Chemical Modification of Silk Fibroin through Serine Amino Acid Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of SF
2.3. Characterization by FTIR, 1H NMR, and UV-Vis
2.4. Amino Acid Analyses and Determination of Sulfonic Group Content
2.5. X-ray Diffraction (XRD) Analysis
2.6. Hemolytic Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. FTIR Spectrum
3.2. Structure Characteristics
3.3. 1H-NMR Analysis
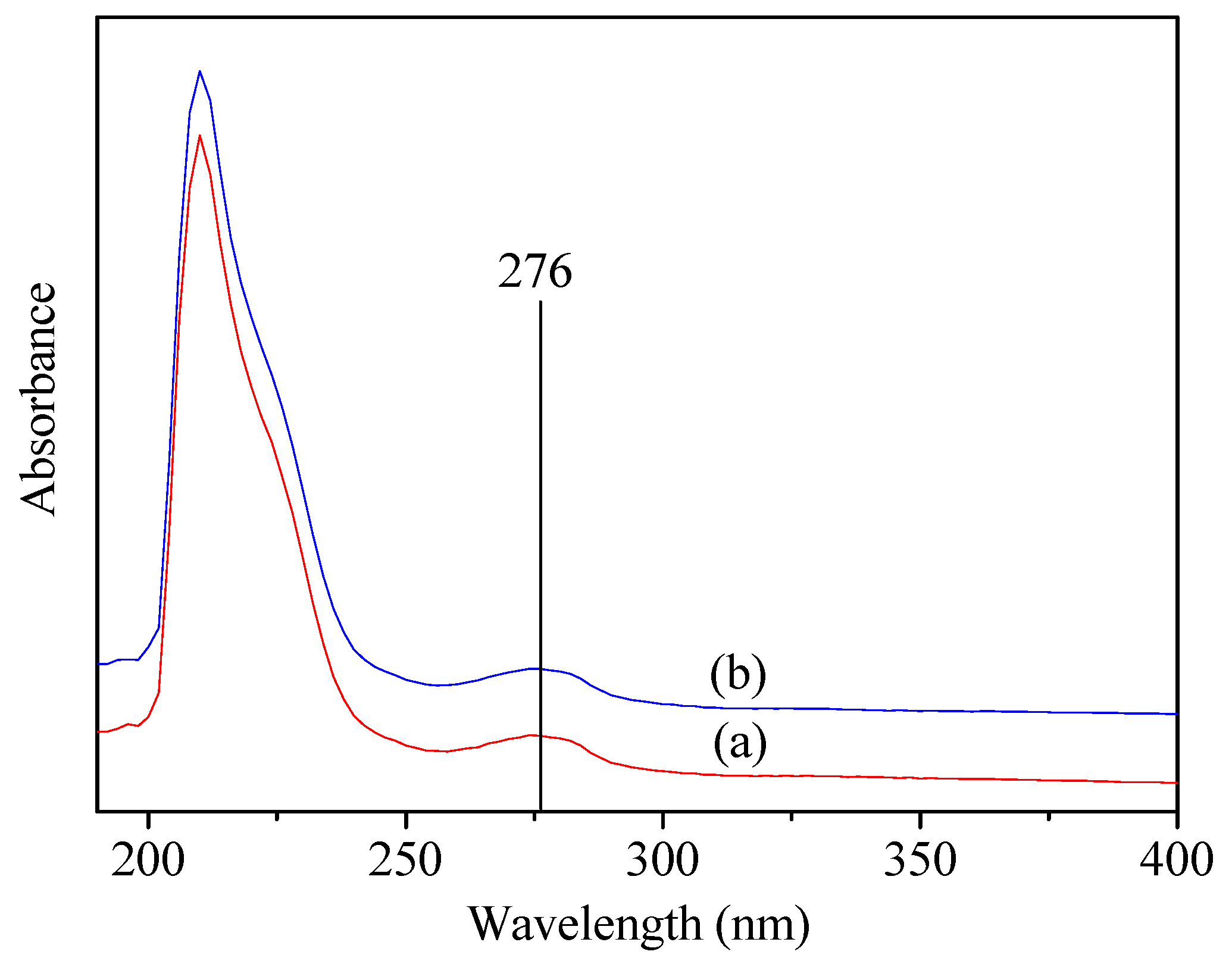
3.4. UV-Vis Spectrum
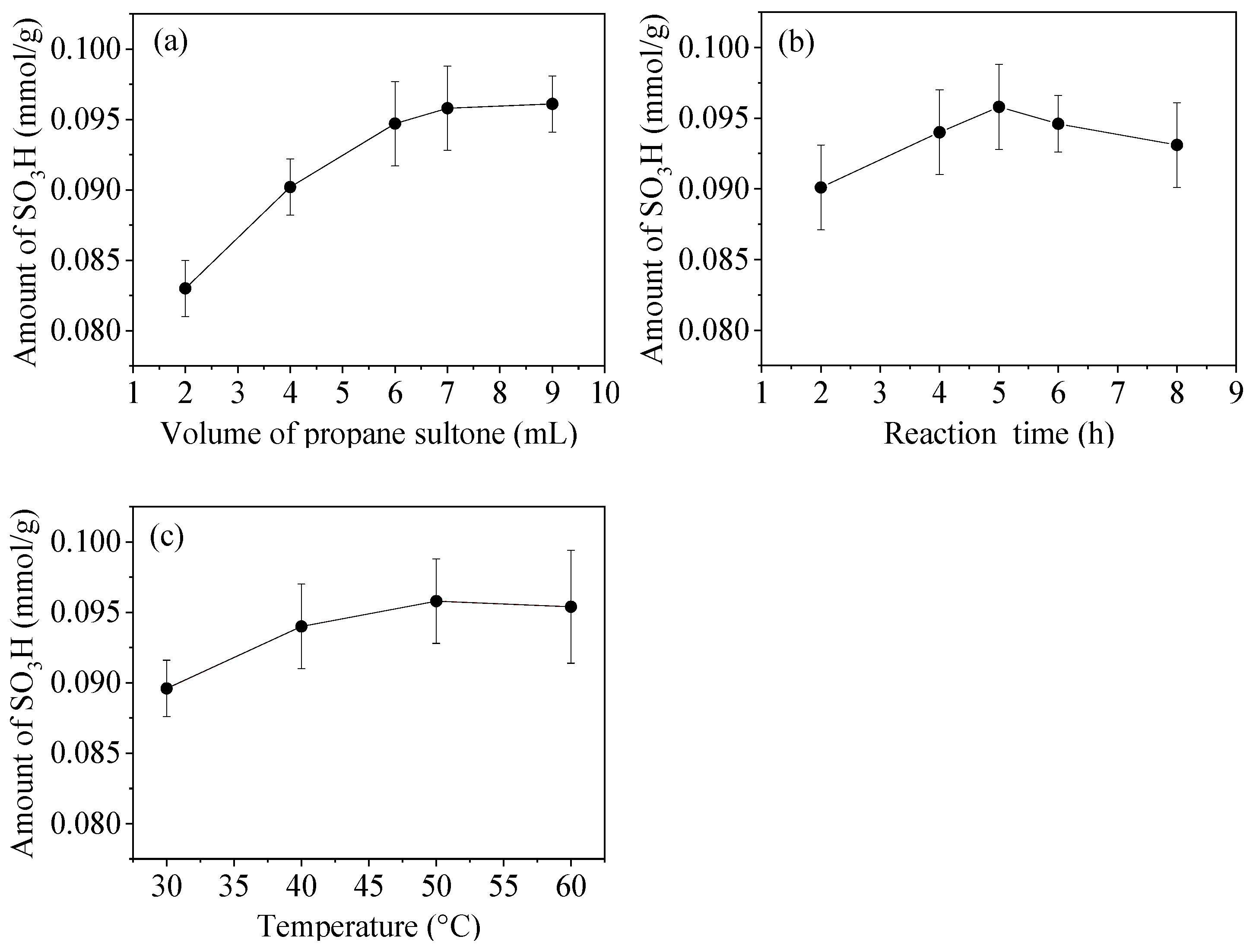
3.5. Optimization of Modification Conditions
3.6. Modification Extent
3.7. Hemolysis Percent
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.H.; Le, T.H.; Huynh, V.Q.N.; Vo, D.V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk fibroin-based biomaterials for biomedical applications: A review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, Y. Functionalization of silk fibroin-based biomaterials for tissue engineering. Polym. J. 2021, 53, 1345–1351. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized silk fibroin nanofiber as drug carriers: Advantages and challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Ghalei, S.; Handa, H. A review on antibacterial silk fibroin-based biomaterials: Current state and prospects. Mater. Today Chem. 2022, 23, 100673. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Katashima, T.; Malay, A.D.; Numata, K. Chemical modification and biosynthesis of silk-like polymers. Curr. Opin. Chem. Eng. 2019, 24, 61–68. [Google Scholar] [CrossRef]
- Chen, J.; Venkatesan, H.; Hu, J. Chemically Modified Silk Proteins. Adv. Eng. Mater. 2018, 20, 1700961. [Google Scholar] [CrossRef]
- Aigner, T.B.; Desimone, E.; Scheibel, T. Biomedical applications of recombinant silk-based materials. Adv. Mater. 2018, 30, e1704636. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, H.; Fan, Y. Silk fibroin for vascular regeneration. Microsc. Res. Technol. 2017, 80, 280–290. [Google Scholar] [CrossRef]
- Fu, C.; Shao, Z.; Fritz, V. Animal silks: Their structures, properties and artificial production. Chem. Commun. 2009, 43, 6515–6529. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, M.; Holland, G.P.; Kaplan, D.L. Experimental methods for characterizing the secondary structure and thermal properties of silk proteins. Macromol. Rapid Commun. 2018, 40, 1800390. [Google Scholar] [CrossRef] [PubMed]
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Silk I and Silk II studied by fast scanning calorimetry. Acta Biomater. 2017, 55, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Sashina, E.S.; Bochek, A.M.; Novoselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 869–876. [Google Scholar] [CrossRef]
- Ajisawa, A. Dissolution of silk fibroin with calcium chloride/ethanol aqueous solution. J. Seric. Sci. Jpn. 1998, 67, 91–94. [Google Scholar] [CrossRef]
- Mobika, J.; Rajkumar, M.; Nithya Priya, V.; Linto Sibi, S.P. Effect of chitosan reinforcement on properties of hydroxyapatite/silk fibroin composite for biomedical application. Physica E 2021, 131, 114734. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Zhang, S.; Yin, Z.; Xing, T.; Kaplan, D.L. The influence of the hydrophilic-lipophilic environment on the structure of silk fibroin protein. J. Mater. Chem. B 2015, 3, 2599–2606. [Google Scholar] [CrossRef]
- He, S.J.; Valluzzi, R.; Gido, S.P. Silk I structure in Bombyx Mori silk foams. Int. J. Biol. Macromol. 1999, 24, 187–195. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Furuzono, T.; Ishihara, K.; Nakabayashi, N.; Tamada, Y. Chemical modification of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine. 1 Graft-polymerization onto fabric using ammonium persulfate and interaction between fabric and platelets. J. Appl. Polym. Sci. 1999, 73, 2541–2544. [Google Scholar] [CrossRef]
- Gotoh, Y.; Tsukada, M.; Minoura, N. Chemical modification of silk fibroin with cyanuric chloride activated poly(ethylene glycol): Analyses of reaction site by 1H-NMR spectroscopy and conformation of the conjugates. Bioconjugate Chem. 1993, 4, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Goto, Y.; Freddi, G.; Shiozaki, H.J. Chemical modification of silk with aromatic acid anhydrides. Appl. Polym. Sci. 1992, 45, 1189–1194. [Google Scholar] [CrossRef]
- Tsukada, M.; Shiozaki, H. Chemical and property modification of silk with dibasic acid anhydrides. Appl. Polym. Sci. 1989, 37, 2637–2644. [Google Scholar] [CrossRef]
- Murphy, A.R.; St John, P.; Kaplan, D.L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 2008, 29, 2829–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Yang, X.; Zhu, H. Surface sulfonation of silk fibroin film by plasma treatment and in vitro antithrombogenicity study. Mater. Sci. Eng. C 2002, 20, 199–202. [Google Scholar] [CrossRef]
- Freddi, G.; Anghileri, A.; Sampaio, S.; Buchert, J.; Monti, P.; Taddei, P. Tyrosinase-catalyzed modification of bombyx mori silk fibroin: Grafting of chitosan under heterogeneous reaction conditions. J. Biotechnol. 2006, 125, 281–294. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Zhang, C.; Liu, H.; Fang, J. Homogeneous sulfation of silk fibroin in an ionic liquid. Mater. Lett. 2015, 143, 302–304. [Google Scholar] [CrossRef]
- Tamada, Y. Sulfation of silk fibroin by chlorosulfonic acid and the anticoagulant activity. Biomaterials 2004, 25, 377–383. [Google Scholar] [CrossRef]
- Tamada, Y. Sulfation of silk fibroin by sulfuric acid and anticoagulant activity. J. Appl. Polym. Sci. 2003, 87, 2377–2382. [Google Scholar] [CrossRef]
- Rong, L.; Liu, Z.; Ma, M.; Liu, J.; Xu, Z.; Lim, L.W.; Takeuchi, T. Simultaneous determination of inorganic cations by capillary ion chromatography with a non-suppressed contactless conductivity detector. Anal. Sci. 2012, 28, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.F. Propanesultone. Ind. Eng. Chem. 1964, 56, 41–45. [Google Scholar] [CrossRef]
- Rozema, D.; Gellman, S.H. Artificial chaperone-assisted refolding of carbonic anhydrase B. J. Biol. Chem. 1996, 271, 3478–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Huang, H.; Ju, R.; Chen, K.; Li, S.; Wang, W.; Yan, Y. In vivo biocompatibility and hemocompatibility of a polytetrafluoroethylene small diameter vascular graft modified with sulfonated silk fibroin. Am. J. Surg. 2017, 213, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhang, X.; Lu, P.; Wu, Y.; Wang, Q.; Sun, H.; Heng, B.C.; Bunpetch, V.; Zhang, S.; Ouyang, H. A gelatin-sulfonated silk composite scaffold based on 3d printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci. Rep. 2017, 7, 4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Cao, C.; Zhu, H. The biocompatibility of silk fibroin films containing sulfonated silk fibroin. J. Biomed. Mater. Res. Part B 2006, 78, 89–96. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Liu, Q.; Zhang, S. Dual-functional composite with anticoagulant and antibacterial properties based on heparinized silk fibroin and chitosan. Colloids Surf. B 2011, 85, 241–247. [Google Scholar] [CrossRef]
- Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformation characterization of Bombyx mori silk fibroin in the solid state by high-frequency 13C cross polarization-magic angle spinning NMR, X-ray diffraction, and infrared spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Shao, H.; Hu, X. A study on the flow stability of regenerated silk fibroin aqueous solution. Int. J. Biol. Macromol. 2005, 36, 66–70. [Google Scholar] [CrossRef]
- Xing, T.; Hu, W.; Li, S.; Chen, G. Preparation, structure and properties of multi-functional silk via ATRP method. Appl. Surf. Sci. 2012, 258, 3208–3213. [Google Scholar] [CrossRef]
- Qu, J.; Wang, L.; Niu, L.; Lin, J.; Huang, Q.; Jiang, X.; Li, M. Porous Silk Fibroin Microspheres Sustainably Releasing Bioactive Basic Fibroblast Growth Factor. Materials 2018, 11, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, L.; Dai, F.; Zhang, H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Huang, A.; Ma, L.; Huang, Z.; Huang, X.; Qiang, P.; Gong, Z.; Zhang, L. Structure regulation of silk fibroin Films for controlled drug release. J. Appl. Polym. Sci. 2012, 125, E477–E484. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, C.; Ma, X.; Hu, L.; Chen, L.; Wang, C. In vitro and in vivo degradation behavior of aqueous-derived electrospun silk fibroin scaffolds. Polym. Degrad. Stab. 2010, 95, 1679–1685. [Google Scholar] [CrossRef]
- Zainuddin; Le, T.T.; Park, Y.; Chirila, T.V.; Halley, P.J.; Whittaker, A.K. The behavior of aged regenerated Bombyx mori silk fibroin solutions studied by 1H NMR and rheology. Biomaterials 2008, 29, 4268–4274. [Google Scholar] [CrossRef]
- Anderson, J.M.B. 9.19-Biocompatibility. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier Ltd.: Oxford, UK, 2012; Volume 9, pp. 363–383. [Google Scholar] [CrossRef]
- Sánchez, J.; Rodríguez-Reyes, M.; Cortés-Hernández, D.A.; Ávila-Orta, C.A.; Reyes-Rodríguez, P.Y. Heating capacity and biocompatibility of Pluronic-coated manganese gallium ferrites for magnetic hyperthermia treatment. Colloids Surf. A 2021, 612, 125986. [Google Scholar] [CrossRef]
- Lv, J.; Jin, J.; Chen, J.; Cai, B.; Jiang, W. Antifouling and antibacterial properties constructed by quaternary ammonium and benzyl ester derived from lysine methacrylamide. ACS Appl. Mater. Interfaces 2019, 11, 25556–25568. [Google Scholar] [CrossRef] [PubMed]

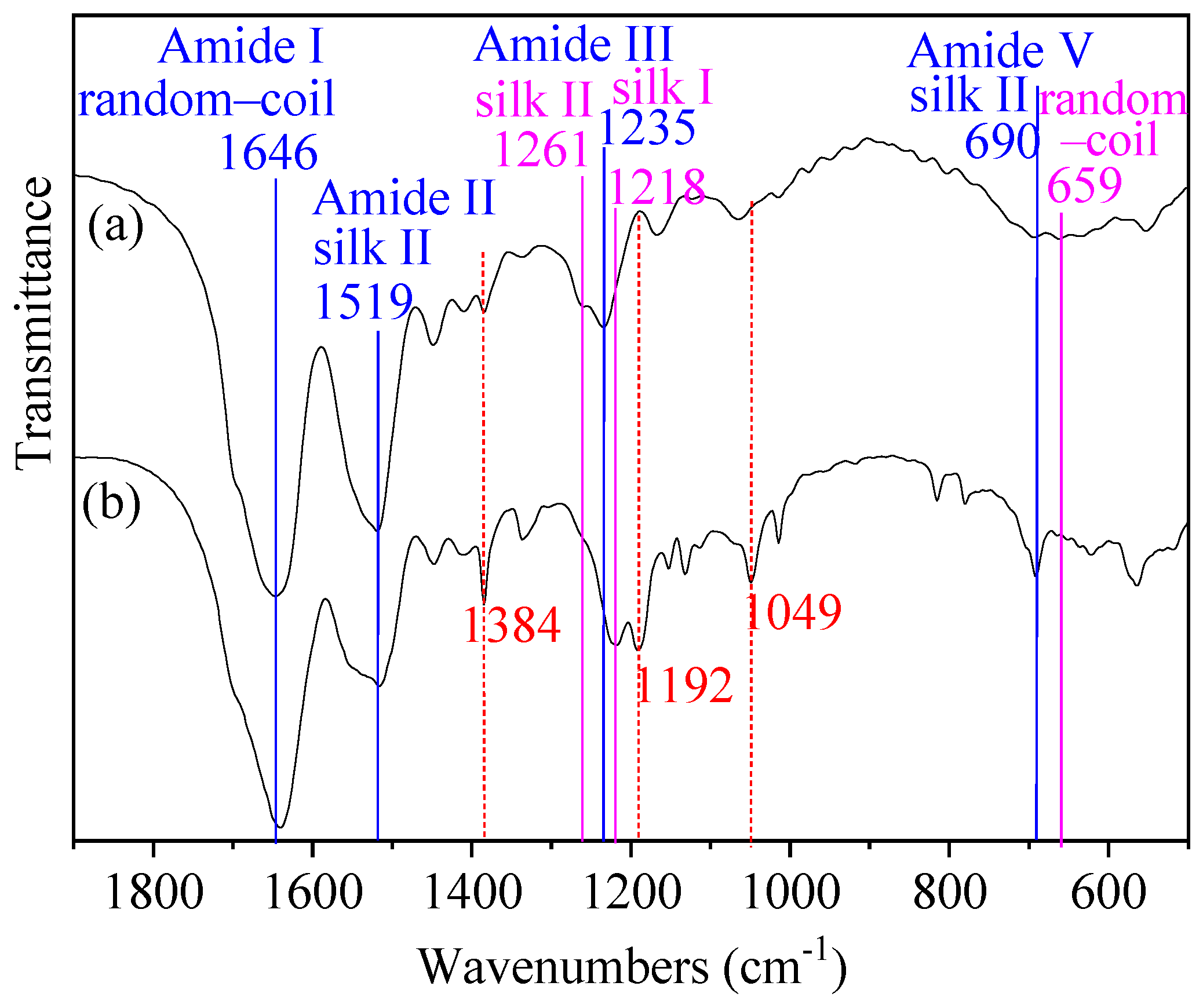
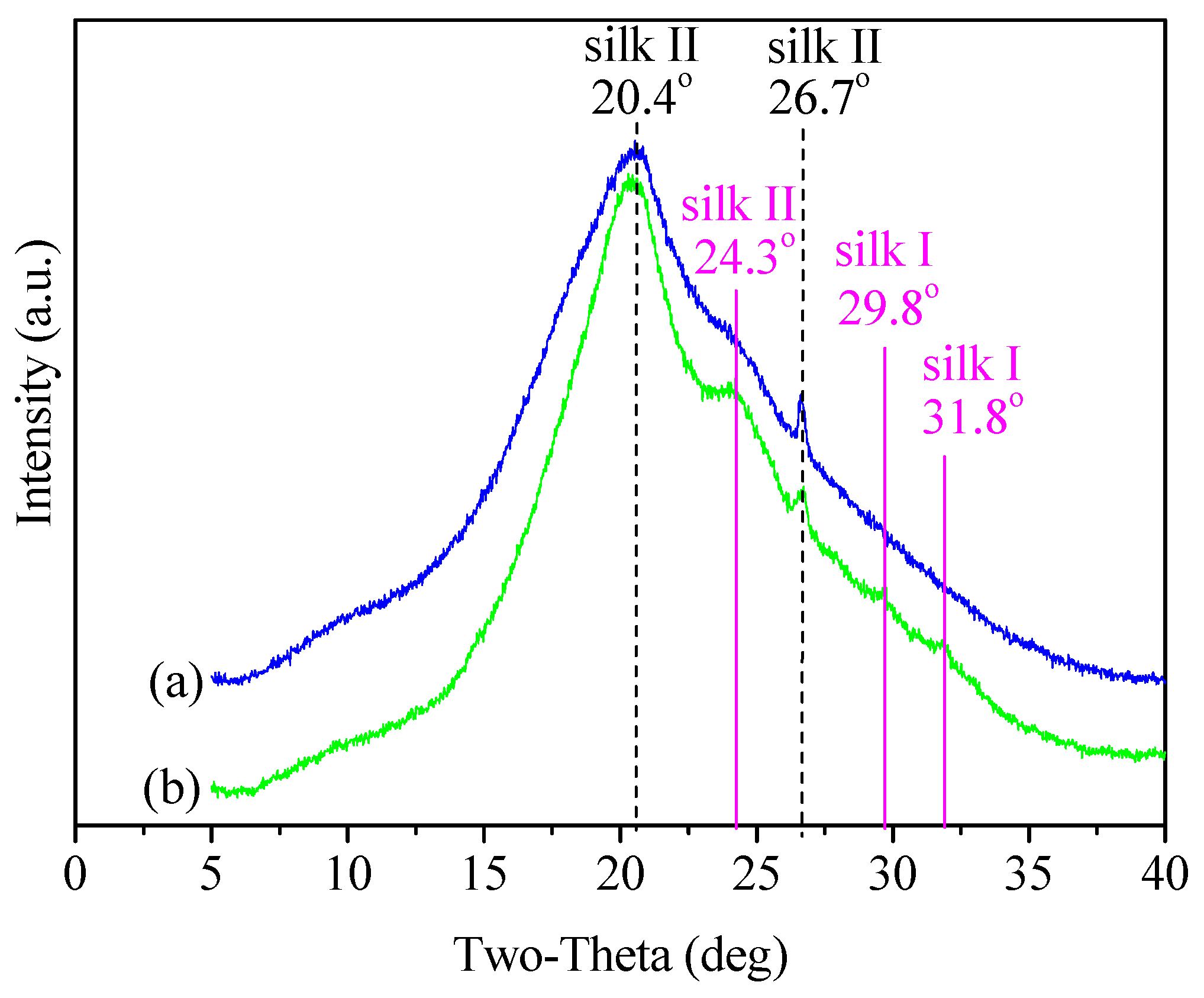
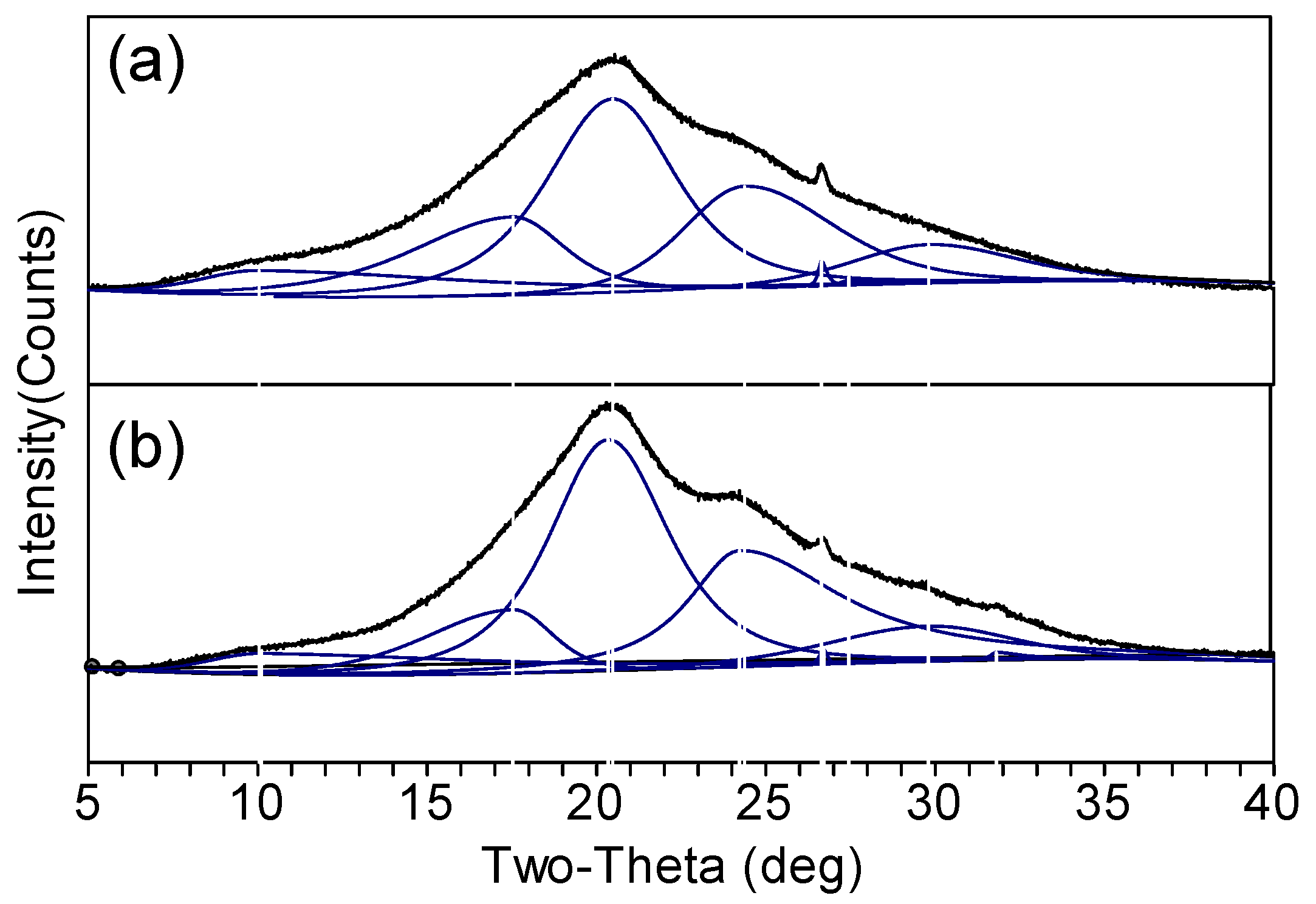
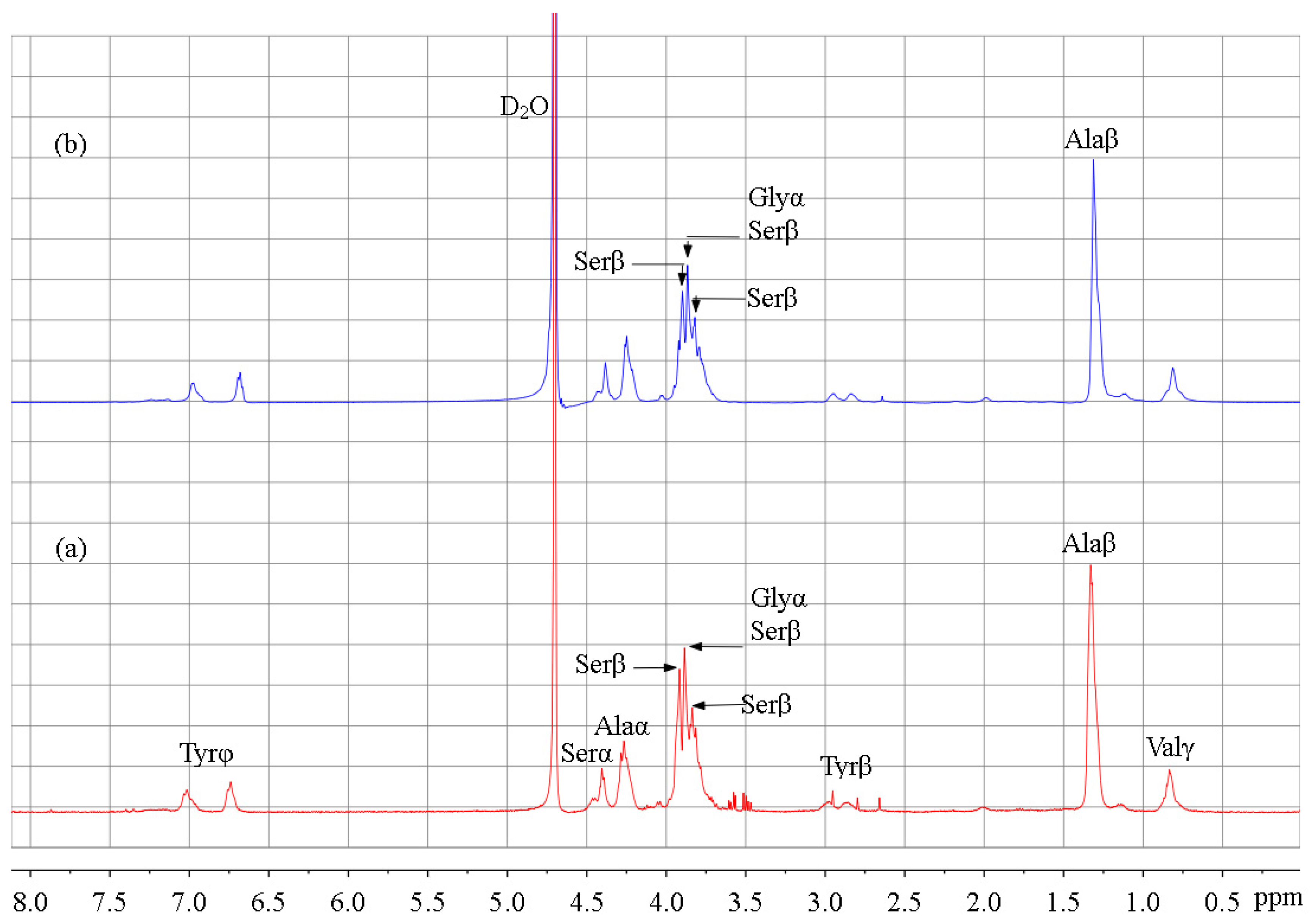
| Peak Position (°) | d Spacing (Å) | Structure | Area Percentage (%) | Total Area Percentage of Non-Crystalline/Silk II/Silk I Structures (%) | ||
|---|---|---|---|---|---|---|
| SF | SSF | SF | SSF | |||
| 10.1 | 8.76 | non-crystalline structures | 10.0 | 8.3 | 28.1/62.4/9.5 | 19.3/71.3/9.4 |
| 17.5 | 5.05 | non-crystalline structures | 18.1 | 11.0 | ||
| 20.4 | 4.35 | silk II | 39.7 | 41.8 | ||
| 24.3 | 3.66 | silk II | 22.3 | 29.3 | ||
| 26.7 | 3.34 | silk II | 0.4 | 0.2 | ||
| 27.4 | 3.25 | silk I | 0.3 | 0 | ||
| 29.8 | 3.00 | silk I | 9.2 | 8.9 | ||
| 31.8 | 2.81 | silk I | 0 | 0.5 | ||
| Amino Acid | Content (mmol/g ± SD) | Amino Acid | Content (mmol/g ± SD) | Amino Acid | Content (mmol/g ± SD) |
|---|---|---|---|---|---|
| Gly | 3.9596 ± 0.0986 | Glu | 0.1708 ± 0.0026 | Lys | 0.0366 ± 0.0018 |
| Ala | 2.7310 ± 0.0138 | Thr | 0.1048 ± 0.0006 | Met | 0.0103 ± 0.0002 |
| Ser | 1.0549 ± 0.0208 | Ile | 0.0787 ± 0.0012 | His | 0.0188 ± 0.0005 |
| Tyr | 0.5280 ± 0.0117 | Phe | 0.0786 ± 0.0016 | Arg | 0.0643 ± 0.0002 |
| Val | 0.2257 ± 0.0017 | Leu | 0.0772 ± 0.0027 | Cys | 0 |
| Asp | 0.1810 ± 0.0007 | Pro | 0.0760 ± 0.0036 |
| Sample | Concentration (μg/mL) | Hemolytic Rate (% ± SD) (n = 3, p < 0.05) |
|---|---|---|
| Positive control | - | 100 |
| Negative control | - | 0 |
| SF | 100 | 3.170 ± 0.239 |
| 500 | 3.275 ± 0.143 | |
| SSF | 100 | 2.552 ± 0.195 |
| 500 | 2.667 ± 0.121 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xia, Q.; Zhou, J.; Zhang, Y.; Ju, H.; Deng, Z. Chemical Modification of Silk Fibroin through Serine Amino Acid Residues. Materials 2022, 15, 4399. https://doi.org/10.3390/ma15134399
Liu X, Xia Q, Zhou J, Zhang Y, Ju H, Deng Z. Chemical Modification of Silk Fibroin through Serine Amino Acid Residues. Materials. 2022; 15(13):4399. https://doi.org/10.3390/ma15134399
Chicago/Turabian StyleLiu, Xiuying, Qianna Xia, Jiao Zhou, Yanbo Zhang, Haiyan Ju, and Zhongmin Deng. 2022. "Chemical Modification of Silk Fibroin through Serine Amino Acid Residues" Materials 15, no. 13: 4399. https://doi.org/10.3390/ma15134399
APA StyleLiu, X., Xia, Q., Zhou, J., Zhang, Y., Ju, H., & Deng, Z. (2022). Chemical Modification of Silk Fibroin through Serine Amino Acid Residues. Materials, 15(13), 4399. https://doi.org/10.3390/ma15134399






