Distribution Law of Corrosion Products in a Marine Chloride Environment
Abstract
:1. Introduction
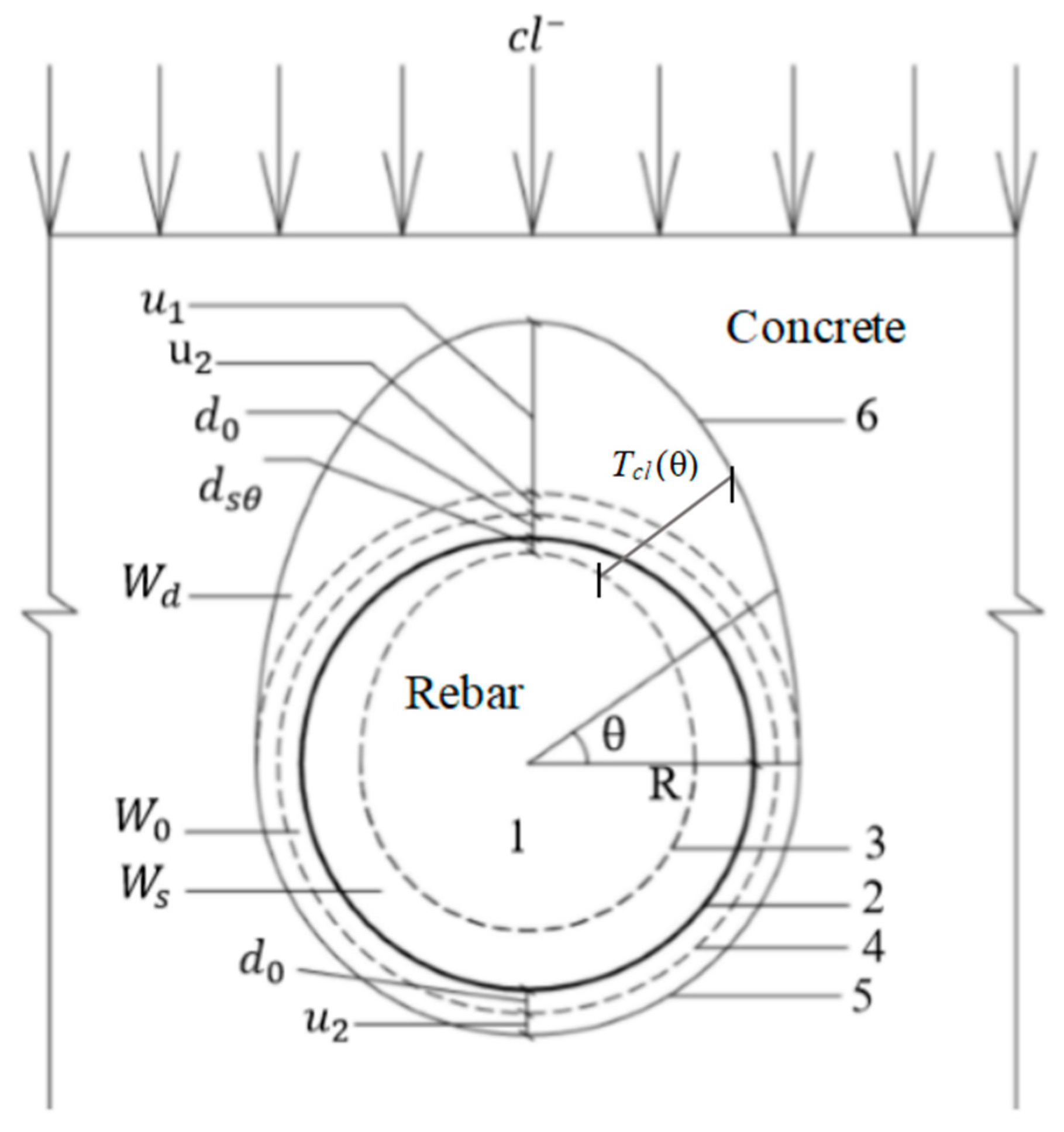
2. Distribution of Corrosion Products and Radial Displacement of Reinforced Concrete
2.1. Relationship between the Amount of Corrosion and Thickness of the Porous Zone
2.2. Corrosion Rate of Reinforcement upon Cracking of the Concrete Cover
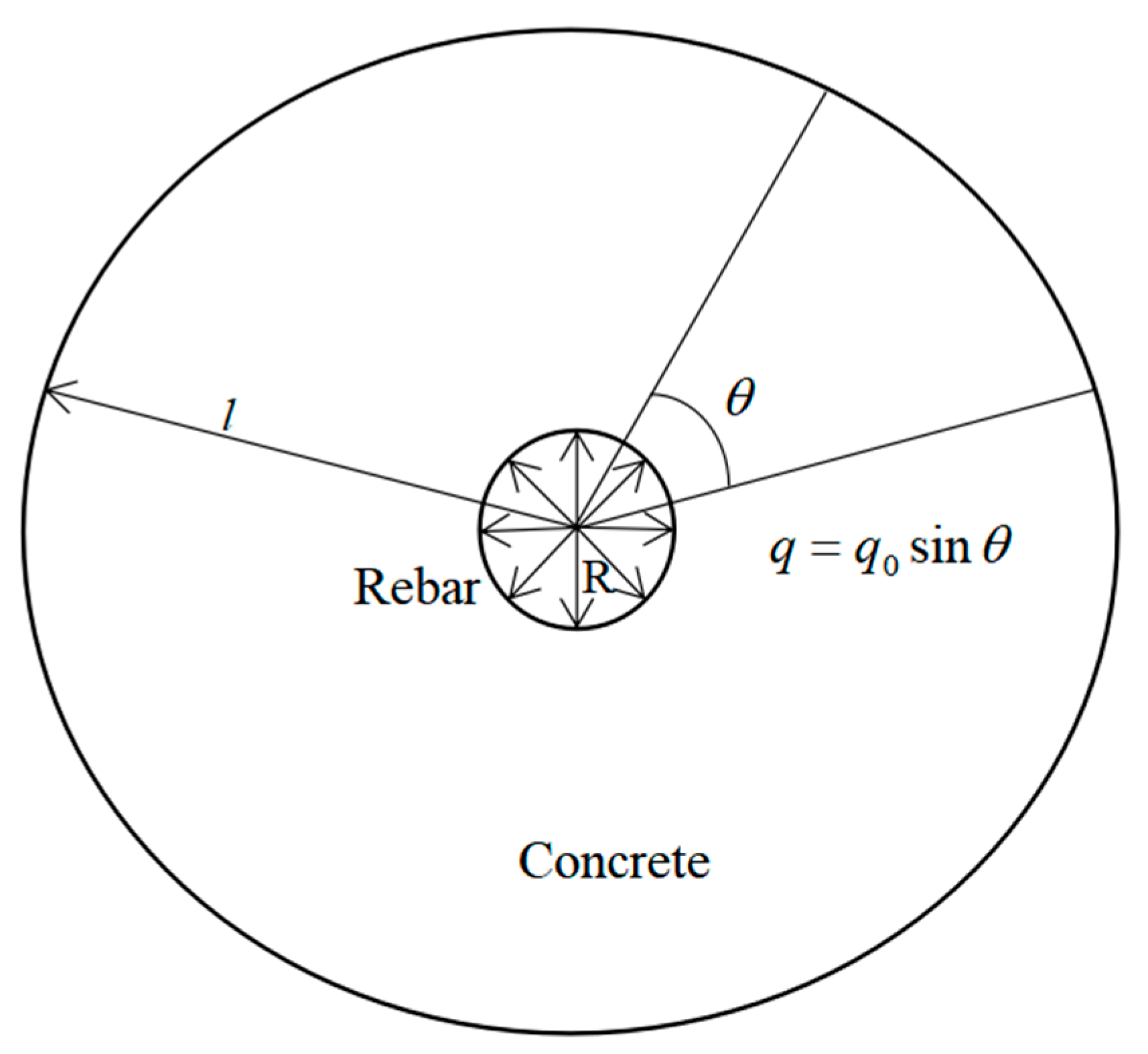
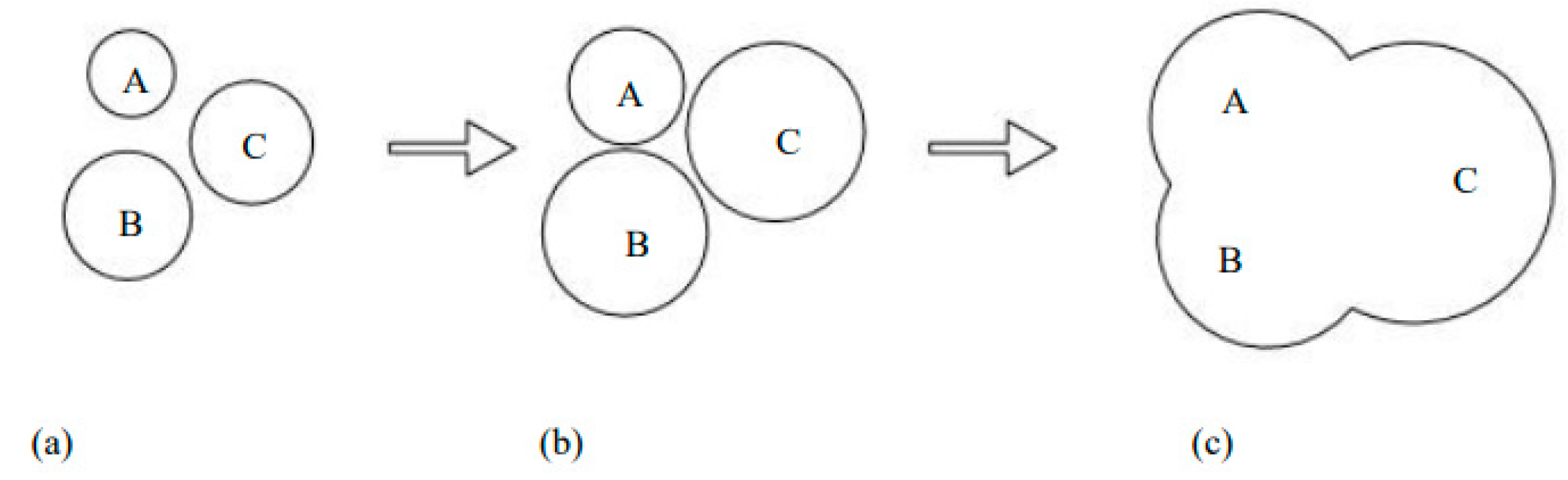
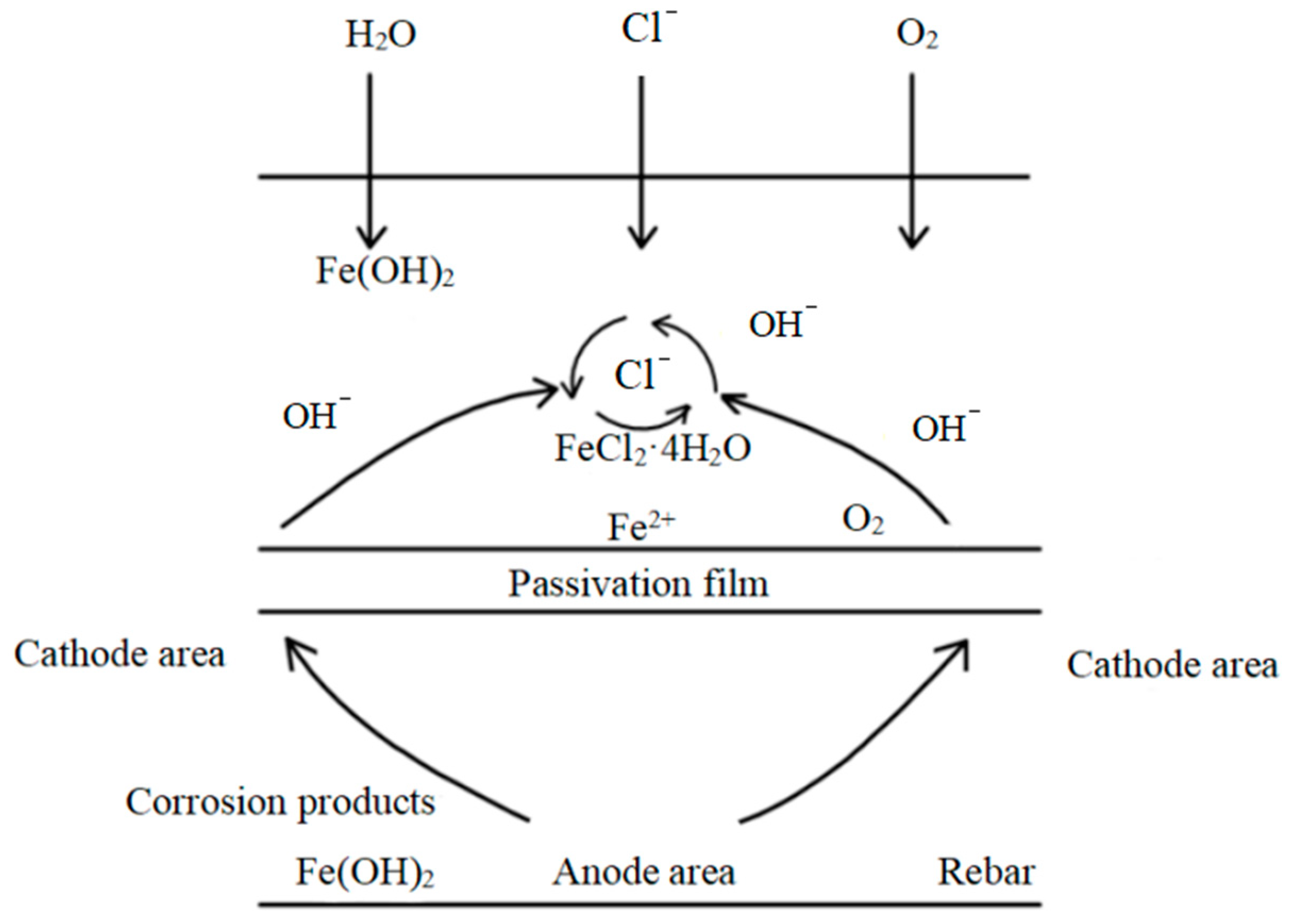
2.3. Distribution of Corrosion Products of Steel Bars
2.4. Radial Displacement of Corroded Reinforced Concrete
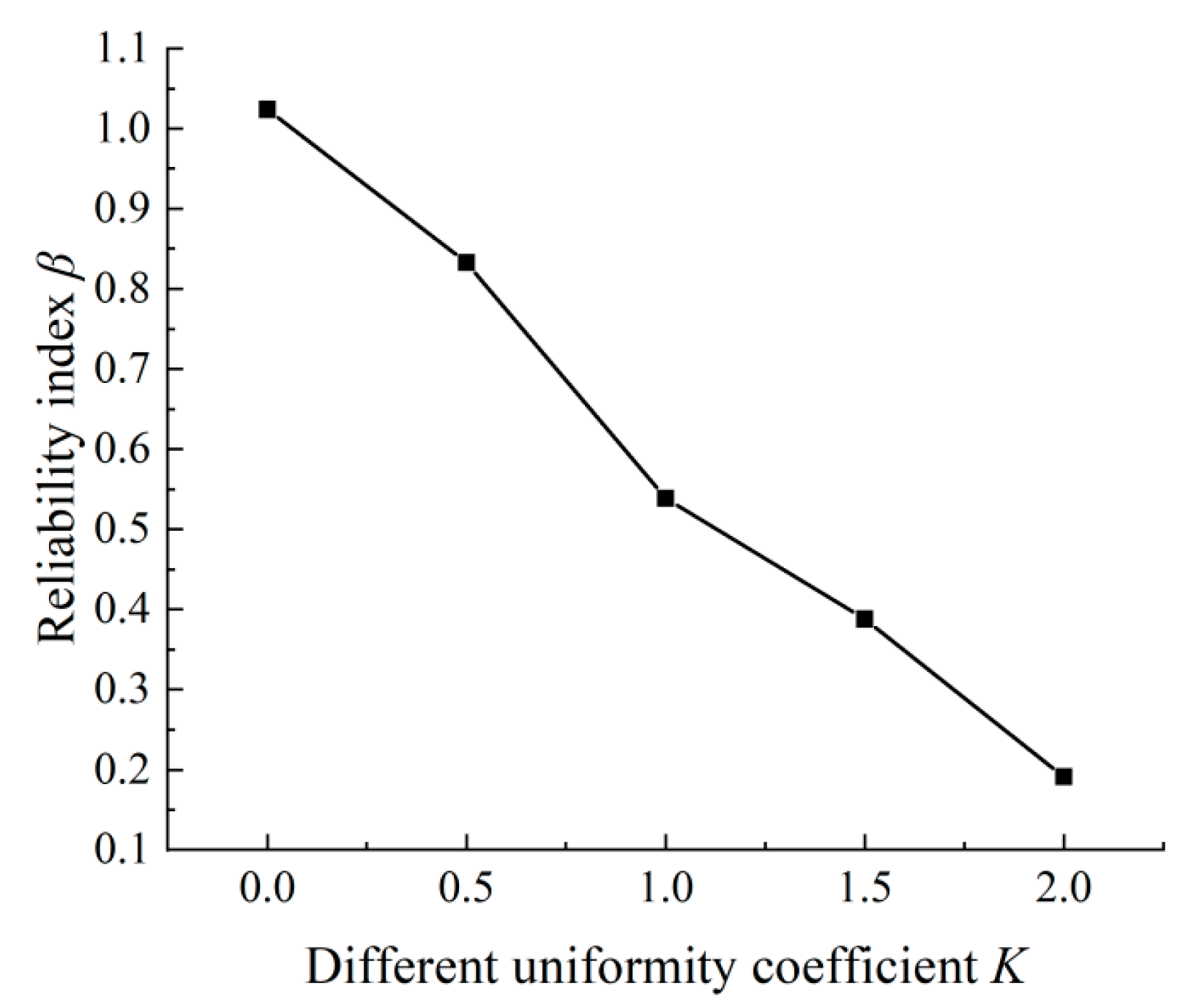
3. Reliability Analysis of Concrete Cracking
4. Experimental Section
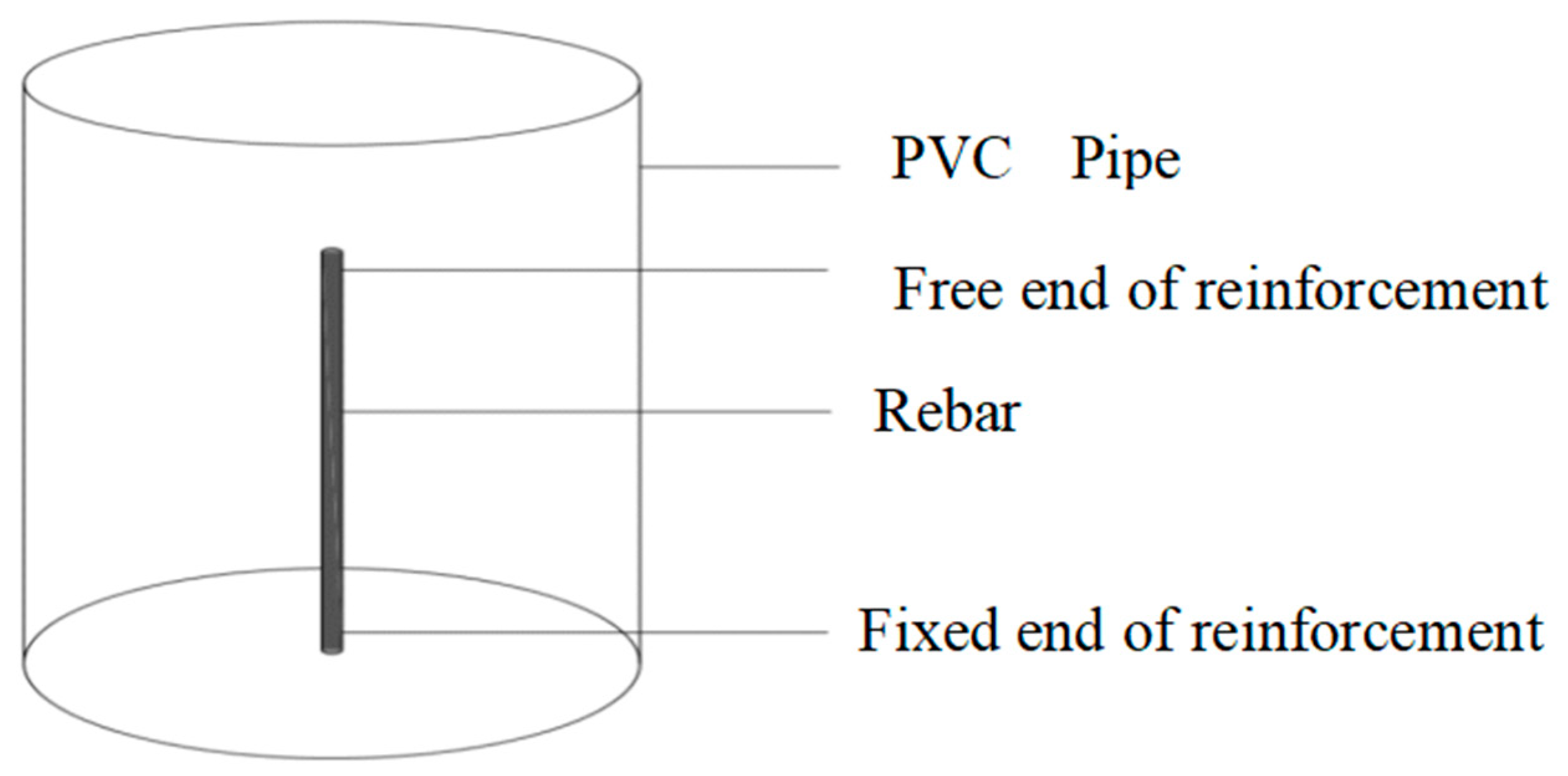
4.1. Preparation of Materials and Specimens
4.2. Corrosion Acceleration
4.3. Experimental Results and Analysis
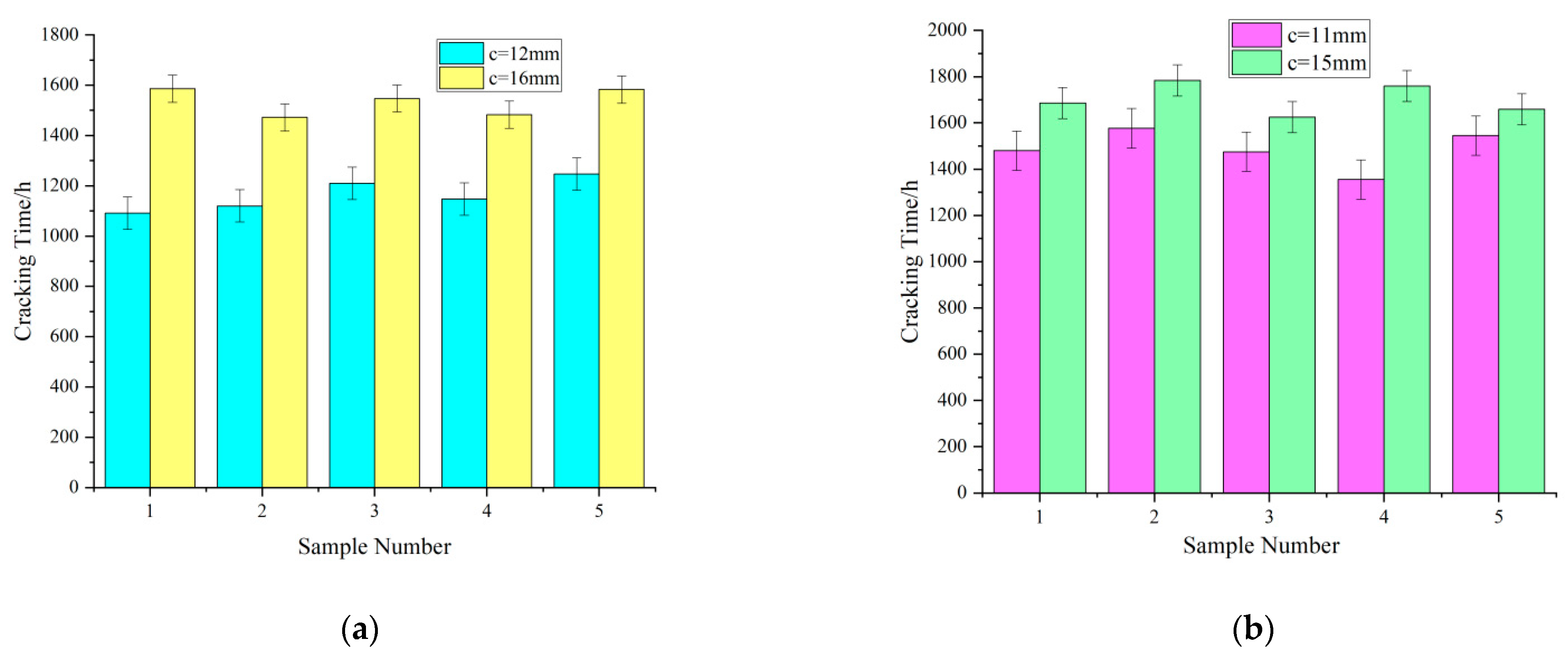
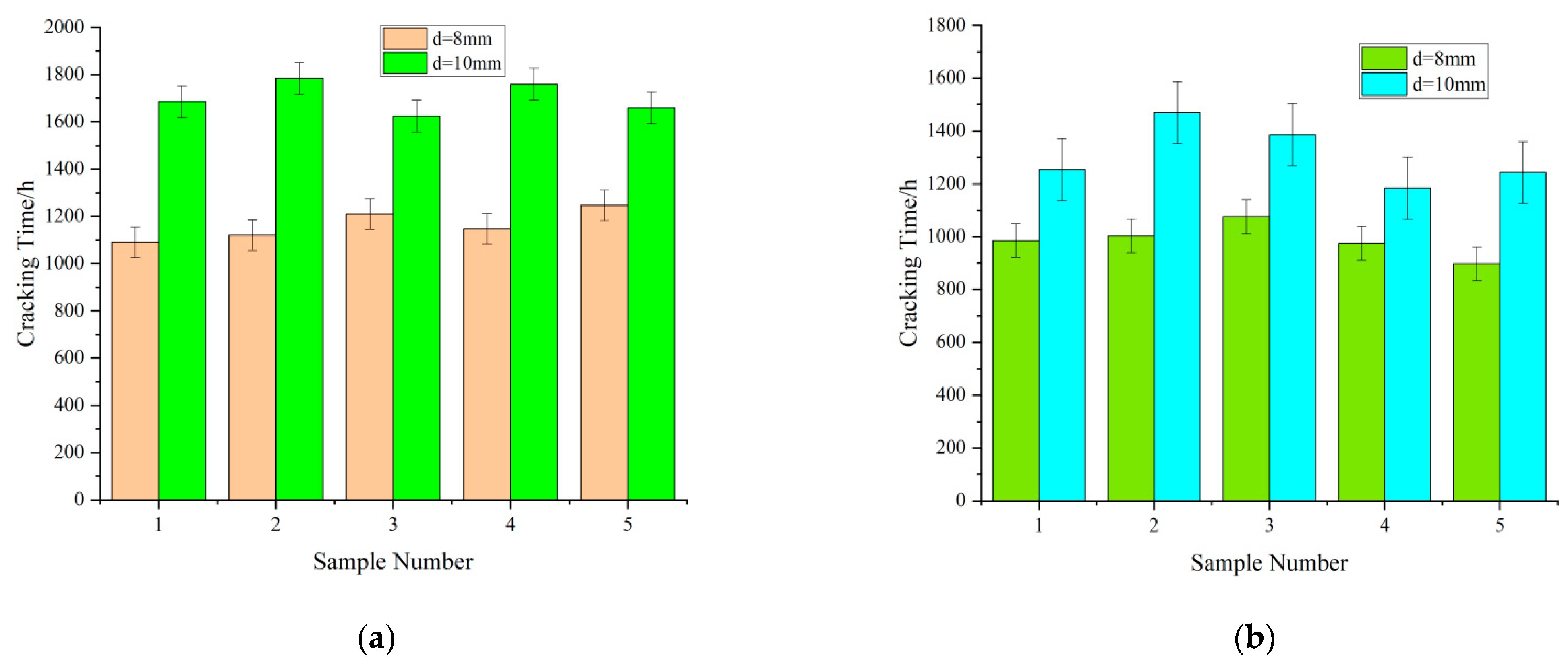
- Influence of the thickness of the concrete cover on the cracking time
- Influence of the reinforcement diameter on the cracking time
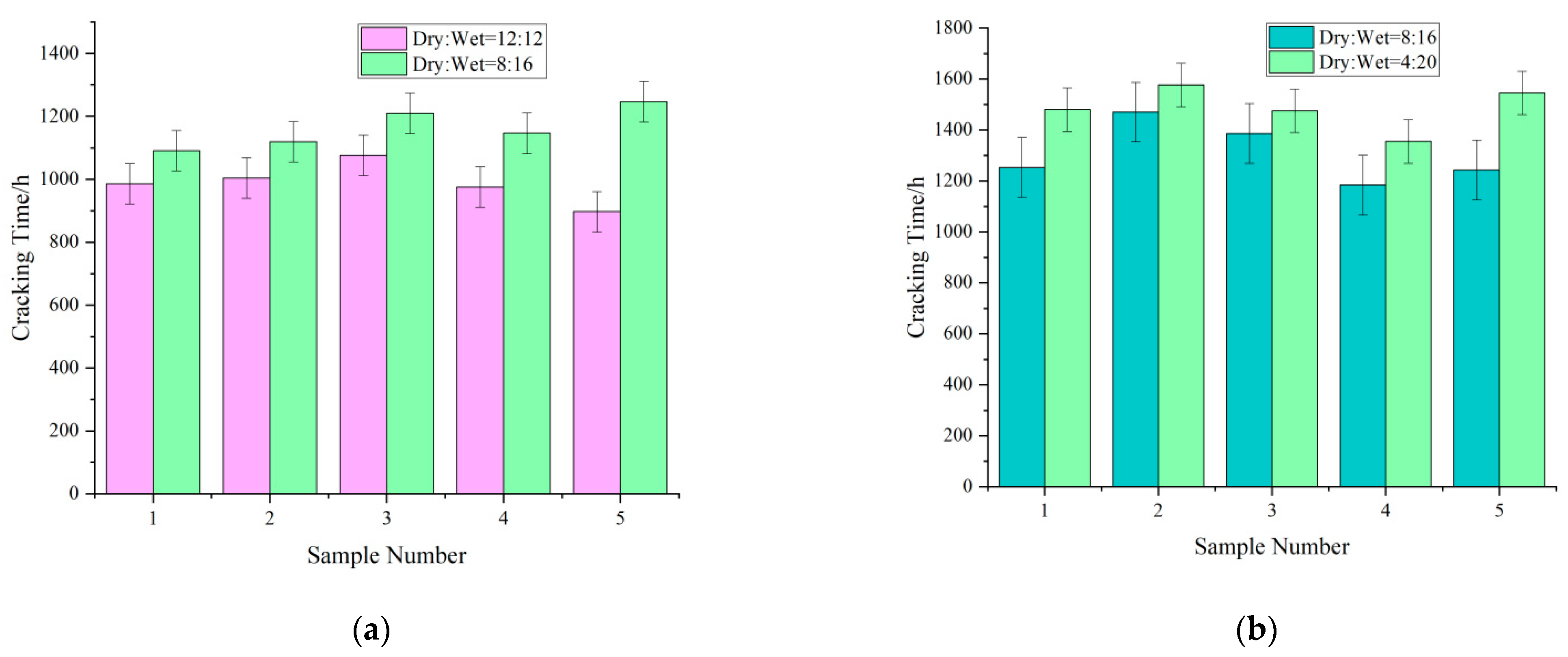
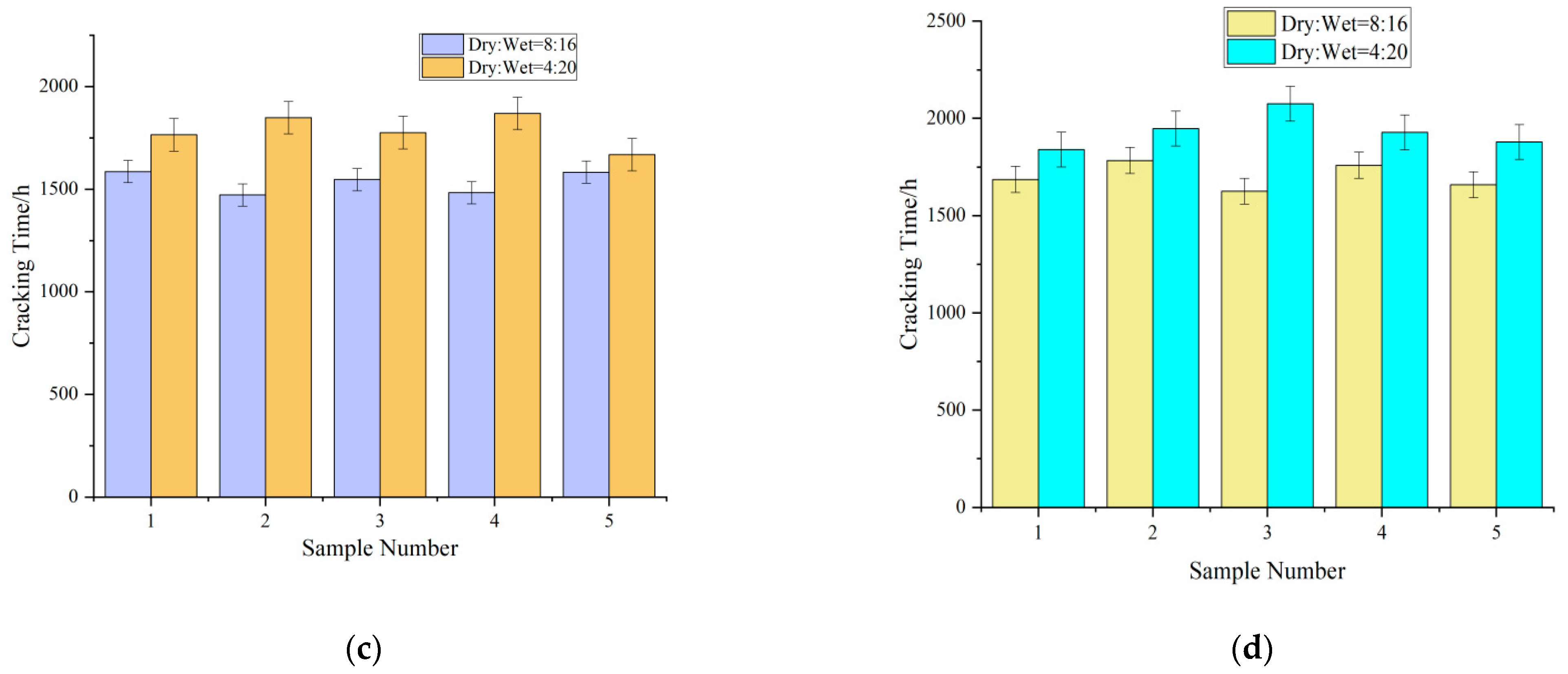
- Effect of the ratio of dry–wet cycles on the cracking time
5. Macroscopic Characteristics of Samples under an Optical Microscope
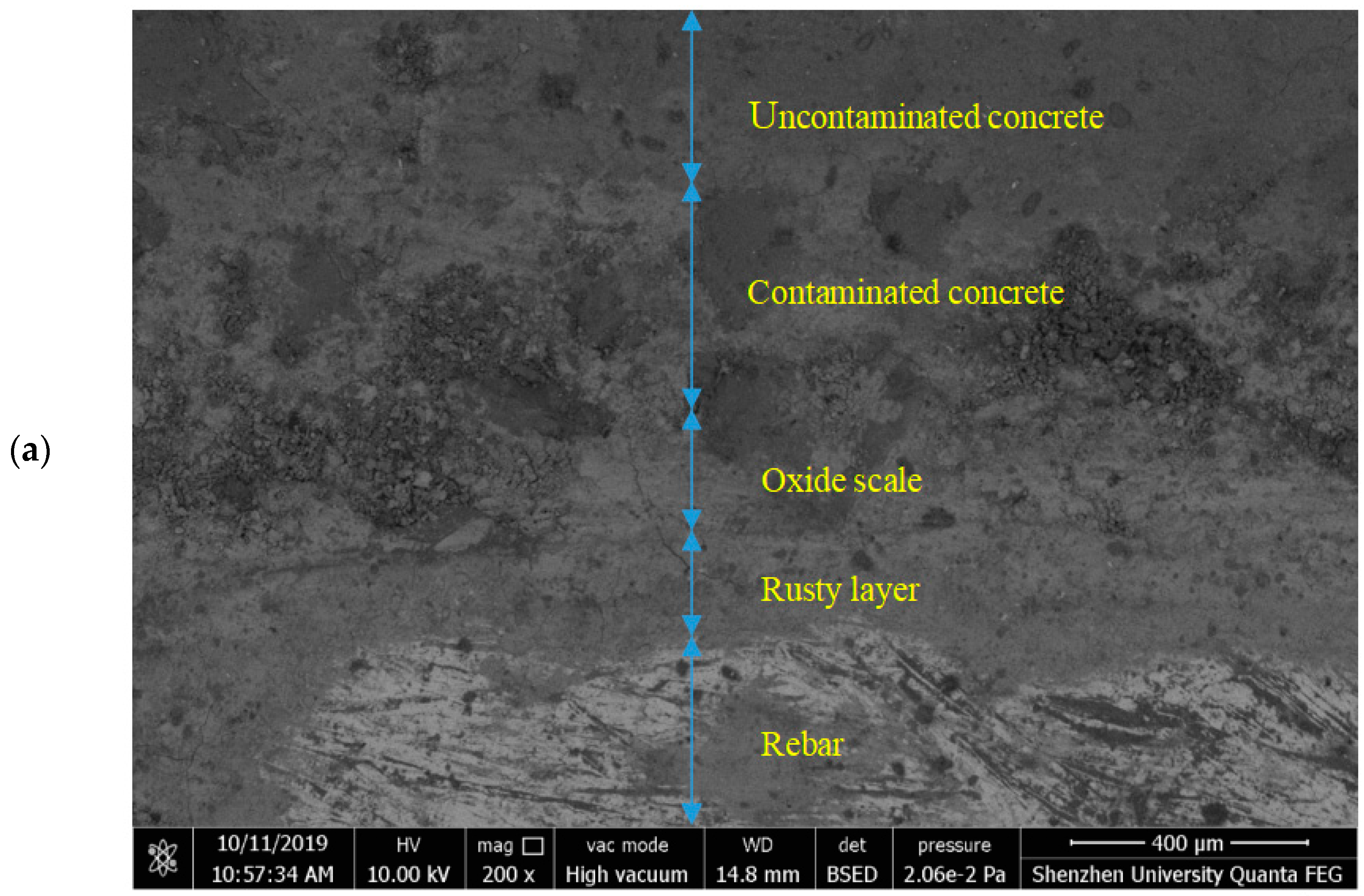
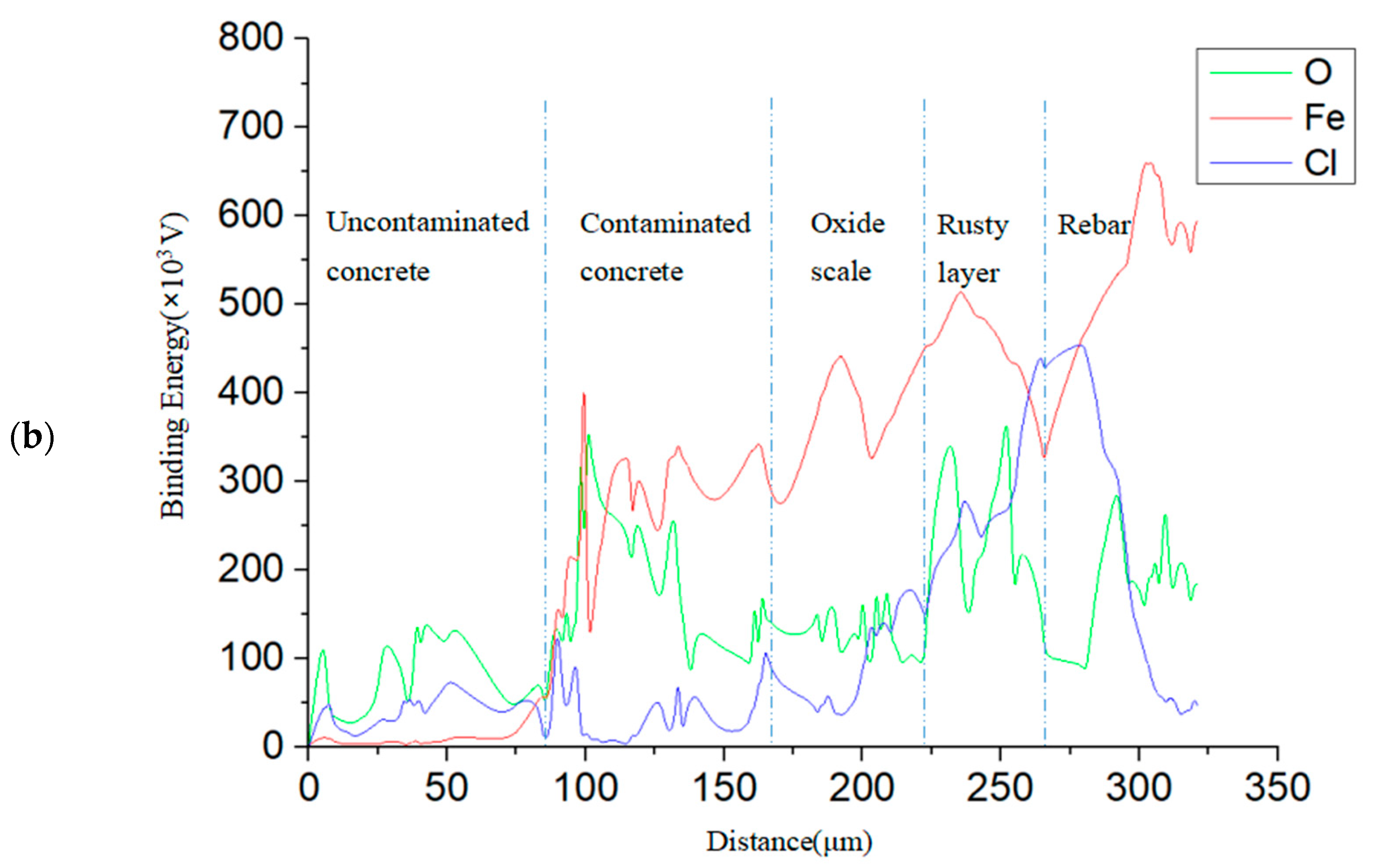
5.1. Distribution of Corrosion Products in Cross-Sections of the Samples
5.2. Rust Pits on the Surface of Corroded Reinforcement
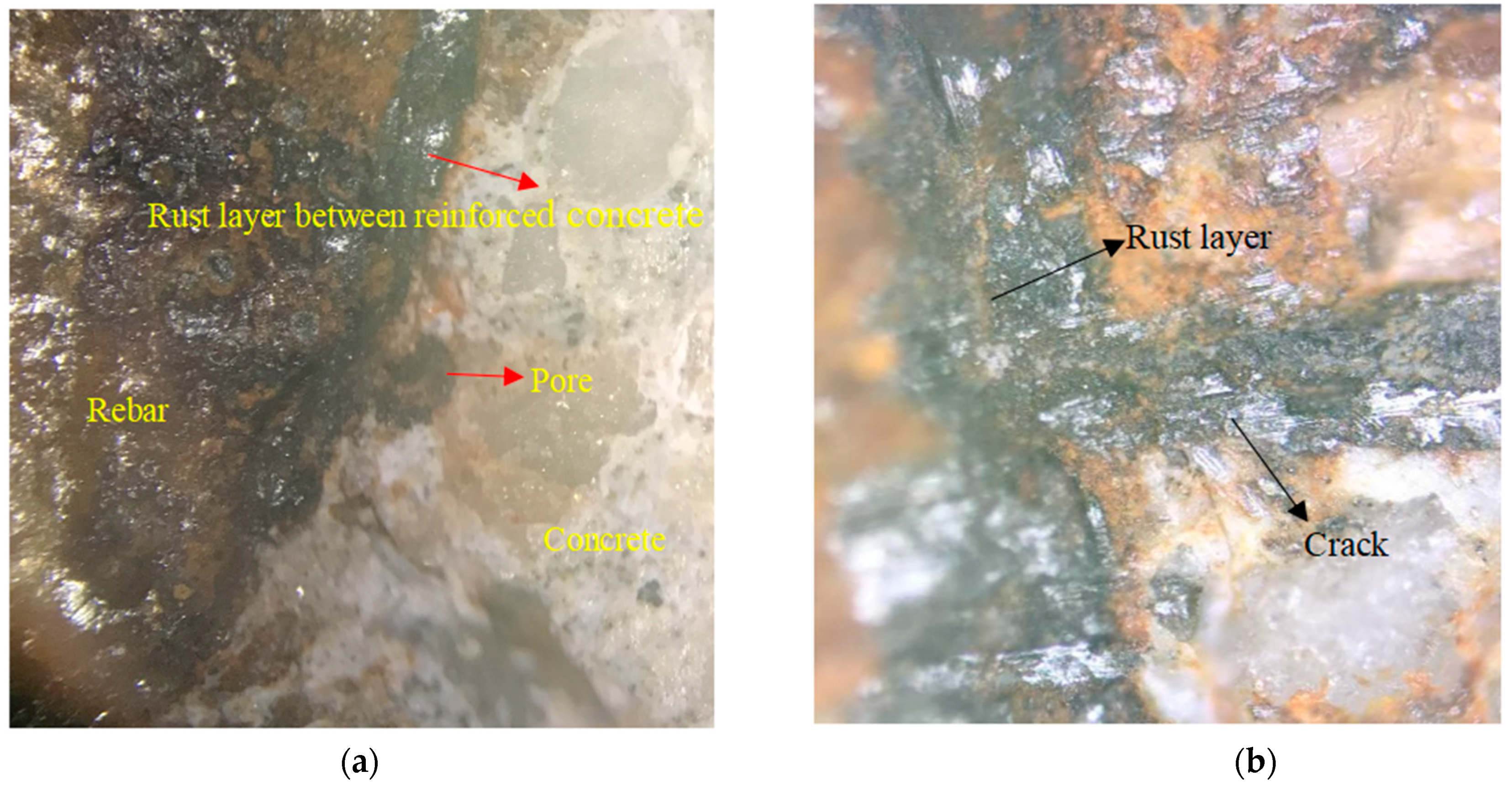
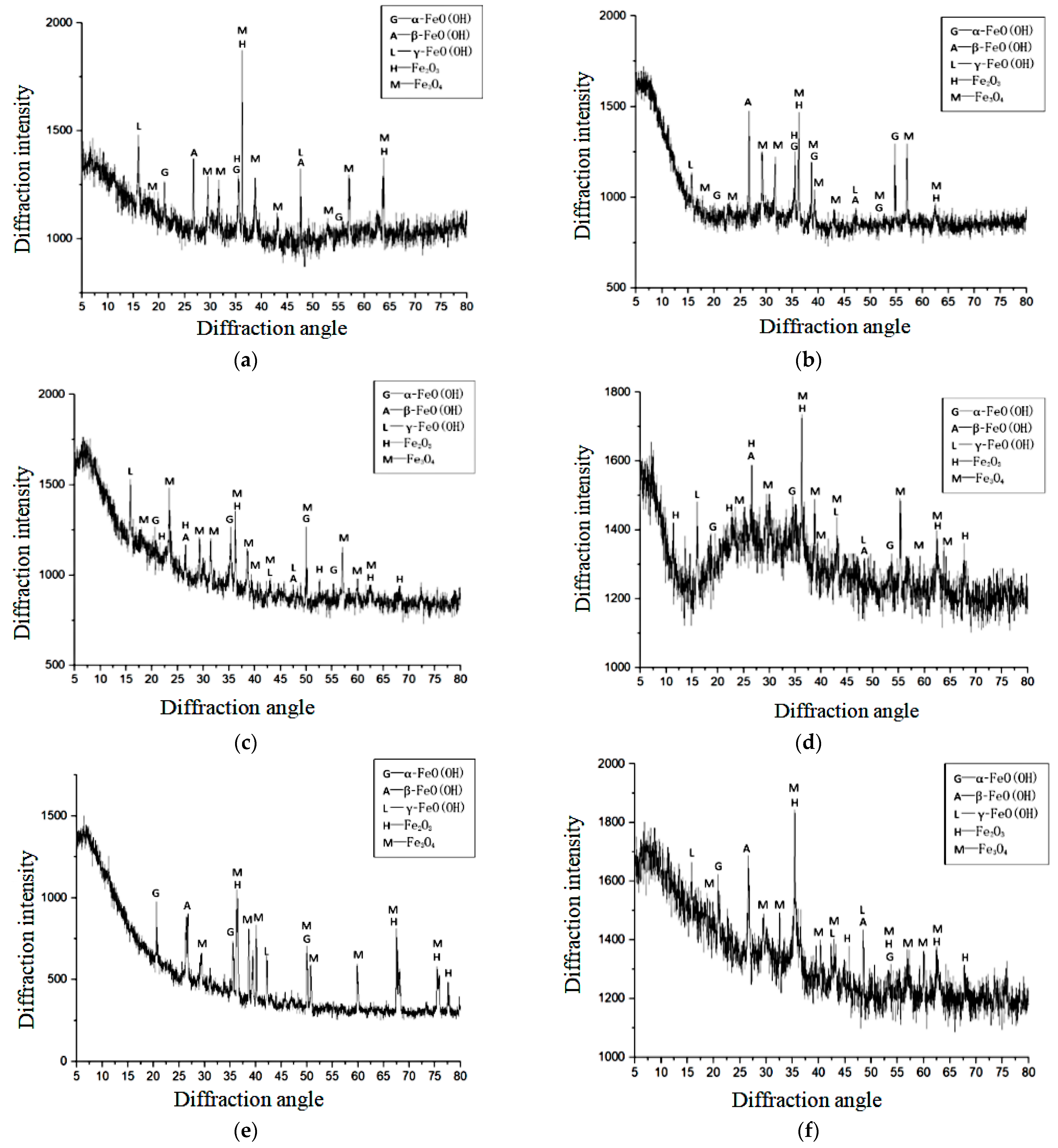
5.3. Corrosion Products of Reinforced Concrete Interface
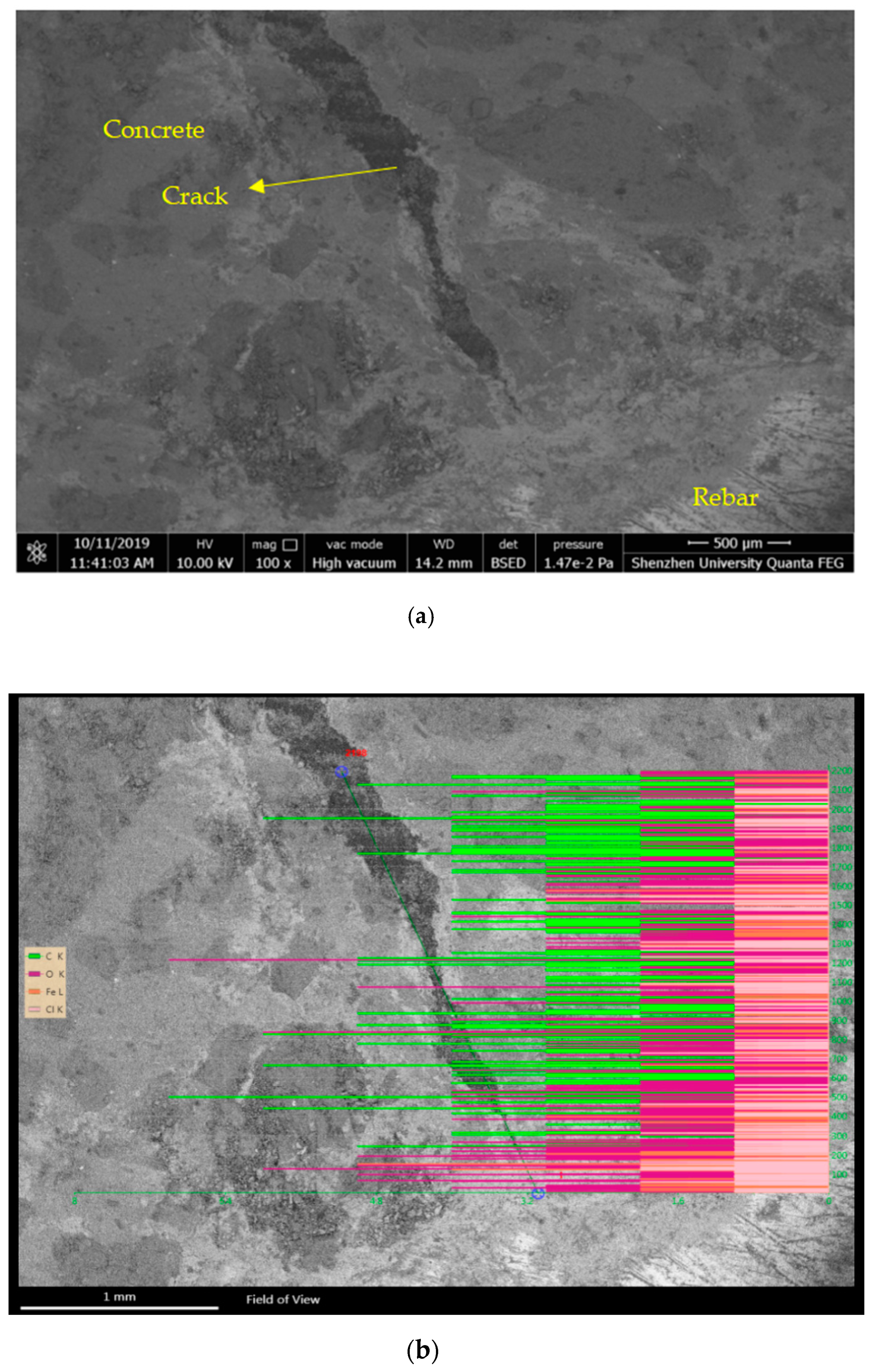
5.4. Corrosion Products of Concrete Cracks
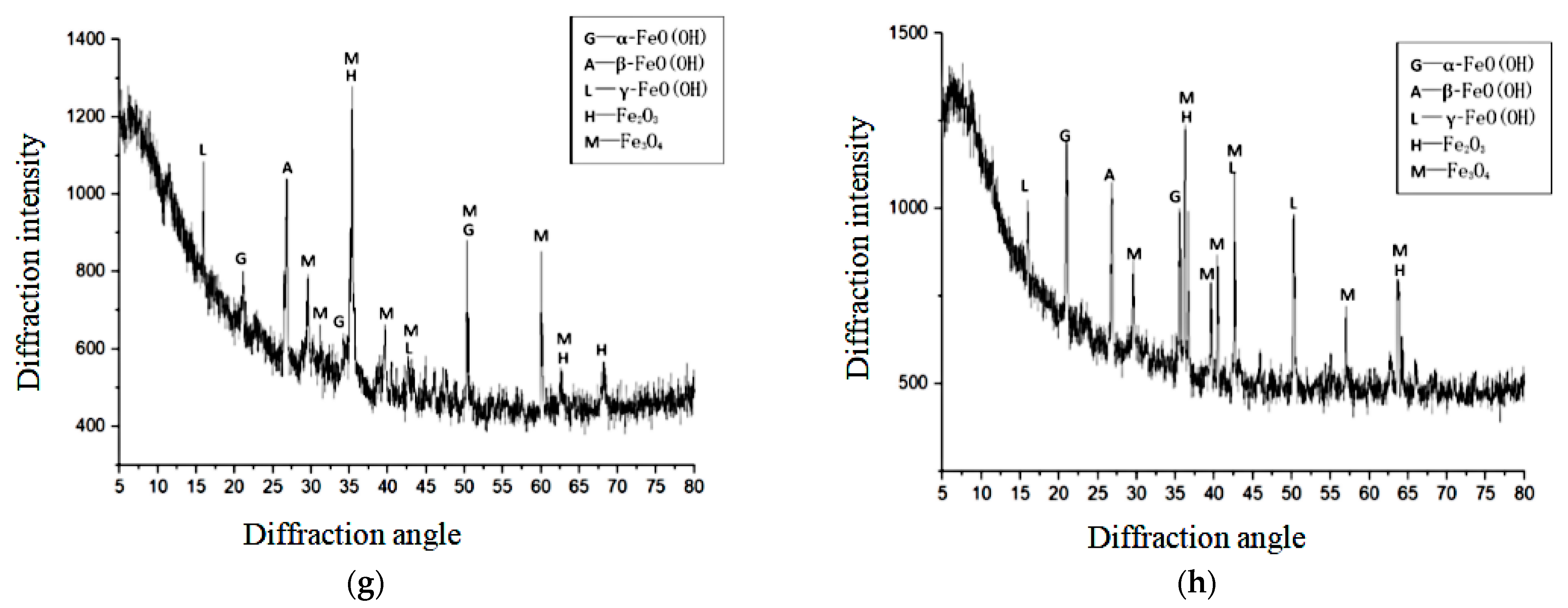
5.5. Composition of Rust Products Studied Using X-ray Diffraction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Dai, L.; Bian, H.; Ma, Y.; Zhang, J. Concrete cracking prediction under combined prestress and strand corrosion. Struct. Infrastruct. Eng. 2019, 15, 285–295. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Martin, U.; Bastidas, J.M.; Ress, J. Corrosion Inhibition Mechanism of Steel Reinforcements in Mortar Using Soluble Phosphates: A Critical Review. Materials 2021, 14, 6168. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Jin, W. Damage analysis and cracking model of reinforced concrete structures with rebar corrosion. Corros. Sci. 2011, 53, 3388–3397. [Google Scholar] [CrossRef]
- Chun-Qing, L.; Melchers, R.E.; Jian-Jun, Z. Analytical model for corrosion-induced crack width in reinforced concrete structures. ACI Struct. J. 2006, 103, 479. [Google Scholar]
- Papakonstantinou, K.G.; Shinozuka, M. Probabilistic model for steel corrosion in reinforced concrete structures of large dimensions considering crack effects. Eng. Struct. 2013, 57, 306–326. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: Experimental and theoretical investigation. Corros. Sci. 2010, 52, 198–204. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Li, W.; Gong, M.; Yin, L. Experimental and theoretical studies of two imidazolium-based ionic liquids as inhibitors for mild steel in sulfuric acid solution. Corros. Sci. 2015, 95, 168–179. [Google Scholar] [CrossRef]
- Melchers, R.E. Corrosion uncertainty modelling for steel structures. J. Constr. Steel Res. 1999, 52, 3–19. [Google Scholar] [CrossRef]
- Yuan, Y.; Yong-sheng, J.; Mu, Y. Propagation and model of distribution for corrosion of steel bars in concrete. China Civ. Eng. J. 2007, 40, 5–10, 24. [Google Scholar]
- Deba, L. The development of rust expansion force of steel bar in concrete and reinforcement corrosion rate’s time-varying property. Sichuan Constr. Sci. Res. 2013, 39, 98–103. [Google Scholar]
- Xuesong, W.; Xianyu, J.; Ye, T.; Bei, L.; Nanguo, J. Applicability of accelerated corrosion method of steel bars in cracked concrete structure. J. Zhejiang Univ. 2013, 47, 565–574. [Google Scholar]
- Liu, R.G.; Yu, M.X.; Jiang, H. Analysis of elliptical non-uniform expansion stress due to rebar corrosion at the cover cracking moment in RC members. Bull. Chin. Ceram. Soc. 2015, 34, 1769–1774. [Google Scholar]
- Tepfers, R. Cracking of concrete cover along anchored deformed reinforcing bars. Mag. Concr. Res. 1979, 31, 3–12. [Google Scholar] [CrossRef]
- El Maaddawy, T.; Soudki, K. A model for prediction of time from corrosion initiation to corrosion cracking. Cem. Concr. Compos. 2007, 29, 168–175. [Google Scholar] [CrossRef]
- Bazán, A.M.; Gálvez, J.C.; Reyes, E.; Galé-Lamuela, D. Study of the rust penetration and circumferential stresses in reinforced concrete at early stages of an accelerated corrosion test by means of combined SEM, EDS and strain gauges. Constr. Build. Mater. 2018, 184, 655–667. [Google Scholar] [CrossRef]
- Shilang, X.; Guofan, Z. A double-K fracture criterion for the crack propagation in concrete structures. China Civ. Eng. J. 1992, 25, 32–38. [Google Scholar]
- Xu, S.; Reinhardt, H.W. Determination of double-K criterion for crack propagation in quasi-brittle fracture, Part I: Experimental investigation of crack propagation. Int. J. Fract. 1999, 98, 111–149. [Google Scholar] [CrossRef]
- Sutton, M.A.; Yan, J.H.; Deng, X.; Cheng, C.S.; Zavattieri, P. Three-dimensional digital image correlation to quantify deformation and crack-opening displacement in ductile aluminum under mixed-mode I/III loading. Opt. Eng. 2007, 46, 051003. [Google Scholar] [CrossRef]
- Zhimin, W.; Shiyun, X.; Jinlai, W. Double K fracture parameters of concrete based on virtual crack model. J. Water Conserv. 1999, 7, 12–16. [Google Scholar]
- Ping, W.; Kun, D.; Yidong, X.; Wei, C.; Lei, H. Meso-scale numerical simulation of corrosion cracking process of reinforced concrete based on brittle cracking. Bull. Chin. Ceram. Soc. 2018, 37, 2372–2377. [Google Scholar]
- Smarzewski, P. Influence of silica fume on mechanical and fracture properties of high performance concrete. Procedia Struct. Integr. 2019, 17, 5–12. [Google Scholar] [CrossRef]
- Qing, L.; Cheng, Y.; Fan, X.; Ru, M.; Shaoqiang, D. An arc bending notched specimen for determining the mechanical and fracture parameters of concrete based on the FET. Eng. Fract. Mech. 2019, 220, 106639. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Lotfi-Omran, O.; Nikbin, I.M. On the fracture parameters of heavy-weight magnetite concrete with different water-cement ratios through three methods. Eng. Fract. Mech. 2019, 219, 106615. [Google Scholar] [CrossRef]
- Jang, B.S.; Oh, B.H. Effects of non-uniform corrosion on the cracking and service life of reinforced concrete structures. Cem. Concr. Res. 2010, 40, 1441–1450. [Google Scholar] [CrossRef]
- Wong, H.S.; Zhao, Y.X.; Karimi, A.R.; Buenfeld, N.R.; Jin, W.L. On the penetration of corrosion products from reinforcing steel into concrete due to chloride-induced corrosion. Corros. Sci. 2010, 52, 2469–2480. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.X.; Zhang, X.; Ding, H.; Jin, W. Non-uniform distribution of a corrosion layer at a steel/concrete interface described by a Gaussian model. Corros. Sci. 2016, 112, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.X.; Jin, W.L. Modeling the amount of steel corrosion at the cracking of concrete cover. Adv. Struct. Eng. 2006, 9, 687–696. [Google Scholar] [CrossRef]
- Forbes, C.; Evans, M.; Hastings, N.; Peacock, B. Statistical Distributions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Xi, X.; Yang, S.; Li, C. A non-uniform corrosion model and mesoscale fracture modelling of concrete. Cem. Concr. Res. 2018, 108, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Guofan, Z. Crack Control of Reinforced Concrete Structure; Ocean Press: Beijing, China, 1991. [Google Scholar]
- Wang, Q.Q.; Jiang, J.H. Method for predicting service life of concrete materials and structures based on climate environmental action. J. Archit. Civ. Eng. 2015, 32, 67–73. [Google Scholar]
- Jun, C. Study on degradation regularity of bearing capacity of RC beams under corrosive conditions. J. Disaster Prev. Mitig. Eng. 2013, 33 (Suppl. S1), 83–87. [Google Scholar]
- Zhong, X.; Jin, W. Reliability design method of reinforced concrete structure based on durability. China Civ. Eng. J. 2016, 49, 31–39. [Google Scholar]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Physical and chemical characteristics affecting the durability of concrete. ACI Mater. J. 1991, 88, 186–196. [Google Scholar]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction a review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.P.; Ferreira, M.G.S. Chloride-induced corrosion on reinforcing steel: From the fundamentals to the monitoring techniques. Cem. Concr. Compos. 2003, 25, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Hansson, C.M. Comments on electrochemical measurements of the rate of corrosion of steel in concrete. Cem. Concr. Res. 1984, 14, 574–584. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Hu, J.Y.; Li, S.; Tang, W.S. Active and passive protection of steel reinforcement in concrete column using carbon fibre reinforced polymer against corrosion. Electrochim. Acta 2018, 278, 124–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Lixue, J.; Weiping, Z. Introduction to Concrete Durability; Shanghai Science and Technology Press: Shanghai, China, 2003. [Google Scholar]



| Parameter | fc (MPa) | c (mm) | d (mm) | ρ |
|---|---|---|---|---|
| Average value | 28.66 | 33.8 | 32 | 0.806 |
| Standard deviation | 5.52 | 13.1 | 0.79 | 250.23 |
| Distribution type | Normal distribution | Normal distribution | Normal distribution | Normal distribution |
| Water–Cement Ratio | Water (kg) | Cement (kg) | Sand (kg) | Strength Grade of Concrete |
|---|---|---|---|---|
| 0.6 | 250 | 417 | 1734 | C30 |
| NaCl Concentration (%) | d (mm) | l (mm) | h (mm) | Soaking Time (h) | Drying Time (h) | Number of Specimens |
|---|---|---|---|---|---|---|
| 7 | 8 | 32 | 100 | 12 | 12 | 5 |
| 16 | 8 | 5 | ||||
| 40 | 16 | 8 | 5 | |||
| 20 | 4 | 5 | ||||
| 10 | 32 | 12 | 12 | 5 | ||
| 16 | 8 | 5 | ||||
| 40 | 16 | 8 | 5 | |||
| 20 | 4 | 5 |
| Sample Number | Crack Width (mm) | Cracking Time (h) | Sample Number | Crack Width (mm) | Cracking Time (h) |
|---|---|---|---|---|---|
| 32-8-12-1 | 0.14 | 986 | 40-8-16-1 | 0.10 | 1586 |
| 32-8-12-2 | 0.12 | 1004 | 40-8-16-2 | 0.14 | 1472 |
| 32-8-12-3 | 0.11 | 1076 | 40-8-16-3 | 0.12 | 1547 |
| 32-8-12-4 | 0.18 | 975 | 40-8-16-4 | 0.10 | 1483 |
| 32-8-12-5 | 0.10 | 897 | 40-8-16-5 | 0.10 | 1583 |
| 32-8-16-1 | 0.14 | 1091 | 40-8-20-1 | 0.12 | 1765 |
| 32-8-16-2 | 0.13 | 1120 | 40-8-20-2 | 0.10 | 1849 |
| 32-8-16-3 | 0.16 | 1210 | 40-8-20-3 | 0.10 | 1776 |
| 32-8-16-4 | 0.10 | 1147 | 40-8-20-4 | 0.14 | 1869 |
| 32-8-16-5 | 0.14 | 1247 | 40-8-20-5 | 0.16 | 1669 |
| 32-10-12-1 | 0.18 | 1254 | 40-10-16-1 | 0.12 | 1686 |
| 32-10-12-2 | 0.20 | 1470 | 40-10-16-2 | 0.18 | 1784 |
| 32-10-12-3 | 0.14 | 1386 | 40-10-16-3 | 0.10 | 1625 |
| 32-10-12-4 | 0.21 | 1184 | 40-10-16-4 | 0.16 | 1759 |
| 32-10-12-5 | 0.14 | 1243 | 40-10-16-5 | 0.14 | 1659 |
| 32-10-16-1 | 0.12 | 1480 | 40-10-20-1 | 0.20 | 1840 |
| 32-10-16-2 | 0.14 | 1577 | 40-10-20-2 | 0.16 | 1948 |
| 32-10-16-3 | 0.10 | 1475 | 40-10-20-3 | 0.10 | 2076 |
| 32-10-16-4 | 0.13 | 1355 | 40-10-20-4 | 0.14 | 1929 |
| 32-10-16-5 | 0.14 | 1545 | 40-10-20-5 | 0.10 | 1879 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ye, X.; Li, L.; Liu, P. Distribution Law of Corrosion Products in a Marine Chloride Environment. Materials 2022, 15, 4339. https://doi.org/10.3390/ma15124339
Wang J, Ye X, Li L, Liu P. Distribution Law of Corrosion Products in a Marine Chloride Environment. Materials. 2022; 15(12):4339. https://doi.org/10.3390/ma15124339
Chicago/Turabian StyleWang, Jiao, Xinying Ye, Ling Li, and Peng Liu. 2022. "Distribution Law of Corrosion Products in a Marine Chloride Environment" Materials 15, no. 12: 4339. https://doi.org/10.3390/ma15124339
APA StyleWang, J., Ye, X., Li, L., & Liu, P. (2022). Distribution Law of Corrosion Products in a Marine Chloride Environment. Materials, 15(12), 4339. https://doi.org/10.3390/ma15124339







