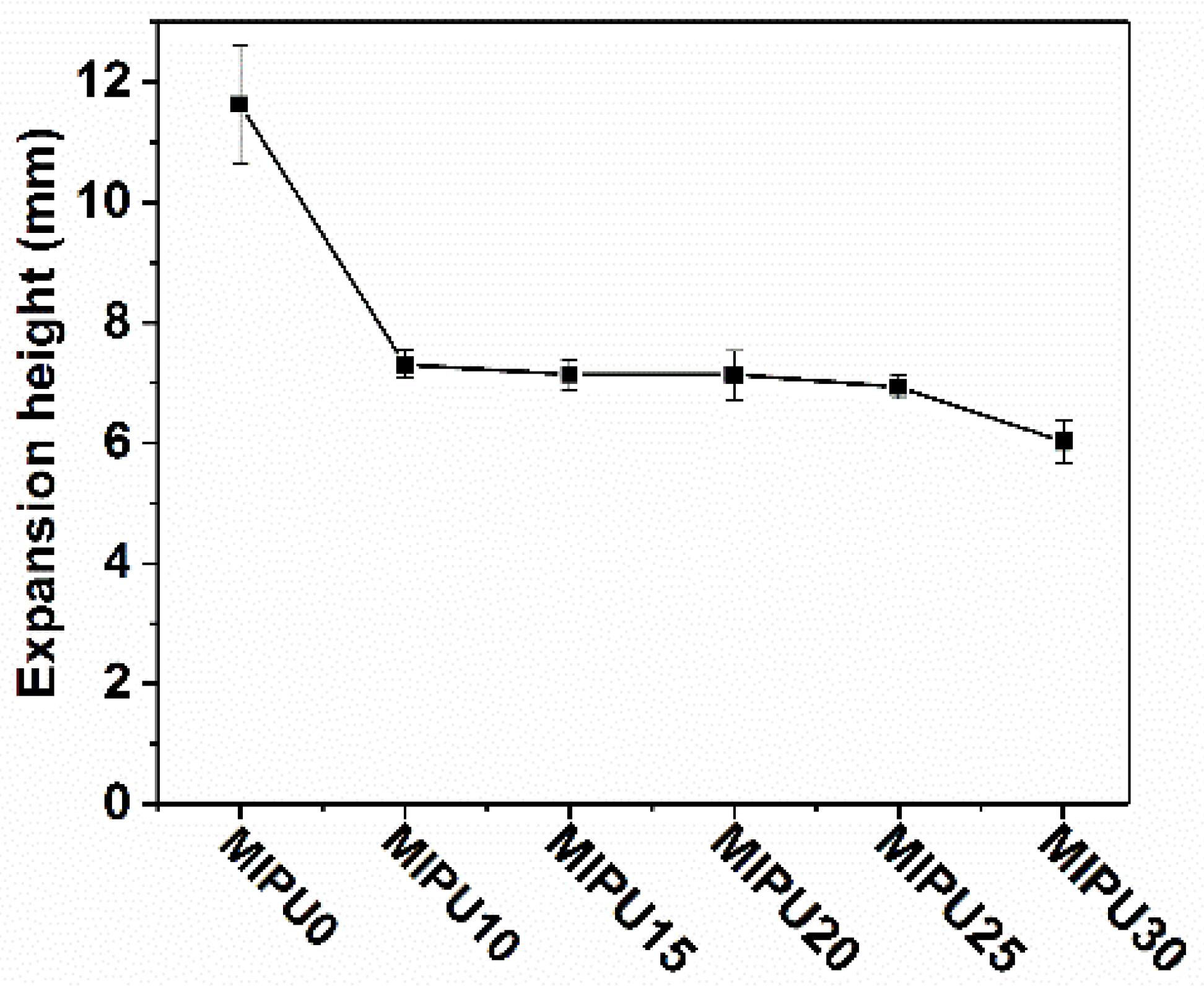



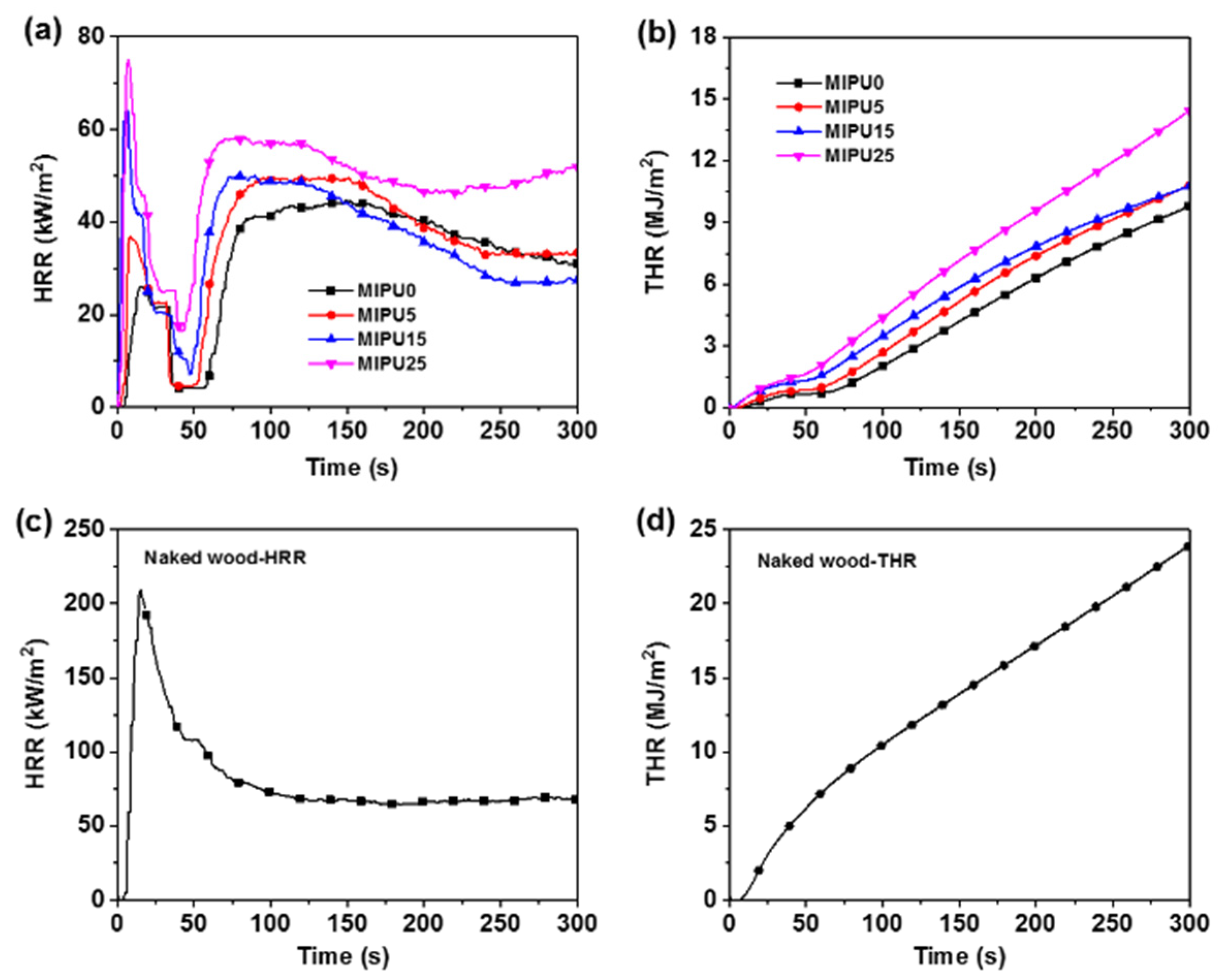
| Sample | Tonset (°C) | Tmax (°C) | Char Residue at (%) | |||
|---|---|---|---|---|---|---|
| a | b | 200 °C | 350 °C | 800 °C | ||
| MIPU0 | 196.0 ± 3.0 | 286.2 ± 3.3 | 634.4 ± 1.1 | 94.5 ± 1.3 | 57.6 ± 1.1 | 7.4 ± 1.2 |
| MIPU5 | 151.1 ± 3.4 | 273.5 ± 3.0 | 570.7 ± 2.0 | 90.2 ± 0.9 | 54.6 ± 0.7 | 8.0 ± 1.0 |
| MIPU15 | 189.7 ± 2.1 | 285.5 ± 2.8 | 588.4 ± 1.6 | 92.5 ± 1.2 | 55.9 ± 1.4 | 5.6 ± 1.1 |
| MIPU25 | 180.7 ± 2.6 | 277.8 ± 3.1 | 597.0 ± 1.4 | 93.2 ± 1.0 | 55.3 ± 1.2 | 3.8 ± 1.0 |
| MIPU30 | 185.5 ± 3.1 | 274.1 ± 3.5 | 595.7 ± 1.1 | 92.5 ± 1.2 | 54.7 ± 1.1 | 4.3 ± 0.8 |
| Sample | pHRR1 (kW/m2) | TTI2 (s) | AveHRR300s (kW/m2) | THR300s (MJ/m2) | TSP300s (m2) |
|---|---|---|---|---|---|
| Naked wood | 209.4 ± 3.5 | - | 79.9 ± 4.9 | 23.9 ± 2.1 | 1.57 ± 0.05 |
| MIPU0 | 26.0 ± 2.8 | 58 ± 1 | 33.4 ± 3.6 | 9.8 ± 1.7 | 0.46 ± 0.02 |
| MIPU5 | 36.8 ± 3.6 | 51 ± 2 | 36.7 ± 3.1 | 10.9 ± 2.0 | 0.50 ± 0.07 |
| MIPU15 | 63.9 ± 4.1 | 48 ± 3 | 36.0 ± 4.4 | 10.8 ± 1.9 | 0.43 ± 0.04 |
| MIPU25 | 75.1 ± 3.8 | 43 ± 1 | 48.7 ± 3.2 | 14.4 ± 1.5 | 0.52 ± 0.07 |
| MIPU30 | 76.2 ± 2.7 | 41 ± 2 | 48.8 ± 3.8 | 14.6 ± 1.9 | 0.31 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wu, Y.; Qu, W.; Zhang, J. Crack-Resistant Amino Resin Flame-Retardant Coatings Using Waterborne Polyurethane as a Co-Binder Resin. Materials 2022, 15, 4122. https://doi.org/10.3390/ma15124122
He Y, Wu Y, Qu W, Zhang J. Crack-Resistant Amino Resin Flame-Retardant Coatings Using Waterborne Polyurethane as a Co-Binder Resin. Materials. 2022; 15(12):4122. https://doi.org/10.3390/ma15124122
Chicago/Turabian StyleHe, Yanrong, Yuzhang Wu, Wei Qu, and Jingpeng Zhang. 2022. "Crack-Resistant Amino Resin Flame-Retardant Coatings Using Waterborne Polyurethane as a Co-Binder Resin" Materials 15, no. 12: 4122. https://doi.org/10.3390/ma15124122
APA StyleHe, Y., Wu, Y., Qu, W., & Zhang, J. (2022). Crack-Resistant Amino Resin Flame-Retardant Coatings Using Waterborne Polyurethane as a Co-Binder Resin. Materials, 15(12), 4122. https://doi.org/10.3390/ma15124122







