Dynamic Adsorption Properties of Insoluble Humic Acid/Tourmaline Composite Particles for Iron and Manganese in Mine Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Water Quality
2.2. Preparation and Characterization of Materials
2.2.1. Preparation of the IHA/TM Composite Particles
2.2.2. Micro Test Analysis
2.3. Dynamic Adsorption Experimental Equipment
2.4. Experimental Data Analysis
2.5. Penetration Curve Model Fitting
2.6. Regeneration of IHA/TM
3. Results and Discussion
3.1. Preparation of IHA/TM Composite Particles
3.1.1. Effect of Mixing Ratio
3.1.2. Effect of Mixing Time
3.1.3. Effect of Calcination Temperature
3.1.4. Effect of Calcination Time
3.1.5. Comparison of Adsorption Effects of Different Adsorbents on Fe2+ and Mn2+
3.1.6. Effect of Coexisting Ions
3.2. Composite Material Characterization
3.3. Analysis of Dynamic Adsorption Influence Factors
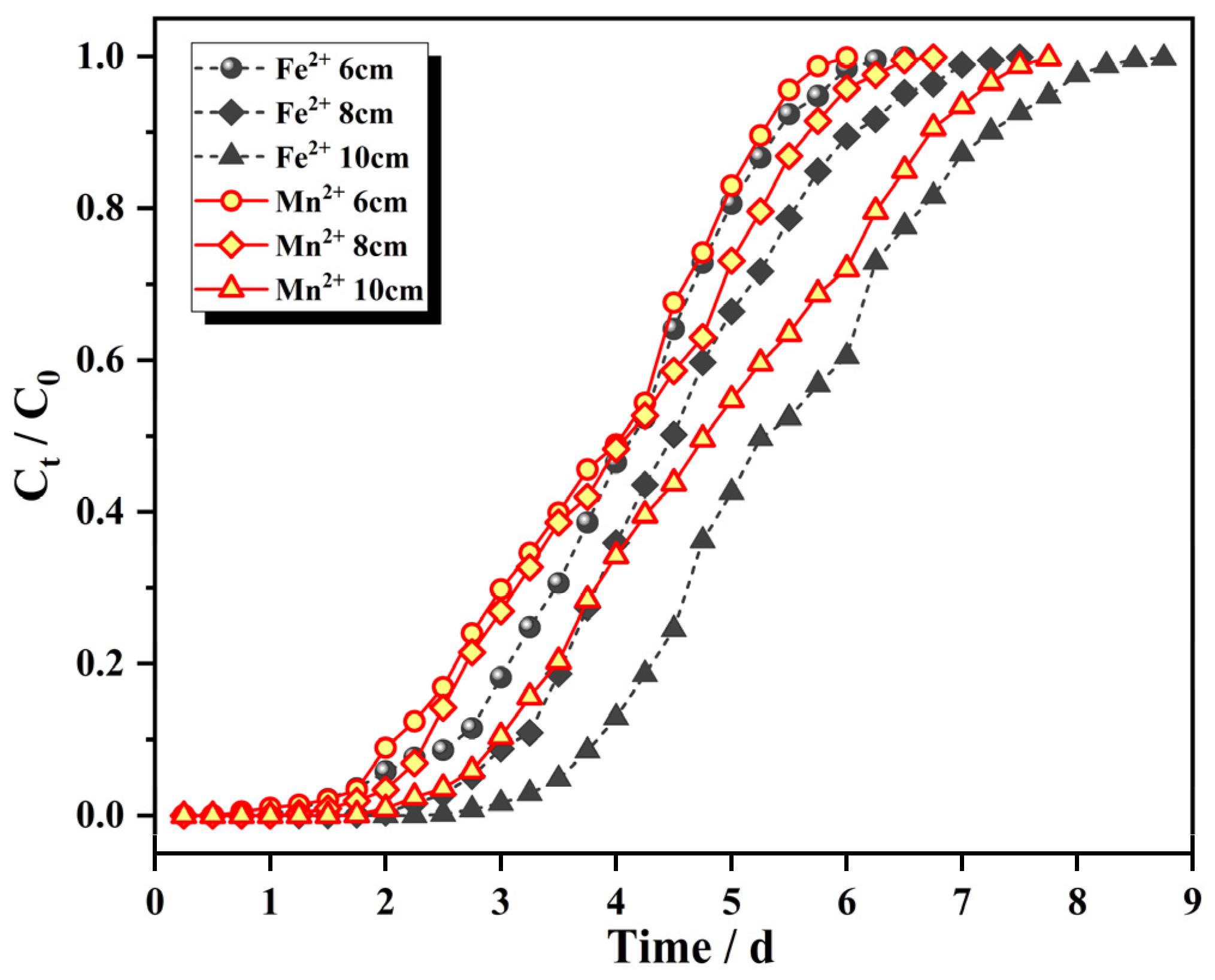
3.3.1. Effect of the Adsorption Column Height on the Penetration Curve
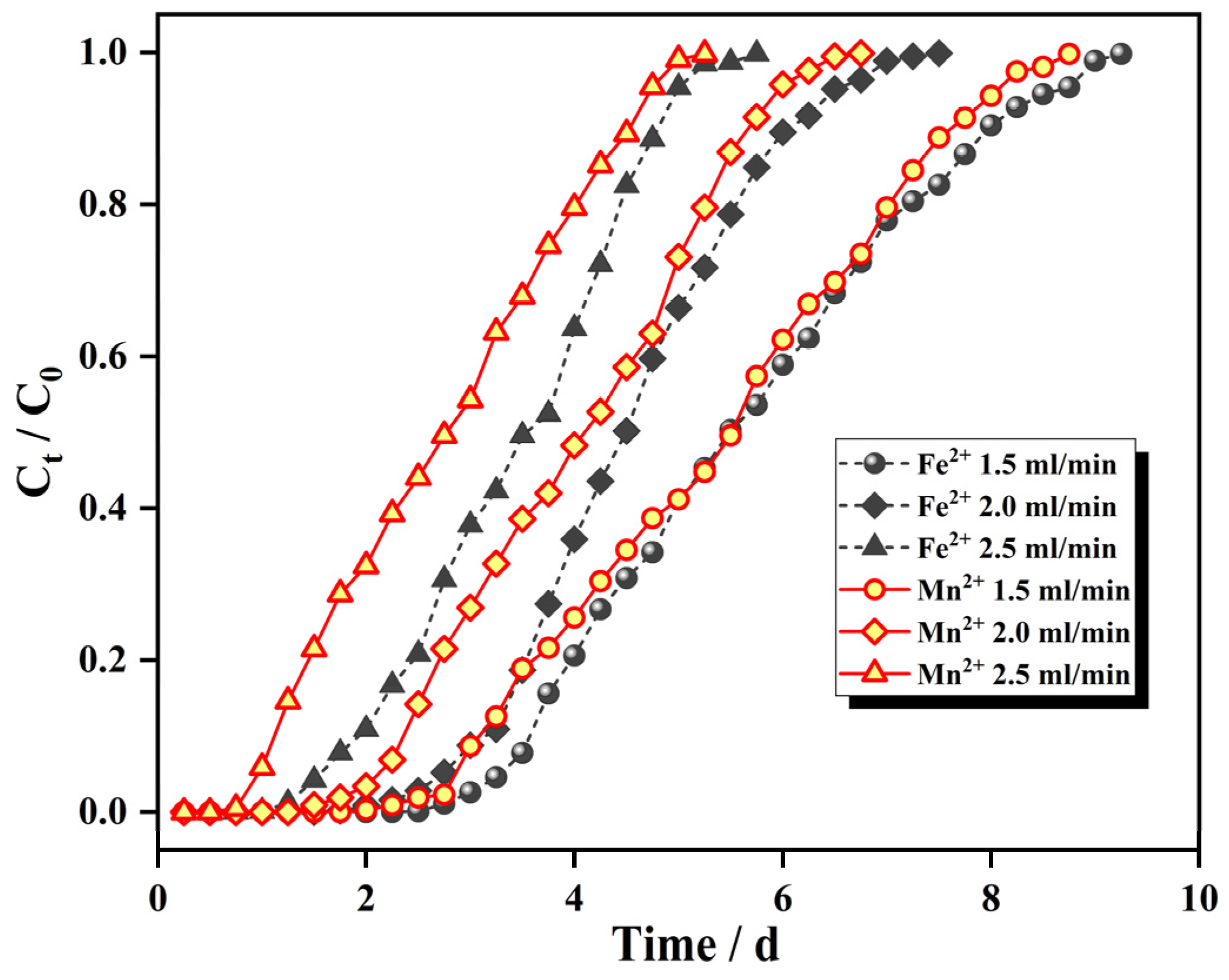
3.3.2. Effect of Inlet Flow Rate on the Penetration Curve
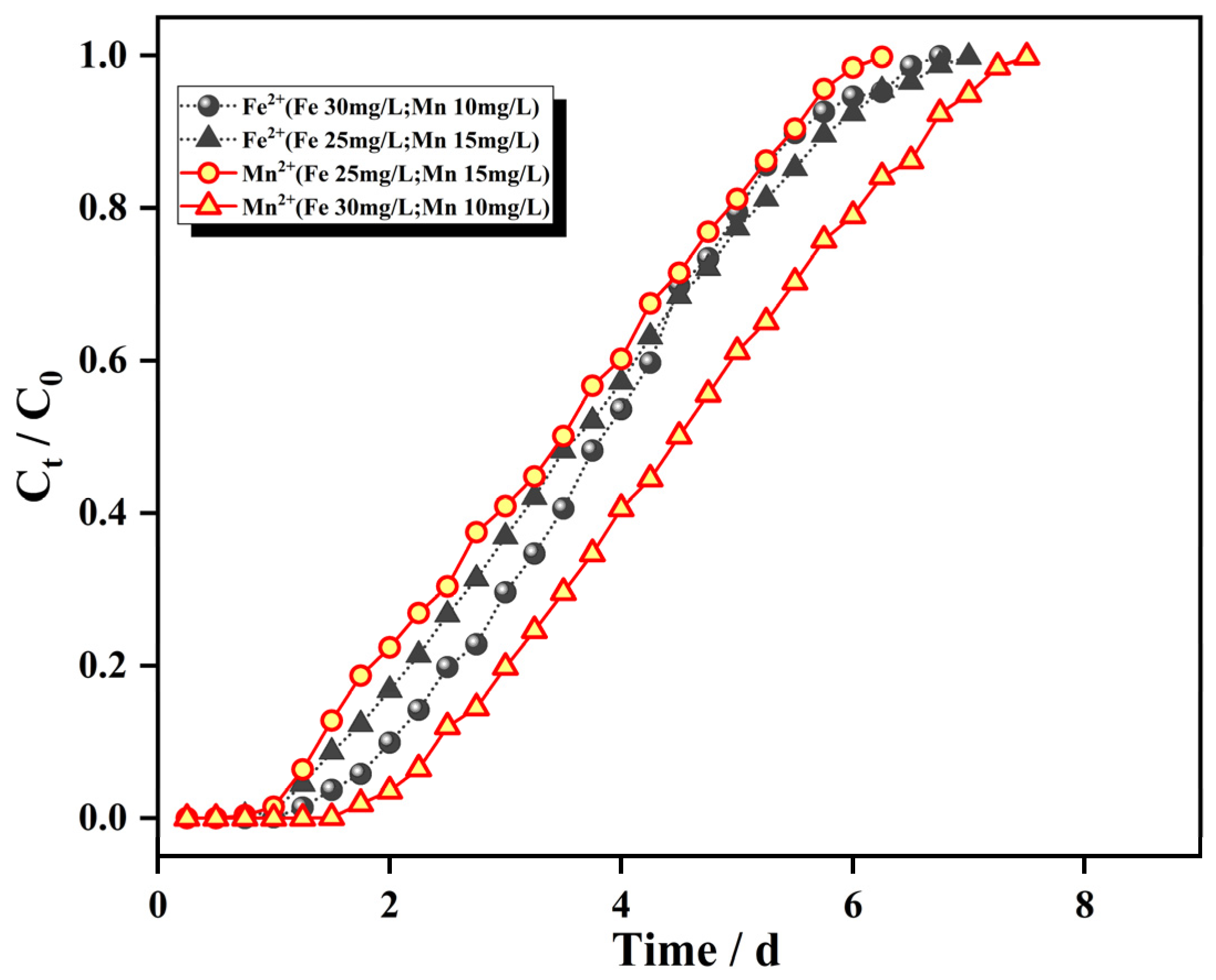
3.3.3. Influence of Inlet Water Concentration on the Penetration Curve
3.4. Adsorption Kinetic Analysis
3.5. Desorption and Reusability of Insoluble Humic Acid/Tourmaline Composite Particles
4. Conclusions
- (1)
- The optimal preparation conditions of the IHA/TM composite adsorbent were as follows: the mixing ratio of IHA and TM was 2:3, the mixing time was 18 h, the calcination temperature was 330 °C and the calcination time was 90 min. The prepared IHA/TM composite adsorbent not only has the multifunctional groups properties of organic insoluble humic acid, but also has the thermoelectric and spontaneous polarization effects of inorganic silicate tourmaline, and has a good adsorption efficiency for high-concentration iron- and manganese-contaminated mine wastewater.
- (2)
- The filling height of the IHA/TM in the dynamic column, the inlet water flow rate and the initial concentrations of Fe2+ and Mn2+ all affected the penetration process of the dynamic adsorption of the IHA/TM composite particles. With an increase in the column bed height, the decrease in the flow rate, and the decrease in the initial concentration of Fe2+ and Mn2+, the penetration curves of each ion moved from left to right and the adsorption equilibration time lengthened in turn. At a filling height of 8 cm and an inlet water flow rate of 2 mL/min, the maximum dynamic adsorption capacities of the dynamic column for Fe2+ and Mn2+ with the initial concentrations of 25 mg/L and 10 mg/L, respectively, were 2.348 mg/g and 1.025 mg/g.
- (3)
- For the dynamic adsorption of Fe2+ and Mn2+ by the IHA/TM composite adsorbent, the correlation coefficient R2 (>0.96) of the Thomas model after the adsorption of Fe2+ and Mn2+ and the theoretical unit adsorption capacity obtained from the analysis were closer to the actual value, so the Thomas model is more suitable for describing the dynamic adsorption process of the IHA/TM composite particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Yu, X.; Liu, L.; Yang, J.; Liu, S.; Zhang, T. Preparation, characterization serpentine-loaded hydroxyapatite and its simultaneous removal performance for fluoride, iron and manganese. RSC Adv. 2021, 11, 16201–16215. [Google Scholar] [CrossRef] [PubMed]
- Farrag, A.E.H.A.; Abdel, M.T.; Mohamed, A.M.G.; Saleem, S.S.; Fathy, M. Abu zenima synthetic zeolite for removing iron and manganese from assiut governorate groundwater, egypt. Appl. Water. Sci. 2017, 7, 3087–3094. [Google Scholar] [CrossRef] [Green Version]
- Akbar, N.A.; Aziz, H.A.; Adlan, M.N. Potential of high quality limestone as adsorbent for iron and manganese removal in groundwater. Appl. Mech. Mater. 2015, 802, 460–465. [Google Scholar] [CrossRef]
- Jusoh, A.B.; Cheng, W.H.; Low, W.M.; Nora’Aini, A.; Noor, M. Study on the removal of iron and manganese in groundwater by granular activated carbon. Desalination 2005, 182, 347–353. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, T. Drought over East Asia: A Review. J. Clim. 2015, 28, 3375–3399. [Google Scholar] [CrossRef]
- Sung, B.; Chu, K.; Yun, S. Removal of iron and manganese ions from abandoned neutral or alkaline mine drainage via ozone oxidation and micro-sand filtration: A pilot-scale operation. Desalin. Water Treat. 2015, 53, 2354–2362. [Google Scholar] [CrossRef]
- Melo, C.; Riella, H.; Kuhnen, N. Adsorption of iron and manganese from acid mine drainage using 4A-zeolite synthesised from waste with high kaolin concentrations. Int. J. Environ. Pollut. 2014, 56, 79–93. [Google Scholar] [CrossRef]
- Bai, Z. Engineering application of the removal of iron and manganese from mine water. Ind. Water Treat. 2014, 34, 2. [Google Scholar] [CrossRef]
- Ren, H.; Zhu, S.; Wang, X.; Liu, Y.; Cao, L. Study on Issues and Countermeasures in Coal Measures Mine Water Resources Exploitation and Utilization. China Coal Geol. 2020, 32, 9–20. [Google Scholar] [CrossRef]
- Ellis, D.; Bouchard, C.; Lantagne, G. Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination 2000, 130, 255–264. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Liu, L.; Liu, S. The performance of calcined serpentine to simultaneously remove fluoride, iron and manganese. Water Supply 2021, 12, 2750–2766. [Google Scholar] [CrossRef]
- Adekola, F.A.; Hodonou, D.S.S.; Adegoke, H.I. Thermodynamic and kinetic studies of biosorption of iron and manganese from aqueous medium using rice husk ash. Appl. Water Sci. 2016, 6, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Hu, J.; Zhang, J. Investigating the adsorption behavior and mechanisms of insoluble Humic acid/starch composite microspheres for metal ions from water. Colloids Surf. A 2020, 610, 125672. [Google Scholar] [CrossRef]
- Nassar, M. Adsorption of Fe3+ and Mn2+ from GroundWater onto Maize Cobs Using BatchAdsorber and Fixed Bed Column. Sep. Sci. Technol. 2006, 41, 943–959. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Sefa-Ntiri, B.; Von-Kiti, E.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C. Recent Progress in the Application of Hydroxyapatite for the Adsorption of Heavy Metals from Water Matrices. Materials 2021, 14, 6898. [Google Scholar] [CrossRef]
- Panek, R.; Medykowska, M.; Winiewska, M. Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and its Carbon Na-X(C) Composite. Materials 2021, 14, 2832. [Google Scholar] [CrossRef]
- Nakamura, T.; Kubo, T. Tourmaline group crystals reaction with water. Ferroelectrics. 2011, 137, 13–31. [Google Scholar] [CrossRef]
- Liao, G.; Zhao, W.; Li, Q.; Pang, Q.; Xu, Z. Novel Poly(acrylic acid)-modified Tourmaline/Silver Composites for Adsorption Removal of Cu(II) ions and Catalytic Reduction of Methylene Blue in Water. Chem. Lett. 2017, 46, 1631–1634. [Google Scholar] [CrossRef]
- Wei, Z.; Jin, C.; Wang, Y.; Lin, H.; Sythari, P.; Wei, D. Preparation of tourmaline bamboo-charcoal ceramic composites and its adsorption properties for Cr(VI). New Chem. Mater. 2020, 48, 279–283. [Google Scholar] [CrossRef]
- Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D.; Salvestrini, S. Sorption of organic pollutants by humic acids: A review. Molecules 2020, 25, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, V.; Iovino, P.; Capasso, S.; Trifuoggi, M.; Musmarra, D. Sorption of benzene derivatives onto insolubilized humic acids. Chem. Pap. 2018, 72, 929–935. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, F.; Zhou, Y.; Zhang, H.; Ke, X. Effect of humic acid load hydroxyapatite on the adsorption of Cd2+ in wastewater. Acta Sci. Circumstantiae 2019, 39, 4022–4030. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Wu, Z.; Zhang, L.; Zeng, G.; Zhou, C. Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloids Surf. A. 2013, 435, 85–90. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Yu, X.; Mkandawire, V.; Ma, J.; Li, X. Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles. Materials 2022, 15, 3130. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H. Competitive adsorption of Pb(II) and Zn(II) from aqueous solution by modified beer lees in a fixed bed column. Process Saf. Environ. Prot. 2017, 111, 263–269. [Google Scholar] [CrossRef]
- Emmanuel, D.; Siewe, J.; Njopwouo, D. A fixed-bed column for phosphate removal from aqueous solutions using an andosol-bagasse mixture. J. Environ. Manag. 2015, 151c, 450–460. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Suganya, E.; Selvaraju, N. Packed bed column investigation on hexavalent chromium adsorption using activated carbon prepared from swietenia mahogani fruit shells. Desalin. Water Treat. 2016, 57, 13048–13055. [Google Scholar] [CrossRef]
- Keshtkar, A.; Kafshgari, F.; Mousavian, M. Binary biosorption of uranium(vi) and nickel(ii) from aqueous solution by ca-pretreated cystoseira indica in a fixed-bed column. J. Radioanal. Nucl. Chem. 2012, 292, 501–512. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Li, Y. Adsorption of Pb(II) by tourmaline-montmorillonite composite in aqueous phase. J. Colloid Interface Sci. 2020, 575, 367–376. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Liang, J.; Fang, B.; Zhang, H.; Zhang, Y. Phase transformation and microstructural evolution of black tourmaline mineral powders during heating and cooling processes. Ceram. Int. 2018, 44, 13253–13258. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.; Liang, J.; Fang, B.; Zhang, H. Insight into the thermal behavior of tourmaline mineral. JOM 2019, 71, 2468–2474. [Google Scholar] [CrossRef]
- Seki, H.; Suzuki, A. Adsorption of heavy metal ions onto insolubilized humic acid. J. Colloid Interface Sci. 1995, 171, 490–494. [Google Scholar] [CrossRef]
- Zhao, W.; Ren, B.; Hursthouse, A.; Jiang, F. The adsorption of Mn(II) by insolubilized humic acid. Water Sci. Technol. 2020, 82, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhao, X.; Ding, J.; Li, G.; Liang, H. Study on removal of manganese from modified silica alumina sand. Water Wastes Eng. 2020, 56, 10–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Zhao, J.; Jiang, Z.; Cao, B.; Song, Q.; Su, G.; Li, M. Dynamic adsorption of iron ions in groundwater by rice husk ash. J. Northeast. Agric. Univ. 2015, 46, 68–73. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Bai, F.; Chen, M. Removal of Fe(III) and Mn(II) from Aqueous Solutions Using Modified Fly Ash. J. Agric. Environ. Sci. 2013, 32, 635–640. [Google Scholar] [CrossRef]
- Zheng, R. Adsorption Characteristic Study of Fe(II) and Mn(II) from Groundwater Using Biosorbents. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2013. [Google Scholar]
- Yang, L.; Wei, Z.; Zhong, W.; Cui, J.; Wei, W. Modifying hydroxyapatite nanoparticles with humic acid for highly efficient removal of Cu(II) from aqueous solution. Colloids Surf. A Physicochem. Eng. Aspects. 2016, 490, 9–21. [Google Scholar] [CrossRef]
- Xue, G.; Luo, X.; Srinivasakannan, C.; Zheng, L.; Miao, Y.; Duan, X. Effective removal of organic dye and heavy metal from wastewater by tourmaline/graphene oxide composite nano material. Mater. Res. Express 2019, 6, 115618. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Shanthakumar, S. Column adsorption studies on nickel and cobalt removal from aqueous solution using native and biochar form of Tectona grandis. Environ. Prog. Sustain. Energy. 2017, 36, 1030–1038. [Google Scholar] [CrossRef]
- Du, Z.; Jia, M.; Men, J. Dynamic performance of Cs+ adsorption from aqueous solution with polyacrylonitrile-potassium titanium hexacyanoferrate(II) spherical composite adsorbent. J. Cent. South Univ. (Sci. Technol.) 2014, 45, 4100–4104. [Google Scholar]
- Kizito, S.; Wu, S.; Wandera, S.M.; Guo, L.; Dong, R. Evaluation of ammonium adsorption in biochar-fixed beds for treatment of anaerobically digested swine slurry: Experimental optimization and modeling. Sci. Total Environ. 2016, 563–564, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Bajpai, A.K. Dynamic Column Adsorption Studies of Toxic Cr(VI) Ions onto Iron Oxide Loaded Gelatin Nanoparticles. J. Dispers. Sci. Technol. 2011, 32, 1353–1362. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of lead(II) from aqueous solution using date seed-derived biochar: Batch and column studies. Appl. Water Sci. 2018, 8, 181. [Google Scholar] [CrossRef] [Green Version]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Jellali, S.; Diamantopoulos, E.; Haddad, K.; Anane, M.; Durner, W.; Mlayah, A. Lead removal from aqueous solutions by raw sawdust and magnesium pretreated biochar: Experimental investigations and numerical modelling. J. Environ. Manag. 2016, 180, 439–449. [Google Scholar] [CrossRef]

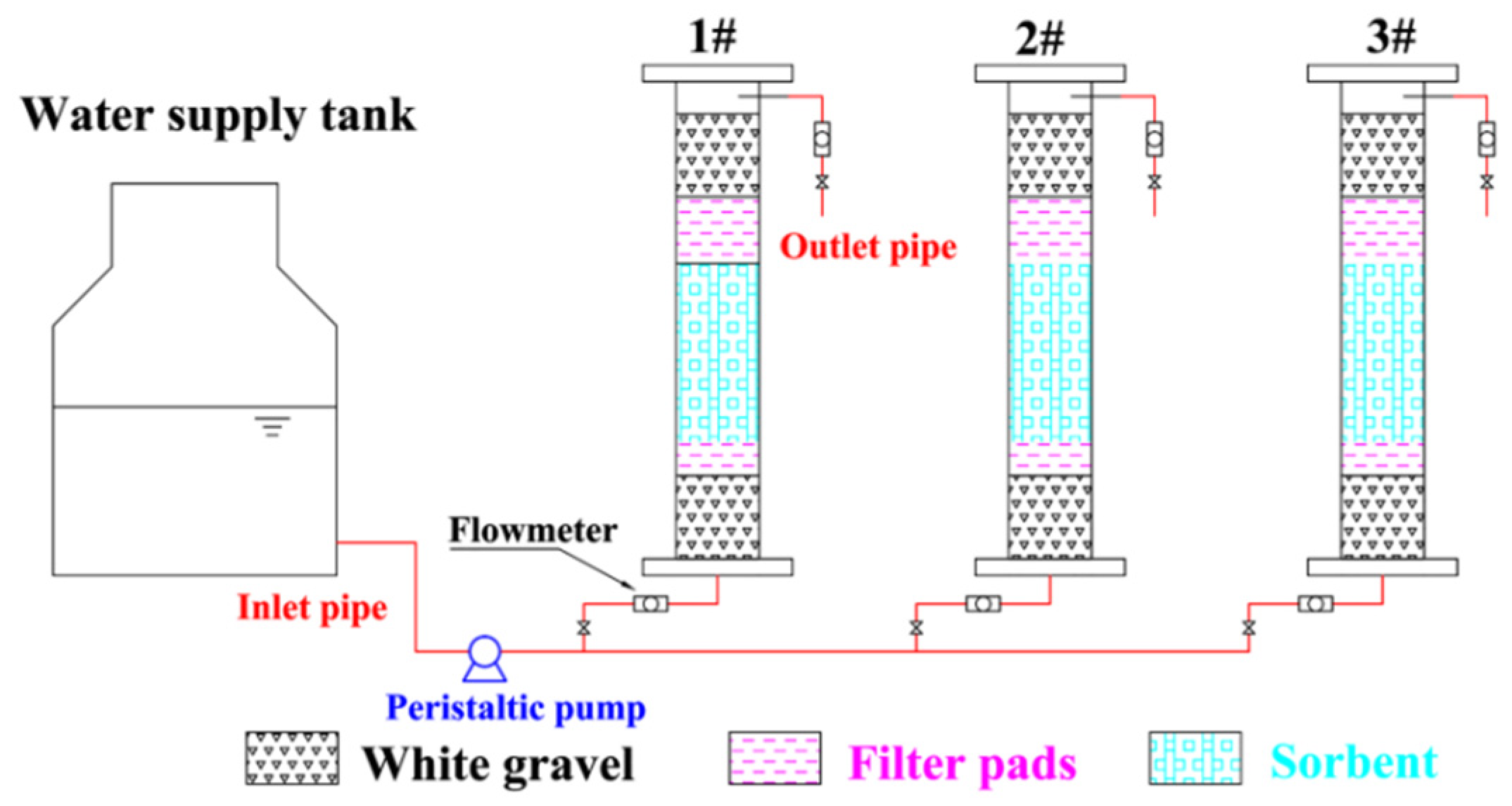



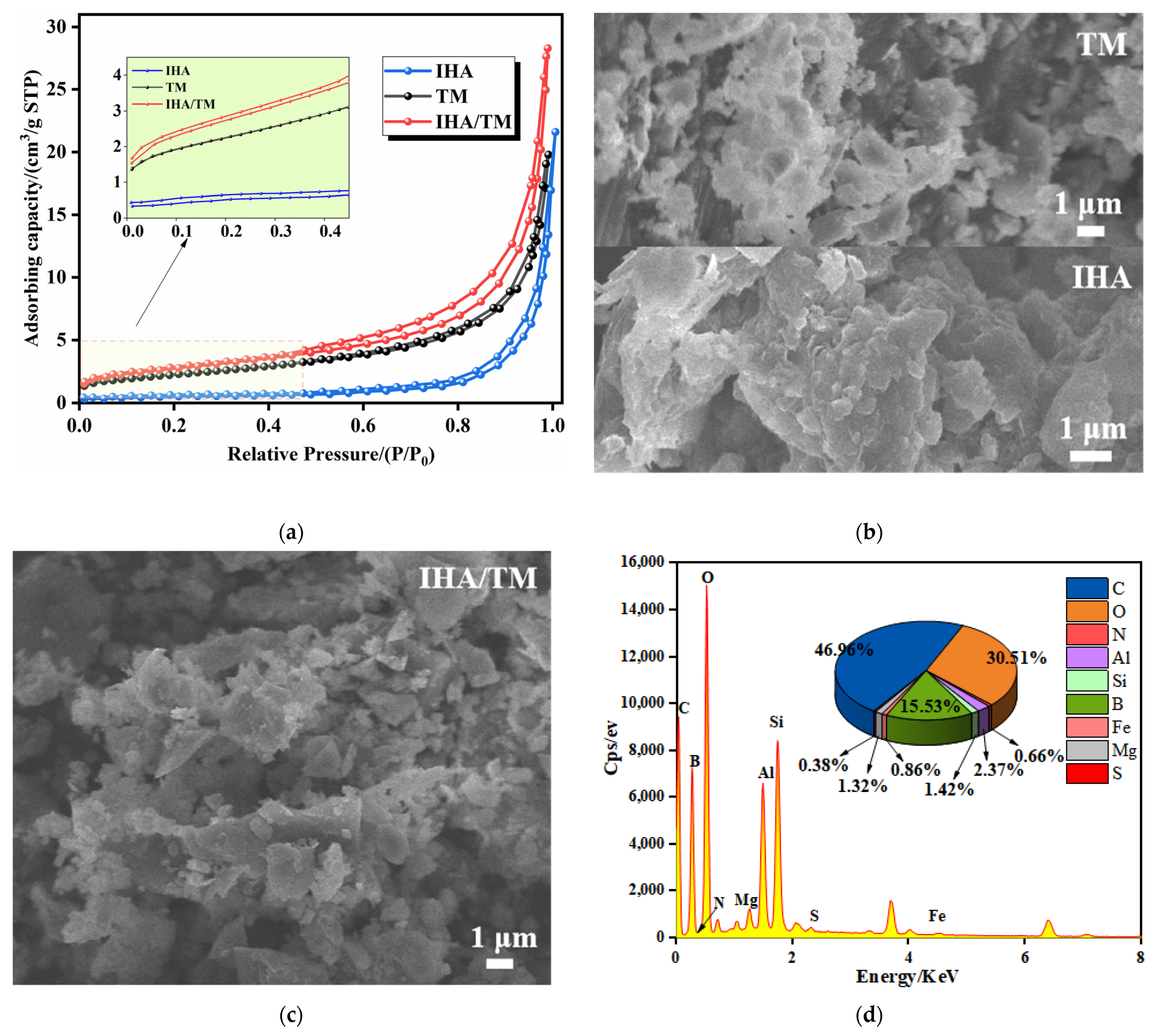
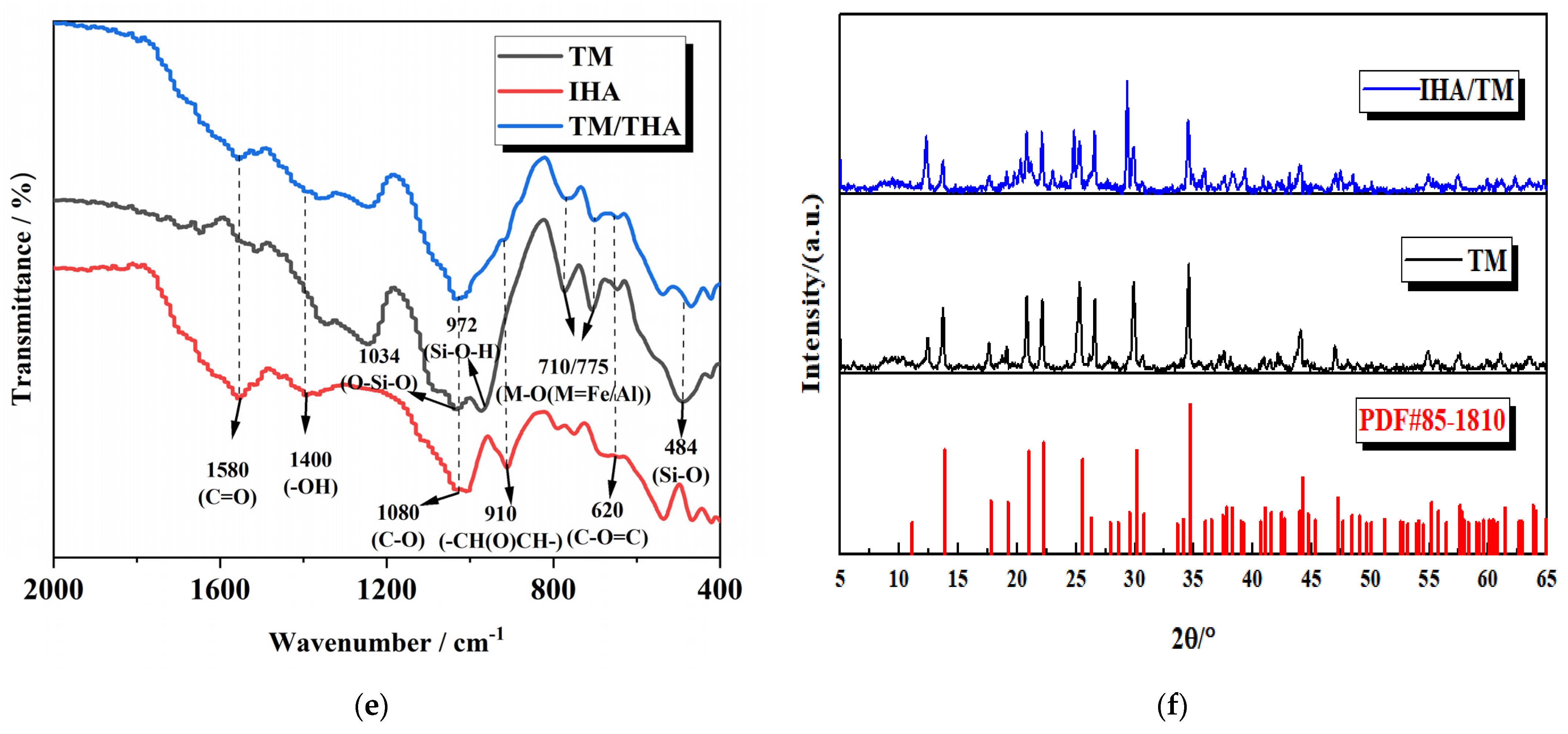
| Adsorbent Material | pH | qe of Fe2+ (mg/g) | qe of Mn2+ (mg/g) | References |
|---|---|---|---|---|
| Modified silica alumina sand | 6.5 | 0.319 | [35] | |
| Rice husk ash | 5.0 | 4.19 | [36] | |
| Modified fly ash | 3.0 | 4.8 | 0.81 | [37] |
| Corn cob | 5.5 | 2.5 | 2.3 | [14] |
| Y-type zeolite | 6.5 | 0.023 | 0.015 | [15] |
| Yeast | 5–6 | 4.46 | 2.23 | [38] |
| IHA/TM composite particles | 6.0 | 5.318 | 3.106 | This research |
| Ions | H (cm) | Q (mL/min) | C (mg/L) | Tb (d) | Te (d) | qtotal (mg) | qe (mg/g) |
|---|---|---|---|---|---|---|---|
| Fe2+ | 6 | 2 | 25 | 2.0 | 5.75 | 196.24 | 2.726 |
| 8 | 2 | 25 | 2.75 | 6.5 | 225.38 | 2.348 | |
| 10 | 2 | 25 | 3.5 | 7.75 | 264.49 | 2.204 | |
| 8 | 1.5 | 25 | 3.5 | 7.75 | 222.77 | 2.321 | |
| 8 | 2.5 | 25 | 2.25 | 6.25 | 214.65 | 2.236 | |
| 8 | 2 | 30 | 1.75 | 6.25 | 257.04 | 2.678 | |
| Mn2+ | 6 | 2 | 10 | 2.0 | 5.5 | 75.72 | 1.052 |
| 8 | 2 | 10 | 2.25 | 6.0 | 95.66 | 1.025 | |
| 10 | 2 | 10 | 2.75 | 7.25 | 100.85 | 0.840 | |
| 8 | 1.5 | 10 | 3 | 7.5 | 83.12 | 0.866 | |
| 8 | 2.5 | 10 | 1.5 | 5.75 | 81.65 | 0.851 | |
| 8 | 2 | 15 | 1.25 | 5.75 | 118.74 | 1.237 |
| Test Parameters | Adams–Bohart Model | Thomas Model | Yoon–Nelson Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C0/ (mg/L) | Q/ (mL/min) | H/ cm | KAB/ ×10−5 | N0/ ×104 mg/L | R2 | KTh | q0/ (mg/g) | R2 | KYN× 10−2/ min−1 | τ/ min | qYN/ (mg/g) | R2 |
| 25 | 2 | 6 | 2.582 | 6.529 | 0.837 | 0.052 | 2.477 | 0.983 | 0.129 | 5572 | 3.869 | 0.955 |
| 25 | 2 | 8 | 2.432 | 5.607 | 0.7744 | 0.050 | 2.184 | 0.989 | 0.126 | 6512 | 3.392 | 0.970 |
| 25 | 2 | 10 | 2.158 | 5.292 | 0.749 | 0.044 | 2.126 | 0.987 | 0.111 | 7975 | 3.323 | 0.967 |
| 25 | 1.5 | 8 | 1.773 | 5.231 | 0.645 | 0.035 | 2.077 | 0.964 | 0.088 | 8311 | 3.247 | 0.903 |
| 25 | 2.5 | 8 | 2.815 | 5.360 | 0.707 | 0.057 | 2.029 | 0.983 | 0.142 | 4870 | 3.171 | 0.932 |
| 30 | 2 | 8 | 1.781 | 5.960 | 0.690 | 0.037 | 2.200 | 0.976 | 0.111 | 5501 | 3.458 | 0.917 |
| Test Parameters | Adams–Bohart Model | Thomas Model | Yoon–Nelson Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C0/ (mg/L) | Q/ (mL/min) | H/ cm | KAB/ ×10−5 | N0/ ×104 mg/L | R2 | KTh | q0/ (mg/g) | R2 | KYN× 10−2/ min−1 | τ/ min | qYN/ (mg/g) | R2 |
| 10 | 2 | 6 | 6.586 | 2.436 | 0.862 | 0.123 | 0.876 | 0.987 | 0.123 | 5258 | 1.461 | 0.928 |
| 10 | 2 | 8 | 5.919 | 2.258 | 0.665 | 0.112 | 0.871 | 0.992 | 0.119 | 5740 | 1.196 | 0.937 |
| 10 | 2 | 10 | 5.554 | 1.871 | 0.718 | 0.105 | 0.746 | 0.984 | 0.105 | 6995 | 1.166 | 0.924 |
| 10 | 1.5 | 8 | 4.463 | 1.987 | 0.728 | 0.087 | 0.759 | 0.987 | 0.087 | 7795 | 1.186 | 0.930 |
| 10 | 2.5 | 8 | 6.045 | 1.958 | 0.623 | 0.127 | 0.660 | 0.971 | 0.126 | 3958 | 1.031 | 0.901 |
| 15 | 2 | 8 | 3.155 | 2.791 | 0.681 | 0.066 | 0.986 | 0.982 | 0.099 | 4928 | 1.540 | 0.912 |
| Cycles | Fe2+ | Mn2+ | ||
|---|---|---|---|---|
| Adsorption Capacity (mg/g) | Regeneration Rate (%) | Adsorption Capacity (mg/g) | Regeneration Rate (%) | |
| 1 | 2.310 | 98.4 | 1.002 | 97.8 |
| 2 | 2.270 | 96.7 | 0.980 | 95.6 |
| 3 | 2.188 | 93.2 | 0.951 | 92.8 |
| 4 | 2.146 | 91.4 | 0.916 | 89.4 |
| 5 | 2.073 | 88.3 | 0.872 | 85.1 |
| Cycles | C | O | N | Al | Si | B | Fe | Mg | S | Mn |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46.13 | 30.95 | 0.74 | 2.28 | 1.54 | 15.57 | 0.94 | 1.43 | 0.37 | 0.05 |
| 2 | 46.88 | 30.48 | 0.77 | 2.37 | 1.49 | 15.26 | 1.02 | 1.27 | 0.36 | 0.10 |
| 3 | 46.29 | 31.64 | 0.79 | 2.18 | 1.38 | 14.97 | 1.17 | 1.02 | 0.38 | 0.18 |
| 4 | 47.32 | 30.65 | 0.80 | 2.26 | 1.11 | 15.08 | 1.24 | 0.91 | 0.35 | 0.28 |
| 5 | 46.91 | 30.86 | 0.76 | 2.08 | 0.97 | 15.47 | 1.38 | 0.86 | 0.36 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Ma, J.; Yu, X.; Zhang, T.; Mkandawire, V.; Li, X. Dynamic Adsorption Properties of Insoluble Humic Acid/Tourmaline Composite Particles for Iron and Manganese in Mine Wastewater. Materials 2022, 15, 4338. https://doi.org/10.3390/ma15124338
Liu L, Ma J, Yu X, Zhang T, Mkandawire V, Li X. Dynamic Adsorption Properties of Insoluble Humic Acid/Tourmaline Composite Particles for Iron and Manganese in Mine Wastewater. Materials. 2022; 15(12):4338. https://doi.org/10.3390/ma15124338
Chicago/Turabian StyleLiu, Ling, Jiadi Ma, Xiaowan Yu, Tianyi Zhang, Vitumbiko Mkandawire, and Xilin Li. 2022. "Dynamic Adsorption Properties of Insoluble Humic Acid/Tourmaline Composite Particles for Iron and Manganese in Mine Wastewater" Materials 15, no. 12: 4338. https://doi.org/10.3390/ma15124338
APA StyleLiu, L., Ma, J., Yu, X., Zhang, T., Mkandawire, V., & Li, X. (2022). Dynamic Adsorption Properties of Insoluble Humic Acid/Tourmaline Composite Particles for Iron and Manganese in Mine Wastewater. Materials, 15(12), 4338. https://doi.org/10.3390/ma15124338







