Multi-Scale Modeling of Plastic Waste Gasification: Opportunities and Challenges
Abstract
:1. Introduction
2. Plastic Waste Gasification: A Promising, but Less Mature Recycling Route
2.1. Opportunities and Challenges of PWG

2.2. Numerical Modeling

2.3. Multi-Scale Modeling of Plastic Waste Gasification
- The plastic, in the solid phase, is fed into the reactor
- The plastic is melted first and then fed into the reactor to cover the fluidization agent or to be present as liquid droplets. The latter is reported very rarely [66].
- The plastic is melted first but fed as a layer into a falling film reactor [67].
3. Thermophysical Properties
3.1. Individual Species
3.1.1. Conventional Methods
3.1.2. Advanced Numerical Methods
3.2. Effective Properties
- Structural effects
- The presence of impurities, or
- Internal motions (in the liquid phase)
3.3. Mixture Properties
4. Reaction Kinetics
4.1. Challenges Faced in Gasification and/vs. Pyrolysis
4.1.1. Diverse Micro-Scale Characteristics of Plastics
4.1.2. Coupling of Available Kinetic Models
4.1.3. Presence of Char
4.2. Global vs. Detailed Kinetic Models
4.2.1. Global Kinetic Models
4.2.2. Detailed Kinetic Models
Feedstock Description
Devolatilization
Gasification
Challenges and Opportunities
- Coupling
- The mechanistic models are feedstock independent. Hence, they are supposed to perform properly for different compositions and feedstock characteristics. Moreover, the similarities in the polymer segments and reaction families make it less burdensome to introduce new polymer types.
- The presence of different gasification agents with different concentrations can be taken into account in a single model. This may also reflect the synergistic effects as a result of gasification with multiple gasification agents. As it can be seen in the developed detailed kinetic models [137], all the gasification agents are present and based on their concentration, their contribution to the overall gasification process is accounted for.
- To introduce new species, only the initial propagation and decomposition steps should be defined [107]. Hence, reliable modification of the model can be done easily in this approach.
- Size of the Reaction Network
| Global Modeling | Mechanistic (Detailed) Modeling | ||
|---|---|---|---|
| MOM | kMC | ||
| Requires detailed feedstock description | No (pre-defined lumps) | Yes | |
| Degree of complexity | Low | Medium | High |
| Degree of details on the product description | Low | Medium (average properties) [98] | High (full molecular detail) [98] |
| Computational cost | Low | Medium | High |
| OM of number of species | 50 | 100–1000 [154] (Reduced: 10–100) | 1000–10,000 [154] (Reduced: 10–100) |
| OM of number of reactions | 50 | 1000–50,000 [154] (Reduced: 100–1000) | 1000-50,000 [154] (Reduced: 100–1000) |
| Common application | CFD/1D Models | 1D Models (Reduced: CFD) | |
| Feedstock independent | No | Yes | |
| Reliable coupling to other kinetic models | No | Yes | |
| Adaptability to new species (and gasification agents) | No | Yes | |
| Reliable temperature extrapolation | No | Yes | |
| Needs reaction network generator (extra complexity) | No | Yes | |
| Ability to consider dynamic char activity | No | Yes | |
4.2.3. Validation Challenges
- TGA data include the evaporation rates, which are not equal to the degradation rates. So, if a kinetic model is validated against it, in FB regimes with higher evaporation rates, it is supposed to underestimate the devolatilization rate (if the evaporation and degradation models are not decoupled).
- The reactive environment affects the degradation and the evaporation rate of polymers, as was discussed in Section 4.1.2.
- It can include the internal heat and mass transfer limitations, which are not considered in the kinetic models. For large sample sizes [161], providing the isothermal conditions is not possible, and for samples with weak mass transfer properties, concentration gradients are observed within them [162]. Even if in a kinetic model, the effect of diffusion limits on the kinetic parameters is considered [111], two other problems can be raised: First, this shows the incapability in deriving the pure intrinsic kinetic data; and second, the mixing degree and mass transfer limitations can be different from the conditions in which this kinetic model is derived. Hence, this increases the uncertainty in using this kinetic model in different conditions.
- It is not possible to measure the concentration of reacting species in the liquid phase, or the products right after being produced in the gas phase. Hence, secondary reactions can and will happen.
- The uncertainty related to enough sensitivity of the balance used in the TGA instrument is another challenge [162].
- The effect of radiation on the sample in high temperatures is different for the samples with different absorption properties [162].
5. Internal Transport Limitations
5.1. Internal Mass Transfer
5.1.1. Solid Phase
5.1.2. Liquid Phase
Simplifying Assumptions
5.2. Internal Heat Transfer
5.2.1. Solid Phase
5.2.2. Liquid Phase
- A weaker effect of Marangoni convection (and hence weaker internal motions or circulative heat transfer); and
- Monotonically decreasing temperature profile toward the center of the droplet.
6. Phase Transformations and Interfacial Transport Phenomena
6.1. Melting
6.1.1. Melting Phenomenon
6.1.2. Melting Models
Extrusion Models
Enthalpy-Based Models
Phase-Field Models
Reaction-Type Models
6.1.3. Application in the Multi-Scale Framework
6.2. Evaporation
6.2.1. From 0D to 1D Models
6.2.2. Modeling Complexities for PWG
Multiple Components
Mass Fraction at the Interface
Non-Ideal Behavior
Role of Radiation
Role of Surface Area
6.2.3. Simplifying Assumptions
- Considering the liquid as a spherical droplet
- The presence of an inert atmosphere
- Negligible diffusion of the gas to the liquid
- Negligible mass diffusion due to temperature and pressure gradients
- The heaviest component that has been implemented in these simulations is C20. Although the table doesn’t cover all the available studies in this regard, it can demonstrate that in general, not all the components available in the liquid phase of PW during the pyrolysis have been assessed extensively. Hence, one of the main areas to be focused on is the assessment of the cases that, from the components’ point of view, are closer to what is happening in PWG.
- The shape of the liquid phase is important in simulations. In each study, either spherical or film shape is assessed. This is while different shapes can be simultaneously present in PWG, e.g., it can be droplet, agglomerate, or the liquid film on the wall. Besides, in all cases in the table, a uniform characteristic length of the liquid phase is considered, while the shapes that are present in the PWG are not perfect spheres or liquid film. This demonstrates the complexity that is faced in PWG due to the shape imperfections.
- Most of the cases consider the ideal gas assumptions and this can be true due to the high temperature and low pressure [172,219]. However, for the liquid phase, due to the presence of multiple components with different properties, this is not necessarily true. Implementing the non-ideal conditions for a large number of components is a challenge itself.
- Many of the studies use the DMC approach. This demonstrates that the simulation of the evaporation in the PWG can also be done in this approach at a logical computational expense and hence, can be coupled to the available detailed kinetic models for the plastic pyrolysis.
6.3. Interfacial Heat and Mass Transfer
6.3.1. Empirical-Based Correlations
6.3.2. Numerical-Based Correlations
- Each of them is derived for a specific range of void fraction and Reynolds and Prandtl numbers
- For the particles with different shapes, the Nusselt correlations have been developed, including the incident angle of the particles [233]
- Depending on the direction of heat flow, the Nusselt correlation is different, due to the different behavior of water properties in the heating and cooling process at supercritical conditions [234]
- The application of the classical empirical correlations for the complex systems is in doubt because it has been shown that for each case, a different correlation (which has been validated against the experimental data) should be developed
- The numerical tools have been advanced enough to be used for developing new correlations for each specific condition of PWG process. This way, it is possible to increase the precision of the interfacial heat and mass transfer models used in this process.
| Feedstock | Liquid Shape | Ideality | Spherical Droplet/Uniform Film Thickness | 0D/1D | Internal Heat/Mass Transfer | External Heat/Mass Transfer | Reactive | Radiation | Equilibrium | Approach | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O, CH3OH, C2H5OH, 1-C4H9OH, n-C7H16, n-C10H22 | Droplet | Real fluid (UNIFAC), ideal gas | Yes | 0D | No/No | No/No | No | No | Yes | DMC | [175] |
| C2H5OH, n-C5H12, cyclo-C5H10, 1-C6H12, n-C7H16, C7H8, iso-C8H18 | Droplet | Real fluid (UNIFAC), Ideal mixture for the gas phase | Yes | 1D | Yes/Yes | Yes/Yes | Yes | Yes | Yes | DMC | [172] |
| iso-C6H14, n-C7H16, iso-C8H18, cyclo-C9H18, n-C10H22, ben-C10H14, n-C11H24, n-C12H26, ben-C12H18, n-C13H28, n-C14H30, n-C15H32, n-C16H34, n-C17H36, n-C18H38, n-C19H40, n-C20H42, n-C21H44, n-C22H46, n-C30H62 | Droplet | Real fluid, Real gas | Yes | 1D | Yes/Yes | Yes/Yes | No | - | No | DMC | [216] |
| n-C6H14, n-C7H16, iso-C8H18, n-C10H22 | Film | Ideal fluid, Ideal gas | Yes | 1D | Yes/No | - | No | - | Yes | DMC | [176] |
| C4H9OH, C7H8, n-C10H22 | Droplet | Non-Ideal fluid (UNIFAC) | Yes | 1D | Yes/Yes | Yes/Yes | No | Yes | Yes | DMC | [219] |
| n-C7H16, n-C16H34 | Film | Ideal gas | Yes | 1D | Yes/Yes (polynomial expressions) | Yes/Yes | No | - | Yes | DMC | [181] |
| C10H22, C16H34 | Film | Ideal and Non-Ideal Gas | Yes | 1D /Quasi-Dimensional | Yes/Yes (polynomial expressions) | Yes/Yes | No | - | Yes | DMC | [213] |
| C7H16, C10H22, C16H34 | Droplet | Ideal Gas | Yes | 1D | Yes/Yes | Yes/Yes | No | - | Yes | DMC | [173] |
| n-C5H12, iso-C5H12, C7H16, iso-C8H18, C9H20, C10H22, C12H18, C12H26, C16H34, C20H42 | Droplet | Ideal and Non-Ideal Gas | Yes | 1D /Quasi-Dimensional | Yes/Yes (polynomial expressions) | Yes/Yes | No | Yes | Yes | DMC | [209] |
| H2O, CH3OH, C2H5OH, C3H6O, C4H9OH, 3-C5H10O, C8H18, C10H22, C12H26, C14H30, C16H34 | Droplet | - | 1D | No/No | No (Isothermal)/Yes (Stefan-Maxwell approach) | No | - | - | DMC | [177] | |
| Air, H2O | Droplet | Ideal Gas | Yes (Including the number of droplets) | 1D | Yes/Yes | Yes/Yes | No | - | - | DMC | [221] |
| C7H8, tr-C10H18, C12H26, iso-C16H34 | Droplet | Ideal/Real Gas/Liquid | Yes | 0D | Yes/Yes | Yes/Yes | Yes | No | Yes | DMC | [235] |
| n-Paraffin, Iso-Paraffin, Cyclo-Paraffin, Aromatics, Olefin | Droplet | Real Fluid, Ideal Gas (Modified) | Yes | 1D | Yes/No | Yes/Yes | No | No | Yes | DMC | [179] |
| C2H6O (DME), C7H16 | Droplet | Real Fluid (UNIFAC), Ideal Gas | - | 0D | - | - | No | - | No (LK) | DMC | [217] |
| C2H5OH, iso-C5H12, iso-C6H14, iso-C7H16, iso-C8H18, C9H20, C10H22, C12H26 | Droplet | Real Fluid (Wilson equation), Ideal Gas | Yes | 1D | Yes/Yes | Yes/Yes | No | No | Yes | DMC | [182] |
| C7H16, C10H22 | Droplet | Real/Ideal Gas | Yes | 1D | No/No | Yes/Yes | No | No | Yes | DMC | [178] |
| iso-C5H12, iso-C6H14, iso-C7H16, C7H8, iso-C8H18, C9H20, C10H22, C12H26, C14H30, C16H32, C18H34 | Droplet | Ideal Fluid | Yes | 1D (Implemented in multi-dimensional CFD) | Yes/No | Yes/Yes | No | No | Yes | DMC (Derived from CMC) | [236] |
6.3.3. Determining the Limiting Step
6.4. Momentum Transfer
- The role that it plays in the interaction between the particle/droplet/bubbles and change in the interfacial area and shapes as the result of agglomeration, coalescence, and breakup
- The drag force is the main contributing force in the momentum transfer, which acts against the fluid flow direction to resist the motion of a particle, droplet, or bubble. This force is a function of fluid density, dispersed phase diameter, the slip velocity (difference between the velocity of the continuous and discrete phase), and a drag coefficient.
- The lift force acts perpendicular to the flow direction and is the result of turning of the fluid because of the presence of the discrete phase.
- The virtual mass force is the result of acceleration of the discrete phase, i.e., change of its relative motion compared to the fluid phase. This imposes an extra force as an extra mass or “added mass” in the acceleration force.
- The buoyancy force acts against the gravity force as the result of the difference between the density of the fluid and the discrete phase
6.4.1. Drag Force
| Correlation | Method | Limit | Year | Ref | |||
|---|---|---|---|---|---|---|---|
| Shape/Conditions | |||||||
| DNS | 0.4–0.9 | 10–100 | 1.0 | Spherical | 2014 | [255] | |
| PR-DNS | 0.5–0.9 | 1–100 | 0.7 | Spherical | 2015 | [256] | |
| PR-DNS | 0.351–0.367 | 9–180 | 0.5–1.0 | Spherical | 2017 | [257] | |
| PR-DNS | 0.418–0.526 | 9–180 | 0.5–1.0 | Cylindrical | 2017 | [258] | |
| PR-DNS | 0.65–0.9 | 10–200 | 0.74 | Ellipsoidal | 2017 | [259] | |
| DNS | 0.877–0.948 | 0–550 | 1 | Cellular porous media | 2018 | [260] | |
| LBM | 0.5–0.9 | 1–100 | 0.7 | Sphere | 2019 | [261] | |
| DNS-LBM | 0.6–1.0 | 20–500 | 0.5–1.5 | Sphere | 2019 | [262] | |
| PR-DNS | - | 10–200 | 3.07 | Spheroid (Ar = 0.5–2.5)/SCW | 2019 | [232] | |
| PR-DNS | - | 10–200 | 0.744, 3.07 | Spheroid (Ar = 0.5–2.5)/SCW | 2019 | [233] | |
| PR-DNS | - | 10–200 | 0.7–380 | Spherical/SCW/Cold particle | 2020 | [234] | |
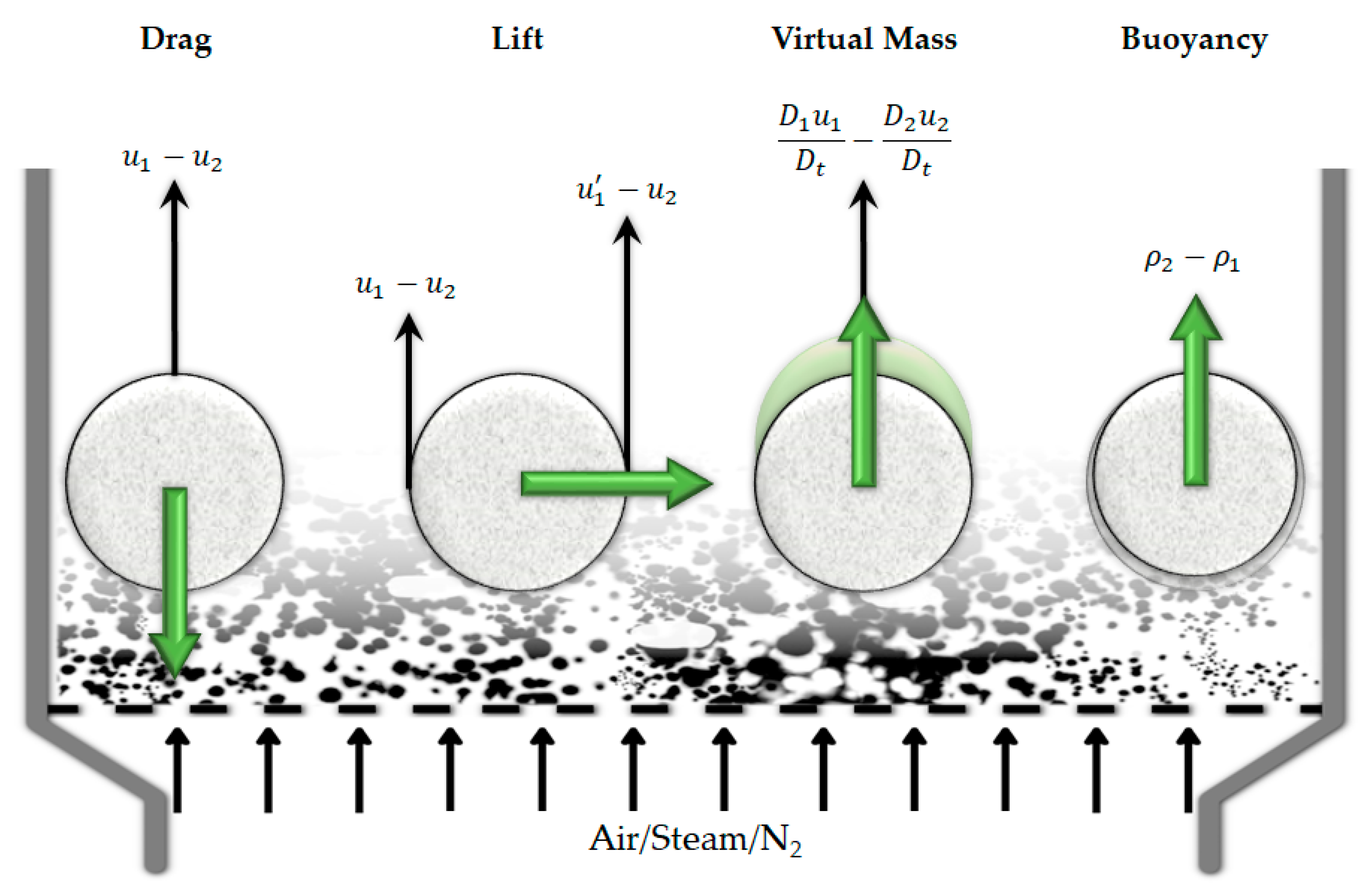
6.4.2. Non-Drag Forces
7. Multi-Phase Flow Modeling
7.1. Reactor Modeling Approaches
7.1.1. Complex vs. Ideal Models
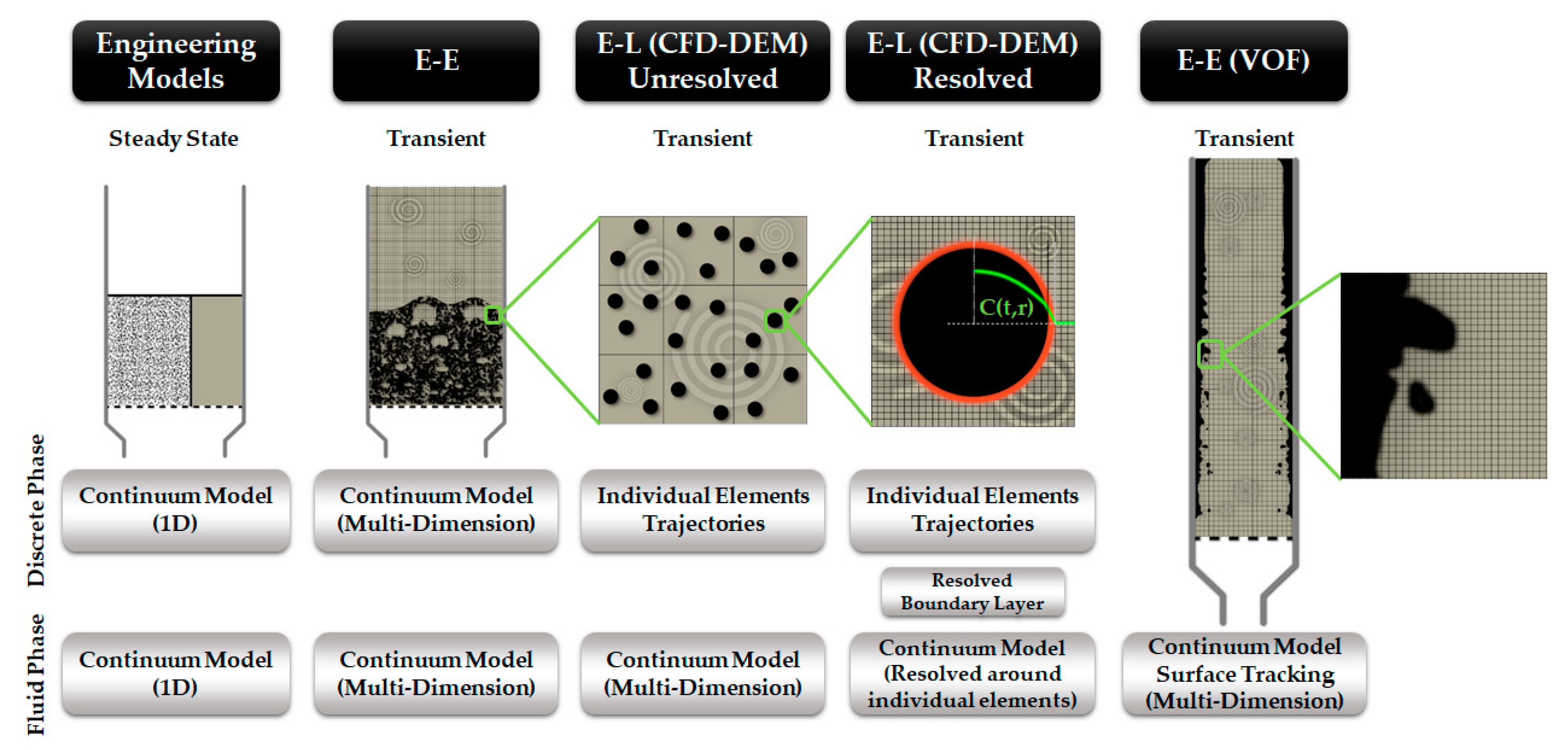
7.1.2. Engineering Models
7.1.3. 3D Computational Fluid Dynamics
Eulerian-Eulerian
Eulerian-Langrangian
| Feed | Gasification Agent | Time (Reaction/ Space/Residence) (s) | Plant Size (OM-m3) | Bed Material | Temperature (°C) | Kinetic | Software/Code | Ref |
|---|---|---|---|---|---|---|---|---|
| Plastic (PVC) | Steam | - | Lab | Alumina | ~900 | - | Inhouse | [299] |
| Plastic (Poly Olefin) | Air-Steam | Pyrolysis: 0.02 Mixing: 5.4 | Pilot (0.02 & 0.67) | - | 700–850 | Global | Inhouse | [122] |
| Coal, petcoke | Oxygen-Steam | - | Commercial (72) | - | 1100 (Non-isothermal) | Global | Inhouse | [314] |
| Coal, limestone, inert material | Air-Steam-Carbon Dioxide | Devolatilization: <10 | Pilot (0.07) | Limestone, Sand | 600–1000 | Global | Inhouse (FORTRAN) | [315] |
| Coal | Air-Steam | - | Lab & Pilot (2.6) | Dolomite | 750–950 (Non-isothermal) | Global | Inhouse | [296] |
| Coal | Oxygen-Steam | Particle residence: 3600 | - | - | 700–900 (Isothermal) | Global | Inhouse | [295] |
| Biomass (Wood) | Air-Steam | - | Pilot (0.57) | - | 900–950 | Global | Inhouse | [297] |
| Biomass (Wood powder) | Air-Steam | - | - | - | 700–900 | Global | Inhouse (MATLAB) | [316] |
| Biomass (Straw) | Air-Steam | - | - | - | - | Equilibrium | Inhouse (FORTRAN) | [317] |
| Biomass (Sawdust) | Air | - | - | Sand | 600–1600 | Global | Inhouse | [318] |
| Biomass (Sawdust) | Air-Oxygen-Steam | Reaction: 140–3000 | Pilot (0.06 & 2) | Ofite, Quartz & Silica Sand | 700–900 | Global | Inhouse | [319] |
| Biomass (Pine Sawdust, Rice husk) | Air-Steam | - | Lab (0.003) & Pilot (0.2) | - | 665–900 | Global | Inhouse (FORTRAN) | [298] |
| Biomass (Beech Wood) | Air-Steam | Gas residence time in the freebord: 2–4 | Pilot (0.02) | Silica Sand | 800–815 | Global | Inhouse | [320] |
| Feed | Type | Gasification Agent/Process Gas | Gas Residence/ Space Time (s) | Plant Size (OM-m3) | Bed Material | Temperature (°C) | Phase | Software/Code | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Plastic (Waste) | Circulating FB | Air | 1–3 | 0.1 m3 | Sand | - | GS | MFIX | [313] |
| Plastic (PE) | Conical Spouted Bed | Air-Steam | ~3 | Lab (0.001) Pilot (0.03) | Sand | 800–900 | GS | Fluent + Aspen Plus | [61] |
| Molten Plastics (mix PE, PP, and PS) 1 | Falling Film | Nitrogen | - | Lab (0.002) | - | 550–650 | GL | OpenFOAM | [169] |
| Molten Plastic (PP) 1 | Falling Film | Nitrogen | - | Lab (0.00004) | - | 460–500 | GL | Fluent | [311] |
| Molten Plastic (PE, PP, PS, mix) 1 | Falling Film | Nitrogen | - | Lab (0.002) | - | 550–625 | GL | - | [67] |
| MSW, RDF | Plasma (Fixed Bed) | Air-Steam | - | - | - | ~2200 (max) | GS | Inhouse (COMMENT) | [330] |
| MSW, Biomass (Coffee husk, Forest residues, Vines pruning) | Bubbling FB | Air-Steam | - | Semi-Industrial (0.8) | Dolomite (Experimentally) | 500–1000 | GS | Fluent | [278] |
| MSW | Bubbling FB | Steam | - | Semi-Industrial | - | 850 | GS | - | [331] |
| MSW | Bubbling FB | Air | - | Semi-Industrial (0.8) | Dolomite (Experimentally) | 700–900 | GS | Fluent | [332] |
| MSW | Bubbling FB | Air-Carbon Dioxide | - | Semi-Industrial (0.8) | Dolomite (Experimentally) | 500–900 | GS | Fluent | [279] |
| MSW | Bubbling FB | Air-Steam-Carbon Dioxide | - | Semi-Industrial (0.8) | Dolomite, NiO/MD Catalyst | 700–900 | GS | Fluent | [333] |
| MSW | Bubbling FB | Air | - | Semi-Industrial (0.8) | - | 500–700 | GS | Fluent | [280] |
| MSW | Bubbling FB | Air | - | Semi-Industrial (0.8) | Dolomite (Experimentally) | ~ 500–700 | GS | Fluent | [224] |
| MSW | Plasma/Melting | Air-Steam | - | Pilot (2.7) | - | ~2200 max | GS | Fluent | [225] |
| MSW | Plasma/Melting | Air | - | - | - | - | GS | - | [65] |
| Biomass & Plastic (Wood, PE) 1 | Rotary Kiln | - | - | - | - | - | GS | Fluent | [310] |
| Biomass | Circulating FB | Air | - | Pilot (0.2) | - | ~400–1000 | GS | Fluent | [334] |
| Biomass (Bagasse, Rice husk, Switchgrass) | Bubbling FB | Nitrogen | - | Lab (0.006) | Sand | 400–600 | GS | Fluent | [335] |
| Biomass (Coffee husk) | Bubbling FB | Air | - | Pilot | - | ~600–1400 | GS | Inhouse (COMMENT) | [336] |
| Biomass (Forest residues) | Bubbling FB | Air | - | Pilot (1) | Dolomite | ~ 800 | GS | Fluent | [337] |
| Biomass (Forest residues, Peach Pits, Ground Coffee) | Plasma | Air-Steam | - | - | - | 1000–2000 | GS | Inhouse (COMMENT) | [338] |
| Biomass (Pinewood) | Vortex Reactor | Nitrogen | <1 (order of ms) | Lab (0.0001) | - | 500–600 | GS | Fluent | [339] |
| Biomass (Wood) | Bubbling FB | Air | - | Lab (0.0004) | Sand | ~ 900 | GS | Inhouse (FORTRAN) | [340] |
| Biomass (Wood) | Bubbling FB | Air | - | Lab-Pilot (0.01) | - | 700–750 | GS | ModifiedK-FIX | [341] |
| Biomass (Wood) | Bubbling FB | Air | - | Lab (0.0004) | Sand | 850 | GS | MFIX-based | [342] |
| Biomass (Wood) | Bubbling FB | Air | - | Lab-Pilot (0.01) | - | ~400–800 | GS | - | [343] |
| Biomass (Wood) | Fixed Bed | Air-Steam | - | Pilot (0.22) | - | ~450–1000 | GS | Fluent | [344] |
| Biomass (Wood) | Fixed Bed | Air-Steam | <1 (order of ms) | - | - | ~650–1300 | GS | - | [345] |
| Coal | Bubbling FB | Air-Steam | - | Pilot (0.07) | - | ~400 | GS | OpenFOAM | [306] |
| Coal | Bubbling FB | Air-Oxygen-Steam | - | Pilot (1) | Silica Sand | ~900 | GS | Fluent | [346] |
| Coal | Bubbling FB | Air | - | Lab (0.1) | - | ~600–1000 | GS | Fluent | [347] |
| Coal | Bubbling FB | Air-Steam | - | Lab (0.07) | Limestone | 812, 855 | GS | ANSYS | [270] |
| Coal | Bubbling FB | Air-Steam | - | Lab (0.07) | Sand | 821, 846, 855 | GS | - | [348] |
| Coal | Bubbling FB | Air-Steam | - | Lab (0.07) | - | 812-866 | GS | - | [349] |
| Coal | Entrained Flow | Air | - | Commercial (15) | - | ~370–2000 | GS | CFC code PHOENICS (Inhouse) | [350] |
| Coal | Fixed Bed | Air-Steam | - | Lab (0.01) | - | ~600–1300 | GS | MFIX | [351] |
| Glycerin solutions containing xanthan gum 2 | Bubble Column | Air | - | Lab (0.01) | - | 25 | GL | CFX (MUSIG) | [352] |
| Glycerol | FB | Steam | - | 0.001 | Sand | 600–750 | GS | Fluent | [353] |
| Manure Slurry 2 | Anaerobic Digester | - | - | Industrial (791) | - | 35 | GL | Fluent | [354] |
| Water | Bubble Column | Air | - | - | - | - | GL | OpenFOAM | [355] |
| Water | Bubble Column | Air | - | Lab (0.01) | - | Room | GL | OpenFOAM | [286] |
| Water 3 | Bubble Column | Air | - | Lab (0.007) Pilot (4) | - | - | GL | OpenFOAM (OpenQBMM) | [356] |
| Water | Laboratory Tank | Air | - | Lab (0.02) | - | 22 | GL | OpenFOAM | [274] |
| Water 3 | Vertical Tube | Air | - | Lab (0.01) | - | - | GL | OpenFOAM (twoWayGPBEFoam) | [357] |
| Water 4 | Bubble Column | Air | - | Lab (0.07) | - | - | GL | - | [358] |
| Water 4 | Bubble Column | - | - | - | - | - | GL | - | [359] |
| Water 4 | Bubble Column | Air | - | Lab (0.007) Pilot (0.27) | - | 30 | GL | CFX | [360] |
7.2. Multi-Phase Flow Modeling Challenges and Possible Solutions
7.2.1. Irregular Shape
| Feed | Type | Gasification Agent/Process Gas | SRT (s) | Plant Size (OM-m3) | Bed Material | Temperature (K) | Lagrangian Approach | Software | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Plastic (PE, PP, PS, mix) | Entrained Flow | Air | - | Lab (0.005) | - | ~50–1100 | - | Fluent | [29] |
| Pitch-water slurry | Entrained Flow | Oxygen | 0–50 | Pilot (0.2)/Industrial (33) | - | ~1500 | - | Fluent | [226] |
| - | Conical Spouted Bed | Air | - | Lab (0.001) | ZiO2 | 25 | DEM | Fluent | [363] |
| Biomass | Bubbling FB | Air-Steam | - | Lab (0.003) | Sand | ~800–900 | DEM | - | [323] |
| Biomass | Spouted Bed + DFB | Steam | - | SB Lab (0.01)/DFB Pilot (0.3) | Silica Sand | 820–870 | MP-PIC | OpenFOAM | [364] |
| Biomass (Almond prunings) | DFB | Steam | Up to ~100 | Pilot (0.7) | Sand | ~400–900 | MP-PIC | OpenFOAM | [365] |
| Biomass (Glucose) | FB | Super Critical Water | - | Lab (0.001) | Quartz Sand | ~500–600 | DEM | Fluent | [366] |
| Biomass (Pinewood) | Bubbling FB | Steam-Nitrogen | - | Lab (0.06) | Sand | 820–920 | CGM & DEM | STAR-CGM+12.02 | [367] |
| Biomass (Pinewood) | Bubbling FB | Steam-Nitrogen | - | Lab (0.0005) | Sand | 820–920 | DEM | OpenFOAM | [368] |
| Biomass (Pine, Beech, Holm oak, Eucalyptus) | Conical Spouted Bed | Steam-Argon | - | Lab (0.01) | Sand | 770–920 | MP-PIC | OpenFOAM | [369] |
| Biomass (Pine, Beech, Holm oak, Eucalyptus) | Entrained Flow | Air-Steam | Up to ~2.5 | Lab (0.01) | - | 1000–1400 | - | OpenFOAM | [370] |
| Biomass (Raw, Torrefied) | FB | Air-Nitrogen-Steam | - | Lab (0.0001) | Olivine | 750–850 | DEM | OpenFOAM | [371] |
| Biomass (Raw, Torrefied) (Forest residues, Spruce) | Entrained Flow | Air-Steam | - | Lab (0.01) | - | 1400 | - | OpenFOAM | [372] |
| Biomass (Rice husk) | Entrained Flow | Oxygen-Steam-Carbon Dioxide | - | Lab (0.01) | - | 1400 | - | OpenFOAM | [373] |
| Biomass (Rice husk, Cotton stalks, Sugarcane bagasse, Sawdust) | Concentric tube entrained flow | Oxygen | - | Pilot (0.25) | - | ~900–2300 | DPM | Fluent | [374] |
| Biomass (Sawdust) | Entrained Flow | Air | Lab (0.015) | - | 800–1000 | DPM | Fluent | [375] | |
| Biomass (Sawdust, Cotton trash) | Entrained Flow | Air-Steam | - | Pilot (4) | - | ~800–1100 | - | CFX | [376] |
| Biomass (Wood pellet) | FB | Steam | Up to ~36 | Lab (0.02) | Sand | ~600–800 | CGM-DEM | Fluent | [377] |
| Biomass (Wood) | Bubbling FB | Steam | - | Lab (0.06) | Sand | 820 | DEM | Inhouse (MFIX-DEM) | [378] |
| Biomass (Wood) | FB | Air | Up to ~84 | Lab (0.01) | Charcoal | ~500–700 | DEM | - | [379] |
| Coal | Bubbling FB | Air-Steam | Up to ~20 | Lab (0.07) | Sand | ~ 800 | MP-PIC | OpenFOAM | [380] |
| Coal | Circulating FB | Air | - | Pilot (0.2) | Sand | ~600–850 | MP-PIC | - | [381] |
| Coal | Circulating FB | Carbon Dioxide-Oxygen-Nitrogen | - | Pilot (0.03) | Sand | ~950 (max) | DPM + MP-PIC | Fluent + CPFD Barracuda | [382] |
| Coal | Entrained Flow | Oxygen-Steam | - | Industrial | - | 1370–1620 | - | Fluent | [383] |
| Coal | Entrained Flow | Air-Steam | - | Lab (0.004) | - | ~200–1850 | - | Fluent | [384] |
| Coal | Entrained Flow | Air | - | Pilot (0.26) | - | ~700–1900 | - | CFX + FORTRAN | [385] |
| Coal | Two-stage Entrained Flow | Oxygen | - | Industrial (32) | - | ~700–2100 | DPM | Fluent | [386] |
| Coal | Updraft gasifier | Air-Steam | - | Industrial (60) | - | ~500 (mean) | DPM | Fluent | [387] |
| Water 1 | Bubble Column | Air | - | Lab (0.01) | - | - | DEM | OpenFOAM | [388] |
| Water 1 | Bubble Column | Air | - | Lab (0.01) | - | - | DEM | OpenFOAM | [389] |
7.2.2. Roughness
7.2.3. Polydispersity
7.2.4. Aggregation, Coalescence, and Breakup
7.2.5. Regime Transition
7.2.6. Non-Newtonian Behavior
7.3. Multi-Scale Frameworks and Computational Efficiency
8. Conclusions
- It does not require extensive sorting of PW.
- It does not necessarily require a catalyst that could be easily deactivated by impurities present in PW.
- The wide variety of plastic types and elements in PW that result in the necessity of substantial upgrading of the syngas, e.g., removal of HCl, or dealing with the fluctuations in the feedstock composition.
- PWG setups are studied and designed based on the existing knowledge when gasifying coal or biomass, and hence are believed to be not optimal for PW. In particular, the presence of liquid is usually neglected.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Acronyms | |
| CFB | Circulating Fluidized Bed |
| CGM | Coarse Grain Model |
| CMC | Continuous Multi-component |
| DAEM | Distributed Activation Energy Model |
| DFB | Dual Fluidized Bed reactor |
| DPM | Discrete Particle Method/Discrete Phase Method |
| DQMOM | Direct Quadrature Method of Moments |
| E-E | Eulerian-Eulerian approach |
| E-L | Eulerian-Lagrangian approach |
| ERN | Equivalent Reactor Network |
| FB | Fluidized Bed |
| FCMOM | Finite-size Domain Complete Set of Trial Functions Method of Moments |
| FTS | Fischer-Tropsch Synthesis |
| GL | Gas-Liquid |
| GS | Gas-Solid |
| KTGF | Kinetic Theory of Granular Flow |
| LBM | Lattice-Boltzmann Method |
| LK | Langmuir-Knudsen Model |
| MD | Molecular Dynamics |
| MOM | Method of Moments |
| MP-PIC | MultiPhase Particle-in-Cell |
| MSW | Municipal Solid Waste |
| OM | Order of Magnitude |
| PBE | Population Balance Equation |
| PR | Particle Resolved |
| PW | Plastic Waste |
| PWG | Plastic Waste Gasification |
| RDF | Refuse-Derived Fuel |
| SB | Spouted Bed |
| SCW | Super Critical Water |
| SRT | Solid Residence Time |
| Roman and Greek Letters | |
| Pre-exponential factor/Surface area | |
| Ar | Aspect ratio of spheroids |
| Biot number | |
| Specific heat capacity | |
| Courant number | |
| Diffusivity coefficient | |
| Damköhler number | |
| E | Activation energy |
| Force | |
| Gravity acceleration | |
| Convective heat transfer coefficient | |
| H | Enthalpy |
| Reaction kinetic constant | |
| L | Latent heat/Characteristic length |
| Mass | |
| Ma | Dimensionless Marangoni number |
| Number of droplets per mass of liquid | |
| Number of particles | |
| Nu | Nusselt number |
| Pressure | |
| The perimeter of the circle equivalent to the maximum projection area of a particle | |
| Maximum projection perimeter | |
| Pr | Prandtl number |
| Py | Pyrolysis number |
| Heat | |
| Generated or consumed heat due to reaction | |
| Radius | |
| R | Production rate/Universal gas constant |
| Re | Reynolds number |
| Solid-liquid interface position | |
| Heat transfer between phases | |
| Momentum transfer between phases | |
| Species transfer between phases | |
| Net mass transfer rate between phases | |
| SP | Particle-based mass source term |
| Sc | Schmidt number |
| Time | |
| T | Temperature |
| Velocity | |
| V | Volume |
| Spatial coordinate/Interface position | |
| Monomer conversion | |
| Mass fraction | |
| Volume fraction | |
| Porosity, void fraction | |
| Incident angle | |
| Thermal conductivity | |
| Dynamic viscosity | |
| Kinematic viscosity | |
| Density | |
| Surface tension | |
| Stress-strain tensor | |
| Sphericity parameter | |
| Circularity | |
| Shape factor/Particle-based species transfer rate between phases | |
| Sub/Superscripts | |
| Initial | |
| First | |
| Second | |
| Bulk | |
| Contact | |
| Conduction | |
| Convection | |
| Drag | |
| Effective | |
| Fluid | |
| The ith species | |
| Liquid | |
| Particle/Particle surface | |
| Pressure gradient | |
| Radiation | |
| Reaction | |
| Solid | |
| Turbulent | |
References
- PlasticsEurope. Plastics—The Facts 2020, an Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope, Association of Plastics Manufacturers: Brussels, Belgium, 2020. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2019, an Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope, Association of Plastics Manufacturers: Brussels, Belgium, 2019. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2018, an Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope, Association of Plastics Manufacturers: Brussels, Belgium, 2018. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2021, an Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope, Association of Plastics Manufacturers: Brussels, Belgium, 2021. [Google Scholar]
- Cordier, M.; Uehara, T. How much innovation is needed to protect the ocean from plastic contamination? Sci. Total Environ. 2019, 670, 789–799. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Arena, U. Olivine as a tar removal catalyst during fluidized bed gasification of plastic waste. AlChE J. 2008, 54, 1656–1667. [Google Scholar] [CrossRef]
- Da Silva, T.R.; De Azevedo, A.R.G.; Cecchin, D.; Marvila, M.T.; Amran, M.; Fediuk, R.; Vatin, N.; Karelina, M.; Klyuev, S.; Szelag, M. Application of Plastic Wastes in Construction Materials: A Review Using the Concept of Life-Cycle Assessment in the Context of Recent Research for Future Perspectives. Materials 2021, 14, 3549. [Google Scholar] [CrossRef] [PubMed]
- Awoyera, P.O.; Adesina, A. Plastic wastes to construction products: Status, limitations and future perspective. Case Stud. Constr. Mater. 2020, 12, e00330. [Google Scholar] [CrossRef]
- Aneke, F.I.; Shabangu, C. Green-efficient masonry bricks produced from scrap plastic waste and foundry sand. Case Stud. Constr. Mater. 2021, 14, e00515. [Google Scholar] [CrossRef]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020, 54, 13282–13293. [Google Scholar] [CrossRef] [PubMed]
- Santander, P.; Cruz Sanchez, F.A.; Boudaoud, H.; Camargo, M. Closed loop supply chain network for local and distributed plastic recycling for 3D printing: A MILP-based optimization approach. Resour. Conserv. Recycl. 2020, 154, 104531. [Google Scholar] [CrossRef] [Green Version]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. (Oxford UK) 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T. Hydrogen and Carbon Nanotubes from Pyrolysis-Catalysis of Waste Plastics: A Review. Waste Biomass Valorization 2020, 12, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Ügdüler, S.; Van Geem, K.M.; Roosen, M.; Delbeke, E.I.P.; De Meester, S. Challenges and opportunities of solvent-based additive extraction methods for plastic recycling. Waste Manag. (Oxford) 2020, 104, 148–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClarivateTM. Web of Science. Available online: https://www.webofknowledge.com (accessed on 1 February 2022).
- Wang, M.; Smith, J.M.; McCoy, B.J. Continuous kinetics for thermal degradation of polymer in solution. AlChE J. 1995, 41, 1521–1533. [Google Scholar] [CrossRef]
- Zolghadr, A.; Sidhu, N.; Mastalski, I.; Facas, G.; Maduskar, S.; Uppili, S.; Go, T.; Neurock, M.; Dauenhauer, P.J. On the Method of Pulse-Heated Analysis of Solid Reactions (PHASR) for Polyolefin Pyrolysis. ChemSusChem 2021, 14, 4214–4227. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Frances, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Pyrolysis-gasification of plastics, mixed plastics and real-world plastic waste with and without Ni-Mg-Al catalyst. Fuel 2010, 89, 3022–3032. [Google Scholar] [CrossRef]
- Na, J.I.; Park, S.J.; Kim, Y.K.; Lee, J.G.; Kim, J.H. Characteristics of oxygen-blown gasification for combustible waste in a fixed-bed gasifier. Appl. Energy 2003, 75, 275–285. [Google Scholar] [CrossRef]
- Tsuji, T.; Hatayama, A. Gasification of waste plastics by steam reforming in a fluidized bed. J. Mater. Cycles Waste Manag. 2009, 11, 144–147. [Google Scholar] [CrossRef]
- Tsuji, T.; Sasaki, A.; Okajima, S.; Masuda, T. Steam reforming of the oils produced from waste plastics. Kagaku Kogaku Ronbunshu 2004, 30, 705–709. [Google Scholar] [CrossRef]
- Janajreh, I.; Adeyemi, I.; Raza, S.S.; Ghenai, C. A review of recent developments and future prospects in gasification systems and their modeling. Renew. Sustain. Energy Rev. 2021, 138, 110505. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Conversion of mixed plastic wastes in a dual fluidized bed steam gasifier. Fuel 2013, 107, 787–799. [Google Scholar] [CrossRef]
- Baloch, H.A.; Yang, T.; Li, R.; Nizamuddin, S.; Kai, X.; Bhutto, A.W. Parametric study of co-gasification of ternary blends of rice straw, polyethylene and polyvinylchloride. Clean Technol. Environ. Policy 2016, 18, 1031–1042. [Google Scholar] [CrossRef]
- Interreg. PSYCHE, Production of Basic Chemicals from Plastic Waste for Reuse in the Chemical Industry. Available online: https://psycheplastics.eu/ (accessed on 10 July 2020).
- Janajreh, I.; Adeyemi, I.; Elagroudy, S. Gasification feasibility of polyethylene, polypropylene, polystyrene waste and their mixture: Experimental studies and modeling. Sustain. Energy Technol. Assess. 2020, 39, 100684. [Google Scholar] [CrossRef]
- Tukker, A.; de Groot, H.; Simons, L.; Wiegersma, S. Chemical Recycling of Plastics Waste (PVC and Other Resins); TNO Institute of Strategy, Technology and Policy: Delft, The Nedtherlands, 1999. [Google Scholar]
- Hoang, Q.N.; Vanierschot, M.; Blondeau, J.; Croymans, T.; Pittoors, R.; Van Caneghem, J. Review of numerical studies on thermal treatment of municipal solid waste in packed bed combustion. Fuel Commun. 2021, 7, 100013. [Google Scholar] [CrossRef]
- Harmon, R.E.; SriBala, G.; Broadbelt, L.J.; Burnham, A.K. Insight into Polyethylene and Polypropylene Pyrolysis: Global and Mechanistic Models. Energy Fuels 2021, 35, 6765–6775. [Google Scholar] [CrossRef]
- Sharma, S.S.; Batra, V.S. Production of hydrogen and carbon nanotubes via catalytic thermo-chemical conversion of plastic waste: Review. J. Chem. Technol. Biotechnol. 2020, 95, 11–19. [Google Scholar] [CrossRef]
- Ciuffi, B.; Chiaramonti, D.; Rizzo, A.M.; Frediani, M.; Rosi, L. A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste. Appl. Sci. 2020, 10, 6307. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Arku, P.; Dutta, A. Gasification of Plastic Solid Waste and Competitive Technologies. In Plastics to Energy; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 269–293. [Google Scholar]
- Ramos, A.; Monteiro, E.; Rouboa, A. Numerical approaches and comprehensive models for gasification process: A review. Renew. Sustain. Energy Rev. 2019, 110, 188–206. [Google Scholar] [CrossRef]
- Dedeyne, J.; Virgilio, M.; Arts, T.; Marin, G.B.; Van Geem, K.M. Design and Optimization of 3D Reactor Technologies for the Production of Light Olefins. In Proceedings of the AIChE Annual Meeting (19AIChE), Orlando, FL, USA, 10–15 November 2019. [Google Scholar]
- Nakhaei, M.; Wu, H.; Grévain, D.; Jensen, L.S.; Glarborg, P.; Clausen, S.; Dam–Johansen, K. Experiments and modeling of single plastic particle conversion in suspension. Fuel Process. Technol. 2018, 178, 213–225. [Google Scholar] [CrossRef]
- Ponzio, A.; Kalisz, S.; Blasiak, W. Effect of operating conditions on tar and gas composition in high temperature air/steam gasification (HTAG) of plastic containing waste. Fuel Process. Technol. 2006, 87, 223–233. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, Y.; Yi, C. Hydrogen-rich gas production from biomass catalytic gasification using hot blast furnace slag as heat carrier and catalyst in moving-bed reactor. Int. J. Hydrogen Energy 2012, 37, 15081–15085. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Barbarias, I.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. HDPE pyrolysis-steam reforming in a tandem spouted bed-fixed bed reactor for H2 production. J. Anal. Appl. Pyrolysis 2015, 116, 34–41. [Google Scholar] [CrossRef]
- Lopez, G.; Erkiaga, A.; Amutio, M.; Bilbao, J.; Olazar, M. Effect of polyethylene co-feeding in the steam gasification of biomass in a conical spouted bed reactor. Fuel 2015, 153, 393–401. [Google Scholar] [CrossRef]
- Alvarez, J.; Kumagai, S.; Wu, C.; Yoshioka, T.; Bilbao, J.; Olazar, M.; Williams, P.T. Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int. J. Hydrogen Energy 2014, 39, 10883–10891. [Google Scholar] [CrossRef]
- Lopez, G.; Erkiaga, A.; Amutio, M.; Alvarez, J.; Barbarias, I.; Bilbao, J.; Olazar, M. Steam gasification of waste plastics in a conical spouted bed reactor. In Proceedings of the the 14th International Conference on Fluidization—From Fundamentals to Products, NH Conference Centre Leeuwenhorst Noordwijkerhout, Noordwijkerhout, The Netherlands, 26–31 May 2013; pp. 945–952. [Google Scholar]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Olazar, M.; Bilbao, J. Operating Conditions for the Pyrolysis of Poly-(ethylene terephthalate) in a Conical Spouted-Bed Reactor. Ind. Eng. Chem. Res. 2010, 49, 2064–2069. [Google Scholar] [CrossRef]
- Vandewalle, L.A.; Gonzalez-Quiroga, A.; Perreault, P.; Van Geem, K.M.; Marin, G.B. Process Intensification in a Gas–Solid Vortex Unit: Computational Fluid Dynamics Model Based Analysis and Design. Ind. Eng. Chem. Res. 2019, 58, 12751–12765. [Google Scholar] [CrossRef]
- De Wilde, J. Gas-solid fluidized beds in vortex chambers. Chem. Eng. Process. 2014, 85, 256–290. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H. Decomposition of polyethylene in radio-frequency nitrogen and water steam plasmas under reduced pressures. Fuel Process. Technol. 2007, 88, 549–556. [Google Scholar] [CrossRef]
- Kikuchi, R.; Sato, H.; Matsukura, Y.; Yamamoto, T. Semi-pilot scale test for production of hydrogen-rich fuel gas from different wastes by means of a gasification and smelting process with oxygen multi-blowing. Fuel Process. Technol. 2005, 86, 1279–1296. [Google Scholar] [CrossRef]
- Lee, J.W.; Yu, T.U.; Lee, J.W.; Moon, J.H.; Jeong, H.J.; Park, S.S.; Yang, W.; Lee, U.D. Gasification of Mixed Plastic Wastes in a Moving-Grate Gasifier and Application of the Producer Gas to a Power Generation Engine. Energy Fuels 2013, 27, 2092–2098. [Google Scholar] [CrossRef]
- Wong, S.-C.; Lin, A.-C. Internal temperature distributions of droplets vaporizing in high-temperature convective flows. J. Fluid Mech. 1992, 237, 671–687. [Google Scholar] [CrossRef]
- Floyd, S.; Choi, K.Y.; Taylor, T.W.; Ray, W.H. Polymerization of olefins through heterogeneous catalysis. III. Polymer particle modelling with an analysis of intraparticle heat and mass transfer effects. J. Appl. Polym. Sci. 1986, 32, 2935–2960. [Google Scholar] [CrossRef] [Green Version]
- Calfa, B.A. Multi-Scale Process Systems Engineering. In Proceedings of the AIChE Annual Meeting, Salt Lake City, UT, USA, 8–13 November 2015. [Google Scholar]
- Fu, Z.; Zhu, J.; Barghi, S.; Zhao, Y.; Luo, Z.; Duan, C. On the two-phase theory of fluidization for Geldart B and D particles. Powder Technol. 2019, 354, 64–70. [Google Scholar] [CrossRef]
- Plehiers, P. Multi-Scale Modeling of Chemical Processes via Machine Learning. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2020. [Google Scholar]
- Alli, R. Performance Prediction of Waste Polyethylene Gasification Using CO2 in a Bubbling Fluidized Bed: A Modelling Study. Chem. Biochem. Eng. Q. 2018, 32, 349–358. [Google Scholar] [CrossRef]
- Horton, S.R.; Woeckener, J.; Mohr, R.; Zhang, Y.; Petrocelli, F.; Klein, M.T. Molecular-Level Kinetic Modeling of the Gasification of Common Plastics. Energy Fuels 2016, 30, 1662–1674. [Google Scholar] [CrossRef]
- Ramos, A.; Tavares, R.; Rouboa, A. Microplastics co-gasification with biomass: Modelling syngas characteristics at low temperatures. AIP Conf. Proc. 2018, 1968, 020016. [Google Scholar] [CrossRef]
- Donskoy, I. Mathematical modelling and optimization of biomass-plastic fixed-bed downdraft co-gasification process. EPJ Web Conf. 2017, 159, 00010. [Google Scholar] [CrossRef] [Green Version]
- Horton, S.R.; Zhang, Y.; Mohr, R.; Petrocelli, F.; Klein, M.T. Implementation of a Molecular-Level Kinetic Model for Plasma-Arc Municipal Solid Waste Gasification. Energy Fuels 2016, 30, 7904–7915. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Q.; Berrouk, A.S.; Yang, C.; Al Shoaibi, A.S. Equivalent Reactor Network Model for Simulating the Air Gasification of Polyethylene in a Conical Spouted Bed Gasifier. Energy Fuels 2014, 28, 6830–6840. [Google Scholar] [CrossRef]
- Grana, R.; Sommariva, S.; Maffei, T.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Pierucci, S.; Ranzi, E. Detailed kinetics in the mathematical model of fixed bed gasifiers. Comput.-Aided Chem. Eng. 2010, 28, 829–834. [Google Scholar]
- Mazzoni, L.; Janajreh, I. Plasma gasification of municipal solid waste with variable content of plastic solid waste for enhanced energy recovery. Int. J. Hydrogen Energy 2017, 42, 19446–19457. [Google Scholar] [CrossRef]
- Janajreh, I.; Raza, S.S. Numerical simulation of waste tyres gasification. Waste Manag. Res. 2015, 33, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dor, L.; Yang, W.; Blasiak, W. CFD modeling of municipal solid waste gasification in a fixed-bed plasma gasification melting reactor. In Proceedings of the International Conference on Thermal Treatment Technologies and Hazardous Waste Combustors, Jacksonville, FL, USA, 10–13 May 2011; pp. 252–278. [Google Scholar]
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J.Y. High efficiency chlorine removal from polyvinyl chloride (PVC) pyrolysis with a gas–liquid fluidized bed reactor. Waste Manag. (Oxford) 2014, 34, 1045–1050. [Google Scholar] [CrossRef]
- Jin, Z.; Yin, L.; Chen, D.; Jia, Y.; Yuan, J.; Yu, B. Heat transfer characteristics of molten plastics in a vertical falling film reactor. Chin. J. Chem. Eng. 2019, 27, 1015–1020. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U. Stepwise pyrolysis for raw material recovery from plastic waste. J. Anal. Appl. Pyrolysis 1998, 46, 1–13. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Mazloum, S.; Awad, S.; Allam, N.; Aboumsallem, Y.; Loubar, K.; Tazerout, M. Modelling plastic heating and melting in a semi-batch pyrolysis reactor. Appl. Energy 2021, 283, 116375. [Google Scholar] [CrossRef]
- Mikulionok, I.; Gavva, O.; Kryvoplias-Volodina, L. Modeling of melting process in a single screw extruder for polymer processing. East.-Eur. J. Enterp. Technol. 2018, 2, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Hossain, A.; Nakamura, Y. Numerical modeling of melting and dripping process of polymeric material subjected to moving heat flux: Prediction of drop time. Proc. Combust. Inst. 2015, 35, 2555–2562. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, C. Modeling of the Heat Capacity of Polymers with the Variable Connectivity Index. Polym. J. 2002, 34, 954–961. [Google Scholar] [CrossRef] [Green Version]
- Algaer, E.A.; Müller-Plathe, F. Molecular Dynamics Calculations of the Thermal Conductivity of Molecular Liquids, Polymers, and Carbon Nanotubes. Soft Mater. 2012, 10, 42–80. [Google Scholar] [CrossRef]
- Kiessling, A.; Simavilla, D.N.; Vogiatzis, G.G.; Venerus, D.C. Thermal conductivity of amorphous polymers and its dependence on molecular weight. Polymer 2021, 228, 123881. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, J.-W.; Wei, N.; Zhang, Y.; Rabczuk, T. Thermal conductivity dependence on chain length in amorphous polymers. J. Appl. Phys. 2013, 113, 184304. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Shukre, R.; Chen, C.-C. Development of a Thermophysical Properties Model for Flowsheet Simulation of Biomass Pyrolysis Processes. ACS Sustain. Chem. Eng. 2019, 7, 9017–9027. [Google Scholar] [CrossRef]
- Ungerer, P.; Nieto-Draghi, C.; Rousseau, B.; Ahunbay, G.; Lachet, V. Molecular simulation of the thermophysical properties of fluids: From understanding toward quantitative predictions. J. Mol. Liq. 2007, 134, 71–89. [Google Scholar] [CrossRef]
- Gavoille, T.; Pannacci, N.; Bergeot, G.; Marliere, C.; Marre, S. Microfluidic approaches for accessing thermophysical properties of fluid systems. React. Chem. Eng. 2019, 4, 1721–1739. [Google Scholar] [CrossRef]
- ANSYS Chemkin Theory Manual 17.0 (15151); Reaction Design: San Diego, CA, USA; ANSYS, Inc.: Carnotsburg, PA, USA, 2015.
- Dadgostar, N.; Shaw, J.M. A predictive correlation for the constant-pressure specific heat capacity of pure and ill-defined liquid hydrocarbons. Fluid Phase Equilib. 2012, 313, 211–226. [Google Scholar] [CrossRef]
- Detar, D.F. Theoretical ab Initio Calculation of Entropy, Heat Capacity, and Heat Content. J. Phys. Chem. A 1998, 102, 5128–5141. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Molecular scale simulations on thermoset polymers: A review. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 103–122. [Google Scholar] [CrossRef]
- Kumar, A.; Sundararaghavan, V.; Browning, A.R. Study of temperature dependence of thermal conductivity in cross-linked epoxies using molecular dynamics simulations with long range interactions. Model. Simul. Mater. Sci. Eng. 2014, 22, 025013. [Google Scholar] [CrossRef] [Green Version]
- Henry, A.; Chen, G. High Thermal Conductivity of Single Polyethylene Chains Using Molecular Dynamics Simulations. Phys. Rev. Lett. 2008, 101, 235502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautenberger, C.; Fernandez-Pello, C. Generalized pyrolysis model for combustible solids. Fire Saf. J. 2009, 44, 819–839. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Zhang, P.; Xian, Y.; Zeng, J.; Shi, B. Effective thermal conductivity of polymer composites: Theoretical models and simulation models. Int. J. Heat Mass Transf. 2018, 117, 358–374. [Google Scholar] [CrossRef]
- Sakiyama, T.; Akutsu, M.; Miyawaki, O.; Yano, T. Effective thermal diffusivity of food gels impregnated with air bubbles. J. Food Eng. 1999, 39, 323–328. [Google Scholar] [CrossRef]
- Yin, C. Transient heating and evaporation of moving mono-component liquid fuel droplets. Appl. Therm. Eng. 2016, 104, 497–503. [Google Scholar] [CrossRef]
- Baghel, V.; Sikarwar, B.S.; Muralidhar, K. Modeling of heat transfer through a liquid droplet. Heat Mass Transf. 2019, 55, 1371–1385. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 5th ed.; McGRAW-HILL: New York, NY, USA, 2001. [Google Scholar]
- Ferkl, P.; Toulec, M.; Laurini, E.; Pricl, S.; Fermeglia, M.; Auffarth, S.; Eling, B.; Settels, V.; Kosek, J. Multi-scale modelling of heat transfer in polyurethane foams. Chem. Eng. Sci. 2017, 172, 323–334. [Google Scholar] [CrossRef]
- Dogu, O.; Plehiers, P.P.; Van De Vijver, R.; D’Hooge, D.R.; Van Steenberge, P.H.M.; Van Geem, K.M. Distribution Changes during Thermal Degradation of Poly(styrene peroxide) by Pairing Tree-Based Kinetic Monte Carlo and Artificial Intelligence Tools. Ind. Eng. Chem. Res. 2021, 60, 3334–3353. [Google Scholar] [CrossRef]
- Dente, M.; Bozzano, G.; Faravelli, T.; Marongiu, A.; Pierucci, S.; Ranzi, E. Kinetic Modelling of Pyrolysis Processes in Gas and Condensed Phase. Adv. Chem. Eng. 2007, 32, 51–166. [Google Scholar]
- Faravelli, T.; Bozzano, G.; Colombo, M.; Ranzi, E.; Dente, M. Kinetic modeling of the thermal degradation of polyethylene and polystyrene mixtures. J. Anal. Appl. Pyrolysis 2003, 70, 761–777. [Google Scholar] [CrossRef]
- Kiran Ciliz, N.; Ekinci, E.; Snape, C.E. Pyrolysis of virgin and waste polypropylene and its mixtures with waste polyethylene and polystyrene. Waste Manag. 2004, 24, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Dogu, O.; Pelucchi, M.; Van De Vijver, R.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Vinu, R.; Broadbelt, L.J. Unraveling reaction pathways and specifying reaction kinetics for complex systems. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 29–54. [Google Scholar] [CrossRef]
- Poutsma, M.L. Fundamental reactions of free radicals relevant to pyrolysis reactions. J. Anal. Appl. Pyrolysis 2000, 54, 5–35. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Thermodynamic modelling and evaluation of a two-stage thermal process for waste gasification. Fuel 2013, 108, 356–369. [Google Scholar] [CrossRef] [Green Version]
- Jarungthammachote, S.; Dutta, A. Equilibrium modeling of gasification: Gibbs free energy minimization approach and its application to spouted bed and spout-fluid bed gasifiers. Energy Convers. Manag. 2008, 49, 1345–1356. [Google Scholar] [CrossRef]
- Zainal, Z.A.; Ali, R.; Lean, C.H.; Seetharamu, K.N. Prediction of performance of a downdraft gasifier using equilibrium modeling for different biomass materials. Energy Convers. Manag. 2001, 42, 1499–1515. [Google Scholar] [CrossRef]
- Melgar, A.; Pérez, J.F.; Laget, H.; Horillo, A. Thermochemical equilibrium modelling of a gasifying process. Energy Convers. Manag. 2007, 48, 59–67. [Google Scholar] [CrossRef]
- Lee, U.; Chung, J.N.; Ingley, H.A. High-Temperature Steam Gasification of Municipal Solid Waste, Rubber, Plastic and Wood. Energy Fuels 2014, 28, 4573–4587. [Google Scholar] [CrossRef]
- Jand, N.; Brandani, V.; Foscolo, P.U. Thermodynamic Limits and Actual Product Yields and Compositions in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2006, 42, 834–843. [Google Scholar] [CrossRef]
- Ranzi, E.; Dente, M.; Goldaniga, A.; Bozzano, G.; Faravelli, T. Lumping procedures in detailed kinetic modeling of gasification, pyrolysis, partial oxidation and combustion of hydrocarbon mixtures. Prog. Energy Combust. Sci. 2001, 27, 99–139. [Google Scholar] [CrossRef]
- Faravelli, T.; Pinciroli, M.; Pisano, F.; Bozzano, G.; Dente, M.; Ranzi, E. Thermal degradation of polystyrene. J. Anal. Appl. Pyrolysis 2001, 60, 103–121. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Van Geem, K.M.; Fathi, M.; Bazgir, H.; Ghadiri, M. The pyrolysis of oak with polyethylene, polypropylene and polystyrene using fixed bed and stirred reactors and TGA instrument. Energy 2021, 232, 121085. [Google Scholar] [CrossRef]
- Singh, P.; Déparrois, N.; Burra, K.G.; Bhattacharya, S.; Gupta, A.K. Energy recovery from cross-linked polyethylene wastes using pyrolysis and CO2 assisted gasification. Appl. Energy 2019, 254, 113722. [Google Scholar] [CrossRef]
- Marongiu, A.; Bozzano, G.; Dente, M.; Ranzi, E.; Faravelli, T. Detailed kinetic modeling of pyrolysis of tetrabromobisphenol A. J. Anal. Appl. Pyrolysis 2007, 80, 325–345. [Google Scholar] [CrossRef]
- Westerhout, R.W.J.; Waanders, J.; Kuipers, J.A.M.; Van Swaaij, W.P.M. Kinetics of the Low-Temperature Pyrolysis of Polyethene, Polypropene, and Polystyrene Modeling, Experimental Determination, and Comparison with Literature Models and Data. Ind. Eng. Chem. Res. 1997, 36, 1955–1964. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, T.; Ohlemiller, T.J. A study of oxygen effects on nonflaming transient gasification of PMMA and PE during thermal irradiation. Symp. (Int.) Combust. 1982, 19, 815–823. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Choi, Y.-K.; Kim, J.-S. Three-stage air gasification of waste polyethylene: In-situ regeneration of active carbon used as a tar removal additive. Energy 2019, 166, 335–342. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Karlsson, M.; Hupa, M.; Frankenhaeuser, M. Combustion and gasification properties of plastics particles. J. Air Waste Manag. Assoc. 1997, 47, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Jin, B.; Zhou, H.; Zhong, Z.; Zhang, M. Air gasification of polypropylene plastic waste in fluidized bed gasifier. Energy Convers. Manag. 2007, 48, 778–786. [Google Scholar] [CrossRef]
- Esmaeili, V.; Ajalli, J.; Faramarzi, A.; Abdi, M.; Gholizadeh, M. Gasification of wastes: The impact of the feedstock type and co-gasification on the formation of volatiles and char. Int. J. Energy Res. 2020, 44, 3587–3606. [Google Scholar] [CrossRef]
- Koo, J.-K.; Kim, S.-W. Reaction Kinetic Model for Optimal Pyrolysis of Plastic Wastes Mixtures. Waste Manag. Res. 1993, 11, 515–529. [Google Scholar] [CrossRef]
- Bradbury, A.G.W.; Sakai, Y.; Shafizadeh, F. A kinetic model for pyrolysis of cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- Chen, S.; Meng, A.; Long, Y.; Zhou, H.; Li, Q.; Zhang, Y. TGA pyrolysis and gasification of combustible municipal solid waste. J. Energy Inst. 2015, 88, 332–343. [Google Scholar] [CrossRef]
- Wang, L.; Chai, M.; Liu, R.; Cai, J. Synergetic effects during co-pyrolysis of biomass and waste tire: A study on product distribution and reaction kinetics. Bioresour. Technol. 2018, 268, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lera, S.; Pallarés Ranz, J. On the development of a polyolefin gasification modelling approach. Fuel 2017, 197, 518–527. [Google Scholar] [CrossRef]
- Conesa, J.A.; Font, R.; Marcilla, A.; Caballero, J.A. Kinetic model for the continuous pyrolysis of two types of polyethylene in a fluidized bed reactor. J. Anal. Appl. Pyrolysis 1997, 40–41, 419–431. [Google Scholar] [CrossRef]
- Hoffmann, A.C.; Janssen, L.P.B.M.; Prins, J. Particle segregation in fluidised binary mixtures. Chem. Eng. Sci. 1993, 48, 1583–1592. [Google Scholar] [CrossRef] [Green Version]
- Sommariva, S.; Grana, R.; Maffei, T.; Pierucci, S.; Ranzi, E. A kinetic approach to the mathematical model of fixed bed gasifiers. Comput. Chem. Eng. 2011, 35, 928–935. [Google Scholar] [CrossRef]
- Hla, S.S.; Lopes, R.; Roberts, D. The CO2 gasification reactivity of chars produced from Australian municipal solid waste. Fuel 2016, 185, 847–854. [Google Scholar] [CrossRef]
- Paviet, F.; Bals, O.; Antonini, G. Kinetic study of various chars steam gasification. Int. J. Chem. React. Eng. 2007, 5. [Google Scholar] [CrossRef]
- ECN.TNO. Phyllis2, Database for Biomass and Waste. Available online: https://phyllis.nl/ (accessed on 18 January 2022).
- Serranti, S.; Gargiulo, A.; Bonifazi, G. Characterization of post-consumer polyolefin wastes by hyperspectral imaging for quality control in recycling processes. Waste Manag. 2011, 31, 2217–2227. [Google Scholar] [CrossRef] [PubMed]
- McGhee, B.; Norton, F.; Snape, C.E.; Hall, P.J. The copyrolysis of poly(vinyl chloride) with cellulose derived materials as a model for municipal waste derived chars. Fuel 1995, 74, 28–31. [Google Scholar] [CrossRef]
- De Oliveira, L.P.; Hudebine, D.; Guillaume, D.; Verstraete, J.J. A Review of Kinetic Modeling Methodologies for Complex Processes. OGST–Revue d’IFP Energies Nouvelles 2016, 71, 45. [Google Scholar] [CrossRef] [Green Version]
- Németh, A.; Blazsó, M.; Baranyai, P.; Vidóczy, T. Thermal degradation of polyethylene modeled on tetracontane. J. Anal. Appl. Pyrolysis 2008, 81, 237–242. [Google Scholar] [CrossRef]
- Mastan, E.; Zhu, S. Method of moments: A versatile tool for deterministic modeling of polymerization kinetics. Eur. Polym. J. 2015, 68, 139–160. [Google Scholar] [CrossRef]
- Nasresfahani, A.; Hutchinson, R.A. Modeling the Distribution of Functional Groups in Semibatch Radical Copolymerization: An Accelerated Stochastic Approach. Ind. Eng. Chem. Res. 2018, 57, 9407–9419. [Google Scholar] [CrossRef]
- Vandewiele, N.M.; Van Geem, K.M.; Reyniers, M.-F.; Marin, G.B. Genesys: Kinetic model construction using chemo-informatics. Chem. Eng. J. 2012, 207–208, 526–538. [Google Scholar] [CrossRef]
- De Smit, K.; Marien, Y.W.; Van Geem, K.M.; Van Steenberge, P.H.M.; D’Hooge, D.R. Connecting polymer synthesis and chemical recycling on a chain-by-chain basis: A unified matrix-based kinetic Monte Carlo strategy. React. Chem. Eng. 2020, 5, 1909–1928. [Google Scholar] [CrossRef]
- CRECK Modeling Lab. Detailed Kinetic Mechanisms and CFD of Reacting Flows. Available online: http://creckmodeling.chem.polimi.it/ (accessed on 10 June 2021).
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Qiao, Y.; Tian, Y. The steam gasification reactivity and kinetics of municipal solid waste chars derived from rapid pyrolysis. Waste Manag. 2018, 80, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.P.; Agnew, J.B.; Zhang, D.K. Gasification of a South Australian low-rank coal with carbon dioxide and steam: Kinetics and reactivity studies. Fuel 1998, 77, 1209–1219. [Google Scholar] [CrossRef]
- Murillo, R.; Navarro, M.V.; López, J.M.; García, T.; Callén, M.S.; Aylón, E.; Mastral, A.M. Activation of pyrolytic tire char with CO2: Kinetic study. J. Anal. Appl. Pyrolysis 2004, 71, 945–957. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AlChE J. 1980, 26, 379–386. [Google Scholar] [CrossRef]
- Senneca, O.; Salatino, P. A semi-detailed kinetic model of char combustion with consideration of thermal annealing. Proc. Combust. Inst. 2011, 33, 1763–1770. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, G.; Liao, Z.; Yang, Y.; Sun, J.; Jiang, B.; Wang, J.; Yang, Y. Kinetic modeling with automatic reaction network generator, an application to naphtha steam cracking. Energy 2020, 207, 118204. [Google Scholar] [CrossRef]
- Froment, G.F. On fundamental kinetic equations for chemical reactions and processes. Curr. Opin. Chem. Eng. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Gao, C.W.; Allen, J.W.; Green, W.H.; West, R.H. Reaction Mechanism Generator: Automatic construction of chemical kinetic mechanisms. Comput. Phys. Commun. 2016, 203, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Vandewiele, N.M.; Van De Vijver, R.; Van Geem, K.M.; Reyniers, M.-F.; Marin, G.B. Symmetry calculation for molecules and transition states. J. Comput. Chem. 2015, 36, 181–192. [Google Scholar] [CrossRef]
- Coley, C.W.; Green, W.H.; Jensen, K.F. RDChiral: An RDKit Wrapper for Handling Stereochemistry in Retrosynthetic Template Extraction and Application. J. Chem. Inf. Model. 2019, 59, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Vandewiele, N.M.; Van De Vijver, R.; Carstensen, H.-H.; Van Geem, K.M.; Reyniers, M.-F.; Marin, G.B. Implementation of Stereochemistry in Automatic Kinetic Model Generation. Int. J. Chem. Kinet. 2016, 48, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Van De Vijver, R.; Van Geem, K.M.; Marin, G.B. On-the-fly ab initio calculations toward accurate rate coefficients. Proc. Combust. Inst. 2019, 37, 283–290. [Google Scholar] [CrossRef]
- Broadbelt, L.J.; Stark, S.M.; Klein, M.T. Computer Generated Pyrolysis Modeling: On-the-Fly Generation of Species, Reactions, and Rates. Ind. Eng. Chem. Res. 1994, 33, 790–799. [Google Scholar] [CrossRef]
- Ohno, K.; Maeda, S. A scaled hypersphere search method for the topography of reaction pathways on the potential energy surface. Chem. Phys. Lett. 2004, 384, 277–282. [Google Scholar] [CrossRef]
- Van De Vijver, R.; Zádor, J. KinBot: Automated stationary point search on potential energy surfaces. Comput. Phys. Commun. 2020, 248, 106947. [Google Scholar] [CrossRef]
- Stagni, A.; Cuoci, A.; Frassoldati, A.; Faravelli, T.; Ranzi, E. Lumping and Reduction of Detailed Kinetic Schemes: An Effective Coupling. Ind. Eng. Chem. Res. 2014, 53, 9004–9016. [Google Scholar] [CrossRef]
- Huang, H.; Fairweather, M.; Griffiths, J.F.; Tomlin, A.S.; Brad, R.B. A systematic lumping approach for the reduction of comprehensive kinetic models. Proc. Combust. Inst. 2005, 30, 1309–1316. [Google Scholar] [CrossRef]
- Wang, H.; Frenklach, M. Detailed reduction of reaction mechanisms for flame modeling. Combust. Flame 1991, 87, 365–370. [Google Scholar] [CrossRef]
- Gascoin, N.; Navarro-Rodriguez, A.; Fau, G.; Gillard, P. Kinetic modelling of High Density PolyEthylene pyrolysis: Part 2. Reduction of existing detailed mechanism. Polym. Degrad. Stab. 2012, 97, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Law, C.K. Linear time reduction of large kinetic mechanisms with directed relation graph: N-Heptane and iso-octane. Combust. Flame 2006, 144, 24–36. [Google Scholar] [CrossRef]
- Briceno, J.; Lemos, M.A.; Lemos, F. Kinetic analysis of the degradation of HDPE+PP polymer mixtures. Int. J. Chem. Kinet. 2021, 53, 660–674. [Google Scholar] [CrossRef]
- Tuffi, R.; D’Abramo, S.; Cafiero, L.M.; Trinca, E.; Ciprioti, S.V. Thermal behavior and pyrolytic degradation kinetics of polymeric mixtures from waste packaging plastics. eXPRESS Polym. Lett. 2018, 12, 82–99. [Google Scholar] [CrossRef]
- Richter, F.; Rein, G. The Role of Heat Transfer Limitations in Polymer Pyrolysis at the Microscale. Front. Mech. Eng. 2018, 4, 18. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental methods in chemical engineering: Thermogravimetric analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Samih, S.; Chaouki, J. Development of a fluidized bed thermogravimetric analyzer. AlChE J. 2015, 61, 84–89. [Google Scholar] [CrossRef]
- Quan, H. Design of Micro-Fluidized Beds by Experiments and Numerical Simulations: Flow Regims Diagonis and Hydrodynamic Study. Ph.D. Thesis, Ecole Centrale de Lille, Villeneuve-d′Ascq, France, 2017. [Google Scholar]
- Leclerc, P.; Doucet, J.; Chaouki, J. Development of a microwave thermogravimetric analyzer and its application on polystyrene microwave pyrolysis kinetics. J. Anal. Appl. Pyrolysis 2018, 130, 209–215. [Google Scholar] [CrossRef]
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of plastic waste as waste-to-energy or waste-to-syngas recovery route. Nat. Sci. 2013, 05, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Kishore, K.; Mohandas, K.; Annakutty, K.S. Is gasification rate controlling step in polymer ignition? Combust. Sci. Technol. 1983, 31, 183–194. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U.; Jakobströer, P. Modelling of isothermal and dynamic pyrolysis of plastics considering non-homogeneous temperature distribution and detailed degradation mechanism. J. Anal. Appl. Pyrolysis 1999, 49, 53–74. [Google Scholar] [CrossRef]
- Yin, L.; Jia, Y.; Guo, X.; Chen, D.; Jin, Z. Flow behaviors and heat transfer characteristics of liquid film during the pyrolysis process of molten plastics using OpenFOAM. Int. J. Heat Mass Transf. 2019, 133, 129–136. [Google Scholar] [CrossRef]
- Simons, G.A. Char Gasification: Part I. Transport Model. Combust. Sci. Technol. 1979, 20, 107–116. [Google Scholar] [CrossRef]
- Schulze, S.; Nikrityuk, P.; Abosteif, Z.; Guhl, S.; Richter, A.; Meyer, B. Heat and mass transfer within thermogravimetric analyser: From simulation to improved estimation of kinetic data for char gasification. Fuel 2017, 187, 338–348. [Google Scholar] [CrossRef]
- Cuoci, A.; Avedisian, C.T.; Brunson, J.D.; Guo, S.; Dalili, A.; Wang, Y.; Mehl, M.; Frassoldati, A.; Seshadri, K.; Dec, J.E.; et al. Simulating combustion of a seven-component surrogate for a gasoline/ethanol blend including soot formation and comparison with experiments. Fuel 2021, 288, 119451. [Google Scholar] [CrossRef]
- Arabkhalaj, A.; Azimi, A.; Ghassemi, H.; Shahsavan Markadeh, R. A fully transient approach on evaporation of multi-component droplets. Appl. Therm. Eng. 2017, 125, 584–595. [Google Scholar] [CrossRef]
- Tanaka, S.; Kastens, S.; Fujioka, S.; Schlüter, M.; Terasaka, K. Mass transfer from freely rising microbubbles in aqueous solutions of surfactant or salt. Chem. Eng. J. 2020, 387, 121246. [Google Scholar] [CrossRef]
- Brenn, G.; Deviprasath, L.J.; Durst, F.; Fink, C. Evaporation of acoustically levitated multi-component liquid droplets. Int. J. Heat Mass Transf. 2007, 50, 5073–5086. [Google Scholar] [CrossRef]
- Mouvanal, S.; Lamiel, Q.; Lamarque, N.; Helie, J.; Burkhardt, A.; Bakshi, S.; Chatterjee, D. Evaporation of thin liquid film of single and multi-component hydrocarbon fuel from a hot plate. Int. J. Heat Mass Transf. 2019, 141, 379–389. [Google Scholar] [CrossRef]
- Tonini, S.; Cossali, G.E. A multi-component drop evaporation model based on analytical solution of Stefan–Maxwell equations. Int. J. Heat Mass Transf. 2016, 92, 184–189. [Google Scholar] [CrossRef]
- Ebrahimian, V.; Habchi, C. Towards a predictive evaporation model for multi-component hydrocarbon droplets at all pressure conditions. Int. J. Heat Mass Transf. 2011, 54, 3552–3565. [Google Scholar] [CrossRef]
- Yi, P.; Long, W.; Jia, M.; Feng, L.; Tian, J. Development of an improved hybrid multi-component vaporization model for realistic multi-component fuels. Int. J. Heat Mass Transf. 2014, 77, 173–184. [Google Scholar] [CrossRef]
- Tong, A.Y.; Sirignano, W.A. Multicomponent droplet vaporization in a high temperature gas. Combust. Flame 1986, 66, 221–235. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Rybdylova, O.; Crua, C. A mathematical model for heating and evaporation of a multi-component liquid film. Int. J. Heat Mass Transf. 2018, 117, 252–260. [Google Scholar] [CrossRef]
- Samimi Abianeh, O.; Chen, C.P. A discrete multicomponent fuel evaporation model with liquid turbulence effects. Int. J. Heat Mass Transf. 2012, 55, 6897–6907. [Google Scholar] [CrossRef]
- Arri, L.E.; Amundson, N.R. An analytical study of single particle char gasification. AlChE J. 1978, 24, 72–87. [Google Scholar] [CrossRef]
- Sefiane, K.; Ward, C.A. Recent advances on thermocapillary flows and interfacial conditions during the evaporation of liquids. Adv. Colloid Interface Sci. 2007, 134–135, 201–223. [Google Scholar] [CrossRef]
- Shinjo, J.; Xia, J.; Megaritis, A.; Ganippa, L.C.; Cracknell, R.F. Modeling Temperature Distribution Inside an Emulsion Fuel Droplet Under Convective Heating: A Key to Predicting Microexplosion and Puffing. At. Sprays 2016, 26, 551–583. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.G.; Desai, P.S. Modeling the Rheology of Polymer Melts and Solutions. Annu. Rev. Fluid Mech. 2015, 47, 47–65. [Google Scholar] [CrossRef]
- Bress, T.J.; Dowling, D.R. Particle image velocimetry in molten plastic. Polym. Eng. Sci. 2011, 51, 730–745. [Google Scholar] [CrossRef]
- Karkri, M.; Jarny, Y.; Mousseau, P. Thermal state of an incompressible pseudo-plastic fluid and Nusselt number at the interface fluid–die wall. Int. J. Therm. Sci. 2008, 47, 1284–1293. [Google Scholar] [CrossRef]
- Philippoff, W.; Gaskins, F.H. Viscosity measurements on molten polyethylene. J. Polym. Sci. 1956, 21, 205–222. [Google Scholar] [CrossRef]
- Haim, M.; Kalman, H. The effect of internal particle heat conduction on heat transfer analysis of turbulent gas–particle flow in a dilute state. Granul. Matter 2008, 10, 341–349. [Google Scholar] [CrossRef]
- Dutil, Y.; Rousse, D.R.; Salah, N.B.; Lassue, S.; Zalewski, L. A review on phase-change materials: Mathematical modeling and simulations. Renew. Sustain. Energy Rev. 2011, 15, 112–130. [Google Scholar] [CrossRef]
- Nagle, J.F.; Gujrati, P.D.; Goldstein, M. Towards better theories of polymer melting. J. Phys. Chem. 1984, 88, 4599–4608. [Google Scholar] [CrossRef]
- Chalid, M.; Fikri, A.I.; Haidar Satrio, H.; Joshua, Y.M.; Fatriansyah, J.F. An Investigation of the Melting Temperature Effect on the Rate of Solidification in Polymer using a Modified Phase Field Model. Int. J. Technol. 2017, 8, 1321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Fall, W.S.; Hall, K.W.; Gehring, G.A.; Zeng, X.; Ungar, G. Quasi-continuous melting of model polymer monolayers prompts reinterpretation of polymer melting. Nat. Commun. 2021, 12, 1710. [Google Scholar] [CrossRef]
- Riedlbauer, D.; Drexler, M.; Drummer, D.; Steinmann, P.; Mergheim, J. Modelling, simulation and experimental validation of heat transfer in selective laser melting of the polymeric material PA12. Comput. Mater. Sci. 2014, 93, 239–248. [Google Scholar] [CrossRef]
- Sommer, J.-U.; Luo, C. Molecular dynamics simulations of semicrystalline polymers: Crystallization, melting, and reorganization. J. Polym. Sci. Part. B Polym. Phys. 2010, 48, 2222–2232. [Google Scholar] [CrossRef]
- Takahashi, N.; Hikosaka, M.; Yamamoto, T. Computer simulation of melting of polymer crystals. Physical B 1996, 219–220, 420–422. [Google Scholar] [CrossRef]
- Lindt, J.T. Mathematical-Modeling of Melting of Polymers in a Single-Screw Extruder—A Critical-Review. Polym. Eng. Sci. 1985, 25, 585–588. [Google Scholar] [CrossRef]
- Donovan, R.C. Theoretical Melting Model for Plasticating Extruders. Polym. Eng. Sci. 1971, 11, 247–257. [Google Scholar] [CrossRef]
- Liu, H.; Luo, Y.; Zhang, G.; Chen, J.; Yang, Z.; Qu, J. Modeling of Pressure-Induced Melt Removal Melting in Vane Extruder for Polymer Processing. Adv. Polym. Tech. 2014, 33, 21452. [Google Scholar] [CrossRef]
- Celik, A.; Bonten, C.; Togni, R.; Kloss, C.; Goniva, C. A Novel Modeling Approach for Plastics Melting within a CFD-DEM Framework. Polymers 2021, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Voller, V.R.; Swaminathan, C.R.; Thomas, B.G. Fixed grid techniques for phase change problems: A review. Int. J. Numer. Methods. Eng. 1990, 30, 875–898. [Google Scholar] [CrossRef]
- He, Y.-L.; Liu, Q.; Li, Q.; Tao, W.-Q. Lattice Boltzmann methods for single-phase and solid-liquid phase-change heat transfer in porous media: A review. Int. J. Heat Mass Transf. 2019, 129, 160–197. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, X. Coupled solid-liquid phase change and thermal flow simulation by particle method. Int. Commun. Heat Mass Transf. 2020, 113, 104519. [Google Scholar] [CrossRef]
- Truex, M. Numerical Simulation of Liquid-Solid, Solid-Liquid Phase Change Using Finite Element Method in h,p,k Framework with Space-Time Variationally Consistent Integral Forms. Ph.D. Thesis, University of Kansas, Lawrence, KS, USA, 2010. [Google Scholar]
- Schawe, J.E.K.; Bergmann, E. Investigation of polymer melting by temperature modulated differential scanning calorimetry and it’s description using kinetic models. Thermochim. Acta 1997, 304–305, 179–186. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Yuan, J. Numerical simulation of polymer crystal growth under flow field using a coupled phase-field and lattice Boltzmann method. Appl. Math. Comput. 2020, 387, 124302. [Google Scholar] [CrossRef]
- Ansys® Fluent, Release 18.0, Theory Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2017.
- Yi, P.; Long, W.; Jia, M.; Tian, J.; Li, B. Development of a quasi-dimensional vaporization model for multi-component fuels focusing on forced convection and high temperature conditions. Int. J. Heat Mass Transf. 2016, 97, 130–145. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley and Sons, Inc.: New York, NY, USA, 1960. [Google Scholar] [CrossRef]
- Frank-Kamenetskii, D.A. Diffusion and Heat Transfer in Chemical Kinetics, 2nd ed.; Plenum Press: New York, NY, USA, 1969. [Google Scholar]
- Abramzon, B.; Sirignano, W.A. Droplet vaporization model for spray combustion calculations. Int. J. Heat Mass Transf. 1989, 32, 1605–1618. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, M.; Yi, P.; Liu, H.; Xie, M. An efficient liquid film vaporization model for multi-component fuels considering thermal and mass diffusions. Appl. Therm. Eng. 2017, 112, 534–548. [Google Scholar] [CrossRef]
- Saufi, A.E.; Frassoldati, A.; Faravelli, T.; Cuoci, A. DropletSMOKE++: A comprehensive multiphase CFD framework for the evaporation of multidimensional fuel droplets. Int. J. Heat Mass Transf. 2019, 131, 836–853. [Google Scholar] [CrossRef]
- Tamim, J.; Hallett, W.L.H. A continuous thermodynamics model for multicomponent droplet vaporization. Chem. Eng. Sci. 1995, 50, 2933–2942. [Google Scholar] [CrossRef] [Green Version]
- Yi, P.; Zhang, H.; Yang, S. Evaluation of a non-equilibrium multi-component evaporation model for blended diesel/alcohol droplets. In Proceedings of the AIAA Scitech 2020 Forum, Orlando, FL, USA, 6–10 January 2020. [Google Scholar]
- Ju, D.; Xiao, J.; Geng, Z.; Huang, Z. Effect of mass fractions on evaporation of a multi-component droplet at dimethyl ether (DME)/n-heptane-fueled engine conditions. Fuel 2014, 118, 227–237. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M.; Swihart, M.T. Introduction to Chemical Engineering Thermodynamics, 8th ed.; McGraw-Hill: New York, NY, USA, 1959. [Google Scholar]
- Fang, B.; Chen, L.; Li, G.; Wang, L. Multi-component droplet evaporation model incorporating the effects of non-ideality and thermal radiation. Int. J. Heat Mass Transf. 2019, 136, 962–971. [Google Scholar] [CrossRef]
- Govindaraju, P.B.; Ihme, M. Group contribution method for multicomponent evaporation with application to transportation fuels. Int. J. Heat Mass Transf. 2016, 102, 833–845. [Google Scholar] [CrossRef]
- Furfaro, D.; Saurel, R. Modeling droplet phase change in the presence of a multi-component gas mixture. Appl. Math. Comput. 2016, 272, 518–541. [Google Scholar] [CrossRef]
- CRECK Modeling Lab. OpenSMOKE++. Available online: https://www.opensmokepp.polimi.it/ (accessed on 2 June 2021).
- Zhu, L.-T.; Liu, Y.-X.; Luo, Z.-H. An enhanced correlation for gas-particle heat and mass transfer in packed and fluidized bed reactors. Chem. Eng. J. 2019, 374, 531–544. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Monteiro, E.; Teixeira, S.; Chacartegui, R.; Bouziane, K.; Brito, P.S.D.; Rouboa, A. Numerical and experimental analysis of municipal solid wastes gasification process. Appl. Therm. Eng. 2015, 78, 185–195. [Google Scholar] [CrossRef]
- Zhang, Q.; Dor, L.; Biswas, A.K.; Yang, W.; Blasiak, W. Modeling of steam plasma gasification for municipal solid waste. Fuel Process Technol. 2013, 106, 546–554. [Google Scholar] [CrossRef]
- Zhong, H.; Lan, X.; Gao, J. Numerical simulation of pitch–water slurry gasification in both downdraft single-nozzle and opposed multi-nozzle entrained-flow gasifiers: A comparative study. J. Ind. Eng. Chem. 2015, 27, 182–191. [Google Scholar] [CrossRef]
- Ranz, W.E.; Marshall, W.R., Jr. Evaporation From Drops, Part I. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- Gunn, D.J.; De Souza, J.F.C. Heat transfer and axial dispersion in packed beds. Chem. Eng. Sci. 1974, 29, 1363–1371. [Google Scholar] [CrossRef]
- Deen, N.G.; Peters, E.A.J.F.; Padding, J.T.; Kuipers, J.A.M. Review of direct numerical simulation of fluid–particle mass, momentum and heat transfer in dense gas–solid flows. Chem. Eng. Sci. 2014, 116, 710–724. [Google Scholar] [CrossRef]
- Hardy, B.; De Wilde, J.; Winckelmans, G. A penalization method for the simulation of weakly compressible reacting gas-particle flows with general boundary conditions. Comput. Fluids 2019, 190, 294–307. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Wang, Q.; Zou, J.; Zhang, H.; Jin, H.; Li, X. Experimental investigation on gasification characteristics of plastic wastes in supercritical water. Renew. Energy 2019, 135, 32–40. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, B.; An, X.; Ke, C.; Wei, G. Prediction on drag force and heat transfer of spheroids in supercritical water: A PR-DNS study. Powder Technol. 2019, 342, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, B.; An, X.; Ke, C.; Wei, G. Numerical investigation on the effect of the incident angle on momentum and heat transfer of spheroids in supercritical water. Comput. Fluids 2019, 179, 533–542. [Google Scholar] [CrossRef]
- Wu, Z.; Ou, G.; Ren, Y.; Jin, H.; Guo, L. Particle-resolved numerical study of the forced convection heat transfer characteristics of an endothermic-biomass particle placed in supercritical water crossflow. Renew. Energy 2020, 158, 271–279. [Google Scholar] [CrossRef]
- Chen, X.; Khani, E.; Chen, C.P. A unified jet fuel surrogate for droplet evaporation and ignition. Fuel 2016, 182, 284–291. [Google Scholar] [CrossRef]
- Ra, Y.; Reitz, R.D. A vaporization model for discrete multi-component fuel sprays. Int. J. Multiph. Flow 2009, 35, 101–117. [Google Scholar] [CrossRef]
- Dgheim, J.; Chesneau, X.; Pietri, L.; Zeghmati, B. Heat and mass transfer correlations for liquid droplet of a pure fuel in combustion. Heat Mass Transf. 2002, 38, 543–550. [Google Scholar] [CrossRef]
- Tong, A.Y.; Chen, S.J. Heat transfer correlations for vaporizing liquid droplet arrays in a high-temperature gas at intermediate Reynolds number. Int. J. Heat Fluid Flow 1988, 9, 118–130. [Google Scholar] [CrossRef]
- Dabir, B.; Riazi, M.R.; Davoudirad, H.R. Modelling of falling film reactors. Chem. Eng. Sci. 1996, 51, 2553–2558. [Google Scholar] [CrossRef]
- Pyle, D.L.; Zaror, C.A. Heat transfer and kinetics in the low temperature pyrolysis of solids. Chem. Eng. Sci. 1984, 39, 147–158. [Google Scholar] [CrossRef]
- Pecha, M.B.; Arbelaez, J.I.M.; Garcia-Perez, M.; Chejne, F.; Ciesielski, P.N. Progress in understanding the four dominant intra-particle phenomena of lignocellulose pyrolysis: Chemical reactions, heat transfer, mass transfer, and phase change. Green Chem. 2019, 21, 2868–2898. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. Two-Fluid Modeling of Gas-Solid and Gas-Liquid Flows: Solver Development and Application. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2014. [Google Scholar]
- Papadikis, K.; Bridgwater, A.V.; Gu, S. CFD modelling of the fast pyrolysis of biomass in fluidised bed reactors, Part A: Eulerian computation of momentum transport in bubbling fluidised beds. Chem. Eng. Sci. 2008, 63, 4218–4227. [Google Scholar] [CrossRef]
- Ullah, A.; Hong, K.; Gao, Y.; Gungor, A.; Zaman, M. An overview of Eulerian CFD modeling and simulation of non-spherical biomass particles. Renew. Energy 2019, 141, 1054–1066. [Google Scholar] [CrossRef]
- Kolev, N.I. Drag, Lift, and Virtual Mass Forces; Springer: Berlin/Heidelberg, Germany, 2011; pp. 31–85. [Google Scholar]
- Gidaspow, D. Multiphase Flow and Fluidization; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Syamlal, M.; Rogers, W.; O’Brien, T.J. MFIX Documentation Theory Guide; Office of Scientific and Technical Information (OSTI): Washington, DC, USA, 1993. [Google Scholar]
- Yang, N.; Wang, W.; Ge, W.; Wang, L.; Li, J. Simulation of Heterogeneous Structure in a Circulating Fluidized-Bed Riser by Combining the Two-Fluid Model with the EMMS Approach. Ind. Eng. Chem. Res. 2004, 43, 5548–5561. [Google Scholar] [CrossRef]
- Jiang, X.; Siamas, G.A.; Jagus, K.; Karayiannis, T.G. Physical modelling and advanced simulations of gas–liquid two-phase jet flows in atomization and sprays. Prog. Energy Combust. Sci. 2010, 36, 131–167. [Google Scholar] [CrossRef]
- Shu, S.; Vidal, D.; Bertrand, F.; Chaouki, J. Multiscale multiphase phenomena in bubble column reactors: A review. Renew. Energy 2019, 141, 613–631. [Google Scholar] [CrossRef]
- Van Melkebeke, M.; Janssen, C.; De Meester, S. Characteristics and Sinking Behavior of Typical Microplastics Including the Potential Effect of Biofouling: Implications for Remediation. Environ. Sci. Technol. 2020, 54, 8668–8680. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; He, Y.; Tang, T.; Wang, T. Drag coefficient prediction for non-spherical particles in dense gas–solid two-phase flow using artificial neural network. Powder Technol. 2019, 354, 115–124. [Google Scholar] [CrossRef]
- Yow, H.N.; Pitt, M.J.; Salman, A.D. Drag correlations for particles of regular shape. Adv. Powder Technol. 2005, 16, 363–372. [Google Scholar] [CrossRef]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Tavassoli Estahbanati, H. Direct Numerical Simulation of Dense Gas-Solid Non-Isothermal Flows; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2014. [Google Scholar]
- Sun, B.; Tenneti, S.; Subramaniam, S. Modeling average gas–solid heat transfer using particle-resolved direct numerical simulation. Int. J. Heat Mass Transf. 2015, 86, 898–913. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Heat transfer to a gas from densely packed beds of monodisperse spherical particles. Chem. Eng. J. 2017, 314, 27–37. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Heat transfer to a gas from densely packed beds of cylindrical particles. Chem. Eng. Sci. 2017, 172, 1–12. [Google Scholar] [CrossRef]
- He, L.; Tafti, D.K. Heat transfer in an assembly of ellipsoidal particles at low to moderate Reynolds numbers. Int. J. Heat Mass Transf. 2017, 114, 324–336. [Google Scholar] [CrossRef]
- Das, S.; Sneijders, S.; Deen, N.G.; Kuipers, J.A.M. Drag and heat transfer closures for realistic numerically generated random open-cell solid foams using an immersed boundary method. Chem. Eng. Sci. 2018, 183, 260–274. [Google Scholar] [CrossRef]
- Chen, Y.; Müller, C.R. Lattice Boltzmann simulation of gas-solid heat transfer in random assemblies of spheres: The effect of solids volume fraction on the average Nusselt number for Re ≤ 100. Chem. Eng. J. 2019, 361, 1392–1399. [Google Scholar] [CrossRef]
- Kravets, B.; Rosemann, T.; Reinecke, S.R.; Kruggel-Emden, H. A new drag force and heat transfer correlation derived from direct numerical LBM-simulations of flown through particle packings. Powder Technol. 2019, 345, 438–456. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, L. Effects of Interphase Forces on Multiphase Flow and Bubble Distribution in Continuous Casting Strands. Metall. Mater. Trans. B 2021, 52, 528–547. [Google Scholar] [CrossRef]
- Hölzer, A.; Sommerfeld, M. New simple correlation formula for the drag coefficient of non-spherical particles. Powder Technol. 2008, 184, 361–365. [Google Scholar] [CrossRef]
- Dioguardi, F.; Mele, D.; Dellino, P. A New One-Equation Model of Fluid Drag for Irregularly Shaped Particles Valid Over a Wide Range of Reynolds Number. J. Geophys. Res. Solid Earth 2018, 123, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Dellino, P.; Mele, D.; Bonasia, R.; Braia, G.; La Volpe, L.; Sulpizio, R. The analysis of the influence of pumice shape on its terminal velocity. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, K.; Haugen, N.E.L.; Mao, C.; Fan, J. Drag force for a burning particle. Combust. Flame 2020, 217, 188–199. [Google Scholar] [CrossRef]
- Sanjeevi, S.K.P.; Kuipers, J.A.M.; Padding, J.T. Drag, lift and torque correlations for non-spherical particles from Stokes limit to high Reynolds numbers. Int. J. Multiph. Flow 2018, 106, 325–337. [Google Scholar] [CrossRef]
- Papadikis, K.; Gu, S.; Bridgwater, A.V. CFD modelling of the fast pyrolysis of biomass in fluidised bed reactors. Part B. Chem. Eng. Sci. 2009, 64, 1036–1045. [Google Scholar] [CrossRef]
- Armstrong, L.M.; Gu, S.; Luo, K.H. Effects of limestone calcination on the gasification processes in a BFB coal gasifier. Chem. Eng. J. 2011, 168, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Lu, L.; He, W.; Min, Y. Fundamentals and applications of CFD technology on analyzing falling film heat and mass exchangers: A comprehensive review. Appl. Energy 2020, 261, 114473. [Google Scholar] [CrossRef]
- Pan, H.; Chen, X.-Z.; Liang, X.-F.; Zhu, L.-T.; Luo, Z.-H. CFD simulations of gas–liquid–solid flow in fluidized bed reactors—A review. Powder Technol. 2016, 299, 235–258. [Google Scholar] [CrossRef]
- Ling, H.; Zhang, L. Numerical Simulation of Gas and Liquid Two-Phase Flow in the RH Process. Metall. Mater. Trans. B 2019, 50, 2017–2028. [Google Scholar] [CrossRef]
- Duguay, J.; Lacey, J.; Massé, A. Evaluating the Euler-Euler approach for predicting a strongly 3D bubble-induced recirculatory flow with OpenFOAM. Chem. Eng. Sci. 2021, 229, 115982. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sato, H.; Matsukura, Y.; Ujisawa, Y.; Ishida, H.; Sasaki, S.; Hata, Y. Gasification and smelting system using oxygen blowing for plastic waste including polyvinyl chloride. J. Mater. Cycles Waste Manag. 2004, 6, 6–12. [Google Scholar] [CrossRef]
- Narobe, M.; Golob, J.; Klinar, D.; Francetic, V.; Likozar, B. Co-gasification of biomass and plastics: Pyrolysis kinetics studies, experiments on 100 kW dual fluidized bed pilot plant and development of thermodynamic equilibrium model and balances. Bioresour. Technol. 2014, 162, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wilk, V.; Schmid, J.C.; Hofbauer, H. Influence of fuel feeding positions on gasification in dual fluidized bed gasifiers. Biomass Bioenergy 2013, 54, 46–58. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Assessment on steam gasification of municipal solid waste against biomass substrates. Energy Convers. Manag. 2016, 124, 92–103. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Rouboa, A. Municipal solid waste gasification in semi-industrial conditions using air-CO2 mixtures. Energy (Oxford UK) 2016, 104, 42–52. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Monteiro, E.; Rouboa, A. Assessment of municipal solid wastes gasification in a semi-industrial gasifier using syngas quality indices. Energy 2015, 93, 864–873. [Google Scholar] [CrossRef]
- Arena, U.; Mastellone, M.L. Defluidization phenomena during the pyrolysis of two plastic wastes. Chem. Eng. Sci. 2000, 55, 2849–2860. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Arena, U. Carbon attrition during the circulating fluidized bed combustion of a packaging-derived fuel. Combust. Flame 1999, 117, 562–573. [Google Scholar] [CrossRef]
- Gollwitzer, F.; Rehberg, I.; Kruelle, C.A.; Huang, K. Coefficient of restitution for wet particles. Phys. Rev. E 2012, 86, 011303. [Google Scholar] [CrossRef] [Green Version]
- Yong, S.Z.; Ghoniem, A. Modeling the slag layer in solid fuel gasification and combustion—Two-way coupling with CFD. Fuel 2012, 97, 457–466. [Google Scholar] [CrossRef]
- Punčochář, M.; Ruj, B.; Chatterj, P.K. Development of Process for Disposal of Plastic Waste Using Plasma Pyrolysis Technology and Option for Energy Recovery. Procedia Eng. 2012, 42, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Christian, H. Simulation of bubbly flows with special numerical treatments of the semi-conservative and fully conservative two-fluid model. Chem. Eng. Sci. 2017, 174, 25–39. [Google Scholar] [CrossRef]
- Bal, N.; Rein, G. Relevant model complexity for non-charring polymer pyrolysis. Fire Saf. J. 2013, 61, 36–44. [Google Scholar] [CrossRef]
- Norouzi, H.R.; Zarghami, R.; Sotudeh-Gharebagh, R.; Mostoufi, N. Coupled CFD-DEM Modeling: Formulation, Implementation and Application to Multiphase Flows, 1st ed.; WILEY: Hoboken, NJ, USA, 2016. [Google Scholar]
- Rodrigues, A.E. Residence time distribution (RTD) revisited. Chem. Eng. Sci. 2021, 230, 116188. [Google Scholar] [CrossRef]
- Shen, L.; Xiao, J.; Niklasson, F.; Johnsson, F. Biomass mixing in a fluidized bed biomass gasifier for hydrogen production. Chem. Eng. Sci. 2007, 62, 636–643. [Google Scholar] [CrossRef]
- Bezzo, F.; Macchietto, S.; Pantelides, C.C. A general methodology for hybrid multizonal/CFD models, Part I. Theoretical framework. Comput. Chem. Eng. 2004, 28, 501–511. [Google Scholar] [CrossRef]
- Bezzo, F.; Macchietto, S. A general methodology for hybrid multizonal/CFD models, Part II. Automatic zoning. Comput. Chem. Eng. 2004, 28, 513–525. [Google Scholar] [CrossRef]
- Davidson, J.; Harrison, D. Fluidised Particles; Cambridge University Press: Cambridge, UK, 1963. [Google Scholar]
- Kunii, D.; Levenspiel, O. Fluidization Engineering; John Wiley: New York, NY, USA, 1969. [Google Scholar]
- Yan, H.-m.; Heidenreich, C.; Zhang, D.-k. Mathematical modelling of a bubbling fluidised-bed coal gasifier and the significance of ‘net flow’. Fuel 1998, 77, 1067–1079. [Google Scholar] [CrossRef]
- Ross, D.; Yan, H.; Zhong, Z.; Zhang, D. A non-isothermal model of a bubbling fluidised-bed coal gasifier. Fuel 2005, 84, 1469–1481. [Google Scholar] [CrossRef]
- Jennen, T.; Hiller, R.; Köneke, D.; Weinspach, P.-M. Modeling of Gasification of Wood in a Circulating Fluidized Bed. Chem. Eng. Technol. 1999, 22, 822–826. [Google Scholar] [CrossRef]
- Gungor, A. Modeling the effects of the operational parameters on H2 composition in a biomass fluidized bed gasifier. Int. J. Hydrogen Energy 2011, 36, 6592–6600. [Google Scholar] [CrossRef]
- Slapak, M.J.P.; Van Kasteren, J.M.N.; Drinkenburg, A.A.H. Design of a process for steam gasification of PVC waste. Resour. Conserv. Recycl. 2000, 30, 81–93. [Google Scholar] [CrossRef]
- de Souza-Santos, M.L. Solid Fuels Combustion and Gasification: Modeling, Simulation, and Equipment Operations, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gómez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sci. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- And, S.D.; Dudukovic, M.P.; Toseland, B.A.; Bhatt, B.L. A Two-Compartment Convective-Diffusion Model for Slurry Bubble Column Reactors. Ind. Eng. Chem. Res. 1997, 36, 4670–4680. [Google Scholar] [CrossRef]
- Zhao, W.; Buffo, A.; Alopaeus, V.; Han, B.; Louhi-Kultanen, M. Application of the compartmental model to the gas-liquid precipitation of CO2-Ca(OH)2aqueous system in a stirred tank. AlChE J. 2017, 63, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Rieth, I.; Grünewald, M. Auslegung von Blasensäulen mithilfe von Compartmentmodellen. Chem. Ing. Tech. 2019, 91, 1049–1058. [Google Scholar] [CrossRef]
- Shimizu, K.; Takada, S.; Minekawa, K.; Kawase, Y. Phenomenological model for bubble column reactors: Prediction of gas hold-ups and volumetric mass transfer coefficients. Chem. Eng. J. 2000, 78, 21–28. [Google Scholar] [CrossRef]
- Cubero, A.; Sánchez-Insa, A.; Fueyo, N. The effect of particle polydispersion in a gasifier bed dynamics using Eulerian-Eulerian models. Fuel Process. Technol. 2020, 198, 106216. [Google Scholar] [CrossRef]
- Ding, J.; Gidaspow, D. A bubbling fluidization model using kinetic theory of granular flow. AlChE J. 1990, 36, 523–538. [Google Scholar] [CrossRef]
- Van Wachem, B. Derivation, Implementation, and Validation of Computer Simulation Models for Gas-Solid Fluidized Beds. Ph.D. Thesis, Delft University of Technology, Amsterdam, The Netherlands, 2000. [Google Scholar]
- Johnson, P.C.; Jackson, R. Frictional–collisional constitutive relations for granular materials, with application to plane shearing. J. Fluid Mech. 1987, 176, 67–93. [Google Scholar] [CrossRef]
- Lam, K.L.; Oyedun, A.O.; Hui, C.W. Numerical study of mixed-feedstock pyrolysis. In Computer Aided Chemical Engineering; Karimi, I.A., Srinivasan, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 31, pp. 1311–1315. [Google Scholar]
- Yin, L.J.; Chen, D.Z.; Wang, H.; Ma, X.B.; Zhou, G.M. Simulation of an innovative reactor for waste plastics pyrolysis. Chem. Eng. J. 2014, 237, 229–235. [Google Scholar] [CrossRef]
- Reddy, R.; Banerjee, R. GPU accelerated VOF based multiphase flow solver and its application to sprays. Comput. Fluids 2015, 117, 287–303. [Google Scholar] [CrossRef]
- Lee, J.E.; Choi, H.S.; Seo, Y.C. Study of hydrodynamic characteristics in a circulating fluidized bed gasifier for plastic waste by computational fluid dynamics modeling and simulation. J. Mater. Cycles Waste Manage. 2014, 16, 665–676. [Google Scholar] [CrossRef]
- Goyal, A.; Pushpavanam, S.; Voolapalli, R.K. Modeling and simulation of co-gasification of coal and petcoke in a bubbling fluidized bed coal gasifier. Fuel Process. Technol. 2010, 91, 1296–1307. [Google Scholar] [CrossRef]
- Chejne, F.; Hernandez, J.P. Modelling and simulation of coal gasification process in fluidised bed. Fuel 2002, 81, 1687–1702. [Google Scholar] [CrossRef]
- Lü, P.; Kong, X.; Wu, C.; Yuan, Z.; Ma, L.; Chang, J. Modeling and simulation of biomass air-steam gasification in a fluidized bed. Front. Chem. Eng. China 2008, 2, 209–213. [Google Scholar] [CrossRef]
- Sadaka, S.S.; Ghaly, A.E.; Sabbah, M.A. Two phase biomass air-steam gasification model for fluidized bed reactors: Part I—Model development. Biomass Bioenergy 2002, 22, 439–462. [Google Scholar] [CrossRef]
- Fiaschi, D.; Michelini, M. A two-phase one-dimensional biomass gasification kinetics model. Biomass Bioenergy 2001, 21, 121–132. [Google Scholar] [CrossRef]
- Kaushal, P.; Abedi, J.; Mahinpey, N. A comprehensive mathematical model for biomass gasification in a bubbling fluidized bed reactor. Fuel 2010, 89, 3650–3661. [Google Scholar] [CrossRef]
- Radmanesh, R.; Chaouki, J.; Guy, C. Biomass gasification in a bubbling fluidized bed reactor: Experiments and modeling. AlChE J. 2006, 52, 4258–4272. [Google Scholar] [CrossRef]
- Kravets, B.; Schulz, D.; Jasevičius, R.; Reinecke, S.R.; Rosemann, T.; Kruggel-Emden, H. Comparison of particle-resolved DNS (PR-DNS) and non-resolved DEM/CFD simulations of flow through homogenous ensembles of fixed spherical and non-spherical particles. Adv. Powder Technol. 2021, 32, 1170–1195. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M. Semi-resolved CFD–DEM for thermal particulate flows with applications to fluidized beds. Int. J. Heat Mass Transf. 2020, 159, 120150. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Wei, Y.; Hu, J.; Chew, J.W. Eulerian-Lagrangian simulation of air-steam biomass gasification in a three-dimensional bubbling fluidized gasifier. Energy 2019, 181, 1075–1093. [Google Scholar] [CrossRef]
- Song, C.; Liu, D.; Ma, J.; Chen, X. CFD-DEM simulation of flow pattern and particle velocity in a fluidized bed with wet particles. Powder Technol. 2017, 314, 346–354. [Google Scholar] [CrossRef]
- Sun, X.; Sakai, M. Three-dimensional simulation of gas–solid–liquid flows using the DEM–VOF method. Chem. Eng. Sci. 2015, 134, 531–548. [Google Scholar] [CrossRef]
- Zhang, X.; Ahmadi, G. Eulerian–Lagrangian simulations of liquid–gas–solid flows in three-phase slurry reactors. Chem. Eng. Sci. 2005, 60, 5089–5104. [Google Scholar] [CrossRef]
- Wen, J.; Lei, P.A.N.; Huang, L.I.N. Modeling and Simulation of Gas-Liquid-Solid Three-Phase Fluidization. Chem. Eng. Commun. 2005, 192, 941–955. [Google Scholar] [CrossRef]
- Vångö, M.; Pirker, S.; Lichtenegger, T. Unresolved CFD–DEM modeling of multiphase flow in densely packed particle beds. Appl. Math. Model. 2018, 56, 501–516. [Google Scholar] [CrossRef]
- CFD-DEM Coupled to VOF. Available online: https://www.cfdem.com/cfd-dem-coupled-vof (accessed on 18 December 2020).
- Ismail, T.M.; Ramos, A.; Abd El-Salam, M.; Monteiro, E.; Rouboa, A. Plasma fixed bed gasification using an Eulerian model. Int. J. Hydrogen Energy 2019, 44, 28668–28684. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Monteiro, E.; Rouboa, A. Exergy analysis of Portuguese municipal solid waste treatment via steam gasification. Energy Convers. Manag. 2017, 134, 235–246. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Thermodynamic Evaluation of Portuguese municipal solid waste gasification. J. Clean. Prod. 2016, 139, 622–635. [Google Scholar] [CrossRef]
- Couto, N.; Monteiro, E.; Silva, V.; Rouboa, A. Hydrogen-rich gas from gasification of Portuguese municipal solid wastes. Int. J. Hydrogen Energy 2016, 41, 10619–10630. [Google Scholar] [CrossRef]
- Liu, H.; Elkamel, A.; Lohi, A.; Biglari, M. Computational Fluid Dynamics Modeling of Biomass Gasification in Circulating Fluidized-Bed Reactor Using the Eulerian–Eulerian Approach. Ind. Eng. Chem. Res. 2013, 52, 18162–18174. [Google Scholar] [CrossRef]
- Sia, S.Q.; Wang, W.-C. Numerical simulations of fluidized bed fast pyrolysis of biomass through computational fluid dynamics. Renew. Energy 2020, 155, 248–256. [Google Scholar] [CrossRef]
- Ismail, T.M.; Abd El-Salam, M.; Monteiro, E.; Rouboa, A. Eulerian—Eulerian CFD model on fluidized bed gasifier using coffee husks as fuel. Appl. Therm. Eng. 2016, 106, 1391–1402. [Google Scholar] [CrossRef]
- Sousa Cardoso, J.; Silva, V.; Eusébio, D.; Lima Azevedo, I.; Tarelho, L.A.C. Techno-economic analysis of forest biomass blends gasification for small-scale power production facilities in the Azores. Fuel 2020, 279, 118552. [Google Scholar] [CrossRef]
- Ismail, T.M.; Monteiro, E.; Ramos, A.; El-Salam, M.A.; Rouboa, A. An Eulerian model for forest residues gasification in a plasma gasifier. Energy 2019, 182, 1069–1083. [Google Scholar] [CrossRef]
- Kulkarni, S.R.; Vandewalle, L.; Gonzalez Quiroga, A.; Perreault, P.; Heynderickx, G.J.; Van Geem, K.M.; Marin, G.B. CFD-assisted Process Intensification Study for Biomass Fast Pyrolysis in a Gas-Solid Vortex Reactor. Energy Fuels 2018, 32, 10169–10183. [Google Scholar] [CrossRef]
- Xue, Q.; Fox, R.O. Multi-fluid CFD modeling of biomass gasification in polydisperse fluidized-bed gasifiers. Powder Technol. 2014, 254, 187–198. [Google Scholar] [CrossRef]
- Chen, J.; Yu, G.; Dai, B.; Liu, D.; Zhao, L. CFD Simulation of a Bubbling Fluidized Bed Gasifier Using a Bubble-Based Drag Model. Energy Fuels 2014, 28, 6351–6360. [Google Scholar] [CrossRef]
- Xue, Q.; Fox, R. An Euler-Euler CFD model for biomass gasification in fluidized bed. In Proceedings of the NETL Conference on Multiphase Flow Science, Morgantown, WV, USA, 22–24 May 2012. [Google Scholar]
- Oevermann, M.; Gerber, S.; Behrendt, F. Numerical simulation of wood gasification in a fluidized bed reactor using Euler-Euler modeling. In Proceedings of the European Combustion Meeting, Vienna, Austria, 11–17 April 2009. [Google Scholar]
- Wu, Y.; Zhang, Q.; Yang, W.; Blasiak, W. Two-Dimensional Computational Fluid Dynamics Simulation of Biomass Gasification in a Downdraft Fixed-Bed Gasifier with Highly Preheated Air and Steam. Energy Fuels 2013, 27, 3274–3282. [Google Scholar] [CrossRef]
- Gerun, L.; Paraschiv, M.; Vîjeu, R.; Bellettre, J.; Tazerout, M.; Gøbel, B.; Henriksen, U. Numerical investigation of the partial oxidation in a two-stage downdraft gasifier. Fuel 2008, 87, 1383–1393. [Google Scholar] [CrossRef]
- Sahu, A.K.; Raghavan, V.; Prasad, B.V.S.S.S. Numerical simulation of gas-solid flows in fluidized bed gasification reactor. Adv. Powder Technol. 2019, 30, 3050–3066. [Google Scholar] [CrossRef]
- Laugwitz, A.; Rößger, P.; Schurz, M.; Richter, A.; Meyer, B. ‘Towards a validated CFD setup for a range of fluidized beds’. Powder Technol. 2017, 318, 558–568. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Zhong, W. Three-dimensional simulation of fluidized bed coal gasification. Chem. Eng. Process. 2009, 48, 695–705. [Google Scholar] [CrossRef]
- Yu, L.; Lu, J.; Zhang, X.; Zhang, S. Numerical simulation of the bubbling fluidized bed coal gasification by the kinetic theory of granular flow (KTGF). Fuel 2007, 86, 722–734. [Google Scholar] [CrossRef]
- Vicente, W.; Ochoa, S.; Aguillón, J.; Barrios, E. An Eulerian model for the simulation of an entrained flow coal gasifier. Appl. Therm. Eng. 2003, 23, 1993–2008. [Google Scholar] [CrossRef]
- Murgia, S.; Vascellari, M.; Cau, G. Comprehensive CFD model of an air-blown coal-fired updraft gasifier. Fuel 2012, 101, 129–138. [Google Scholar] [CrossRef]
- Anastasiou, A.D.; Passos, A.D.; Mouza, A.A. Bubble columns with fine pore sparger and non-Newtonian liquid phase: Prediction of gas holdup. Chem. Eng. Sci. 2013, 98, 331–338. [Google Scholar] [CrossRef]
- Dou, B.; Wang, K.; Jiang, B.; Song, Y.; Zhang, C.; Chen, H.; Xu, Y. Fluidized-bed gasification combined continuous sorption-enhanced steam reforming system to continuous hydrogen production from waste plastic. Int. J. Hydrogen Energy 2016, 41, 3803–3810. [Google Scholar] [CrossRef]
- Wu, B. CFD simulation of gas and non-Newtonian fluid two-phase flow in anaerobic digesters. Water Res. 2010, 44, 3861–3874. [Google Scholar] [CrossRef]
- Marschall, H.; Mornhinweg, R.; Kossmann, A.; Oberhauser, S.; Langbein, K.; Hinrichsen, O. Numerical Simulation of Dispersed Gas/Liquid Flows in Bubble Columns at High Phase Fractions using OpenFOAM®. Part I—Modeling Basics. Chem. Eng. Technol. 2011, 34, 1311–1320. [Google Scholar] [CrossRef]
- Heylmun, J.C.; Kong, B.; Passalacqua, A.; Fox, R.O. A quadrature-based moment method for polydisperse bubbly flows. Comput. Phys. Commun. 2019, 244, 187–204. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Marchisio, D.; Hasse, C.; Lucas, D. Comparison of Eulerian QBMM and classical Eulerian–Eulerian method for the simulation of polydisperse bubbly flows. AlChE J. 2019, 65, e16732. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Jin, Y. Population Balance Model for Gas−Liquid Flows: Influence of Bubble Coalescence and Breakup Models. Ind. Eng. Chem. Res. 2005, 44, 7540–7549. [Google Scholar] [CrossRef]
- Nayak, A.K.; Borka, Z.; Patruno, L.E.; Sporleder, F.; Dorao, C.A.; Jakobsen, H.A. A Combined Multifluid-Population Balance Model for Vertical Gas−Liquid Bubble-Driven Flows Considering Bubble Column Operating Conditions. Ind. Eng. Chem. Res. 2011, 50, 1786–1798. [Google Scholar] [CrossRef]
- Deju, L.; Cheung, S.C.P.; Yeoh, G.H.; Tu, J.Y. Capturing coalescence and break-up processes in vertical gas–liquid flows: Assessment of population balance methods. Appl. Math. Model. 2013, 37, 8557–8577. [Google Scholar] [CrossRef]
- Athanassiadis, A.G.; Miskin, M.Z.; Kaplan, P.; Rodenberg, N.; Lee, S.H.; Merritt, J.; Brown, E.; Amend, J.; Lipson, H.; Jaeger, H.M. Particle shape effects on the stress response of granular packings. Soft Matter 2014, 10, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horabik, J.; Molenda, M. Parameters and contact models for DEM simulations of agricultural granular materials: A review. Biosyst. Eng. 2016, 147, 206–225. [Google Scholar] [CrossRef]
- Breuninger, P.; Weis, D.; Behrendt, I.; Grohn, P.; Krull, F.; Antonyuk, S. CFD–DEM simulation of fine particles in a spouted bed apparatus with a Wurster tube. Particuology 2019, 42, 114–125. [Google Scholar] [CrossRef]
- Yan, L.; Lim, C.J.; Yue, G.; He, B.; Grace, J.R. Simulation of biomass-steam gasification in fluidized bed reactors: Model setup, comparisons and preliminary predictions. Bioresour. Technol. 2016, 221, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Yang, S.; Sun, Y.; Wei, Y.; Hu, J.; Wang, H. Distribution and particle-scale thermochemical property of biomass in the gasifier of a dual fluidized bed. Energy Convers. Manag. 2020, 209, 112672. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, Y. Hydrogen production by biomass gasification in a supercritical water fluidized bed reactor: A CFD-DEM study. J. Supercrit. Fluids 2018, 131, 26–36. [Google Scholar] [CrossRef]
- Qi, T.; Lei, T.; Yan, B.; Chen, G.; Li, Z.; Fatehi, H.; Wang, Z.; Bai, X.-S. Biomass steam gasification in bubbling fluidized bed for higher-H2 syngas: CFD simulation with coarse grain model. Int. J. Hydrogen Energy 2019, 44, 6448–6460. [Google Scholar] [CrossRef]
- Ku, X.; Li, T.; Løvås, T. CFD–DEM simulation of biomass gasification with steam in a fluidized bed reactor. Chem. Eng. Sci. 2015, 122, 270–283. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Luo, K.; Hu, C.; Sun, L.; Fan, J. Impact of operating parameters on biomass gasification in a fluidized bed reactor: An Eulerian-Lagrangian approach. Powder Technol. 2018, 333, 304–316. [Google Scholar] [CrossRef]
- Ku, X.; Li, T.; Løvås, T. Eulerian–Lagrangian Simulation of Biomass Gasification Behavior in a High-Temperature Entrained-Flow Reactor. Energy Fuels 2014, 28, 5184–5196. [Google Scholar] [CrossRef] [Green Version]
- Ku, X.; Jin, H.; Lin, J. Comparison of gasification performances between raw and torrefied biomasses in an air-blown fluidized-bed gasifier. Chem. Eng. Sci. 2017, 168, 235–249. [Google Scholar] [CrossRef]
- Ku, X.; Lin, J.; Yuan, F. Influence of Torrefaction on Biomass Gasification Performance in a High-Temperature Entrained-Flow Reactor. Energy Fuels 2016, 30, 4053–4064. [Google Scholar] [CrossRef]
- Ku, X.; Wang, J.; Jin, H.; Lin, J. Effects of operating conditions and reactor structure on biomass entrained-flow gasification. Renew. Energy 2019, 139, 781–795. [Google Scholar] [CrossRef]
- Maitlo, G.; Unar, I.N.; Mahar, R.B.; Brohi, K.M. Numerical simulation of lignocellulosic biomass gasification in concentric tube entrained flow gasifier through computational fluid dynamics. Energy Explor. Exploit. 2019, 37, 1073–1097. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, Y.; Bao, F.; Li, B.; Zhao, Y.; Ke, C.; Jiang, B. CFD modeling of sawdust gasification in a lab-scale entrained flow reactor based on char intrinsic kinetics. Part 1: Model development. Chem. Eng. Process. 2018, 125, 280–289. [Google Scholar] [CrossRef]
- Fletcher, D.F.; Haynes, B.S.; Christo, F.C.; Joseph, S.D. A CFD based combustion model of an entrained flow biomass gasifier. Appl. Math. Model. 2000, 24, 165–182. [Google Scholar] [CrossRef]
- Ostermeier, P.; Fischer, F.; Fendt, S.; Deyoung, S.; Spliethoff, H. Coarse-grained CFD-DEM simulation of biomass gasification in a fluidized bed reactor. Fuel 2019, 255, 115790. [Google Scholar] [CrossRef]
- Wang, S.; Luo, K.; Fan, J. CFD-DEM coupled with thermochemical sub-models for biomass gasification: Validation and sensitivity analysis. Chem. Eng. Sci. 2020, 217, 115550. [Google Scholar] [CrossRef]
- Gerber, S.; Oevermann, M. A two dimensional Euler-Lagrangian model of wood gasification in a charcoal bed—Particle histories. Powder Technol. 2018, 324, 5–15. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.; Wang, S. CFD simulation of air-blown coal gasification in a fluidized bed reactor with continuous feedstock. Energy Convers. Manag. 2020, 213, 112774. [Google Scholar] [CrossRef]
- Tokmurzin, D.; Adair, D. Development of Euler-Lagrangian Simulation of a Circulating Fluidized Bed Reactor for Coal Gasification. Eurasian Chem.-Technol. J. 2019, 21, 45. [Google Scholar] [CrossRef]
- Klimanek, A.; Bigda, J. CFD modelling of CO2 enhanced gasification of coal in a pressurized circulating fluidized bed reactor. Energy 2018, 160, 710–719. [Google Scholar] [CrossRef]
- Su, L.; Feng, S.; Li, P.; Zhang, Y.; Liu, Z.; Li, Z. Study on simulation of pulverized coal gasification process in the GSP gasifier. Can. J. Chem. Eng. 2017, 95, 688–697. [Google Scholar] [CrossRef]
- Ajilkumar, A.; Sundararajan, T.; Shet, U.S.P. Numerical modeling of a steam-assisted tubular coal gasifier. Int. J. Therm. Sci. 2009, 48, 308–321. [Google Scholar] [CrossRef]
- Watanabe, H.; Otaka, M. Numerical simulation of coal gasification in entrained flow coal gasifier. Fuel 2006, 85, 1935–1943. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Y.; Kumar, S.; Li, R.; Mahar, R.B.; Ali, M.; Unar, I.N.; Sultan, U.; Memon, K. Numerical analysis on the influential factors of coal gasification performance in two-stage entrained flow gasifier. Appl. Therm. Eng. 2017, 112, 1601–1611. [Google Scholar] [CrossRef]
- Manek, B.; Javia, M.S.; Harichandan, A.; Ramani, H. A CFD based comprehensive study of coal-fired updraft gasifier in ceramic industry. Therm. Sci. Eng. Prog. 2019, 9, 11–20. [Google Scholar] [CrossRef]
- Xue, J.; Chen, F.; Yang, N.; Ge, W. Eulerian–Lagrangian simulation of bubble coalescence in bubbly flow using the spring-dashpot model. Chin. J. Chem. Eng. 2017, 25, 249–256. [Google Scholar] [CrossRef]
- Peña-Monferrer, C.; Monrós-Andreu, G.; Chiva, S.; Martínez-Cuenca, R.; Muñoz-Cobo, J.L. A CFD-DEM solver to model bubbly flow. Part I: Model development and assessment in upward vertical pipes. Chem. Eng. Sci. 2018, 176, 524–545. [Google Scholar] [CrossRef]
- Kafashan, J.; Wiącek, J.; Abd Rahman, N.; Gan, J. Two-dimensional particle shapes modelling for DEM simulations in engineering: A review. Granul. Matter 2019, 21, 80. [Google Scholar] [CrossRef]
- Markauskas, D.; Ramírez-Gómez, Á.; Kačianauskas, R.; Zdancevičius, E. Maize grain shape approaches for DEM modelling. Comput. Electron. Agric. 2015, 118, 247–258. [Google Scholar] [CrossRef]
- Yang, L.; Padding, J.T.; Kuipers, J.A.M. Modification of kinetic theory of granular flow for frictional spheres, Part I: Two-fluid model derivation and numerical implementation. Chem. Eng. Sci. 2016, 152, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Sundaresan, S. A constitutive model with microstructure evolution for flow of rate-independent granular materials. J. Fluid Mech. 2011, 682, 590–616. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Padding, J.T.; Kuipers, J.A.M. Partial slip boundary conditions for collisional granular flows at flat frictional walls. AlChE J. 2017, 63, 1853–1871. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.L.; Padding, J.T.J.; Kuipers, J.A.M.H. Modification of kinetic theory of granular flow for frictional spheres, part II: Model validation. Chem. Eng. Sci. 2016, 152, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Padding, J.T.; Buist, K.A.; Kuipers, J.A.M. Three-dimensional fluidized beds with rough spheres: Validation of a Two Fluid Model by Magnetic Particle Tracking and discrete particle simulations. Chem. Eng. Sci. 2017, 174, 238–258. [Google Scholar] [CrossRef]
- Mollon, G.; Quacquarelli, A.; Andò, E.; Viggiani, G. Can friction replace roughness in the numerical simulation of granular materials? Granul. Matter 2020, 22, 42. [Google Scholar] [CrossRef]
- Wiącek, J.; Molenda, M.; Horabik, J.; Ooi, J.Y. Influence of grain shape and intergranular friction on material behavior in uniaxial compression: Experimental and DEM modeling. Powder Technol. 2012, 217, 435–442. [Google Scholar] [CrossRef]
- Ghadirian, E.; Abbasian, J.; Arastoopour, H. CFD simulation of particle size change during the coal char gasification process using the population balance model with FCMOM. Powder Technol. 2018, 323, 128–138. [Google Scholar] [CrossRef]
- Mathiesen, V.; Solberg, T.; Hjertager, B.H. Predictions of gas/particle flow with an Eulerian model including a realistic particle size distribution. Powder Technol. 2000, 112, 34–45. [Google Scholar] [CrossRef]
- Syamlal, M. The Particle-Particle Drag Term in a Multiparticle Model of Fluidization; National Technical Information Service: Alexandria, VA, USA, 1987; p. 25. [Google Scholar]
- Ramkrishna, D. Population Balances: Theory and Applications to Particulate Systems in Engineering; Academic Press: New York, NY, USA, 2000. [Google Scholar]
- Marchisio, D.L.; Vigil, R.D.; Fox, R.O. Implementation of the quadrature method of moments in CFD codes for aggregation–breakage problems. Chem. Eng. Sci. 2003, 58, 3337–3351. [Google Scholar] [CrossRef]
- Passalacqua, A.; Fox, R.O.; Garg, R.; Subramaniam, S. A fully coupled quadrature-based moment method for dilute to moderately dilute fluid–particle flows. Chem. Eng. Sci. 2010, 65, 2267–2283. [Google Scholar] [CrossRef]
- Yuan, C.; Laurent, F.; Fox, R.O. An extended quadrature method of moments for population balance equations. J. Aerosol Sci 2012, 51, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Strumendo, M.; Arastoopour, H. Solution of PBE by MOM in finite size domains. Chem. Eng. Sci. 2008, 63, 2624–2640. [Google Scholar] [CrossRef]
- Fan, R.; Fox, R.O. Segregation in polydisperse fluidized beds: Validation of a multi-fluid model. Chem. Eng. Sci. 2008, 63, 272–285. [Google Scholar] [CrossRef]
- Fan, R.; Marchisio, D.L.; Fox, R.O. Application of the direct quadrature method of moments to polydisperse gas–solid fluidized beds. Powder Technol. 2004, 139, 7–20. [Google Scholar] [CrossRef]
- Gräbner, M.; Ogriseck, S.; Meyer, B. Numerical simulation of coal gasification at circulating fluidised bed conditions. Fuel Process. Technol. 2007, 88, 948–958. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, W.; Shao, Y.; Li, K. Coupling of CFD-DEM and reaction model for 3D fluidized beds. Powder Technol. 2019, 353, 72–83. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Arena, U. Bed defluidisation during the fluidised bed pyrolysis of plastic waste mixtures. Polym. Degrad. Stab. 2004, 85, 1051–1058. [Google Scholar] [CrossRef]
- Alobaid, F.; Ströhle, J.; Epple, B. Extended CFD/DEM model for the simulation of circulating fluidized bed. Adv. Powder Technol. 2013, 24, 403–415. [Google Scholar] [CrossRef]
- Trofa, M.; D’Avino, G.; Sicignano, L.; Tomaiuolo, G.; Greco, F.; Maffettone, P.L.; Guido, S. CFD-DEM simulations of particulate fouling in microchannels. Chem. Eng. J. 2019, 358, 91–100. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, W.; Yuan, Z.; Yu, A.B. CFD-DEM study on cohesive particles in a spouted bed. Powder Technol. 2017, 314, 377–386. [Google Scholar] [CrossRef]
- Prince, M.J.; Blanch, H.W. Bubble coalescence and break-up in air-sparged bubble columns. AlChE J. 1990, 36, 1485–1499. [Google Scholar] [CrossRef]
- Eesa, M.; Barigou, M. Horizontal laminar flow of coarse nearly-neutrally buoyant particles in non-Newtonian conveying fluids: CFD and PEPT experiments compared. Int. J. Multiphase Flow 2008, 34, 997–1007. [Google Scholar] [CrossRef]
- Oschmann, T.; Schiemann, M.; Kruggel-Emden, H. Development and verification of a resolved 3D inner particle heat transfer model for the Discrete Element Method (DEM). Powder Technol. 2016, 291, 392–407. [Google Scholar] [CrossRef]
- Maffei, T.; Gentile, G.; Rebughini, S.; Bracconi, M.; Manelli, F.; Lipp, S.; Cuoci, A.; Maestri, M. A multiregion operator-splitting CFD approach for coupling microkinetic modeling with internal porous transport in heterogeneous catalytic reactors. Chem. Eng. J. 2016, 283, 1392–1404. [Google Scholar] [CrossRef]
- POLIMI. CatalyticFOAM. Available online: http://www.catalyticfoam.polimi.it/ (accessed on 13 November 2020).
- Radeke, C.A.; Glasser, B.J.; Khinast, J.G. Large-scale powder mixer simulations using massively parallel GPUarchitectures. Chem. Eng. Sci. 2010, 65, 6435–6442. [Google Scholar] [CrossRef]
- Xu, J.; Qi, H.; Fang, X.; Lu, L.; Ge, W.; Wang, X.; Xu, M.; Chen, F.; He, X.; Li, J. Quasi-real-time simulation of rotating drum using discrete element method with parallel GPU computing. Particuology 2011, 9, 446–450. [Google Scholar] [CrossRef]
- Norouzi, H.R.; Zarghami, R.; Mostoufi, N. New hybrid CPU-GPU solver for CFD-DEM simulation of fluidized beds. Powder Technol. 2017, 316, 233–244. [Google Scholar] [CrossRef]
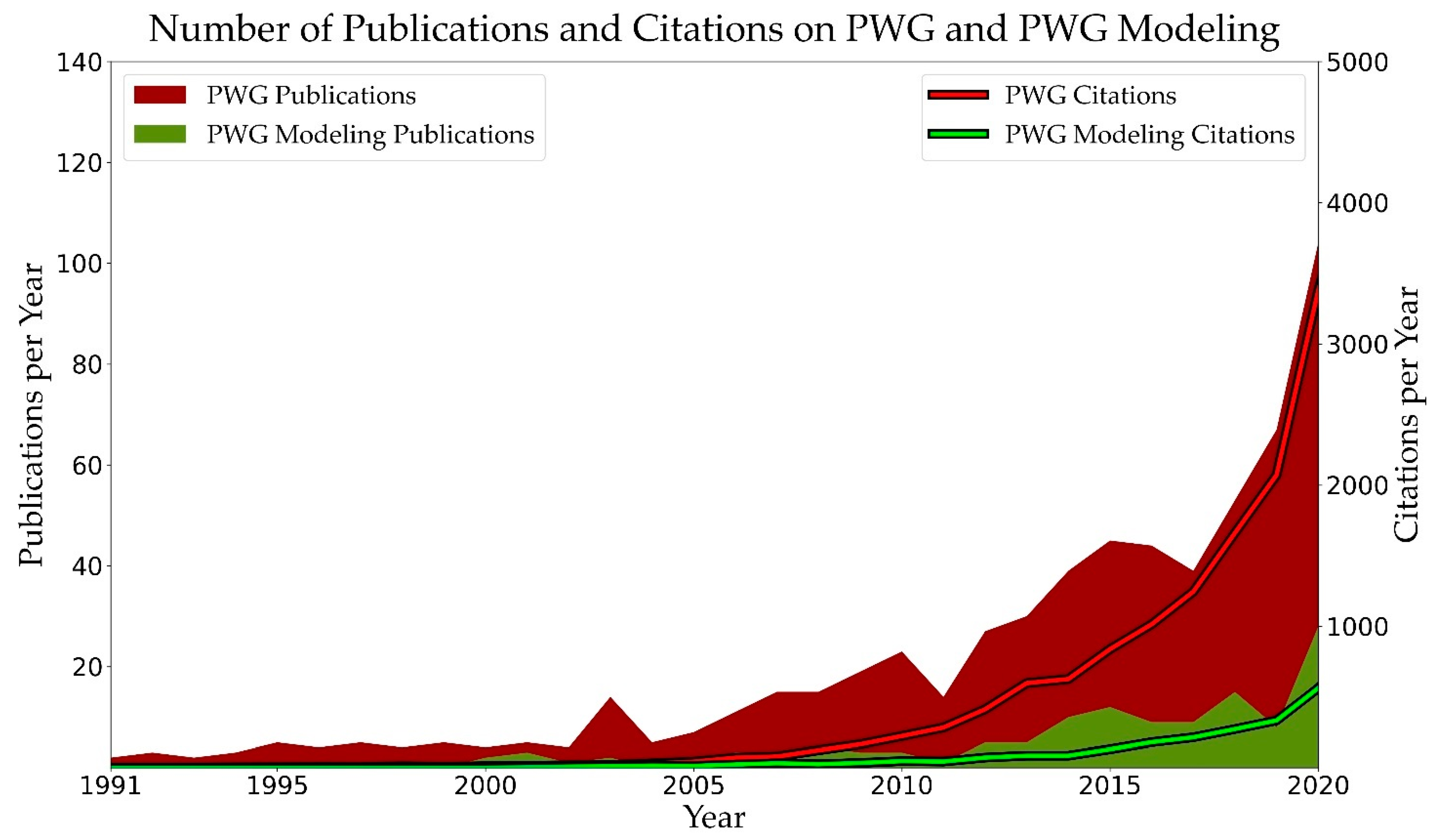
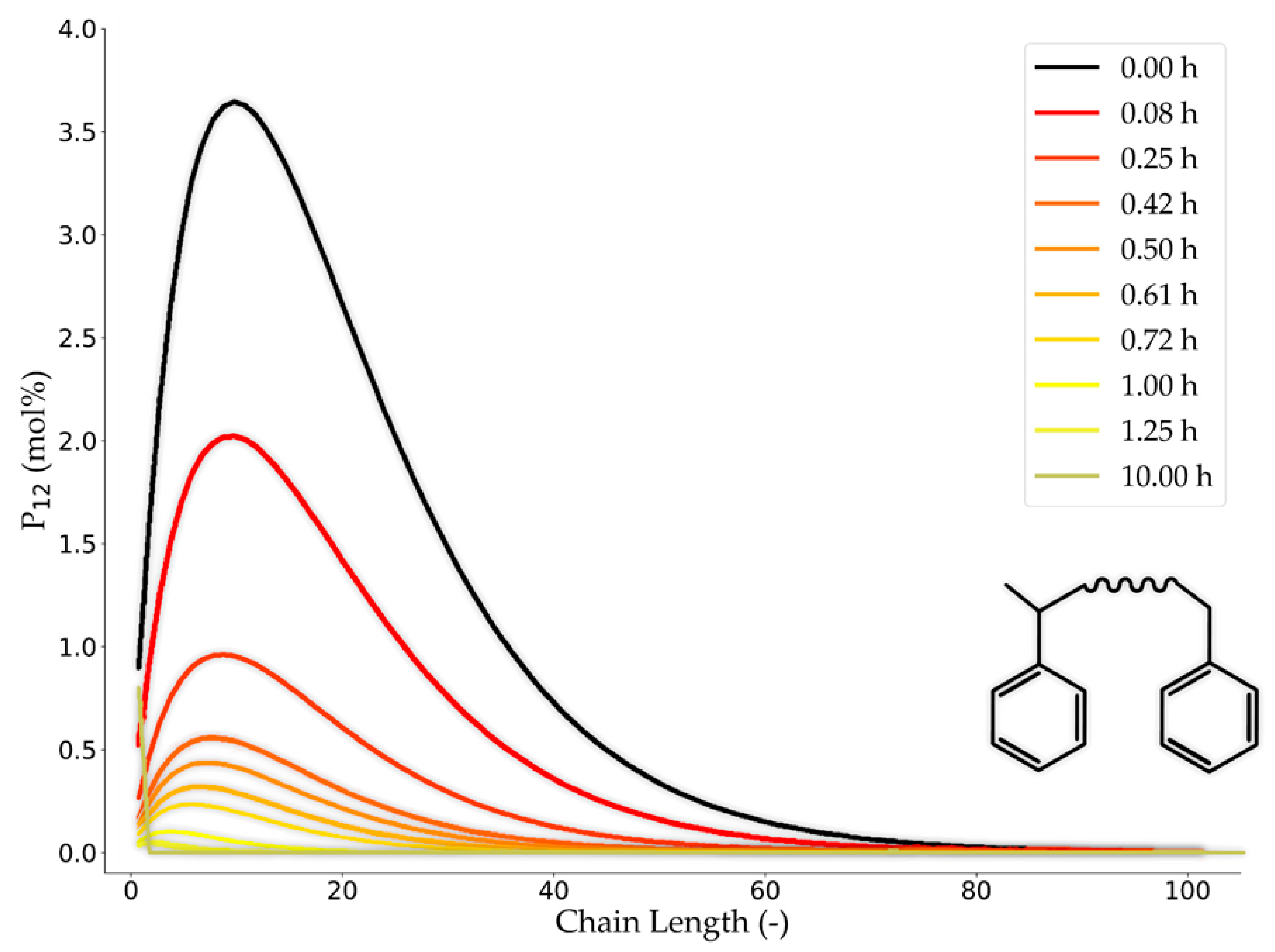
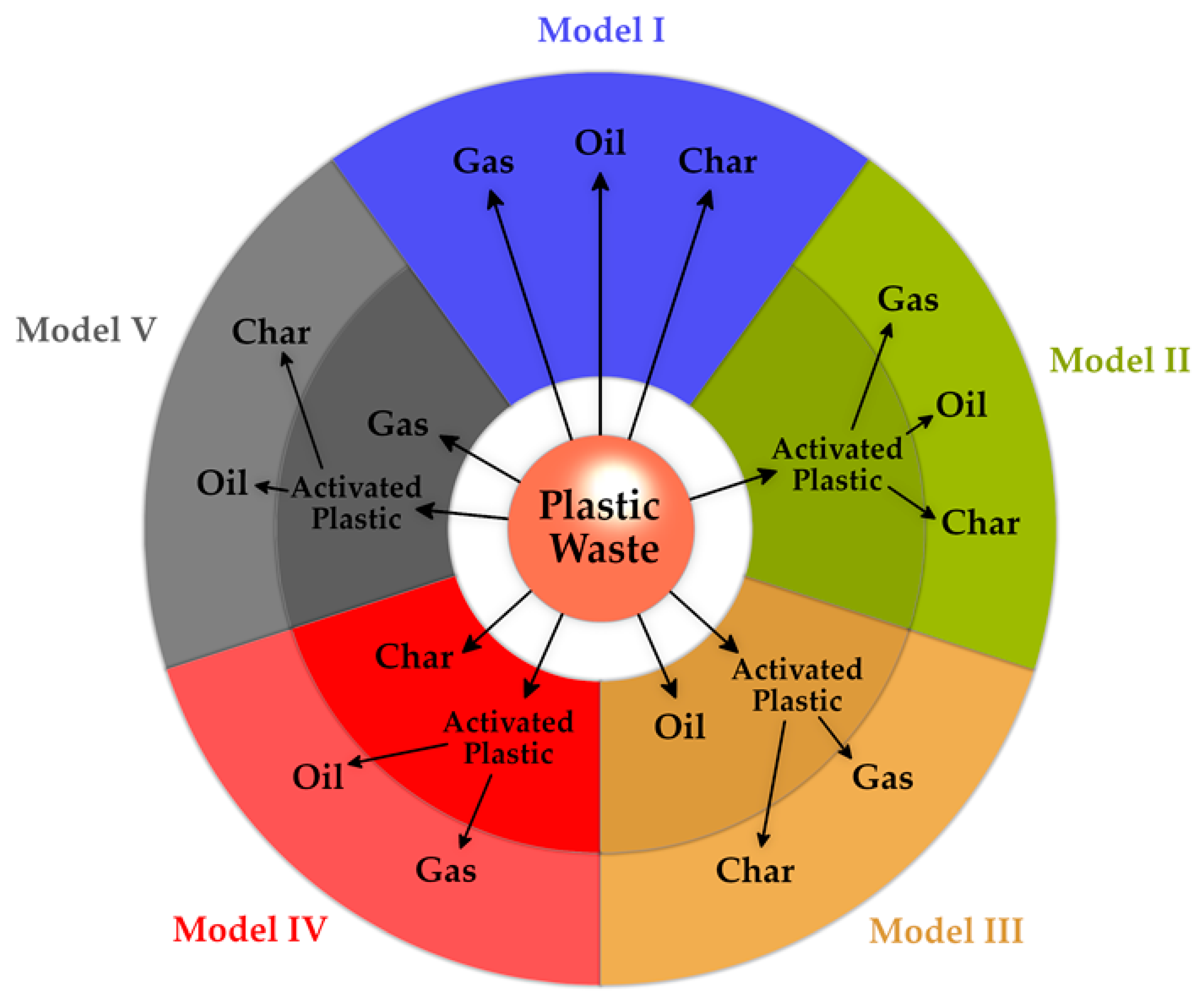


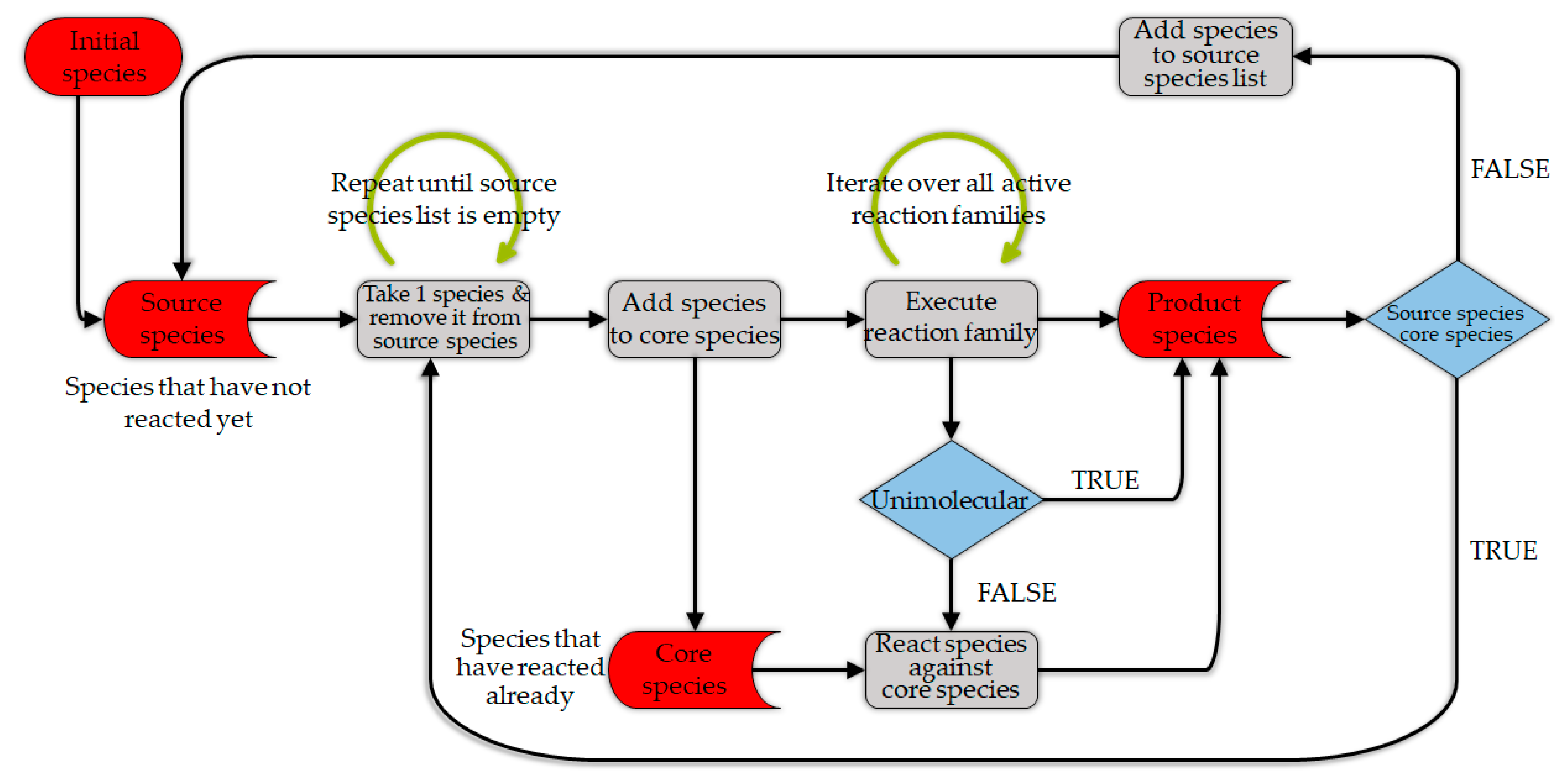
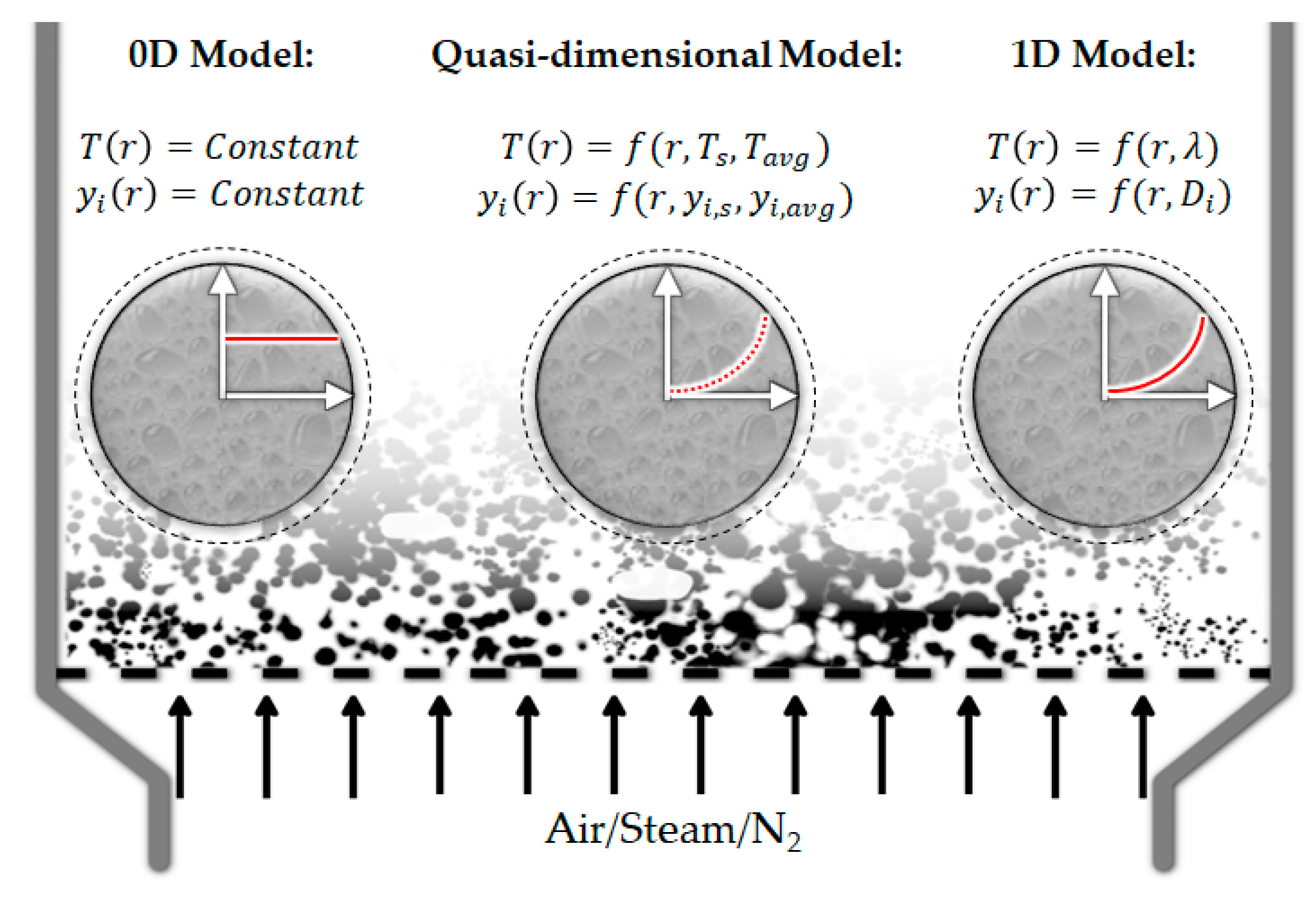
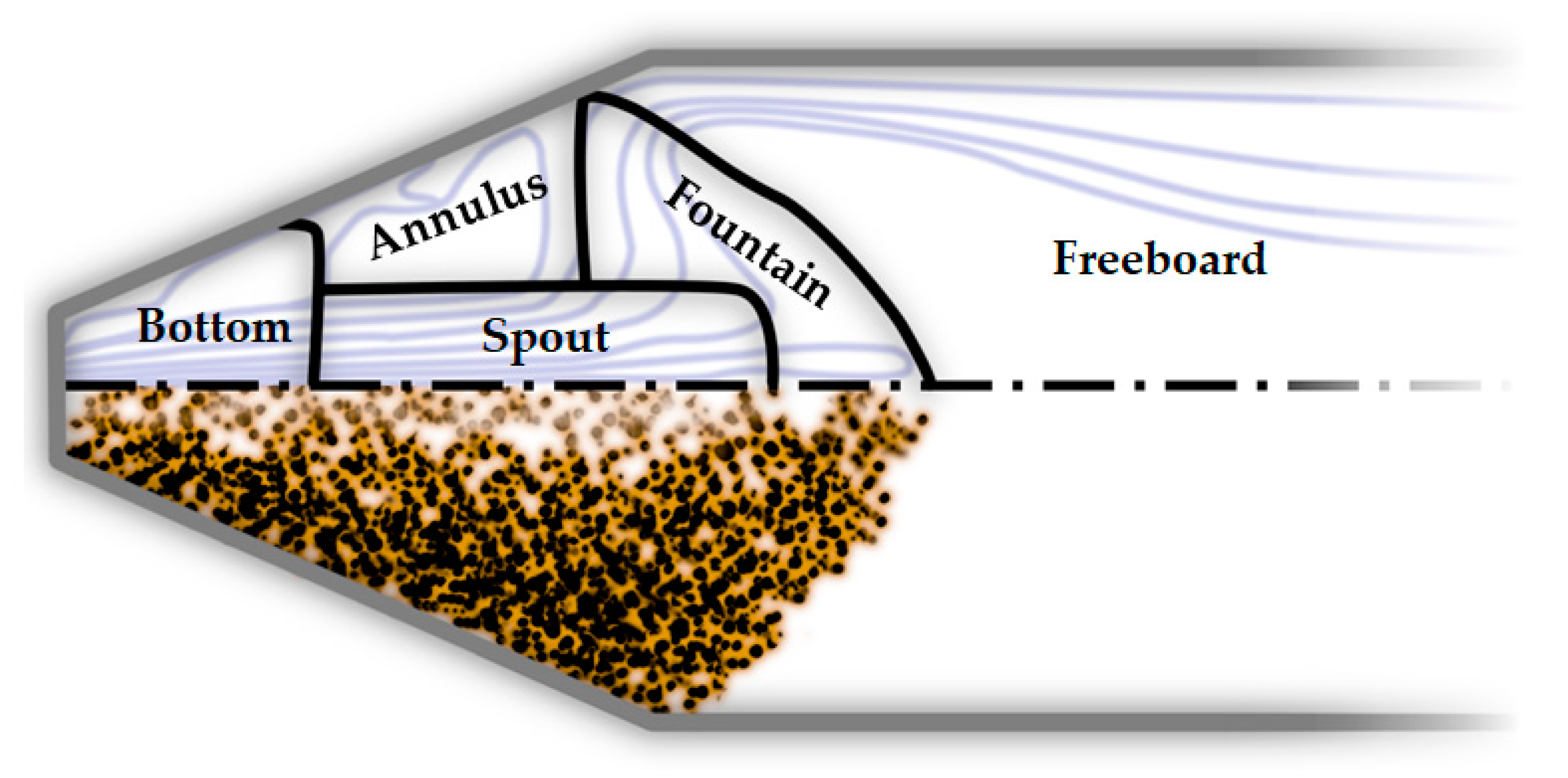

| Gas | Liquid | |
|---|---|---|
| Viscosity |
|
|
| Thermal Conductivity |
|
|
| Diffusion Coefficient |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madanikashani, S.; Vandewalle, L.A.; De Meester, S.; De Wilde, J.; Van Geem, K.M. Multi-Scale Modeling of Plastic Waste Gasification: Opportunities and Challenges. Materials 2022, 15, 4215. https://doi.org/10.3390/ma15124215
Madanikashani S, Vandewalle LA, De Meester S, De Wilde J, Van Geem KM. Multi-Scale Modeling of Plastic Waste Gasification: Opportunities and Challenges. Materials. 2022; 15(12):4215. https://doi.org/10.3390/ma15124215
Chicago/Turabian StyleMadanikashani, Sepehr, Laurien A. Vandewalle, Steven De Meester, Juray De Wilde, and Kevin M. Van Geem. 2022. "Multi-Scale Modeling of Plastic Waste Gasification: Opportunities and Challenges" Materials 15, no. 12: 4215. https://doi.org/10.3390/ma15124215







