Influence of Magnetic Water on Concrete Properties with Different Magnetic Field Exposure Times
Abstract
:1. Introduction
2. Experimental Program
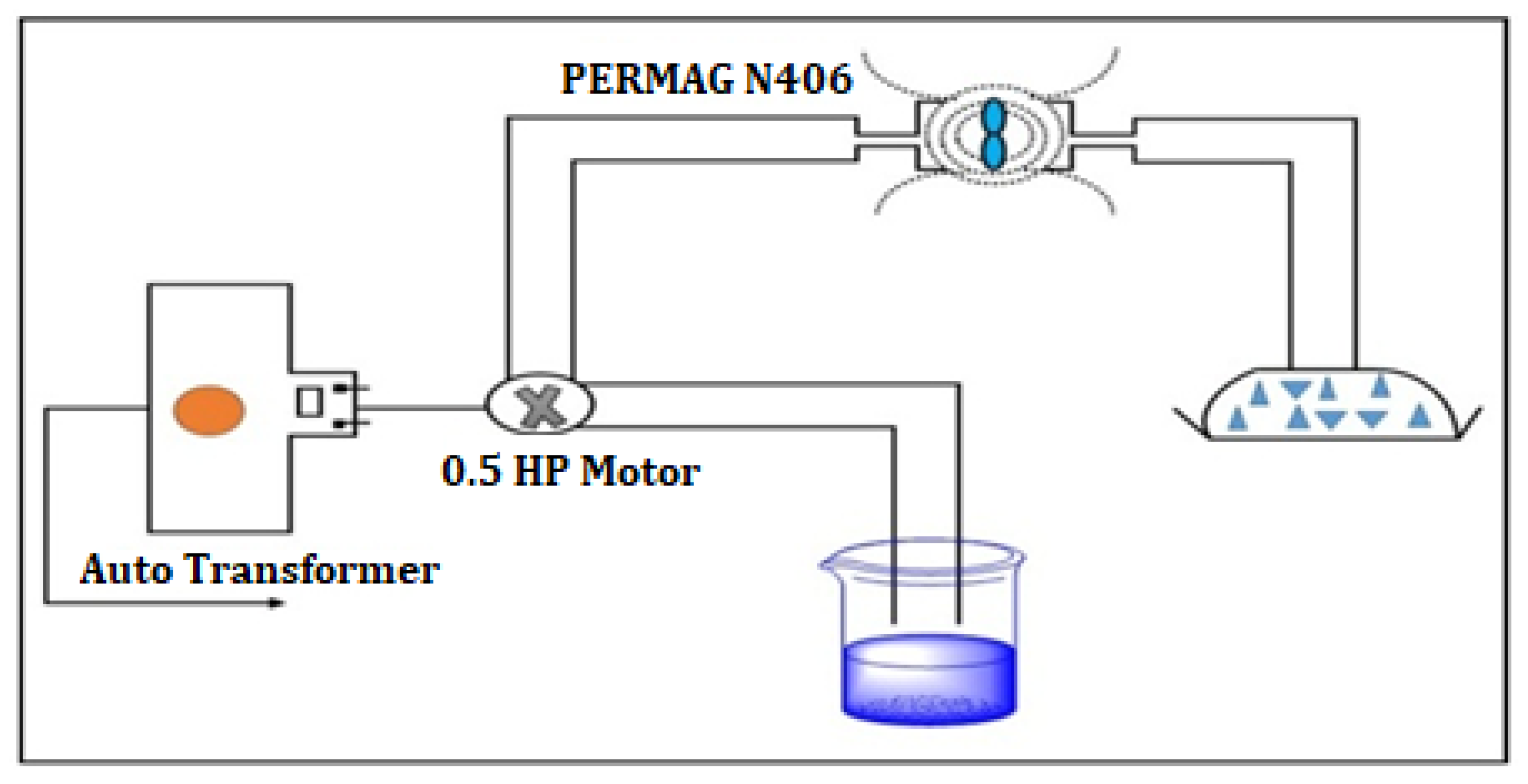
2.1. Magnetic Water Preparation
2.2. Methodology
2.3. Physiochemical Properties
2.3.1. Total Dissolved Solids and Electrical Conductivity
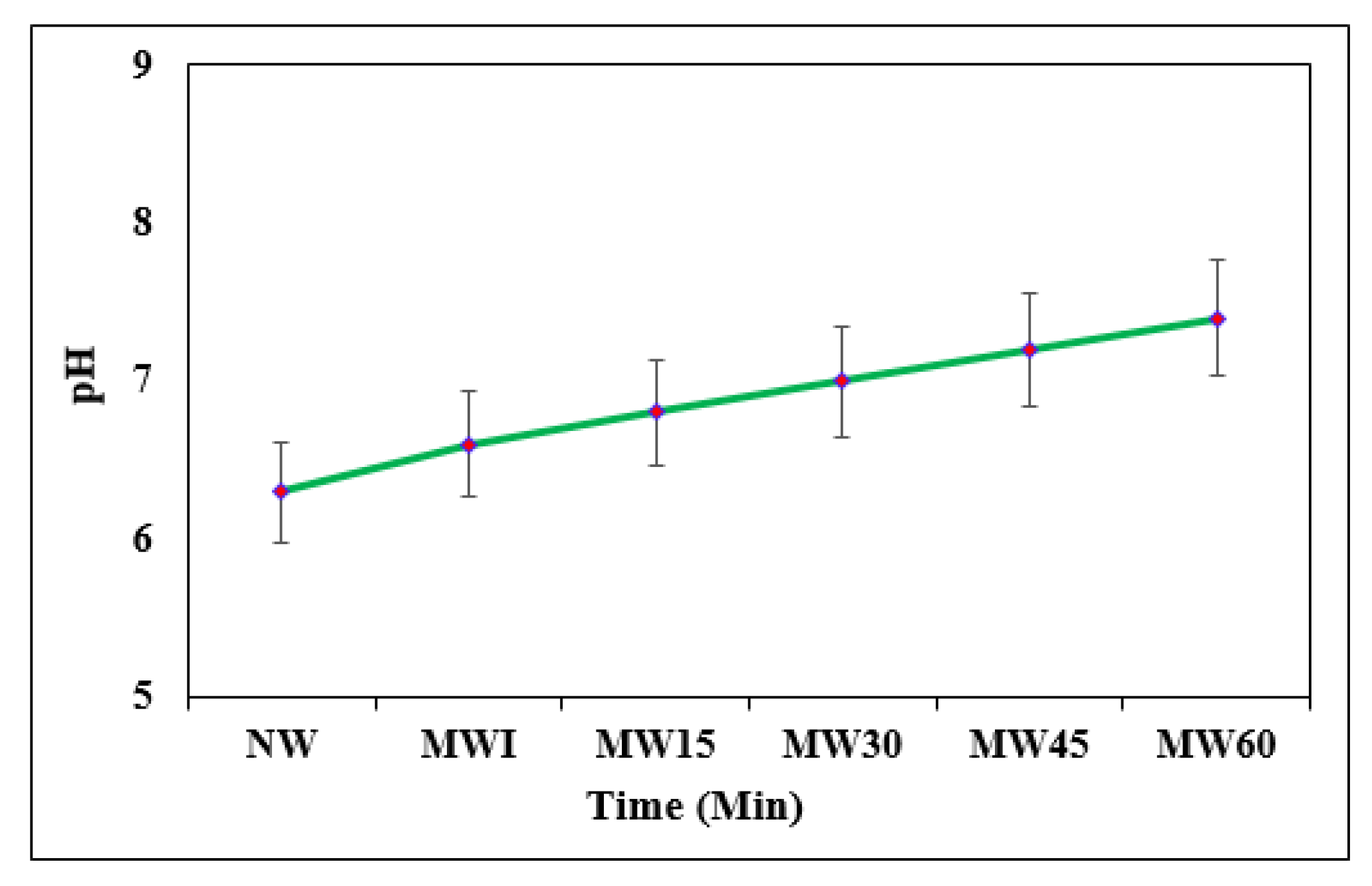
2.3.2. pH
2.3.3. Viscosity
2.4. Optical Properties
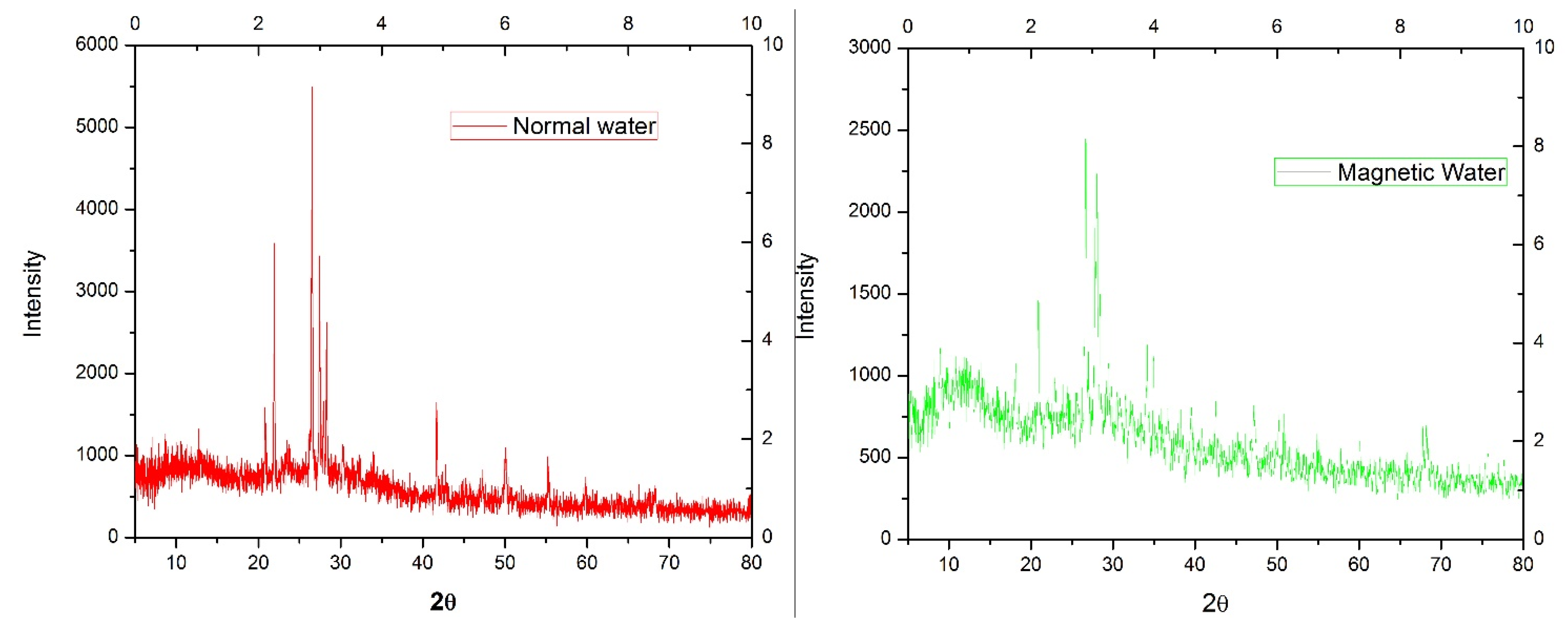
2.4.1. X-ray Diffraction (XRD) Analysis
2.4.2. UV-Visible Spectroscopy
2.5. Properties of Concrete
2.5.1. Materials and Mix Proportioning
2.5.2. Fresh and Hardened Properties of Concrete
2.6. TGA
2.7. FT-IR Analysis
2.8. Scanning Electron Microscope (SEM) Analysis on Concrete Powder Samples
3. Results and Discussion
3.1. Physiochemical Properties
3.1.1. Total Dissolved Solids (TDS) and Electrical Conductivity (EC) of Water
3.1.2. pH
3.1.3. Viscosity of Water
3.2. Optical Properties
3.2.1. X-ray Diffraction
3.2.2. UV-Visible Absorbance
3.3. Fresh and Hardened Properties of Concrete
3.3.1. Effect of Time of Exposure to Magnetic Field on the Slump
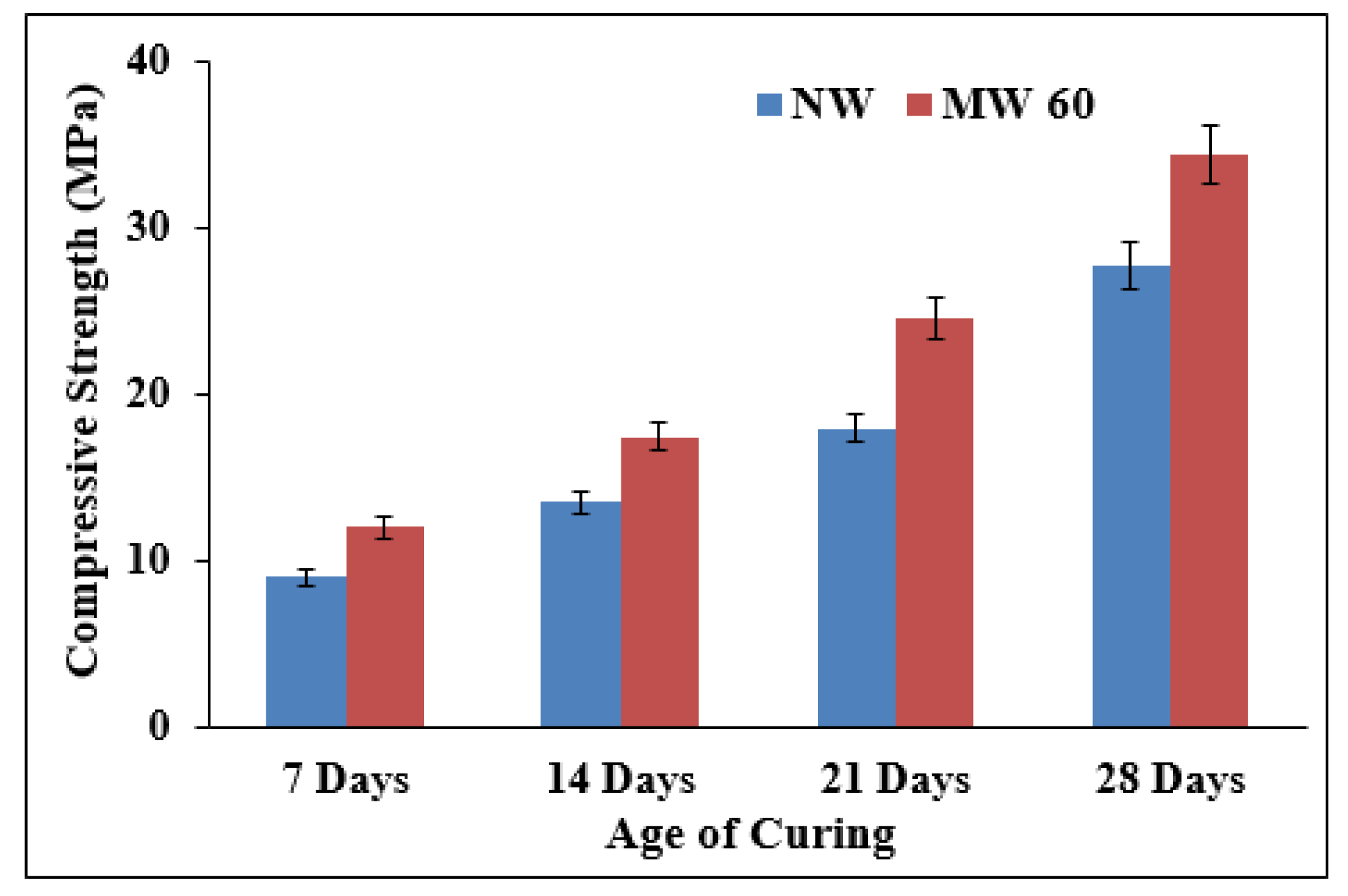
3.3.2. Effect on Compressive Strength
3.4. Thermogravimetric Analysis (TGA)
3.5. Fourier Transform Infrared Spectrophotometer (FT-IR)
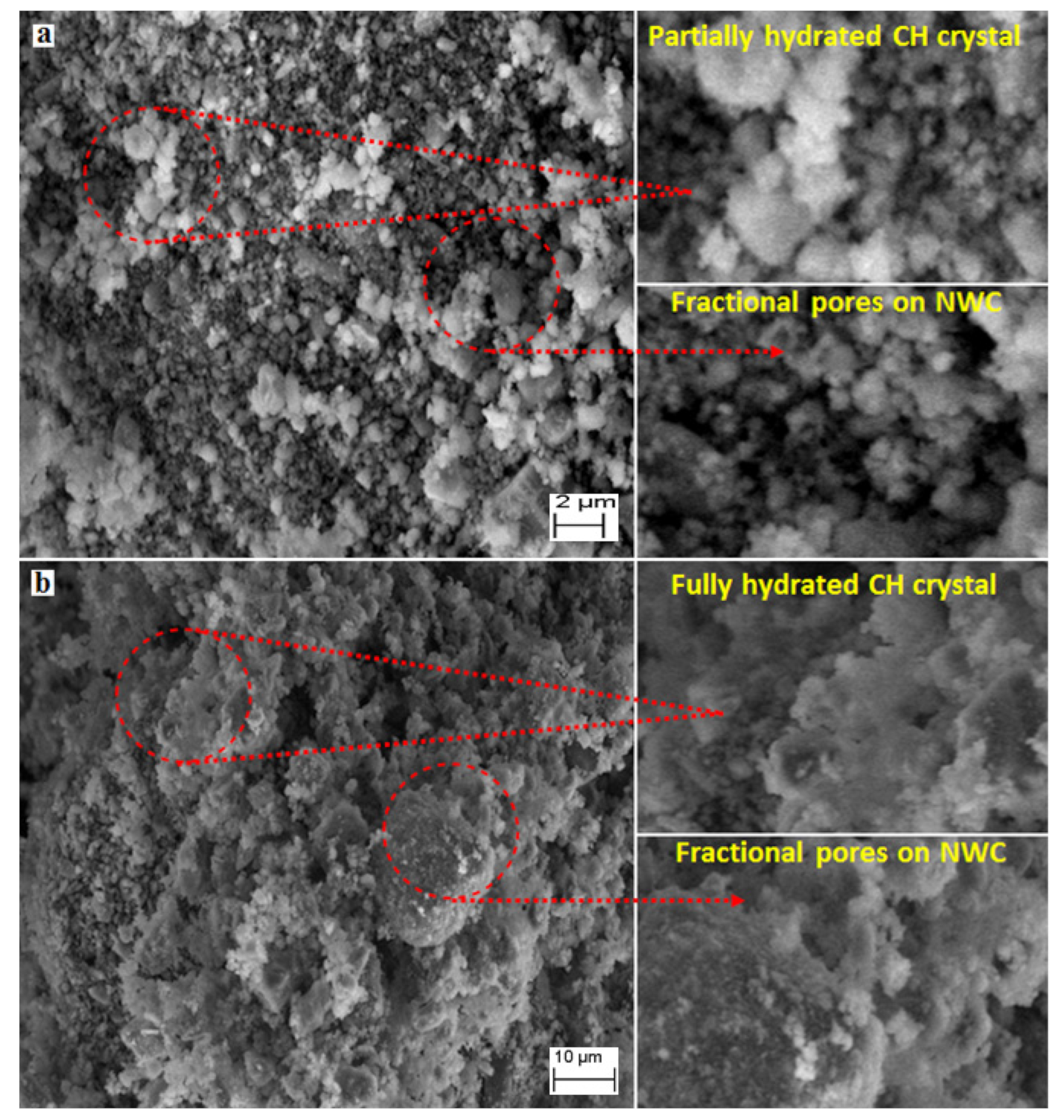
3.6. SEM Analysis
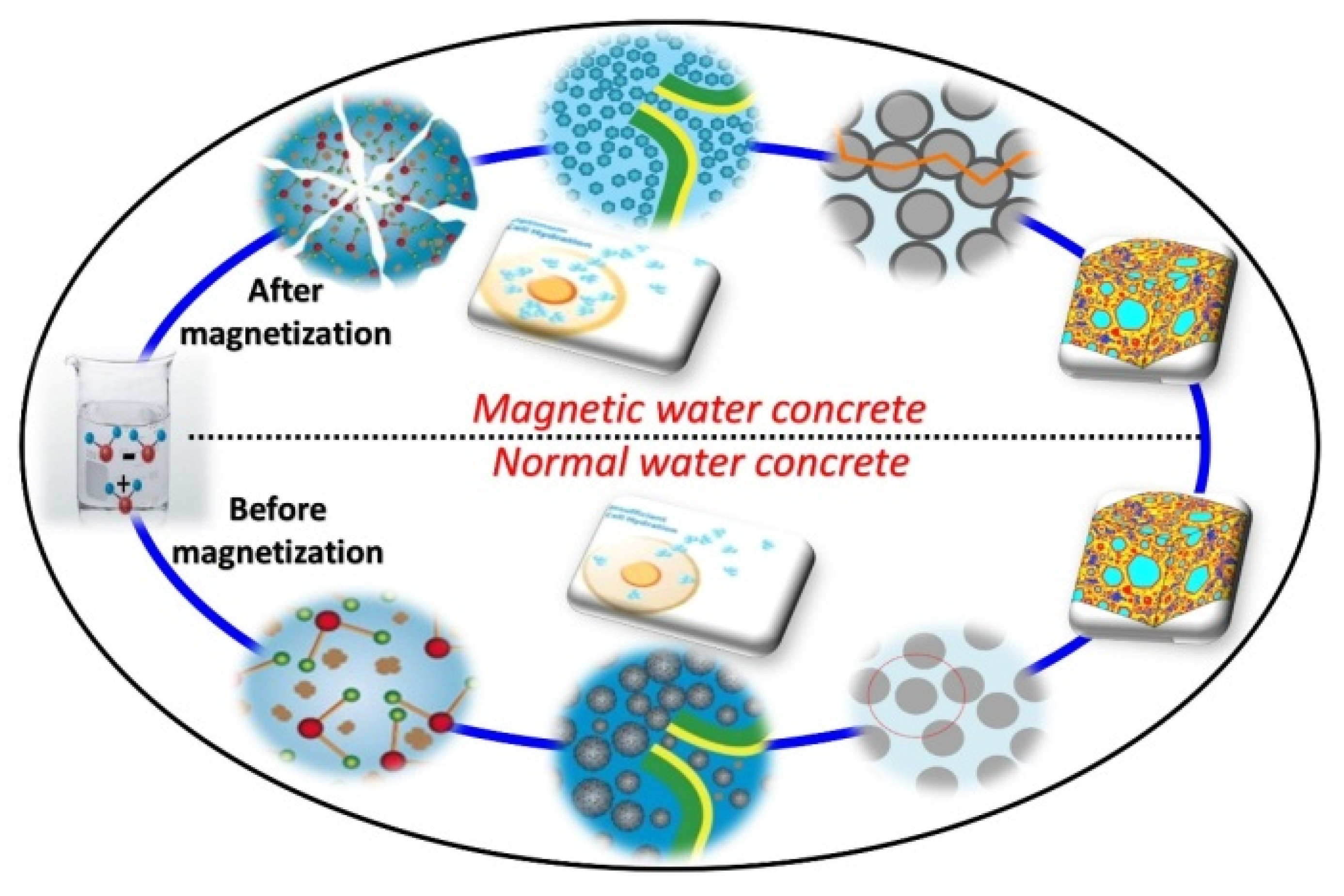
3.7. Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, P.R.M.; Moinuddin, S.; Jagadeesh, P. Effect of treated waste water on the properties of hardened concrete. Int. J. Chem. Sci. 2014, 12, 155–162. [Google Scholar]
- Ramezani, M.; Kim, Y.H.; Sun, Z. Modeling the mechanical properties of cementitious materials containing CNTs. Cem. Concr. Compos. 2019, 104, 103347. [Google Scholar] [CrossRef]
- Ramezani, M.; Kim, Y.H.; Sun, Z. Mechanical properties of carbon-nanotube-reinforced cementitious materials: Database and statistical analysis. Mag. Concr. Res. 2020, 72, 1047–1071. [Google Scholar] [CrossRef]
- Ramezani, M.; Dehghani, A.; Sherif, M.M. Carbon nanotube reinforced cementitious composites: A comprehensive review. Constr. Build. Mater. 2022, 315, 125100. [Google Scholar] [CrossRef]
- Gholhaki, M.; Hajforoush, M.; Kazemi, M. An investigation on the fresh and hardened properties of self-compacting concrete incorporating magnetic water with various pozzolanic materials. Constr. Build. Mater. 2018, 158, 173–180. [Google Scholar] [CrossRef]
- Babu, G.R.; Reddy, B.M.; Ramana, N.V. Quality of mixing water in cement concrete “a review”. Mater. Today Proc. 2018, 5, 1313–1320. [Google Scholar] [CrossRef]
- Kanwal, H.; Arif, S.; Jawaid, M.A.; Farooq, A.; Khan, M.A. Effect on compressive strength of concrete using treated waste water for mixing and curing of concrete. J. Eng. Technol. 2018, 37, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- More, R.A.; Dubey, S. Effect of different types of water on compressive strength of concrete. Int. J. Emerg. Technol. 2014, 5, 40. [Google Scholar]
- IS 456-2000; Plain and Reinforced Concrete-Code of Practice. Bureau of Indian Standards: New Delhi, India, 2000.
- Kucche, K.; Jamkar, S.; Sadgir, P. Quality of water for making concrete: A review of literature. Int. J. Sci. Res. Publ. 2015, 5, 1–10. [Google Scholar]
- Banejad, H.; Abdosalehi, E. The effect of magnetic field on water hardness reducing. In Proceedings of the Thirteenth International Water Technology Conference, IWTC, Hurghada, Egypt, 12–15 March 2009. [Google Scholar]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Peng, Y.; Ma, Z. Effect of magnetic field on optical features of water and KCl solutions. Optik 2016, 127, 6371–6376. [Google Scholar] [CrossRef]
- Holysz, L.; Szczes, A.; Chibowski, E. Effects of a static magnetic field on water and electrolyte solutions. J. Colloid Interface Sci. 2007, 316, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Gong, Z.; Gao, K.; Ou, Y.; Zhang, J. The effect of a static magnetic field on the hydrogen bonding in water using frictional experiments. J. Mol. Struct. 2013, 1052, 102–104. [Google Scholar] [CrossRef]
- Cai, R.; Yang, H.; He, J.; Zhu, W. The effects of magnetic fields on water molecular hydrogen bonds. J. Mol. Struct. 2009, 938, 15–19. [Google Scholar] [CrossRef]
- Toledo, E.J.; Ramalho, T.C.; Magriotis, Z.M. Influence of magnetic field on physical–chemical properties of the liquid water: Insights from experimental and theoretical models. J. Mol. Struct. 2008, 888, 409–415. [Google Scholar] [CrossRef]
- Chang, K.T.; Weng, C.I. The effect of an external magnetic field on the structure of liquid water using molecular dynamics simulation. J. Appl. Phys. 2006, 100, 04391–04397. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Wu, C.F. Effect of magnetic field treated water on mortar and concrete containing fly ash. Cem. Concr. Compos. 2003, 25, 681–688. [Google Scholar] [CrossRef]
- Reddy, B.S.K.; Ghorpade, V.G.; Rao, H.S. Influence of magnetic water on strength properties of concrete. Indian J. Sci. Technol. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Karkush, M.O.; Ahmed, M.D.; Al-Ani, S.M.A. Magnetic field influence on the properties of water treated by reverse osmosis. Eng. Technol. Appl. Sci. Res. 2019, 9, 4433–4439. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Li, Z. Effect of magnetic field on the physical properties of water. Res. Phys. 2018, 8, 262–267. [Google Scholar] [CrossRef]
- Liu, B.; Gao, B.; Xu, X.; Hong, W.; Yue, Q.; Wang, Y.; Su, Y. The combined use of magnetic field and iron-based complex in advanced treatment of pulp and paper wastewater. Chem. Eng. J. 2011, 178, 232–238. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobe, S.; Dražić, G.; McGuiness, P.; Stražišar, J. The influence of the magnetic field on the crystallisation form of calcium carbonate and the testing of a magnetic water-treatment device. J. Magn. Magn. Mater. 2001, 236, 71–76. [Google Scholar] [CrossRef]
- Ghorbani, S.; Gholizadeh, M.; De Brito, J. Effect of magnetized water on the mechanical and durability properties of concrete block pavers. Materials 2018, 11, 1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliassen, R.; Skrinde, R.T.; Davis, W.B. Experimental performance of ‘miracle’ water conditioners. J. Am. Water Work. Assoc. 1958, 50, 1371–1385. [Google Scholar] [CrossRef]
- Hosoda, H.; Mori, H.; Sogoshi, N.; Nagasawa, A.; Nakabayashi, S. Refractive indices of water and aqueous electrolyte solutions under high magnetic fields. J. Phys. Chem. 2004, 108, 1461–1464. [Google Scholar] [CrossRef]
- Bogatin, J.; Bondarenko, N.P.; Gak, E.Z.; Rokhinson, E.E.; Ananyev, I.P. Magnetic treatment of irrigation water: Experimental results and application conditions. Environ. Sci. Technol. 1999, 33, 1280–1285. [Google Scholar] [CrossRef]
- Alimi, F.; Tlili, M.; Ben Amor, M.; Maurin, G.; Gabrielli, C. Influence of magnetic field on calcium carbonate precipitation. Desalination 2007, 206, 163–168. [Google Scholar] [CrossRef]
- Inaba, H.; Saitou, T.; Tozaki, K.-I.; Hayashi, H. Effect of the magnetic field on the melting transition of H2O and D2O measured by a high resolution and supersensitive differential scanning calorimeter. J. Appl. Phys. 2004, 96, 6127–6132. [Google Scholar] [CrossRef]
- Szczes, A.; Chibowski, E.; Rzeznik, E. Magnetic Field Effect on Water Surface Tension in Aspect of Glass and Mica Wettability. Colloid Interface 2020, 4, 37. [Google Scholar] [CrossRef]
- Xiao-Feng, P.; Xing-Chun, Z. The magnetization of water arising from a magnetic-field and its applications in concrete industry. Int. J. Eng. Res. Appl. 2013, 3, 1541–1552. [Google Scholar]
- IS 10262-2009; Recommended Guidelines for Concrete Mix. Bureau of Indian Standards: New Delhi, India, 2009; pp. 1–14.
- Hassan, S.; Rahman, R.A. Effects of exposure to magnetic field on water properties and hatchability of Artemia salina. ARPN J. Agric. Biol. Sci. 2016, 11, 416–423. [Google Scholar]
- Hasaani, A.S.; Hadi, Z.L.; Rasheed, K.A. Experimental study of the interaction of magnetic fields with flowing water. Int. J. Basic Appl. Sci. 2015, 3, 1–8. [Google Scholar]
- Pang, X.F. The experimental evidences of the magnetism of water by magnetic-field treatment. IEEE Trans. Appl. Supercond. 2014, 24, 1–6. [Google Scholar] [CrossRef]
- Deng, B.; Pang, X. Variations of optic properties of water under action of static magnetic field. Chi. Sci. 2007, 52, 3179–3182. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, S.I.; Kim, Y.S.; Lee, S.K. Changes in the electrical conductivity, infrared absorption, and surface tension of partially-degassed and magnetically-treated water. J. Mol. Liq. 2013, 187, 230–237. [Google Scholar] [CrossRef]
- IS 8112-2013; Oridinary Portland Cement, 43 Grade Specification (Second Revision). Bureau of Indian Standards: New Delhi, India, 2013; pp. 1–10.
- IS2386 (Part 1)-1963; Methods for Test for Aggregates for Concrete. Bureau of Indian Standards: New Delhi, India, 2002; pp. 1–17.
- IS 1199-1959; Methods of Sampling and Analysis of Concrete-Guidelines. Bureau of Indian Standards: New Delhi, India, 2004; pp. 1–44.
- IS 516-1959; Methods of Tests for Strength of Concrete. Bureau of Indian Standards: New Delhi, India, 2004; pp. 1–24.
- Ramezani, M.; Kim, Y.H.; Hasanzadeh, B.; Sun, Z. Influence of carbon nanotubes on SCC flowability. In Proceedings of the 8th International RILEM Symposium on Self-Compacting Concrete, Washington, DC, USA, 15–18 May 2016; pp. 397–406. [Google Scholar]
- Ramezani, M.; Kim, Y.H.; Sun, Z.; Sherif, M.M. Influence of carbon nanotubes on properties of cement mortars subjected to alkali-silica reaction. Cem. Concr. Compos. 2022, 131, 104596. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Mishra, G.; Ahalawat, S. Reduction of calcium leaching in cement hydration process using nanomaterials. Mater. Technol. 2012, 27, 233–238. [Google Scholar] [CrossRef]
- Snehal, K.; Das, B.; Akanksha, M. Early age, hydration, mechanical and microstructure properties of nano-silica blended cementitious composites. Constr. Build. Mater. 2020, 233, 117212. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Gao, H. Analysis on microstructure of impermeability of magnetized water concrete. J. Chem. Pharm. Res. 2014, 6, 189–199. [Google Scholar]
- Brameshuber, W.; Raupach, M.; Schröder, P.; Dauberschmidt, C. Non-destructive determination of the water-content in the concrete cover using the multiring-electrode. In Proceedings of the International Symposium (NDT-CE 2003) Non-Destructive Testing in Civil Engineering, Berlin, Germany, 16–19 September 2003. [Google Scholar]
- Amor, H.B.; Elaoud, A.; Hozayn, M. Does magnetic field change water pH? Asian Res. J. Agric. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Čolić, M.; Chien, A.; Morse, D. Synergistic application of chemical and electromagnetic water treatment in corrosion and scale prevention. Croat. Chem. Acta 1998, 71, 905–916. [Google Scholar]
- Sobhnamayan, F.; Sahebi, S.; Alborzi, A.; Ghorbani, S.; Shojaee, N.S. Effect of different pH values on the compressive strength of calcium-enriched mixture cement. Iran. Endod. J. 2015, 10, 26. [Google Scholar] [PubMed]
- Çomak, B. Effects of use of alkaline mixing waters on engineering properties of cement mortars. J. Environ. Civ. Eng. 2018, 22, 736–754. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. Characteristics and applications of magnetized water as a green technology. J. Clean. Prod. 2017, 161, 908–921. [Google Scholar] [CrossRef]
- Neupane, U.; Julnipitawong, P.; Tangtermsirikul, S.; Yusuke, A. Effect of viscosity modifying agents on dewatering under pressure and the performances OF mortar and concrete. ASEAN Eng. J. 2017, 7, 18–29. [Google Scholar] [CrossRef]
- Pang, X.; Deng., B. Investigation of changes in properties of water under the action of a magnetic field. Sci. China Ser. G Phys. Mech. Astron. 2008, 51, 1621–1632. [Google Scholar] [CrossRef]
- Reddy, B.S.K.; Ghorpade, V.G.; Rao, H.S. Effect of magnetic field exposure time on workability and compressive strength of magnetic water concrete. Int. J. Adv. Eng. Technol. 2013, 4, 120–122. [Google Scholar]
- Malathy, R.; Narayanan, K.; Mayakrishnan, P. Performance of prestressed concrete beams using magnetic water for concrete mixing. J. Adhes. Sci. Technol. 2022, 36, 666–684. [Google Scholar] [CrossRef]
- Malathy, R.; Karuppasamy, N.; Vinitha, U. Influence of Contact Time to Magnetic Field of Mixing Water on Fresh and Hardened Properties of Concrete. In Recent Developments in Sustainable Infrastructure (ICRDSI-2020)—Structure and Construction Management; Springer: Singapore, 2022; pp. 693–699. [Google Scholar]
- Malathy, R.; Karuppasamy, N.; Adithya, V.; Gokulapriya, P. Influence of Magnetic Water on Properties of Concrete Paver Blocks. In Smart Technologies for Sustainable Development; Springer: Singapore, 2021; pp. 327–336. [Google Scholar]
- Zhao, K.; Zhang, P.; Wang, B.; Tian, Y.; Xue, S.; Cong, Y. Preparation of Electric- and Magnetic-Activated Water and Its Influence on the Workability and Mechanical Properties of Cement Mortar. Sustainability 2021, 13, 4546. [Google Scholar] [CrossRef]
- Bharath, S.; Subraja, S.; Kumar, P.A. Influence of magnetized water on concrete by replacing cement partially with copper slag. J. Chem. Pharm. Sci. 2016, 9, 2791–2795. [Google Scholar]
- Gholizadeh, M.; Arabshahi, H.; Benam, M. The effect of magnetic field on scale prevention in the industrial boilers. Int. J. Appl. Chem. 2005, 1, 84–89. [Google Scholar]
- Yousry, O.M.; Abdallah, M.A.; Ghazy, M.F.; Taman, M.H.; Kaloop, M.R. A Study for Improving Compressive Strength of Cementitious Mortar Utilizing Magnetic Water. Materials 2020, 13, 1971. [Google Scholar] [CrossRef] [Green Version]
- Soriano, L.; Monzó, J.; Bonilla, M.; Tashima, M.M.; Payá, J.; Borrachero, M.V. Effect of pozzolans on the hydration process of Portland cement cured at low temperatures. Cem. Concr. Compos. 2013, 42, 41–48. [Google Scholar] [CrossRef]
- Singh, L.P.; Goel, A.; Bhattacharyya, S.K.; Sharma, U.; Mishra, G. Hydration studies of cementitious material using silica nano particles. J. Adv. Concr. Technol. 2015, 13, 345–354. [Google Scholar] [CrossRef]
- Mghaiouini, R.; Graich, A.; Elaoud, A.; Garmim, T.; Belghiti, M.E.; Benzbiria, N.; Hozayn, M.; Monkade, M.; El Bouari, A. Formulation and physico-mechanical characterization of an eco-mortar composite based on bottom ash and magnetized water. Indian J. Sci. Technol. 2020, 13, 1172–1187. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared spectroscopy fundamentals and applications. In Analytical Techniques in the Sciences; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Wei, H.; Wang, Y.; Luo, J. Influence of magnetic water on early-age shrinkage cracking of concrete. Const. Build. Mater. 2017, 147, 91–100. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, X.; Yang, Z.K. Study on impermeability mechanism of magnetic water concrete. Appl. Mech. Mater. 2011, 99, 745–748. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramalingam, M.; Narayanan, K.; Masilamani, A.; Kathirvel, P.; Murali, G.; Vatin, N.I. Influence of Magnetic Water on Concrete Properties with Different Magnetic Field Exposure Times. Materials 2022, 15, 4291. https://doi.org/10.3390/ma15124291
Ramalingam M, Narayanan K, Masilamani A, Kathirvel P, Murali G, Vatin NI. Influence of Magnetic Water on Concrete Properties with Different Magnetic Field Exposure Times. Materials. 2022; 15(12):4291. https://doi.org/10.3390/ma15124291
Chicago/Turabian StyleRamalingam, Malathy, Karuppasamy Narayanan, Arivoli Masilamani, Parthiban Kathirvel, Gunasekaran Murali, and Nikolai Ivanovich Vatin. 2022. "Influence of Magnetic Water on Concrete Properties with Different Magnetic Field Exposure Times" Materials 15, no. 12: 4291. https://doi.org/10.3390/ma15124291







