Biochar Synthesis from Mineral- and Ash-Rich Waste Biomass, Part 1: Investigation of Thermal Decomposition Mechanism during Slow Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. TGA-DSC
2.3. FT-IR
2.4. Kinetic Measurements
2.5. Statistics
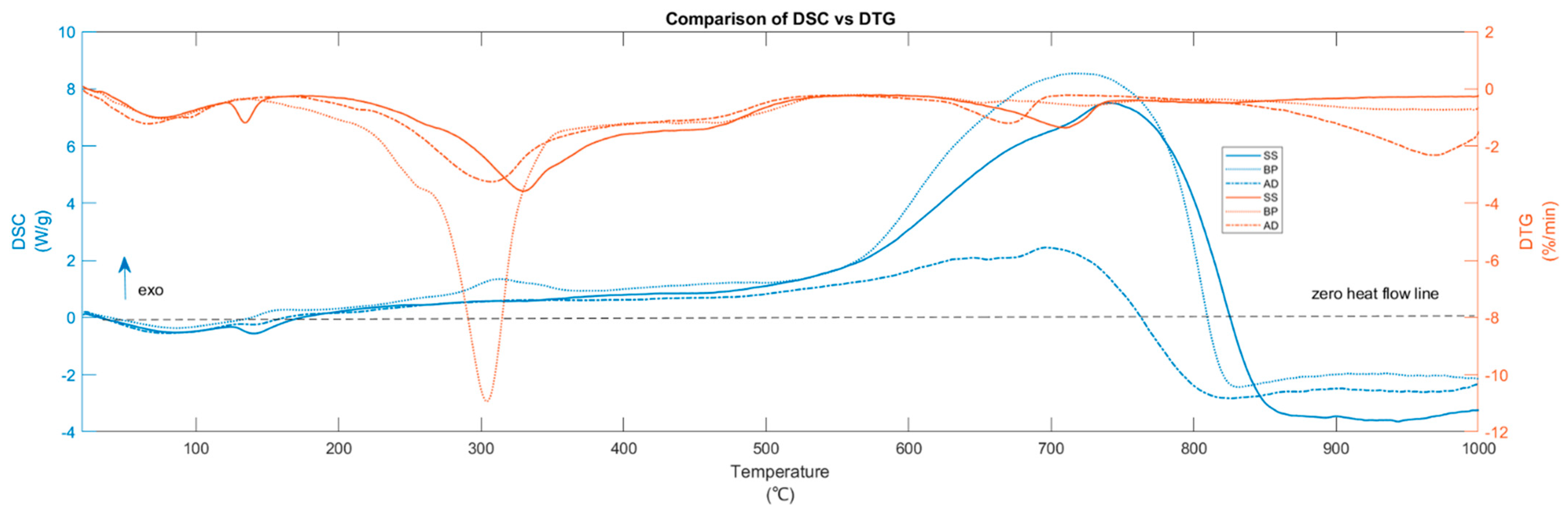
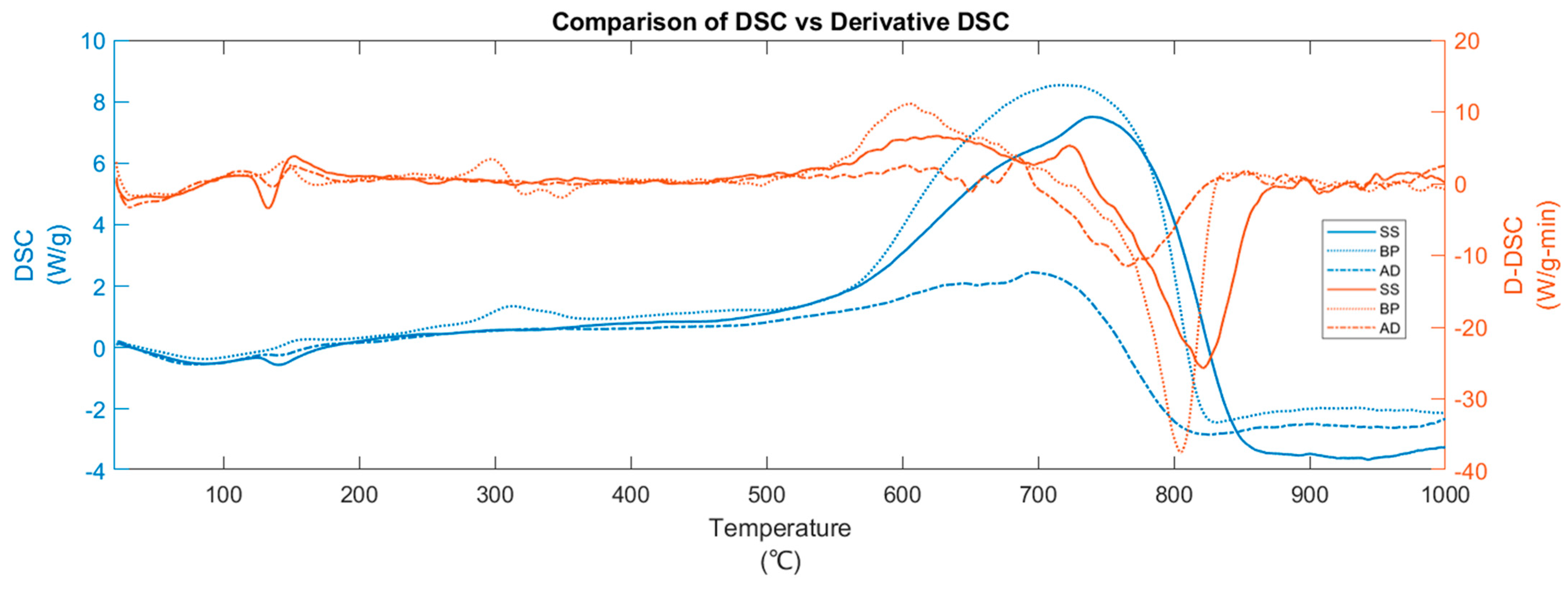
3. Results and Discussion
3.1. Thermal Decomposition Pathway
Catalytic Effect of Inorganics
- Most K may be intercalated with carbon in the biochar matrix due to its high electro positivity and the comparative lack of competing electropositive AAEM species such as Na+, Mg2+, and Ca2+ [60]. This is also an indirect indication of the extent of graphitization of carbon in BP [61]. Furthermore, such intercalated K would be slowly released only at >700 °C [62].
- K also can form loosely and tightly bound metal–oxygen complexes [61]. However, the former is less likely at higher pyrolysis temperatures. The latter is less probable compared to AD due to the lower O/C ratio, which is reduced at higher HTT.
3.2. Evolved Gas Analysis (EGA)
3.2.1. High-Temperature Gasification
3.2.2. NOx Emissions
3.3. Heat of Pyrolysis and Biochar Yield
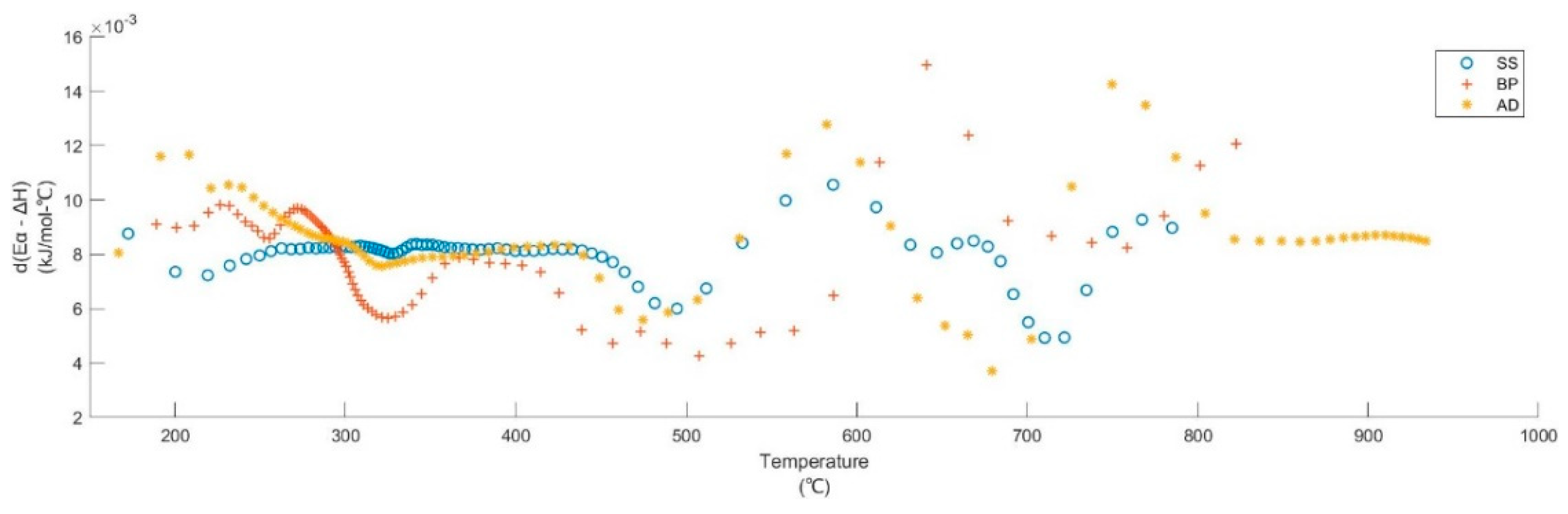
3.4. Thermal Kinetics
3.4.1. Pre-Exponential Factor (A)
3.4.2. Enthalpy
4. Conclusions
- The pyrolysis of BP has the highest heat release and most kinetic favorability. Its biochar yield is the smallest due to high cellulose and low silicate content.
- Amongst the various stages, the highest contribution of exothermicity is from secondary pyrolysis and emission of NOx precursors follows the order SS > AD > BP.
- Based on isoconversional methods, negative apparent activation energies are intrinsic to explain their kinetics after 313, 448, and 339 °C for BP, SS, and AD, respectively.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestate |
| A | Pre-exponential factor corresponding to Eα |
| BP | Banana peduncles |
| D-DSC | Derivative differential scanning calorimetry |
| DSC | Differential scanning calorimetry |
| DTG | Differential thermogravimetry |
| Eα | Apparent activation energy at conversion α |
| FT-IR | Fourier transform infrared spectroscopy |
| HeSTR | Heterogenous secondary tar reactions |
| HHV | Higher heating value |
| HoP | Heat of pyrolysis |
| HoSTR | Homogenous secondary tar reactions |
| HTT | Highest treatment temperature |
| KCE | Kinetic compensation effect |
| MPT | Maximum peak temperature |
| MWB | Mineral- and ash-rich waste biomass |
| NC | Non condensable gases |
| pKCE | Pseudo kinetic compensation effect |
| SS | Sewage sludge |
| TGA | Thermogravimetric analyzer |
| α | Conversion |
References
- Quan, C.; Xu, S.; An, Y.; Liu, X. Co-pyrolysis of biomass and coal blend by TG and in a free fall reactor. J. Therm. Anal. Calorim. 2014, 117, 817–823. [Google Scholar] [CrossRef]
- Marshall, J.; Muhlack, R.; Morton, B.J.; Dunnigan, L.; Chittleborough, D.; Kwong, C.W. Pyrolysis Temperature Effects on Biochar–Water Interactions and Application for Improved Water Holding Capacity in Vineyard Soils. Soil Syst. 2019, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Babu, B.V. Biomass pyrolysis: A state-of-the-art review. Biofuels Bioprod. Biorefin. 2008, 2, 393–414. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Singh, Y.; Gan, Y.Y.; Chen, C.-Y.; Show, P.L. A state-of-the-art review on thermochemical conversion of biomass for biofuel production: A TG-FTIR approach. Energy Convers. Manag. 2020, 209, 112634. [Google Scholar] [CrossRef]
- Rawal, A.; Joseph, S.D.; Hook, J.M.; Chia, C.H.; Munroe, P.R.; Donne, S.; Lin, Y.; Phelan, D.; Mitchell, D.R.; Pace, B.; et al. Mineral-Biochar Composites: Molecular Structure and Porosity. Environ. Sci. Technol. 2016, 50, 7706–7714. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.-H.; Bai, Y.; Hou, D. Progress and future prospects in biochar composites: Application and reflection in the soil environment. Crit. Rev. Environ. Sci. Technol. 2020, 51, 219–271. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Bhattacharya, J. Potassium-iron rice straw biochar composite for sorption of nitrate, phosphate, and ammonium ions in soil for timely and controlled release. Sci. Total Environ. 2020, 712, 136337. [Google Scholar] [CrossRef]
- Cai, T.; Liu, X.; Zhang, J.; Tie, B.; Lei, M.; Wei, X.; Peng, O.; Du, H. Silicate-modified oiltea camellia shell-derived biochar: A novel and cost-effective sorbent for cadmium removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of Electricity and Heat from Biomass Wastes Using a Converted Aircraft Turbine AI-20. Processes 2021, 9, 364. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Vedenyapina, M.D.; Kurmysheva, A.Y.; Weichgrebe, D.; Nair, R.R.; Nguyen, N.P.T.; Kustov, L.M. Modern Carbon-Based Materials for Adsorptive Removal of Organic and Inorganic Pollutants from Water and Wastewater. Molecules 2021, 26, 6628. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.R.; Tariq, R.; Shahbaz, M.; Naqvi, M.; Aslam, M.; Khan, Z.; Mackey, H.; McKay, G.; Al-Ansari, T. Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects. Comput. Chem. Eng. 2021, 150, 107325. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, X.; Wang, K.; Luo, Z. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. [Google Scholar] [CrossRef]
- Meng, A.; Zhou, H.; Qin, L.; Zhang, Y.; Li, Q. Quantitative and kinetic TG-FTIR investigation on three kinds of biomass pyrolysis. J. Anal. Appl. Pyrolysis 2013, 104, 28–37. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. The importance of mineral ingredients in biochar production, properties and applications. Crit. Rev. Environ. Sci. Technol. 2020, 51, 113–139. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Berger, A.; Zobel, N. How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme. Fuel 2014, 123, 230–240. [Google Scholar] [CrossRef]
- BfJ. Bundes-Bodenschutzgesetz vom, Bundessanzeiger Verlag, 17. März 1998 (BGBl. I S. 502). 1998. Available online: http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl198s0502.pdf (accessed on 3 April 2022).
- Chen, X.; Liu, L.; Zhang, L.; Zhao, Y.; Qiu, P. Pyrolysis Characteristics and Kinetics of Coal–Biomass Blends during Co-Pyrolysis. Energy Fuels 2019, 33, 1267–1278. [Google Scholar] [CrossRef]
- Nair, R.R.; Mondal, M.M.; Weichgrebe, D. Biochar from co-pyrolysis of urban organic wastes—Investigation of carbon sink potential using ATR-FTIR and TGA. Biomass Convers. Biorefin. 2020, 1–15. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Czarnecki, J.; Šesták, J. Practical Thermogravimetry. J. Therm. Anal. Calorim. 2000, 60, 759–778. [Google Scholar] [CrossRef]
- Šesták, J. The evaluation of nonisothermal thermoanalytical kinetics is simplified without the description of heat transfers, such as thermal inertia, which is not negligible, as indicated by Vyazovkin. Int. J. Chem. Kinet. 2021, 53, 1050–1057. [Google Scholar] [CrossRef]
- Czarnecki, J.; Sestak, J. From recording balances to thermogravimetric instruments and back. J. Therm. Anal. Calorim. 2015, 120, 157–166. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Wang, C.A.; Feng, Q.Q.; Mao, Q.S.; Wang, C.W.; Li, G.Y.; Che, D.F. Oxy-fuel co-combustion performances and kinetics of bituminous coal and ultra-low volatile carbon-based fuels. Int. J. Energy Res. 2021, 45, 1892–1907. [Google Scholar] [CrossRef]
- Salema, A.A.; Ting, R.M.W.; Shang, Y.K. Pyrolysis of blend (oil palm biomass and sawdust) biomass using TG-MS. Bioresour. Technol. 2019, 274, 439–446. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Zhou, B.; Zhang, Y.; Zhang, M.; Yang, H.; Wang, Z. Experimental investigation of thermal effect in coal pyrolysis process. Fuel Process. Technol. 2020, 200, 106269. [Google Scholar] [CrossRef]
- Vaxelaire, J.; Cezac, P. Moisture distribution in activated sludges: A review. Water Res. 2004, 38, 2214–2229. [Google Scholar] [CrossRef]
- Fisher, T.; Hajaligol, M.; Waymack, B.; Kellogg, D. Pyrolysis behavior and kinetics of biomass derived materials. J. Anal. Appl. Pyrolysis 2002, 62, 331–349. [Google Scholar] [CrossRef]
- Cho, J.; Davis, J.M.; Huber, G.W. The intrinsic kinetics and heats of reactions for cellulose pyrolysis and char formation. ChemSusChem 2010, 3, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, R.; Zhao, B.; Li, Y.; Wang, S.; Wu, H.; Zhuo, Y.; Chen, C. Investigation of heat of biomass pyrolysis and secondary reactions by simultaneous thermogravimetry and differential scanning calorimetry. Fuel 2014, 134, 467–476. [Google Scholar] [CrossRef]
- Mallick, D.; Poddar, M.K.; Mahanta, P.; Moholkar, V.S. Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour. Technol. 2018, 261, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG–FTIR and Py–GC–FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Sebio-Puñal, T.; Naya, S.; López-Beceiro, J.; Tarrío-Saavedra, J.; Artiaga, R. Thermogravimetric analysis of wood, holocellulose, and lignin from five wood species. J. Therm. Anal. Calorim. 2012, 109, 1163–1167. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Dejong, W.; Dinola, G.; Venneker, B.; Spliethoff, H.; Wojtowicz, M. TG-FTIR pyrolysis of coal and secondary biomass fuels: Determination of pyrolysis kinetic parameters for main species and NOx precursors. Fuel 2007, 86, 2367–2376. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.W.; Park, C.; Lee, J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energy Convers. Manag. 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Kopp, J.D.N. The Influence of Free Water Content on Sewage Sludge Dewatering. In Proceedings of the 9th Gothenburg Symposium, Istanbul, Turkey, 2–4 October 2000. [Google Scholar]
- Idris, S.S.; Abd Rahman, N.; Ismail, K.; Alias, A.B.; Abd Rashid, Z.; Aris, M.J. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour. Technol. 2010, 101, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Shukla, S.K.; Upadhyay, S.N.; Mishra, P.K. Analysis of thermal degradation of banana (Musa balbisiana) trunk biomass waste using iso-conversional models. Bioresour. Technol. 2020, 310, 123393. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, S.; Tan, H.; Adeosun, A.; Vujanović, M.; Yang, F.; Duić, N. Synergetic effect of sewage sludge and biomass co-pyrolysis: A combined study in thermogravimetric analyzer and a fixed bed reactor. Energy Convers. Manag. 2016, 118, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Urban, D.; Antaljr, M. Study of the kinetics of sewage sludge pyrolysis using DSC and TGA. Fuel 1982, 61, 799–806. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-pyrolysis of sewage sludge and rice husk by TG–FTIR–MS: Pyrolysis behavior, kinetics, and condensable/non-condensable gases characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Diblasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Zhou, W.; Bai, B.; Chen, G.; Ma, L.; Jing, D.; Yan, B. Study on catalytic properties of potassium carbonate during the process of sawdust pyrolysis. Int. J. Hydrogen Energy 2018, 43, 13829–13841. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C.; Wu, X.; Liang, C.; Chen, X.; Shen, J.; Tang, G.; Wang, Z. Effect of mineral matter on the formation of NOX precursors during biomass pyrolysis. J. Anal. Appl. Pyrolysis 2009, 85, 447–453. [Google Scholar] [CrossRef]
- Sathiamoorthy, S.; Jeyabaskaran, K.J. Potassium management of banana. In Proceedings of the IPI/NARCTT Regional Workshop: Potassium and Water Management in West Asia and North Africa, Amman, Jordan, 5–6 November 2001. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Clery, D.S.; Mason, P.E.; Rayner, C.M.; Jones, J.M. The effects of an additive on the release of potassium in biomass combustion. Fuel 2018, 214, 647–655. [Google Scholar] [CrossRef]
- Jones, J.M.; Darvell, L.I.; Bridgeman, T.G.; Pourkashanian, M.; Williams, A. An investigation of the thermal and catalytic behaviour of potassium in biomass combustion. Proc. Combust. Inst. 2007, 31, 1955–1963. [Google Scholar] [CrossRef]
- Jensen, P.A.; Frandsen, F.J.; Dam-Johansen, K.; Sander, B. Experimental Investigation of the Transformation and Release to Gas Phase of Potassium and Chlorine during Straw Pyrolysis. Energy Fuels 2000, 14, 1280–1285. [Google Scholar] [CrossRef]
- Mason, P.E.; Darvell, L.I.; Jones, J.M.; Williams, A. Observations on the release of gas-phase potassium during the combustion of single particles of biomass. Fuel 2016, 182, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Wornat, M.J.; Hurt, R.H.; Yang, N.Y.C.; Headley, T.J. Structural and Compositional Transformations of Biomass Chars during Combustion. Combust. Flame 1995, 100, 133–145. [Google Scholar] [CrossRef]
- Wigmans, T.; Haringa, H.; Moulijn, J.A. Nature, activity and stability of active sites during alkali metal carbonate-catalysed gasification reactions of coal char. Fuel 1983, 62, 185–189. [Google Scholar] [CrossRef]
- Wood, B.J.; Sancier, K.M. The Mechanism of the Catalytic Gasification of Coal Char: A Critical Review. Catal. Rev. 2006, 26, 233–279. [Google Scholar] [CrossRef]
- Kannan, M.P.; Richards, G.N. Gasification of biomass chars in carbon dioxide: Dependence of gasification rate on the indigenous metal content. Fuel 1990, 69, 747–753. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Progress in Thermochemical Biomass Conversion; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Chen, C.; Luo, Z.; Yu, C.; Wang, T.; Zhang, H. Transformation behavior of potassium during pyrolysis of biomass. RSC Adv. 2017, 7, 31319–31326. [Google Scholar] [CrossRef] [Green Version]
- Lehman, R.L.; Gentry, J.S.; Glumac, N.G. Thermal stability of potassium carbonate near its melting point. Thermochim. Acta 1998, 316, 1–9. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, H.-G. Thermal and carbothermic decomposition of Na2CO3 and Li2CO3. Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Motzfeldt, K. The Thermal Decomposition.of Sodium Carbonate by the Effusion Method. J. Phys. Chem. 2002, 59, 139–147. [Google Scholar] [CrossRef]
- Gomez, C.; Velo, E.; Barontini, F.; Cozzani, V. Influence of Secondary Reactions on the Heat of Pyrolysis of Biomass. Ind. Eng. Chem. Res. 2009, 48, 10222–10233. [Google Scholar] [CrossRef]
- Ferrasse, J.H.; Chavez, S.; Arlabosse, P.; Dupuy, N. Chemometrics as a tool for the analysis of evolved gas during the thermal treatment of sewage sludge using coupled TG–FTIR. Thermochim. Acta 2003, 404, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Scaccia, S. TG–FTIR and kinetics of devolatilization of Sulcis coal. J. Anal. Appl. Pyrolysis 2013, 104, 95–102. [Google Scholar] [CrossRef]
- Calabuig, E.; Juárez-Serrano, N.; Marcilla, A. TG-FTIR study of evolved gas in the decomposition of different types of tobacco. Effect of the addition of SBA-15. Thermochim. Acta 2019, 671, 209–219. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C. Thermal degradation of beech wood with thermogravimetry/Fourier transform infrared analysis. Energy Convers. Manag. 2016, 120, 370–377. [Google Scholar] [CrossRef]
- Li, Y.; Xing, X.; Ma, P.; Zhang, X.; Wu, Y.; Huang, L. Effect of alkali and alkaline earth metals on co-pyrolysis characteristics of municipal solid waste and biomass briquettes. J. Therm. Anal. Calorim. 2019, 139, 489–498. [Google Scholar] [CrossRef]
- Torres-Garcia, E.; Brachi, P. Non-isothermal pyrolysis of grape marc. J. Therm. Anal. Calorim. 2019, 139, 1463–1478. [Google Scholar] [CrossRef]
- Zuo, Z.; Yu, Q.; Xie, H.; Duan, W.; Liu, S.; Qin, Q. Thermogravimetric analysis of the biomass pyrolysis with copper slag as heat carrier. J. Therm. Anal. Calorim. 2017, 129, 1233–1241. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X.; Wu, Z.; Yao, T. TGA–FTIR analysis of co-pyrolysis characteristics of hydrochar and paper sludge. J. Anal. Appl. Pyrolysis 2017, 123, 40–48. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Zhang, D.; Zhu, X.-F. Heat/mass transfer characteristics and nonisothermal drying kinetics at the first stage of biomass pyrolysis. J. Therm. Anal. Calorim. 2011, 109, 847–854. [Google Scholar] [CrossRef]
- Adebisi, J.A.; Agunsoye, J.O.; Bello, S.A.; Kolawole, F.O.; Ramakokovhu, M.M.; Daramola, M.O.; Hassan, S.B. Extraction of Silica from Sugarcane Bagasse, Cassava Periderm and Maize Stalk: Proximate Analysis and Physico-Chemical Properties of Wastes. Waste Biomass Valorization 2017, 10, 617–629. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Trninić, M.R. Modelling and Optimization of Corn Cob Pyrolysis. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2015. [Google Scholar]
- Shi, X.; Ronsse, F.; Pieters, J.G. Finite element modeling of intraparticle heterogeneous tar conversion during pyrolysis of woody biomass particles. Fuel Process. Technol. 2016, 148, 302–316. [Google Scholar] [CrossRef]
- Overend, R.P.; Milne, T.A.; Mudge, L.K. Fundamentals of Thermochemical Biomass Conversion; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Ro, K.S.; Cantrell, K.B.; Hunt, P.G. High-Temperature Pyrolysis of Blended Animal Manures for Producing Renewable Energy and Value-Added Biochar. Ind. Eng. Chem. Res. 2010, 49, 10125–10131. [Google Scholar] [CrossRef]
- Salatino, P.; Senneca, O.; Masi, S. Gasification of a coal char by oxygen and carbon dioxide. Carbon 1998, 36, 443–452. [Google Scholar] [CrossRef]
- Radovic, L.R.; Jiang, H.; Lizzio, A.A. A transient kinetics study of char gasification in carbon dioxide and oxygen. Energy Fuels 1991, 5, 68–74. [Google Scholar] [CrossRef]
- Laurendeau, N.M. Heterogeneous kinetics of coal char gasification and combustion. Prog. Energy Combust. Sci. 1978, 4, 221–270. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Dieguez-Alonso, A.; Zobel, N.; Berger, A.; Kienzl, N.; Behrendt, F. Influence of Heterogeneous Secondary Reactions during Slow Pyrolysis on Char Oxidation Reactivity of Woody Biomass. Energy Fuels 2017, 31, 2335–2344. [Google Scholar] [CrossRef]
- Risnes, H. Calcium addition in straw gasification. Fuel 2003, 82, 641–651. [Google Scholar] [CrossRef]
- Fasina, S.-B.L.a.O. TG-FTIR analysis of switchgrass pyrolysis. In Proceedings of the ASABE Annual International Meeting, Providence, RI, USA, 29 June–2 July 2008. [Google Scholar]
- Zhan, H.; Yin, X.; Huang, Y.; Yuan, H.; Xie, J.; Wu, C.; Shen, Z.; Cao, J. Comparisons of Formation Characteristics of NOx Precursors during Pyrolysis of Lignocellulosic Industrial Biomass Wastes. Energy Fuels 2017, 31, 9557–9567. [Google Scholar] [CrossRef]
- Li, C.-Z.; Tan, L.L. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis. Fuel 2000, 79, 1899–1906. [Google Scholar] [CrossRef]
- Chen, X.; Jeyaseelan, S. Study of Sewage Sludge Pyrolysis Mechanism and Mathematical Modeling. J. Environ. Eng. 2001, 127, 585–593. [Google Scholar] [CrossRef]
- Tian, F.-J.; Yu, J.; McKenzie, L.J.; Hayashi, J.-i.; Li, C.-Z. Conversion of Fuel-N into HCN and NH3 during the Pyrolysis and Gasification in Steam: A Comparative Study of Coal and Biomass†. Energy Fuels 2007, 21, 517–521. [Google Scholar] [CrossRef]
- Werner, K.; Pommer, L.; Broström, M. Thermal decomposition of hemicelluloses. J. Anal. Appl. Pyrolysis 2014, 110, 130–137. [Google Scholar] [CrossRef]
- Roberts, A.F. The heat of reaction during the pyrolysis of wood. Combust. Flame 1971, 17, 79–86. [Google Scholar] [CrossRef]
- Seo, D.K.; Park, S.S.; Kim, Y.T.; Hwang, J.; Yu, T.-U. Study of coal pyrolysis by thermo-gravimetric analysis (TGA) and concentration measurements of the evolved species. J. Anal. Appl. Pyrolysis 2011, 92, 209–216. [Google Scholar] [CrossRef]
- Rath, J. Heat of wood pyrolysis. Fuel 2003, 82, 81–91. [Google Scholar] [CrossRef]
- Bourke, J.; Manley-Harris, M.; Fushimi, C.; Dowaki, K.; Nunoura, T.; Antal, M.J. Do All Carbonized Charcoals Have the Same Chemical Structure? 2. A Model of the Chemical Structure of Carbonized Charcoal. Ind. Eng. Chem. Res. 2007, 46, 5954–5967. [Google Scholar] [CrossRef]
- Yoo, S.; Chung, C.-C.; Kelley, S.S.; Park, S. Graphitization Behavior of Loblolly Pine Wood Investigated by in Situ High Temperature X-ray Diffraction. ACS Sustain. Chem. Eng. 2018, 6, 9113–9119. [Google Scholar] [CrossRef]
- Harris, P.J.F. Non-Graphitizing Carbon: Its Structure and Formation from Organic Precursors. Eurasian Chem. Technol. J. 2019, 21, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Sbirrazzuoli, N. Is the Friedman Method Applicable to Transformations with Temperature Dependent Reaction Heat? Macromol. Chem. Phys. 2007, 208, 1592–1597. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Kinetic Analysis of Thermally Stimulated Processes in Polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Carrier, M.; Auret, L.; Bridgwater, A.; Knoetze, J.H. Using Apparent Activation Energy as a Reactivity Criterion for Biomass Pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Jeong, K.; Kim, S.-S.; Brown, R.C. Kinetic understanding of the effect of Na and Mg on pyrolytic behavior of lignin using a distributed activation energy model and density functional theory modeling. Green Chem. 2019, 21, 1099–1107. [Google Scholar] [CrossRef]
- Arenas, C.N.; Navarro, M.V.; Martinez, J.D. Pyrolysis kinetics of biomass wastes using isoconversional methods and the distributed activation energy model. Bioresour. Technol. 2019, 288, 121485. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qiao, Y.; Zong, P.; Wang, C.; Tian, Y.; Qin, S. Thermogravimetric Analysis and Isoconversional Kinetic Study of Biomass Pyrolysis Derived from Land, Coastal Zone, and Marine. Energy Fuels 2019, 33, 3299–3310. [Google Scholar] [CrossRef]
- Matouq, M.; Amer, M.W.; Al-Ayed, O.S. Variable Activation Energy Principle to Model Oil Shale Pyrolysis Kinetics. Oil Shale 2017, 34, 181–194. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.; Li, G. Pyrolysis characteristic and kinetic analysis of sewage sludge using model-free and master plots methods. Process. Saf. Environ. Prot. 2021, 149, 48–55. [Google Scholar] [CrossRef]
- Wu, W.; Mei, Y.; Zhang, L.; Liu, R.; Cai, J. Effective Activation Energies of Lignocellulosic Biomass Pyrolysis. Energy Fuels 2014, 28, 3916–3923. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Garcia-Hernando, N.; Garcia-Gutierrez, L.M.; Ruiz-Rivas, U. Analysis of biomass and sewage sludge devolatilization using the distributed activation energy model. Energy Convers. Manag. 2013, 65, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhao, B.; Tian, X.; Wang, K.; Tian, Z.; Han, W.; Bian, H. Study on the Pyrolysis Kinetics and Mechanisms of the Tread Compounds of Silica-Filled Discarded Car Tires. Polymers 2020, 12, 810. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, S.; Topcu, Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour. Technol. 2014, 156, 182–188. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Isoconversional determination of the apparent reaction models governing pyrolysis of wood, straw and sewage sludge, with an approach to rate modelling. Renew. Energy 2020, 161, 972–987. [Google Scholar] [CrossRef]
- Ariffen, A.R.; Yusoff, N. Kinetic analysis of co-pyrolysis of biomass/sorbent mixtures at different ratios. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012117. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Ali, I.; Nasir, S.; Ali Ammar Taqvi, S.; Atabani, A.E.; Chen, W.-H. Assessment of agro-industrial residues for bioenergy potential by investigating thermo-kinetic behavior in a slow pyrolysis process. Fuel 2020, 278, 118259. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Analysis of Calorimetric Data on Nonisothermal Crystallization of a Polymer Melt. J. Phys. Chem. B 2002, 107, 882–888. [Google Scholar] [CrossRef]
- Patnaik, A.S.; Goldfarb, J. Continuous activation energy representation of the Arrhenius equation for the pyrolysis of cellulosic materials: Feed corn stover and cocoa shell biomass. Cellul. Chem. Technol. 2016, 50, 311–320. [Google Scholar]
- Scott, S.; Dennis, J.; Davidson, J.; Hayhurst, A. Thermogravimetric measurements of the kinetics of pyrolysis of dried sewage sludge. Fuel 2006, 85, 1248–1253. [Google Scholar] [CrossRef]
- Helfferich, F.G. Kinetics of Multistep Reactions (Comprehensive Chemical Kinetics), 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 40. [Google Scholar]
- Vyazovkin, S.V.; Lesnikovich, A.I. An approach to the solution of the inverse kinetic problem in the case of complex processes. Thermochim. Acta 1990, 165, 273–280. [Google Scholar] [CrossRef]
- Valverde, J.M. On the negative activation energy for limestone calcination at high temperatures nearby equilibrium. Chem. Eng. Sci. 2015, 132, 169–177. [Google Scholar] [CrossRef]
- Völker, S.; Rieckmann, T. Thermokinetic investigation of cellulose pyrolysis—Impact of initial and final mass on kinetic results. J. Anal. Appl. Pyrolysis 2002, 62, 165–177. [Google Scholar] [CrossRef]
- Bichen Liu, Q.F.; Wu, W.; Hu, Y. Structure and Reactivity of Rice Husk Chars under Different Bulk Densities. BioResources 2019, 14, 8289–8303. [Google Scholar]
- Okekunle, P.O.; Watanabe, H.; Pattanotai, T.; Okazaki, K. Effect of Biomass Size and Aspect Ratio on Intra-Particle Tar Decomposition during Wood Cylinder Pyrolysis. J. Therm. Sci. Technol. 2012, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Barrie, P.J. The mathematical origins of the kinetic compensation effect: 1. The effect of random experimental errors. Phys. Chem. Chem. Phys. 2012, 14, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Gelb, R.I.; Alper, J.S. Compensation effects in the complexation reactions of α- and β-cyclodextrin. J. Phys. Org. Chem. 1995, 8, 825–832. [Google Scholar] [CrossRef]
- Flynn, J.H. Temperature dependence of the rate of reaction in thermal analysis. J. Therm. Anal. 1990, 36, 1579–1593. [Google Scholar] [CrossRef]
- Huang, Y.W.; Chen, M.Q.; Li, Y. An innovative evaluation method for kinetic parameters in distributed activation energy model and its application in thermochemical process of solid fuels. Thermochim. Acta 2017, 655, 42–51. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Mohanty, K. Kinetic analysis and pyrolysis behavior of low-value waste lignocellulosic biomass for its bioenergy potential using thermogravimetric analyzer. Mater. Sci. Energy Technol. 2021, 4, 136–147. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Liu, H.; Imran, M.; Snape, C.E. Thermal and kinetic analysis of diverse biomass fuels under different reaction environment: A way forward to renewable energy sources. Energy Convers. Manag. 2020, 203, 112266. [Google Scholar] [CrossRef]
- dos Reis Ferreira, R.A.; da Silva Meireles, C.; Assunção, R.M.N.; Reis Soares, R. Heat required and kinetics of sugarcane straw pyrolysis by TG and DSC analysis in different atmospheres. J. Therm. Anal. Calorim. 2018, 132, 1535–1544. [Google Scholar] [CrossRef]
- Chen, J.; Mu, L.; Jiang, B.; Yin, H.; Song, X.; Li, A. TG/DSC-FTIR and Py-GC investigation on pyrolysis characteristics of petrochemical wastewater sludge. Bioresour. Technol. 2015, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.O.; Araujo, R.O.; Ribeiro, F.C.P.; Queiroz, L.S.; Guimarães, M.N.; Colpani, D.; da Costa, C.E.F.; Chaar, J.S.; de Souza, L.K.C. Non-isothermal kinetics evaluation of buriti and inaja seed biomass waste for pyrolysis thermochemical conversion technology. Biomass Convers. Biorefin. 2021, 1–17. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Liu, C.G.; Tawab, A.; Bai, F.W.; Sakdaronnarong, C.; Xu, J.; Rahimuddin, S.A.; Gull, M. Bioenergy potential of Wolffia arrhiza appraised through pyrolysis, kinetics, thermodynamics parameters and TG-FTIR-MS study of the evolved gases. Bioresour. Technol. 2018, 253, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.A.; Ye, G.; Luo, H.; Liu, C.; Malik, S.; Afzal, I.; Xu, J.; Ahmad, M.S. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour. Technol. 2017, 228, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Luo, H.; Ren, Z.; Ahmad, M.S.; Liu, C.-G.; Tawab, A.; Al-Ghafari, A.B.; Omar, U.; Gull, M.; Mehmood, M.A. Evaluating the bioenergy potential of Chinese Liquor-industry waste through pyrolysis, thermogravimetric, kinetics and evolved gas analyses. Energy Convers. Manag. 2018, 163, 13–21. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Basile, L.; Tugnoli, A.; Stramigioli, C.; Cozzani, V. Thermal effects during biomass pyrolysis. Thermochim. Acta 2016, 636, 63–70. [Google Scholar] [CrossRef]
- Blasi, C.D.; Galgano, A.; Branca, C. Exothermic Events of Nut Shell and Fruit Stone Pyrolysis. ACS Sustain. Chem. Eng. 2019, 7, 9035–9049. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Galiwango, E.; Al-Marzuoqi, A.H.; Khaleel, A.A.; Abu-Omar, M.M. Investigation of Non-Isothermal Kinetics and Thermodynamic Parameters for the Pyrolysis of Different Date Palm Parts. Energies 2020, 13, 6553. [Google Scholar] [CrossRef]
- Mafu, L.D.; Neomagus, H.; Everson, R.C.; Strydom, C.A.; Carrier, M.; Okolo, G.N.; Bunt, J.R. Chemical and structural characterization of char development during lignocellulosic biomass pyrolysis. Bioresour. Technol. 2017, 243, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, Q.; Luo, Z.; Wen, L.; Cen, K. Mechanism study on cellulose pyrolysis using thermogravimetric analysis coupled with infrared spectroscopy. Front. Energy Power Eng. China 2007, 1, 413–419. [Google Scholar] [CrossRef]
- Vyazovkin, S. A time to search: Finding the meaning of variable activation energy. Phys. Chem. Chem. Phys. 2016, 18, 18643–18656. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Órfão, J.J.M. Review and evaluation of the approximations to the temperature integral. AIChE J. 2007, 53, 2905–2915. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2000, 22, 178–183. [Google Scholar] [CrossRef]
- Burnham, A.K.; Dinh, L.N. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J. Therm. Anal. Calorim. 2007, 89, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes, 1st ed.; Springer: Cham, Switzerland, 2015; p. 239. [Google Scholar]
- Sapunov, V.N.; Saveljev, E.A.; Voronov, M.S.; Valtiner, M.; Linert, W. The Basic Theorem of Temperature-Dependent Processes. Thermo 2021, 1, 45–60. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Determination of pre-exponential factors and of the mathematical functions f(α) or G(α) that describe the reaction mechanism in a model-free way. Thermochim. Acta 2013, 564, 59–69. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.X. Isokinetic relationship, isoequilibrium relationship, and enthalpy-entropy compensation. Chem. Rev. 2001, 101, 673–695. [Google Scholar] [CrossRef]
- Koga, N. A review of the mutual dependence of Arrhenius parameters evaluated by the thermoanalytical study of solid-state reactions: The kinetic compensation effect. Thermochim. Acta 1994, 244, 1–20. [Google Scholar] [CrossRef]
- L’vov, B.V.; Galwey, A.K. Interpretation of the kinetic compensation effect in heterogeneous reactions: Thermochemical approach. Int. Rev. Phys. Chem. 2013, 32, 515–557. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Zhang, L.; Zhao, Y.; Zhang, Z.; Xie, X.; Qiu, P.; Chen, G.; Pei, J. Thermogravimetric analysis and kinetics of the co-pyrolysis of coal blends with corn stalks. Thermochim. Acta 2018, 659, 59–65. [Google Scholar] [CrossRef]
- Boehrk, H.; Stokes, J.L. Kinetic Parameters and Thermal Properties of a Cork-Based Material. In Proceedings of the 20th AIAA International Space Planes and Hypersonic Systems and Technologies Conference, Glasgow, Scotland, 6–9 July 2015. [Google Scholar]
- Parthasarathy, P.; Narayanan, K.S.; Arockiam, L. Study on kinetic parameters of different biomass samples using thermo-gravimetric analysis. Biomass Bioenergy 2013, 58, 58–66. [Google Scholar] [CrossRef]
- Celebi, M.C.; Karatepe, N. An Investigation of Thermal Decomposition Behavior of Hazelnut Shells. Int. J. Green Energy 2014, 12, 93–97. [Google Scholar] [CrossRef]
- Pilling, M.J. Low-Temperature Combustion and Autoignition; Elsevier: Amsterdam, The Netherlands, 1997; Volume 35. [Google Scholar]
- McCarty, J.G. Kinetics of PdO combustion catalysis. Catal. Today 1995, 26, 283–293. [Google Scholar] [CrossRef]
- Veser, G.; Wright, A.; Caretta, R. On the oxidation–reduction kinetics of palladium. Catal. Lett. 1999, 58, 199–206. [Google Scholar] [CrossRef]
- Senneca, O.; Ontyd, C.; Cerciello, F.; Schiemann, M.; Scherer, V. Extension of the Thermal Annealing Concepts Developed for Coal Combustion to Conversion of Lignocellulosic Biomass. Energy Fuels 2020, 34, 3661–3670. [Google Scholar] [CrossRef]
- Senneca, O.; Scala, F.; Chirone, R.; Salatino, P. Relevance of structure, fragmentation and reactivity of coal to combustion and oxy-combustion. Fuel 2017, 201, 65–80. [Google Scholar] [CrossRef]
- Senneca, O.; Salatino, P. A semi-detailed kinetic model of char combustion with consideration of thermal annealing. Proc. Combust. Inst. 2011, 33, 1763–1770. [Google Scholar] [CrossRef]




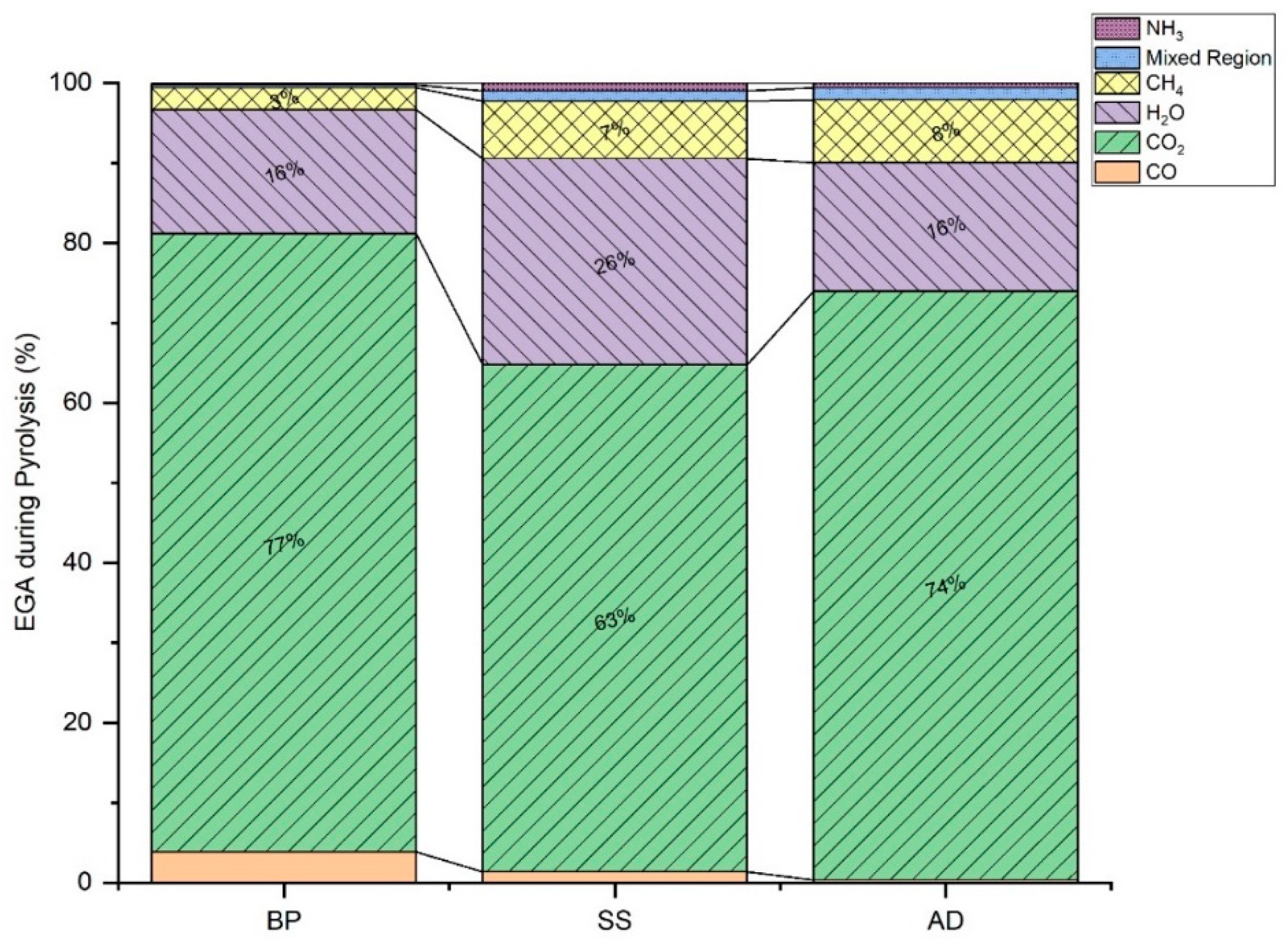
| Evolved Gases | Wavelength (cm−1) |
|---|---|
| CO2 | 2400–2250 |
| CO | 2250–2000 |
| H2O | 3990–3400 |
| CH4 | 3020–2800 |
| Mixed Region | 1200–1000 |
| NH3 | 980–920 |
| Sample | Conversions | R2 of Pseudo KCE at Different Heating Rates | |||||
|---|---|---|---|---|---|---|---|
| α | 5 K/min | 7 K/min | 10 K/min | 12 K/min | 15 K/min | 20 K/min | |
| BP | 0.08 to 0.53 | 0.943 | 0.971 | 0.962 | 0.963 | 0.930 | 0.952 |
| SS | 0.12 to 0.64 | 0.885 | 0.823 | 0.902 | 0.900 | 0.893 | 0.885 |
| AD | 0.08 to 0.39 | 0.849 | 0.895 | 0.894 | 0.826 | 0.793 | 0.763 |
| Substrate | Temperatures of Interest | Predominant Reactions | Weight Loss % | Heat of Pyrolysis | Reaction Progress | MPT | DTG | Predominant Evolved Gases |
|---|---|---|---|---|---|---|---|---|
| °C | % | kJ/kg | % | °C | %/min | |||
| BP | 45–119 | Drying | 3.95 | −99.49 | 5.10 | 76.61 | −1.06 | H2O |
| 156–519 | Active pyrolysis | 57.24 | 1172.9 | 73.83 | 304.32 | −10.93 | CO2, CO, CH4, H2O, volatiles | |
| 519–699 | Secondary Pyrolysis | 3.97 | 3237.8 | 5.12 | NA | −0.49 | CO, CO2, CH4 | |
| 699–744 | Carbonate decomposition + secondary pyrolysis | 1.68 | 1529.5 | 2.16 | 724.23 | −0.59 | CO, CO2 | |
| 869–1000 | Other inorganic decomposition + gasification | 5.79 | −1087.2 | 7.48 | 995.88 | −0.73 | CO, CO2 | |
| SS | 49–120 | Drying | 3.64 | −152.8 | 6.70 | 75.08 | −1.00 | H2O |
| 122–145 | Extended drying | 1.21 | −50.7 | 2.23 | 134.41 | −1.18 | H2O | |
| 221–510 | Active pyrolysis | 32.57 | 663.2 | 59.84 | 329.90 | −3.57 | CO2, CH4, H2O, NH3 | |
| 510–644 | Secondary pyrolysis | 2.64 | 1306.1 | 4.85 | NA | −0.49 | CO2, H2O | |
| 644–737 | Carbonate decomposition + Secondary pyrolysis | 5.76 | 2300.2 | 10.59 | 709.23 | −1.36 | CO, CO2 | |
| 737–1000 | Gasification | 6.65 | −440.6 | 12.21 | 809.09 | −0.49 | CO, CO2, CH4 | |
| AD | 37–120 | Drying | 5.06 | −197.0 | 7.93 | 66.35 | −1.22 | H2O |
| 196–499 | Active pyrolysis | 31.03 | 432.1 | 48.58 | 307.75 | −3.25 | CO2, CH4, H2O | |
| 499–629 | Secondary pyrolysis | 2.73 | 582.6 | 4.28 | NA | −0.49 | CO2, H2O | |
| 629–690 | Carbonate decomposition + Secondary pyrolysis | 3.69 | 469.0 | 5.77 | 669.59 | −1.20 | CO, CO2 | |
| 821–1000 | Other inorganic decomposition + gasification | 16.30 | −1964.1 | 25.52 | 968.93 | −2.32 | CO, CO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, R.R.; Mondal, M.M.; Srinivasan, S.V.; Weichgrebe, D. Biochar Synthesis from Mineral- and Ash-Rich Waste Biomass, Part 1: Investigation of Thermal Decomposition Mechanism during Slow Pyrolysis. Materials 2022, 15, 4130. https://doi.org/10.3390/ma15124130
Nair RR, Mondal MM, Srinivasan SV, Weichgrebe D. Biochar Synthesis from Mineral- and Ash-Rich Waste Biomass, Part 1: Investigation of Thermal Decomposition Mechanism during Slow Pyrolysis. Materials. 2022; 15(12):4130. https://doi.org/10.3390/ma15124130
Chicago/Turabian StyleNair, Rahul Ramesh, Moni Mohan Mondal, Shanmugham Venkatachalam Srinivasan, and Dirk Weichgrebe. 2022. "Biochar Synthesis from Mineral- and Ash-Rich Waste Biomass, Part 1: Investigation of Thermal Decomposition Mechanism during Slow Pyrolysis" Materials 15, no. 12: 4130. https://doi.org/10.3390/ma15124130






