Bonding of Clear Aligner Composite Attachments to Ceramic Materials: An In Vitro Study
Abstract
:1. Introduction
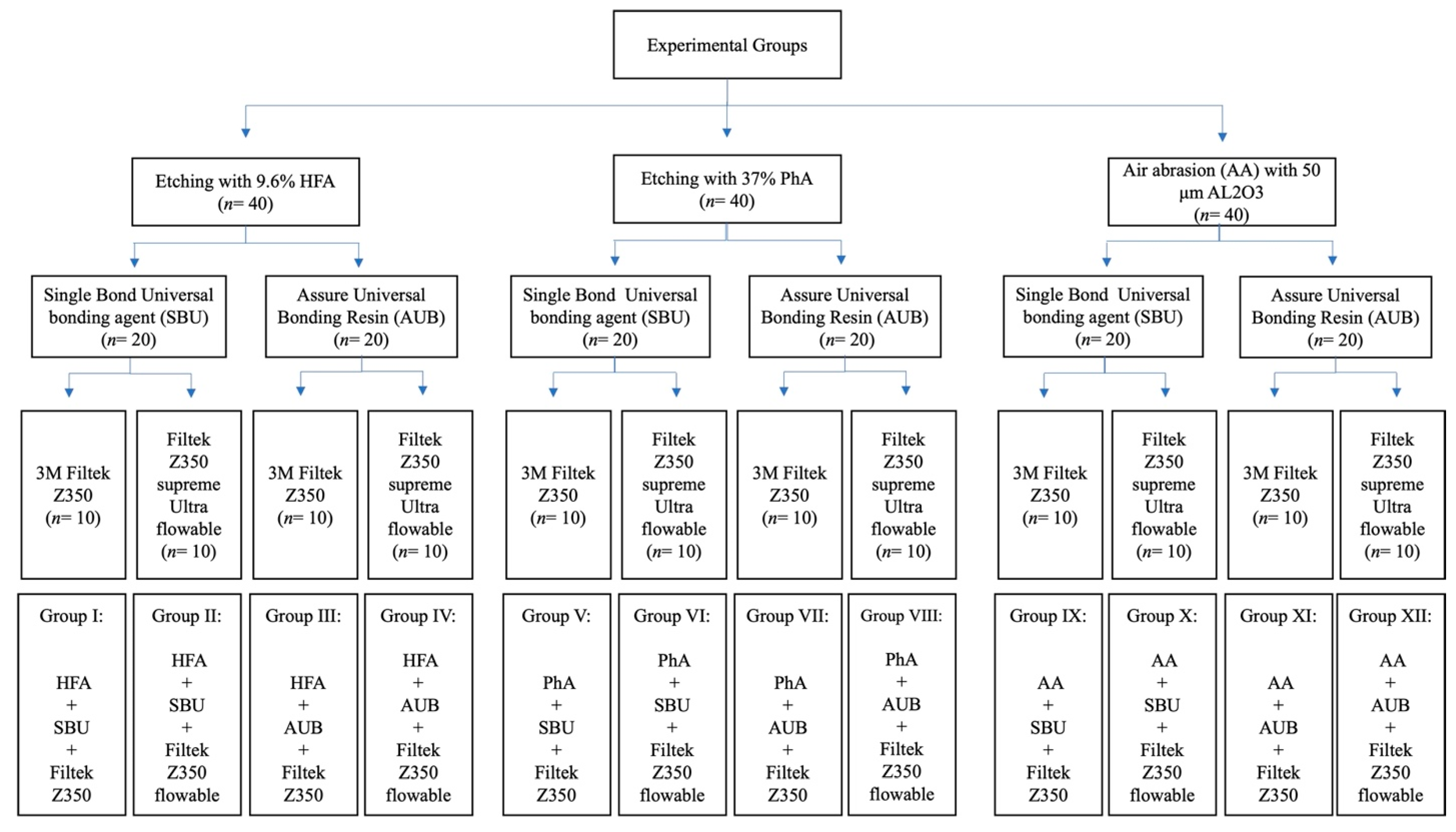
2. Materials and Methods
2.1. Materials
2.2. Sample Size Calculation
2.3. Ceramic Specimen Preparation
2.4. Surface Roughness Test
2.5. Shear Bond Strength (SBS) Test
2.6. Statistical Analysis
3. Results
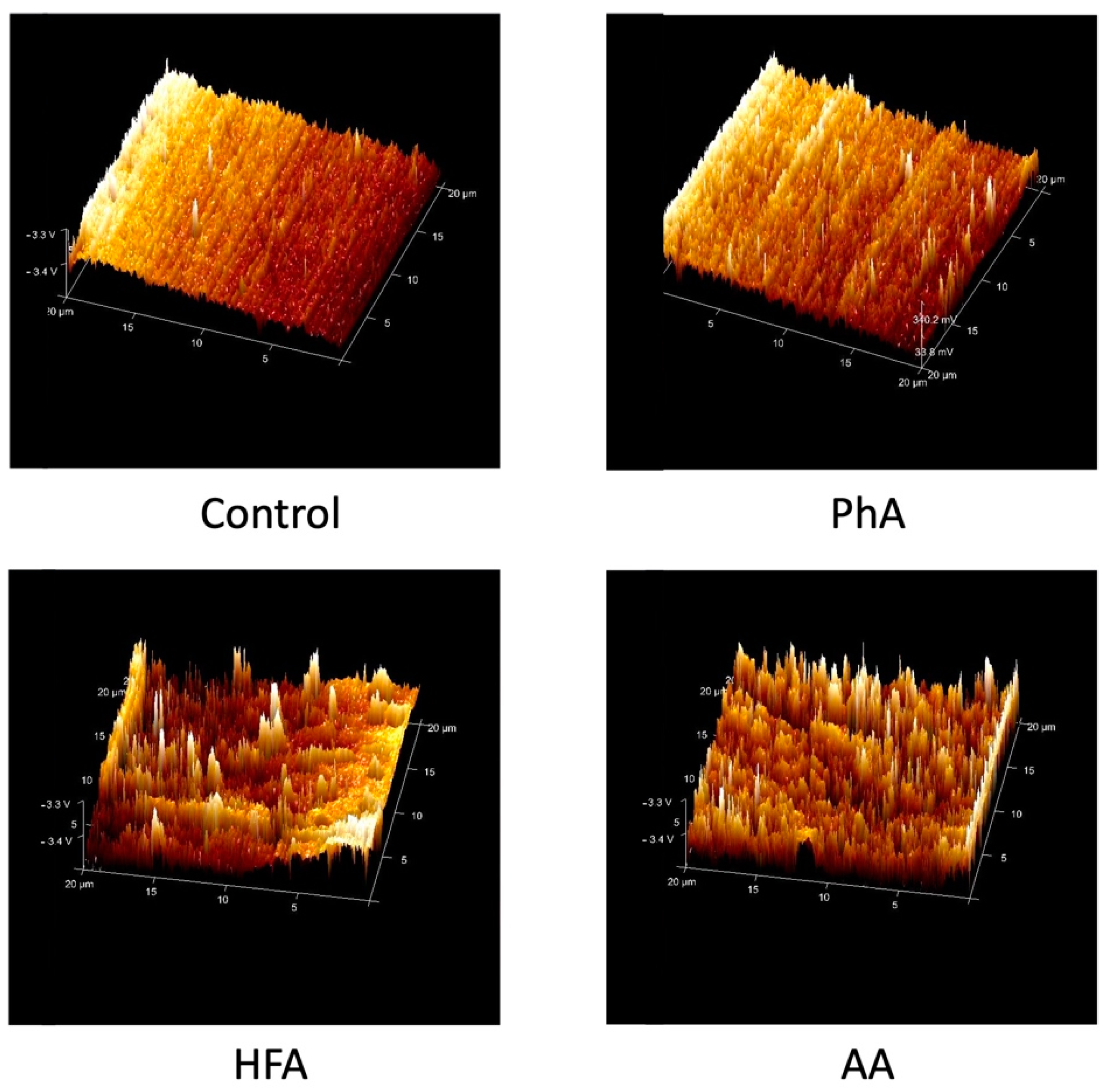
3.1. Surface Roughness
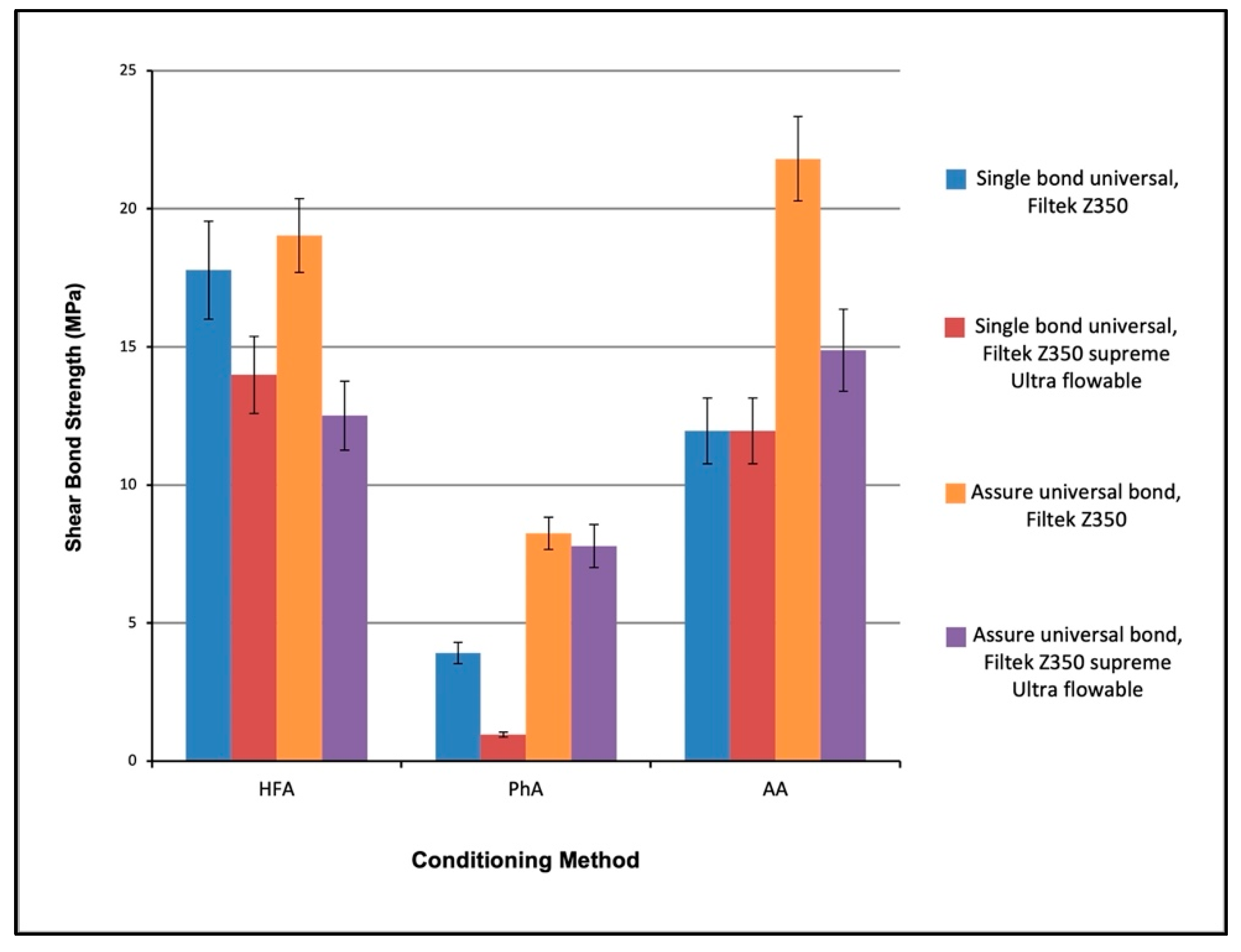
3.2. Shear Bond Strength (SBS)
4. Discussion
5. Conclusions
- AA caused a significant change in the ceramic’s surface microstructure and removed the glazed layer followed by HFA.
- HFA conditioned group had the highest SBS value followed by AA. However, the difference between them was insignificant. On the other hand, the PhA group had the lowest SBS value, which was significantly different from the other groups (p < 0.05).
- Two steps bonding agents such as AUB are more effective and provide significantly higher SBS values than SBU (p < 0.05).
- Using Filtek Z350 composite in attachment bonding provides higher SBS than Filtek supreme Ultra flowable composite.
- The combination of AA conditioning method with AUB and Filtek Z350 composite gave the highest SBS, followed by the combination of HFA etching with AUB and Filtek Z350.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhoundi, M.A.; Kamel, M.R.; Hashemi, S.M.; Imani, M. Tensile bond strength of metal bracket bonding to glazed ceramic surfaces with different surface conditionings. J. Dent. 2011, 8, 201–208. [Google Scholar]
- Chen, W.; Qian, L.; Qian, Y.; Zhang, Z.; Wen, X. Comparative study of three composite materials in bonding attachments for clear aligners. Orthod. Craniofac. Res. 2021, 24, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Efficacy of clear aligners in controlling orthodontic tooth movement: A systematic review. Angle Orthod. 2015, 85, 881–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosvall, M.D.; Fields, H.W.; Ziuchkovski, J.; Rosenstiel, S.F.; Johnston, W.M. Attractiveness, acceptability, and value of orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 276.e1–276.e12. [Google Scholar] [CrossRef]
- Weir, T. Clear aligners in orthodontic treatment. Aust. Dent. J. 2017, 62, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Alajmi, S.; Shaban, A.; Al-Azemi, R. Comparison of Short-Term Oral Impacts Experienced by Patients Treated with Invisalign or Conventional Fixed Orthodontic Appliances. Med. Princ. Pract. 2020, 29, 382–388. [Google Scholar] [CrossRef]
- Beers, A.C.; Choi, W.; Pavlovskaia, E. Computer-assisted treatment planning and analysis. Orthod. Craniofac. Res. 2003, 6, 117–125. [Google Scholar] [CrossRef]
- Barreda, G.J.; Dzierewianko, E.A.; Muñoz, K.A.; Piccoli, G.I. Surface wear of resin composites used for Invisalign® attachments. Acta Odontol. Latinoam. 2017, 30, 90–95. [Google Scholar]
- Tamer, I.; Oztas, E.; Marsan, G. Orthodontic treatment with clear aligners and the scientific reality behind their marketing: A literature review. Turk. J. Orthod. 2019, 32, 241–246. [Google Scholar] [CrossRef]
- Makhija, S.K.; Lawson, N.C.; Gilbert, G.H.; Litaker, M.S.; McClelland, J.A.; Louis, D.R.; Gordan, V.V.; Pihlstrom, D.J.; Meyerowitz, C.; Mungia, R.; et al. Dentist material selection for single-unit crowns: Findings from the National Dental Practice-Based Research Network. J. Dent. 2016, 55, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Zarone, F.; Ferrari, M.; Mangano, F.G.; Leone, R.; Sorrentino, R. “Digitally oriented materials”: Focus on lithium disilicate ceramics. Int. J. Dent. 2016, 2016, 9840594. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Sleem, D. Microtensile bond strength of lithium disilicate ceramics to resin adhesives. J. Adhes. Dent. 2014, 16, 547–552. [Google Scholar] [PubMed]
- Viskic, J.; Jokic, D.; Jakovljevic, S.; Bergman, L.; Ortolan, S.M.; Mestrovic, S.; Mehulic, K. Scanning electron microscope comparative surface evaluation of glazed-lithium disilicate ceramics under different irradiation settings of Nd: YAG and Er: YAG lasers. Angle Orthod. 2018, 88, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Albakry, M.; Guazzato, M.; Swain, M. Biaxial flexural strength, elastic moduli, and x-ray diffraction characterization of three pressable all-ceramic materials. J. Prosthet. Dent. 2003, 89, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Belli, R.; Geinzer, E.; Muschweck, A.; Petschelt, A.; Lohbauer, U. Mechanical fatigue degradation of ceramics versus resin composites for dental restorations. Dent. Mater. 2014, 30, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.M.; Dressler, K.B.; Grenadier, M.R. Direct bonding of orthodontic brackets to esthetic restorative materials using a silane. Am. J. Orthod. 1984, 86, 503–506. [Google Scholar] [CrossRef]
- Gillis, I.; Redlich, M. The effect of different porcelain conditioning techniques on shear bond strength of stainless steel brackets. Am. J. Orthod. Dentofac. Orthop. 1998, 114, 387–392. [Google Scholar] [CrossRef]
- Sabuncuoğlu, F.A.; Ertürk, E. Shear bond strength of brackets bonded to porcelain surface: In vitro study. J. Istanb. Univ. Fac. Dent. 2016, 50, 9–18. [Google Scholar] [CrossRef]
- Bayoumi, R.E.; El-Kabbany, S.M.; Gad, N. Effect of Different Surface Treatment Modalities on Surface Roughness and Shear Bond Strength of Orthodontic Molar Tubes to Lithium Disilicate Ceramics. Egypt. Dent. J. 2019, 65, 641–656. [Google Scholar] [CrossRef] [Green Version]
- Sundfeld Neto, D.; Naves, L.Z.; Costa, A.R.; Correr, A.B.; Consani, S.; Borges, G.A.; Correr-Sobrinho, L. The effect of hydrofluoric acid concentration on the bond strength and morphology of the surface and interface of glass ceramics to a resin cement. Oper. Dent. 2015, 40, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Garboza, C.S.; Berger, S.; Guiraldo, R.D.; Fugolin, A.P.P.; Gonini-Júnior, A.; Moura, S.K.; Lopes, M.B. Influence of Surface Treatments and Adhesive Systems on Lithium Disilicate Microshear Bond Strength. Braz. Dent. J. 2016, 27, 458–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Rifaiy, M.Q. Effect of Erbium-yttrium, scandium, gallium and garnet (Er-YSGG) laser on the bond strength of lithium disilicate ceramics. Pak. J. Med. Sci. 2018, 34, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Alqerban, A. Lithium di silicate ceramic surface treated with Er, Cr: YSGG and other conditioning regimes bonded to orthodontic bracket. Saudi Dent. J. 2021, 33, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.B.; Moura, D.M.D.; Silva, S.E.G.D.; Araújo, G.M.D.; Pinto, R.D.A.S.; Leite, F.P.P.; Özcan, M. Effect of different surface treatments and multimode adhesive application on the Weibull characteristics, wettability, surface topography and adhesion to CAD/CAM lithium disilicate ceramic. J. Appl. Oral Sci. 2020, 28, e20200122. [Google Scholar] [CrossRef]
- Mehmeti, B.; Kelmendi, J.; Iiljazi-Shahiqi, D.; Azizi, B.; Jakovljevic, S.; Haliti, F.; Anić-Milošević, S. Comparison of shear bond strength orthodontic brackets bonded to zirconia and lithium disilicate crowns. Acta Stomatol. Croat. 2019, 53, 17–27. [Google Scholar] [CrossRef]
- Labunet, A.; Kui, A.; Voina-Tonea, A.; Vigu, A.; Sava, S. Orthodontic attachment adhesion to ceramic surfaces. Clin. Cosmet. Investig. Dent. 2021, 13, 83. [Google Scholar] [CrossRef]
- Anadioti, E.; Aquilino, S.A.; Gratton, D.G.; Holloway, J.A.; Denry, I.; Thomas, G.W.; Qian, F. 3D and 2D marginal fit of pressed and CAD/CAM lithium disilicate crowns made from digital and conventional impressions. J. Prosthodont. 2014, 23, 610–617. [Google Scholar] [CrossRef]
- Ji, M.-K.; Park, J.-H.; Park, S.-W.; Yun, K.-D.; Oh, G.-J.; Lim, H.-P. Evaluation of marginal fit of 2 CAD-CAM anatomic contour zirconia crown systems and lithium disilicate glass-ceramic crown. J. Adv. Prosthodont. 2015, 7, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Forster, A.; Ungvári, K.; Györgyey, Á.; Kukovecz, Á.; Turzó, K.; Nagy, K. Human epithelial tissue culture study on restorative materials. J. Dent. 2014, 42, 7–14. [Google Scholar] [CrossRef]
- Dilber, E.; Yavuz, T.; Kara, H.B.; Ozturk, A.N. Comparison of the effects of surface treatments on roughness of two ceramic systems. Photomed. Laser Surg. 2012, 30, 308–314. [Google Scholar] [CrossRef]
- Borges, G.A.; Sophr, A.M.; De Goes, M.F.; Sobrinho, L.C.; Chan, D.C. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J. Prosthet. Dent. 2003, 89, 479–488. [Google Scholar] [CrossRef]
- Kara, H.B.; Dilber, E.; Koc, O.; Ozturk, A.N.; Bulbul, M. Effect of different surface treatments on roughness of IPS Empress 2 ceramic. Lasers Med. Sci. 2012, 27, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ersu, B.; Yuzugullu, B.; Yazici, A.R.; Canay, S. Surface roughness and bond strengths of glass-infiltrated alumina-ceramics prepared using various surface treatments. J. Dent. 2009, 37, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin-ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtulmus-Yilmaz, S.; Cengiz, E.; Ongun, S.; Karakaya, I. The effect of surface treatments on the mechanical and optical behaviors of CAD/CAM restorative materials. J. Prosthodont. 2019, 28, e496–e503. [Google Scholar] [CrossRef]
- Augusti, D.; Gabriele, A.; Francesca, C.; Dino, R. Does sandblasting improve bond strength between nano-ceramic resin and two different luting composites. Bioceram. Dev. Appl. 2015, 5, 2. [Google Scholar]
- Keshvad, A.; Hakimaneh, S.M.R. Microtensile bond strength of a resin cement to silica-based and Y-TZP ceramics using different surface treatments. J. Prosthodont. 2018, 27, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.H.; Matsumura, H.; Atsuta, M. Effect of etchant, etching period, and silane priming on bond strength to porcelain of composite resin. Oper. Dent. 1998, 23, 250–257. [Google Scholar]
- Prochnow, C.; Venturini, A.B.; Grasel, R.; Bottino, M.C.; Valandro, L.F. Effect of etching with distinct hydrofluoric acid concentrations on the flexural strength of a lithium disilicate-based glass ceramic. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 885–891. [Google Scholar] [CrossRef]
- Alao, A.-R.; Stoll, R.; Song, X.-F.; Abbott, J.R.; Zhang, Y.; Abduo, J.; Yin, L. Fracture, roughness and phase transformation in CAD/CAM milling and subsequent surface treatments of lithium metasilicate/disilicate glass-ceramics. J. Mech. Behav. Biomed. Mater. 2017, 74, 251–260. [Google Scholar] [CrossRef]
- de Kok, P.; Pereira, G.K.; Fraga, S.; de Jager, N.; Venturini, A.B.; Kleverlaan, C.J. The effect of internal roughness and bonding on the fracture resistance and structural reliability of lithium disilicate ceramic. Dent. Mater. 2017, 33, 1416–1425. [Google Scholar] [CrossRef]
- Sudré, J.P.; Salvio, L.A.; Baroudi, K.; Sotto-Maior, B.S.; Melo-Silva, C.L.; Assis, N. Influence of surface treatment of lithium disilicate on roughness and bond strength. Int. J. Prosthodont. 2020, 33, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.R. A Review of Direct Orthodontic Bonding. Br. J. Orthod. 1975, 2, 171–178. [Google Scholar] [CrossRef]
- Cardenas, A.M.; Siqueira, F.; Hass, V.; Malaquias, P.; Gutierrez, M.F.; Reis, A.; Perdigao, J.; Loguercio, A. Effect of MDP-containing Silane and Adhesive Used Alone or in Combination on the Long-term Bond Strength and Chemical Interaction with Lithium Disilicate Ceramics. J. Adhes. Dent. 2017, 19, 203–212. [Google Scholar] [PubMed]
- Puppin-Rontani, J.; Sundfeld, D.; Puppin-Rontani, R.; Costa, A.; Correr, A.; Borges, G.; Sinhoreti, M.; Correr-Sobrinho, L. HF concentration and etching times on lithium disilicate glass-ceramic. Dent. Mater. 2016, 32, e83. [Google Scholar] [CrossRef]
- Kalavacharla, V.K.; Lawson, N.; Ramp, L.; Burgess, J. Influence of etching protocol and silane treatment with a universal adhesive on lithium disilicate bond strength. Oper. Dent. 2015, 40, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Murillo-Gómez, F.; Rueggeberg, F.; De Goes, M. Short- and Long-Term Bond Strength Between Resin Cement and Glass-Ceramic Using a Silane-Containing Universal Adhesive. Oper. Dent. 2017, 42, 514–525. [Google Scholar] [CrossRef]
- Yao, C.; Zhou, L.; Yang, H.; Wang, Y.; Sun, H.; Guo, J.; Huang, C. Effect of silane pretreatment on the immediate bonding of universal adhesives to computer-aided design/computer-aided manufacturing lithium disilicate glass ceramics. Eur. J. Oral Sci. 2017, 125, 173–180. [Google Scholar] [CrossRef]
- Ankyu, S.; Nakamura, K.; Harada, A.; Hong, G.; Kanno, T.; Niwano, Y.; Örtengren, U.; Egusa, H. Fatigue analysis of computer-aided design/computer-aided manufacturing resin-based composite vs. lithium disilicate glass-ceramic. Eur. J. Oral Sci. 2016, 124, 387–395. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Han, G.-J.; Chang, J.; Son, H.-H. Bonding of the silane containing multi-mode universal adhesive for lithium disilicate ceramics. Restor. Dent. Endod. 2017, 42, 95–104. [Google Scholar] [CrossRef]
- De Melo, L.A.; Moura, I.D.S.; De Almeida, E.O.; Junior, A.C.F.; Dias, T.G.D.S.; Leite, F.P.P. Efficacy of prostheses bonding using silane incorporated to universal adhesives or applied separately: A systematic review. J. Indian Prosthodont. Soc. 2019, 19, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, H.; Suh, B.I. Effect of incorporating BisGMA resin on the bonding properties of silane and zirconia primers. J. Prosthet. Dent. 2013, 110, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Kim, J.-H.; Son, S.-A.; Park, J.-K. Effect of Silane-Containing Universal Adhesives on the Bonding Strength of Lithium Disilicate. Materials 2021, 14, 3976. [Google Scholar] [CrossRef] [PubMed]
- Dasy, H.; Dasy, A.; Asatrian, G.; Rózsa, N.; Lee, H.F.; Kwak, J.H. Effects of variable attachment shapes and aligner material on aligner retention. Angle Orthod. 2015, 85, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Material | Chemical Composition | Manufacturer |
|---|---|---|
| Ceramic Material | ||
| Lithium disilicate glass ceramic (IPS e.max CAD) | Lithium meta-silicate crystals with approximately 40% crystals by volume. Additional contents: Li2O, K2O, MgO, AL2O3, P2O2 | Ivoclar Vivadent, Schaan, Lichtenstein |
| Surface conditioning methods | ||
| Phosphoric acid (PhA) | 37% PhA, chlorhexidine digluconate, thickener, stain and water | Maquira |
| Hydrofluoric acid (HFA) | 9.6% HFA, 5.3% Ethyl alcohol, thickening agent, dye and water | Pulpdent |
| Aluminum oxide particles (AL2O3) | Al2O3 99.80%, SiO2 0.023%, Fe2O3 0.035%, TiO2 0.006%, CaO 0.01%, Na2O 0.15% | Zhermack |
| Bonding agents | ||
| Single bond universal adhesive (SBU) | MDP phosphate monomer, Dimethacrylate resins, HEMA, Vitrebond copolymer, filler, ethanol, water, initiators, silane. | 3M ESPE |
| Assure universal bonding agent (AUB) | 2-Hydroxyethyl Methacrylate 10–30%, BisGMA 10–30%, 4-Dimethylaminobenzoic Acid 1–5% | Reliance orthodontic |
| Porcelain conditioner (PC) | Ethanol/Denatured 190 PROOF 30–50%, 3-(Trimethoxysilyl) propyl-2-methyl-2-propenoic acid 1–5%, acetone, ACS grade 30–50% | Reliance orthodontic |
| Composite types | ||
| 3M Filtek™ Z350 XT composite | Matrix: Bis-GMA, UDMA, Bis-EMA Filler: Silica, zirconia nanoparticles (20 μm) (72.5 wt%/55.9 vol%) | 3M ESPE |
| 3M Filtek™ Z350 XT flowable composite | Matrix: Bis-GMA, TEGDMA, procrylat resin Filler: yetterbium trifluoride, silica, zirconium oxide (46 vol%/65 wt%) | 3M ESPE |
| Groups | N | Mean (Mpa) | Std Dev | p-Value | |
|---|---|---|---|---|---|
| Conditioning methods | HFA | 40 | 15.82 a | 4.72 | <0.0001 *** |
| AA | 40 | 14.91 a | 5.38 | ||
| PhA | 40 | 5.22 b | 4.03 | ||
| Bonding agent type | Assure universal bond | 60 | 14.04 | 6.04 | 0.001 ** |
| Single bond universal | 60 | 9.93 | 6.80 | ||
| Composite type | Filtek Z350 | 60 | 13.79 | 7.47 | 0.003 ** |
| Filtek Z350 supreme Ultra flowable | 60 | 10.18 | 5.38 | ||
| Interactions | ||||||
|---|---|---|---|---|---|---|
| AA, AUB, Filtek Z350 | A | |||||
| HFA, AUB, Filtek Z350 | A | B | ||||
| HFA, SBU, Filtek Z350 | A | B | ||||
| AA, AUB, Filtek Z350 flowable | B | C | ||||
| HFA, SBU, Filtek Z350 flowable | B | C | ||||
| HFA, AUB, Flowable | C | D | ||||
| AA, SBU, Filtek Z350 | C | D | ||||
| AA, SBU, Filtek Z350 flowable | C | D | ||||
| PhA, AUB, Filtek Z350 | D | E | ||||
| PhA, AUB, Filtek Z350 flowable | D | E | ||||
| PhA, SBU, Filtek Z350 | E | F | ||||
| PhA, SBU, Filtek Z350 flowable | F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaud, B.A.; Hajjaj, M.S.; Masoud, A.I.; Abou Neel, E.A.; Abuelenain, D.A.; Linjawi, A.I. Bonding of Clear Aligner Composite Attachments to Ceramic Materials: An In Vitro Study. Materials 2022, 15, 4145. https://doi.org/10.3390/ma15124145
Alsaud BA, Hajjaj MS, Masoud AI, Abou Neel EA, Abuelenain DA, Linjawi AI. Bonding of Clear Aligner Composite Attachments to Ceramic Materials: An In Vitro Study. Materials. 2022; 15(12):4145. https://doi.org/10.3390/ma15124145
Chicago/Turabian StyleAlsaud, Bashair A., Maher S. Hajjaj, Ahmad I. Masoud, Ensanya A. Abou Neel, Dalia A. Abuelenain, and Amal I. Linjawi. 2022. "Bonding of Clear Aligner Composite Attachments to Ceramic Materials: An In Vitro Study" Materials 15, no. 12: 4145. https://doi.org/10.3390/ma15124145






