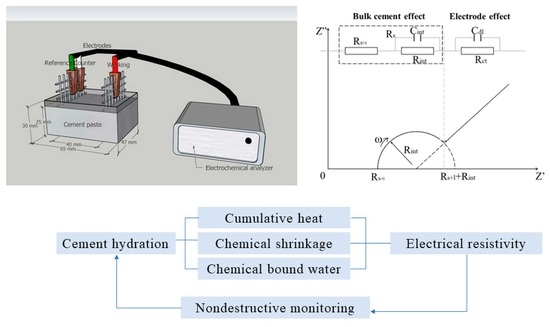
Nondestructive Monitoring Hydration of Belite Calcium Sulfoaluminate Cement by EIS Measurement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydration Heat
2.3. Chemical Shrinkage
2.4. Chemically Bound Water
2.5. Compressive Strength
2.6. Pore Size Distribution
2.7. EIS Measurement
3. Results
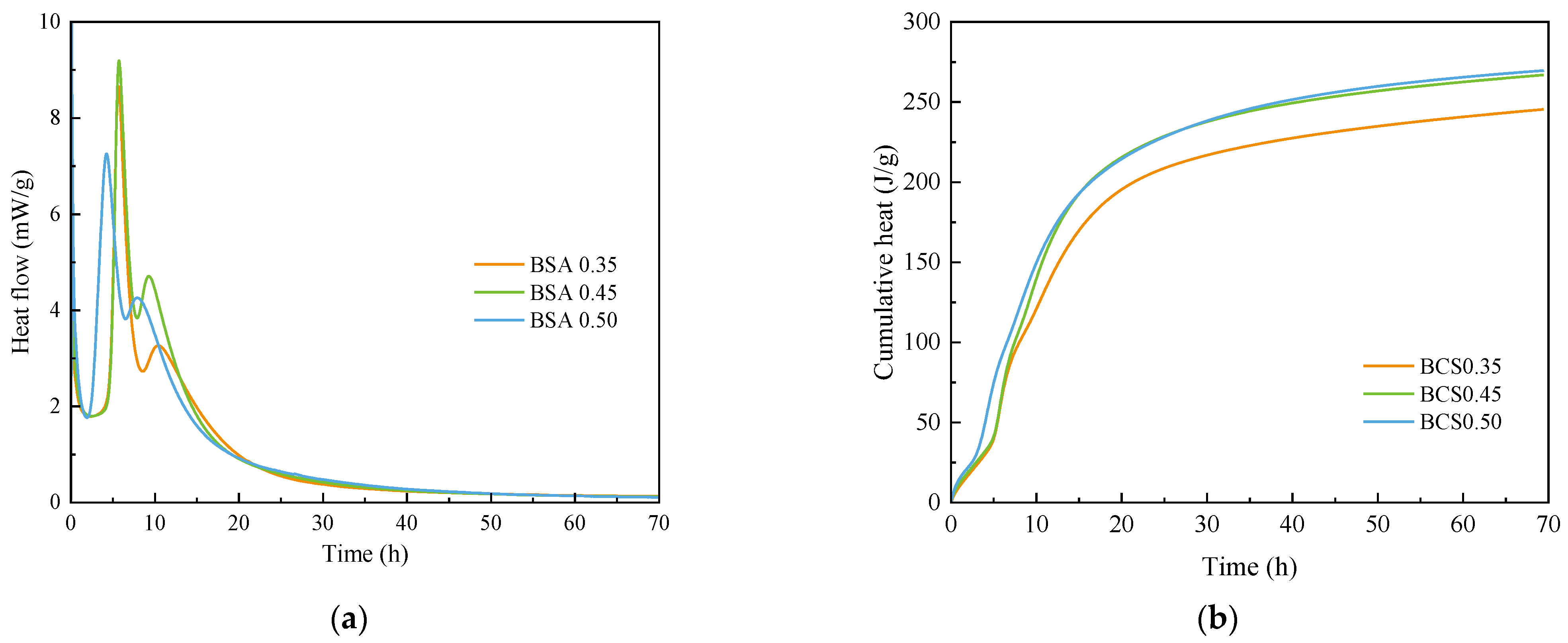
3.1. Hydration Heat Release
3.2. Chemically Bound Water
3.3. Chemical Shrinkage
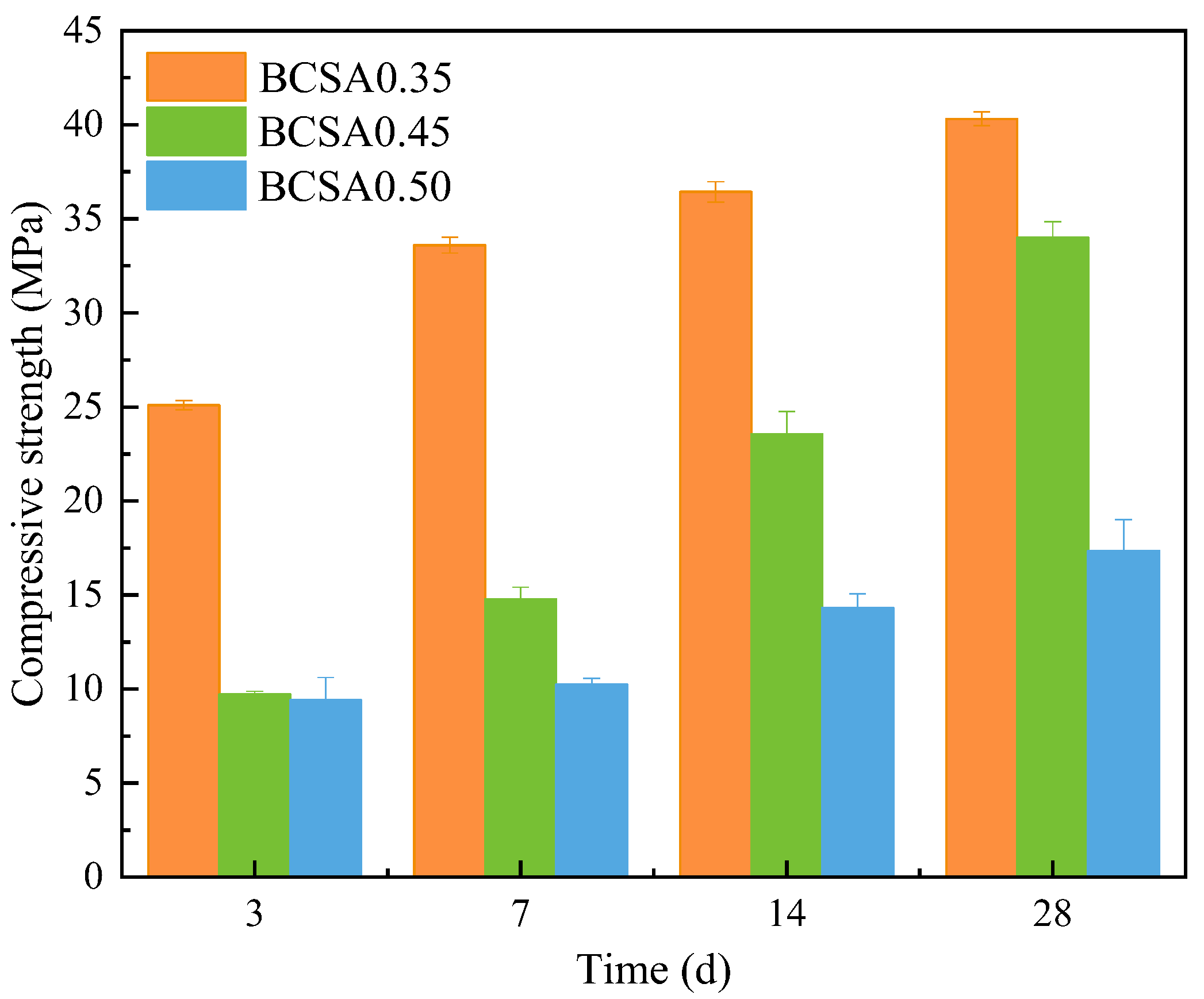
3.4. Compressive Strength
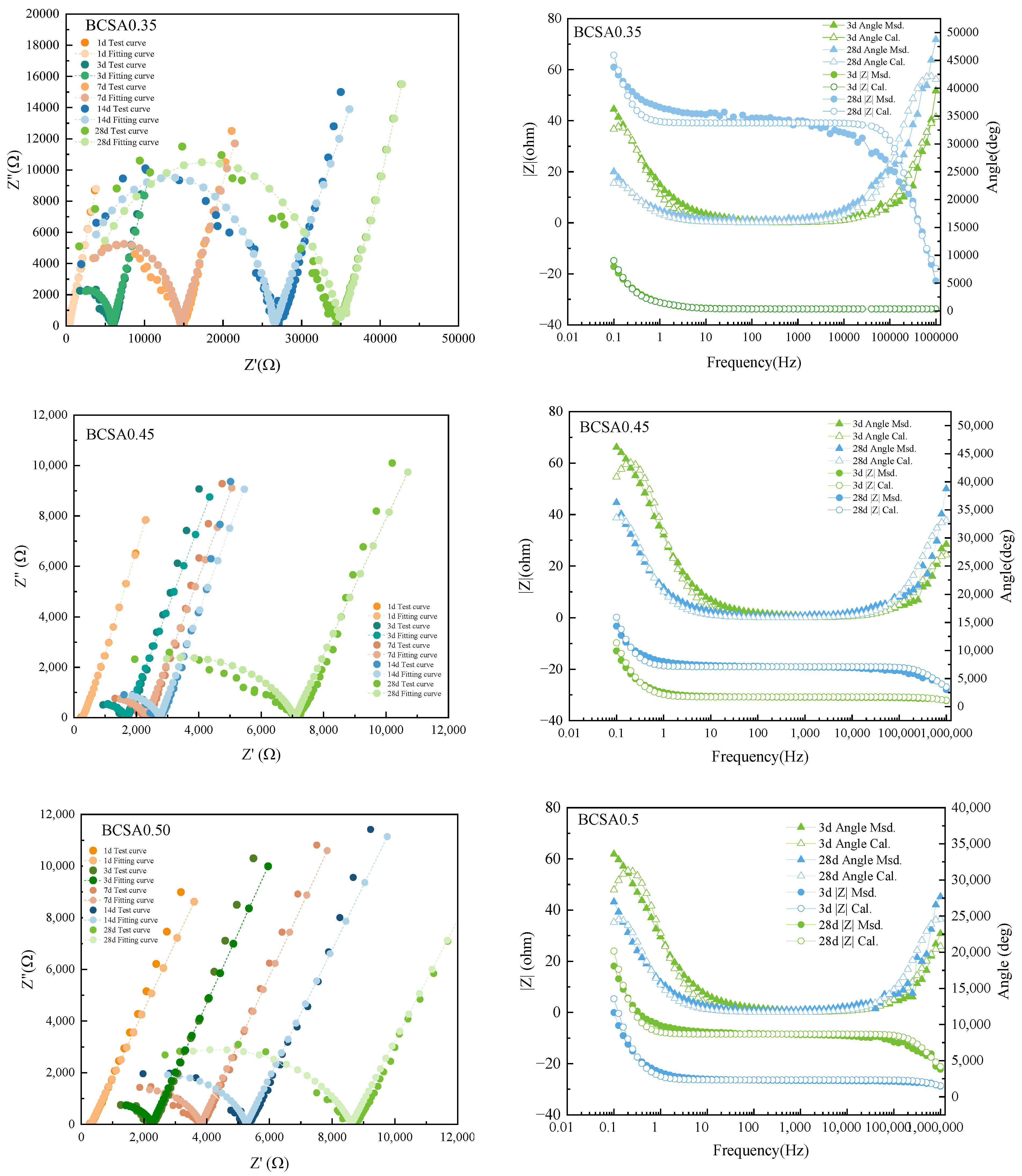
3.5. EIS Measurement
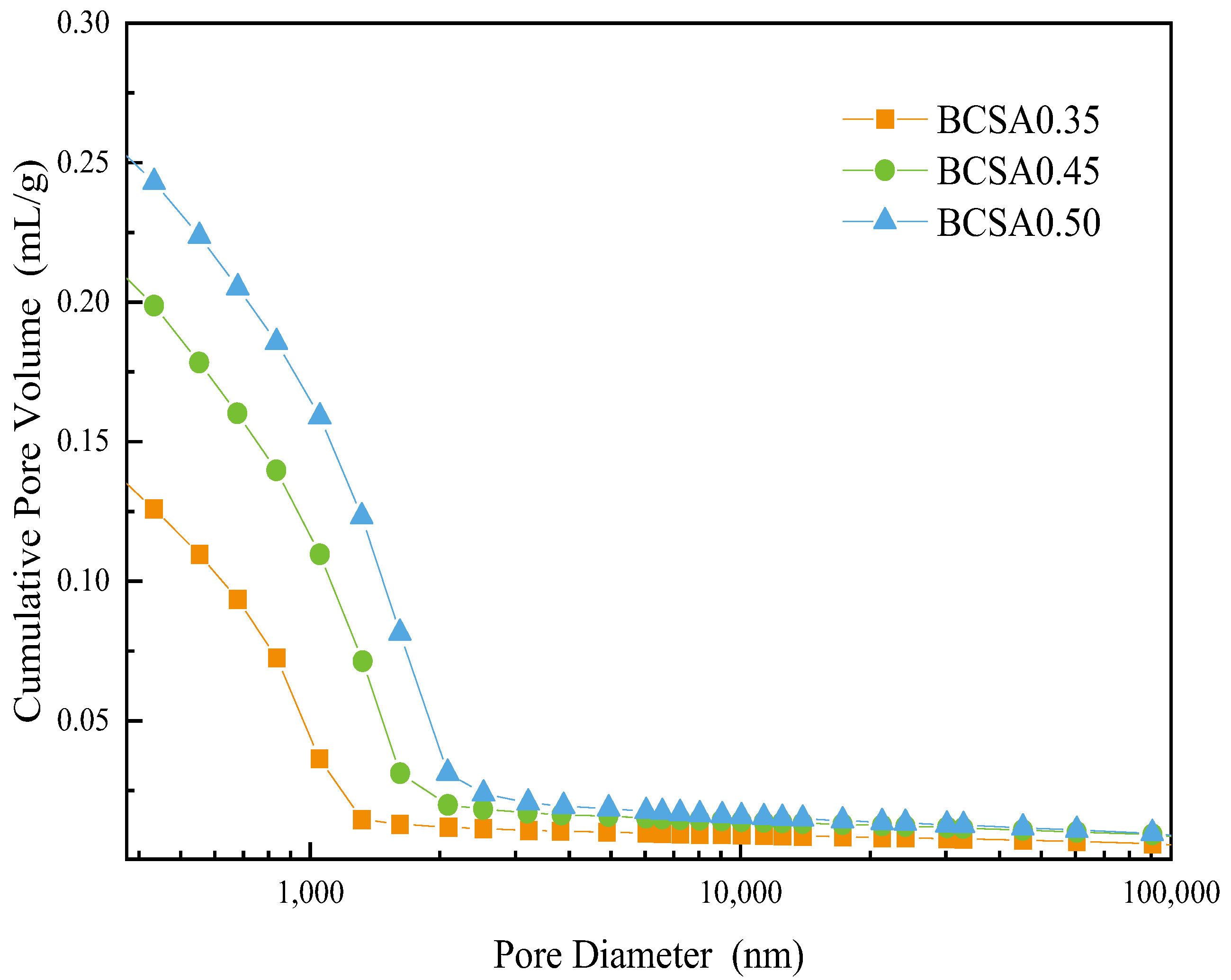
3.6. Porosity Measurement
4. Discussions
4.1. Correlations between Electrical Resistivity and Hydration Heat Release
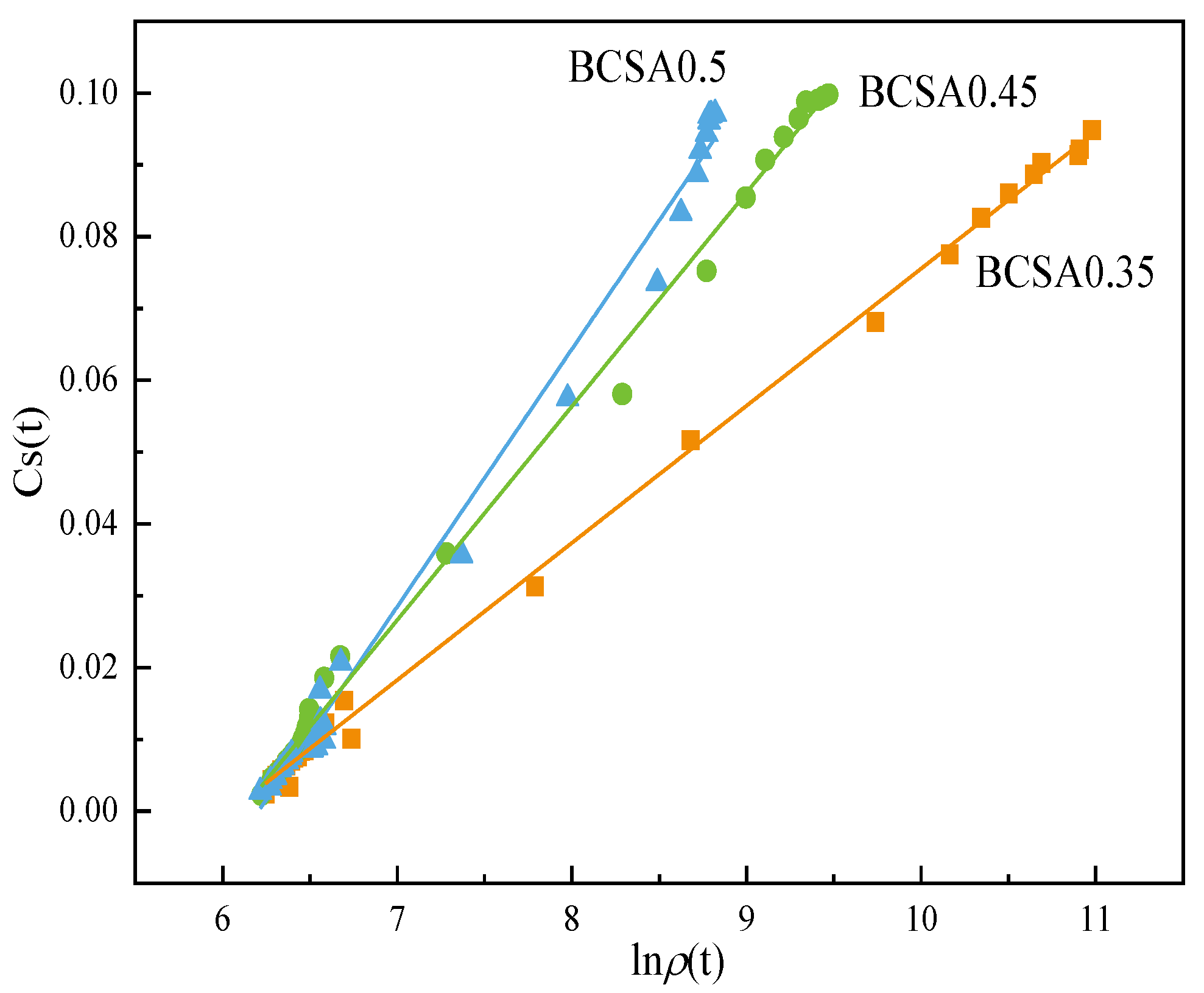
4.2. Correlations between Electrical Resistivity and Chemical Shrinkage
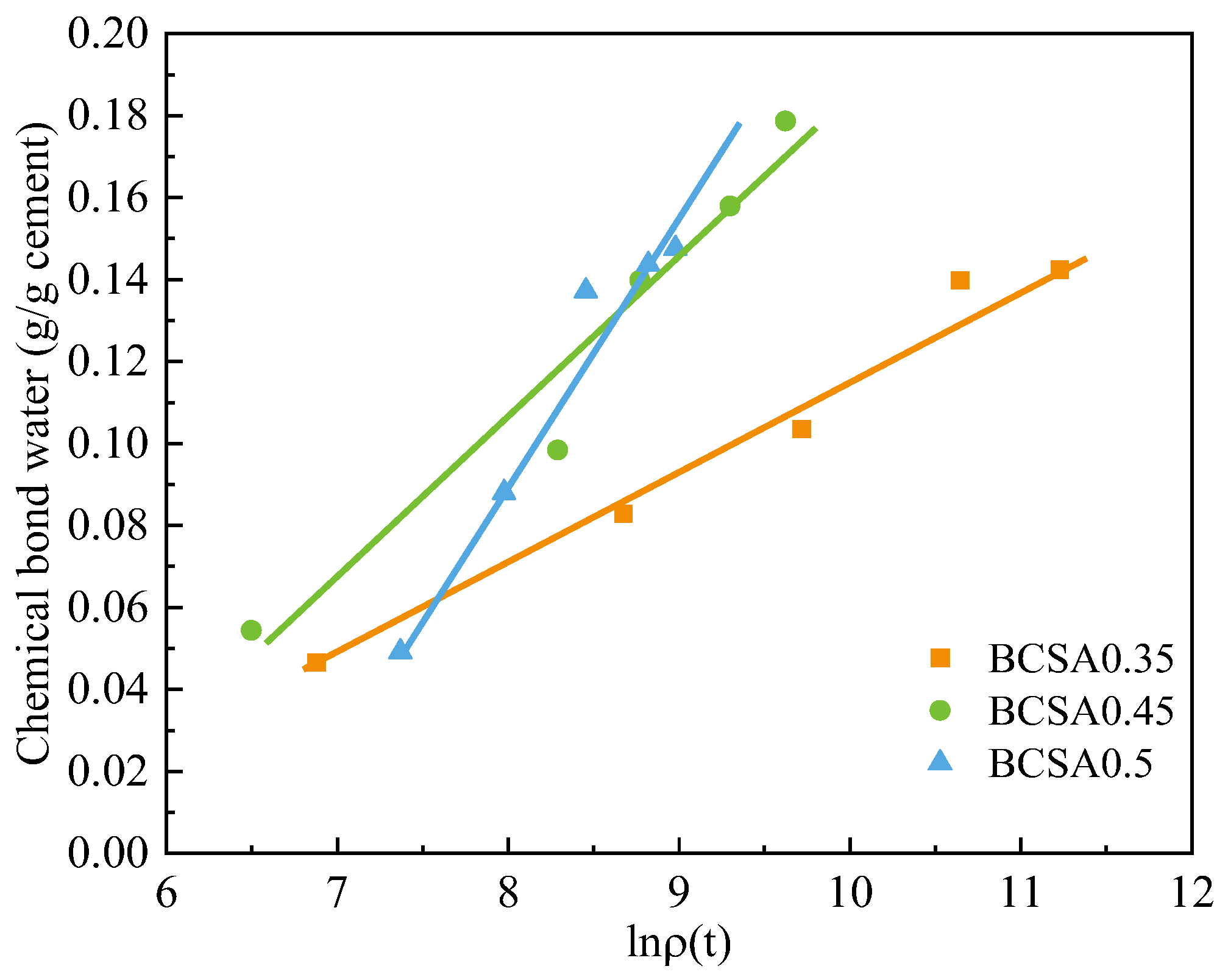
4.3. Correlations between Electrical Resistivity and Chemically Bound Water
4.4. Hydration Degree Evaluation
5. Conclusions
- (1)
- Higher w/c ratios clearly accelerate the hydration of BCSA cement paste. The porosity of BCSA0.5 is 27.5% higher than that of BCSA0.35 and 7.8% higher than that of BCSA0.45.
- (2)
- The electrical resistivity of BCSA0.35 cement paste is more than 4.5 times that of BCSA0.45 and BCSA0.5, due to gradually densified the micropore structure blocking the electrical signal transmission rather than the free charged-ion content. The resistivity is clearly related to the variation in the microstructure, especially for the porosity and pore size distribution during the hydration process.
- (3)
- Linear regression with logarithmic terms of electrical resistivity and classic hydration parameters, such as chemical shrinkage, cumulative hydration heat, and chemically bound water, is built to express the cement hydration degree.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| w/c | Age | R1(ohm) | CPE1(F) | R2(ohm) | CPE2(F) | R3(ohm) |
|---|---|---|---|---|---|---|
| 0.35 | 1 d | 367.4 | 1.379 × 10−4 | 672.3 | 9.489 × 10−5 | 2.433 × 104 |
| 3 d | 1169 | 1.042 × 10−4 | 4857 | 5.659 × 10−11 | 1.893 × 104 | |
| 7 d | 2145 | 8.163 × 10−5 | 1.255 × 104 | 3.684 × 10−11 | 2.125 × 104 | |
| 14 d | 3475 | 6.324 × 10−5 | 2.329 × 104 | 3.277 × 10−11 | 2.413 × 104 | |
| 28 d | 2874 | 5.949 × 10−5 | 3.090 × 104 | 2.401 × 10−11 | 2.491 × 104 | |
| 0.45 | 1 d | 265.4 | 1.654 × 10−4 | 326.5 | 1.244 × 10−4 | 2.718 × 104 |
| 3 d | 720.7 | 1.347 × 10−4 | 1011 | 2.408 × 10−10 | 2.253 × 104 | |
| 7 d | 969.5 | 1.332 × 10−4 | 1412 | 1.633 × 10−10 | 2.401 × 104 | |
| 14 d | 1110 | 1.358 × 10−4 | 1704 | 1.173 × 10−10 | 2.438 × 104 | |
| 28 d | 1718 | 1.245 × 10−4 | 5387 | 5.965 × 10−11 | 2.399 × 104 | |
| 0.55 | 1 d | 323.2 | 8.808 × 10−5 | 524.6 | 1.382 × 10−4 | 2.701 × 104 |
| 3 d | 913.6 | 1.090 × 10−4 | 1419 | 1.628 × 10−10 | 2.174 × 104 | |
| 7 d | 1211 | 1.061 × 10−4 | 2687 | 8.599 × 10−11 | 2.323 × 104 | |
| 14 d | 1730 | 1.012 × 10−4 | 3622 | 7.698 × 10−11 | 2.403 × 104 | |
| 28 d | 2202 | 9.444 × 10−5 | 6469 | 5.248 × 10−11 | 2.517 × 104 |
References
- Schneider, M. The Cement Industry On the Way to a Low-Carbon Future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Julphunthong, P. Synthesizing of Calcium Sulfoaluminate-Belite (CSAB) Cements From Industrial Waste Materials. Mater. Today Proc. 2018, 5 Pt 1, 14933–14938. [Google Scholar] [CrossRef]
- Borštnar, M.; Daneu, N.; Dolenec, S. Phase Development and Hydration Kinetics of Belite-Calcium Sulfoaluminate Cements at Different Curing Temperatures. Ceram. Int. 2020, 46, 29421–29428. [Google Scholar] [CrossRef]
- Popescu, C.D.; Muntean, M.; Sharp, J.H. Industrial Trial Production of Low Energy Belite Cement. Cem. Concr. Comp. 2003, 25, 689–693. [Google Scholar] [CrossRef]
- Wang, D.; Noguchi, T.; Nozaki, T.; Higo, Y. Investigation On the Fast Carbon Dioxide Sequestration Speed of Cement-Based Materials at 300 °C–700 °C. Constr. Build. Mater. 2021, 291, 123392. [Google Scholar] [CrossRef]
- Dolenec, S.; Šter, K.; Borštnar, M.; Nagode, K.; Ipavec, A.; Žibret, L. Effect of the Cooling Regime on the Mineralogy and Reactivity of Belite-Sulfoaluminate Clinkers. Minerals 2020, 10, 910. [Google Scholar] [CrossRef]
- Stanek, T.; Sulovsky, P. Active Low-Energy Belite Cement. Cem. Concr. Res. 2015, 68, 203–210. [Google Scholar] [CrossRef]
- Rungchet, A.; Chindaprasirt, P.; Wansom, S.; Pimraksa, K. Hydrothermal Synthesis of Calcium Sulfoaluminate-Belite Cement From Industrial Waste Materials. J. Clean. Prod. 2016, 115, 273–283. [Google Scholar] [CrossRef]
- Martín-Sedeño, M.C.; Cuberos, A.J.M.; De la Torre, Á.G.; Álvarez-Pinazo, G.; Ordónez, L.M.; Gateshki, M.; Aranda, M.A.G. Aluminum-Rich Belite Sulfoaluminate Cements: Clinkering and Early Age Hydration. Cem. Concr. Res. 2010, 40, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Morin, V.; Termkhajornkit, P.; Huet, B.; Pham, G. Impact of Quantity of Anhydrite, Water to Binder Ratio, Fineness On Kinetics and Phase Assemblage of Belite-Ye’elimite-Ferrite Cement. Cem. Concr. Res. 2017, 99, 8–17. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Müller, C.J.; Lothenbach, B. Using Gypsum to Control Hydration Kinetics of CSA Cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, D.; Zhang, L.; Ouyang, J.; Yu, X.; Cui, X.; Han, B. Comparison of Compressive Strength and Electrical Resistivity of Cementitious Composites with Different Nano- and Micro-Fillers. Arch. Civ. Mech. Eng. 2018, 18, 60–68. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Chen, S.J.; Liu, Y.M.; Duan, W.H.; Shah, S.P. Effects of Graphene Oxide On Early-Age Hydration and Electrical Resistivity of Portland Cement Paste. Constr. Build. Mater. 2017, 136, 506–514. [Google Scholar] [CrossRef]
- Yim, H.J.; Lee, H.; Kim, J.H. Evaluation of of Mortar Setting Time by Using Electrical Resistivity Measurements. Constr. Build. Mater. 2017, 146, 679–686. [Google Scholar] [CrossRef]
- Farzanian, K.; Pimenta Teixeira, K.; Perdigão Rocha, I.; De Sa Carneiro, L.; Ghahremaninezhad, A. The Mechanical Strength, Degree of Hydration, and Electrical Resistivity of Cement Pastes Modified with Superabsorbent Polymers. Constr. Build. Mater. 2016, 109, 156–165. [Google Scholar] [CrossRef]
- Wei, X.; Xiao, L. Kinetics Parameters of Cement Hydration by Electrical Resistivity Measurement and Calorimetry. Adv. Cem. Res. 2014, 26, 187–193. [Google Scholar] [CrossRef]
- Fu, C.; Huang, J.; Dong, Z.; Yan, W.; Gu, X. Experimental and Numerical Study of an Electromagnetic Sensor for Non-Destructive Evaluation of Steel Corrosion in Concrete. Sens. Actuators A Phys. 2020, 315, 112371. [Google Scholar] [CrossRef]
- Bolborea, B.; Baera, C.; Dan, S. Concrete Compressive Strength by Means of Ultrasonic Pulse Velocity and Moduli of Elasticity. Materials 2021, 14, 7018. [Google Scholar] [CrossRef]
- Breysse, D.; Martínez-Fernández, J.L. Assessing Concrete Strength with Rebound Hammer: Review of Key Issues and Ideas for More Reliable Conclusions. Mater. Struct. 2014, 47, 1589–1604. [Google Scholar] [CrossRef]
- Martín-del-Rio, J.J.; Canivell, J.; Falcón, R.M. The Use of Non-Destructive Testing to Evaluate the Compressive Strength of a Lime-Stabilised Rammed-Earth Wall: Rebound Index and Ultrasonic Pulse Velocity. Constr. Build. Mater. 2020, 242, 118060. [Google Scholar] [CrossRef]
- Pucinotti, R. Reinforced Concrete Structure: Non Destructive in Situ Strength Assessment of Concrete. Constr. Build. Mater. 2015, 75, 331–341. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M.; Soni, P.A. Advances and Researches On Non Destructive Testing: A Review. Mater. Today Proc. 2018, 5 Pt 1, 3690–3698. [Google Scholar] [CrossRef]
- Da Silva, G.F.; Martini, S.; Moraes, J.C.B.; Teles, L.K. AC Impedance Spectroscopy (AC-IS) Analysis to Characterize the Effect of Nanomaterials in Cement-Based Mortars. Constr. Build. Mater. 2021, 269, 121260. [Google Scholar] [CrossRef]
- Hu, X.; Shi, C.; Liu, X.; Zhang, J.; de Schutter, G. A Review On Microstructural Characterization of Cement-Based Materials by AC Impedance Spectroscopy. Cem. Concr. Compos. 2019, 100, 1–14. [Google Scholar] [CrossRef]
- Shakir Abbood, I.; Weli, S.S.; Hamid, F.L. Cement-Based Materials for Self-Sensing and Structural Damage Advance Warning Alert by Electrical Resistivity. Mater. Today Proc. 2021, 46, 615–620. [Google Scholar] [CrossRef]
- Da Silveira, G.D.; de Carvalho, L.M.; Montoya, N.; Domenech-Carbó, A. Evaluation of Aging Processes of Petroleum Asphalt Cements by Solid State Electrochemical Monitoring. Electrochim. Acta 2018, 270, 461–470. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Yan, C.; Yin, L.; Wang, X.; Liu, S. Hydration Characteristics of Low Carbon Cementitious Materials with Multiple Solid Wastes. Constr. Build. Mater. 2022, 322, 126366. [Google Scholar] [CrossRef]
- Chi, L.; Wang, Z.; Lu, S.; Zhao, D.; Yao, Y. Development of Mathematical Models for Predicting the Compressive Strength and Hydration Process Using the EIS Impedance of Cementitious Materials. Constr. Build. Mater. 2019, 208, 659–668. [Google Scholar] [CrossRef]
- Chi, L.; Li, W.; Li, Z.; Wang, Z.; Lu, S.; Liu, Q. Investigation of the Hydration Properties of Cement with EDTA by Alternative Current Impedance Spectroscopy. Cem. Concr. Compos. 2022, 126, 104365. [Google Scholar] [CrossRef]
- ASTM C 1608-17; Standard Test Method for Chemical Shrinkage of Hydraulic Cement Paste. American Society for Testing and Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Powers, D.R.; Brace, R.A. Fetal Cardiovascular and Fluid Responses to Maternal Volume Loading with Lactated Ringer Or Hypotonic Solution. Am. J. Obstet. Gynecol. 1991, 165, 1504–1515. [Google Scholar] [CrossRef]
- ASTM C109/C109M-21; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-In. Or [50mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C1702-17; Standard Test Method for Measurement of Heat of Hydration of Hydraulic Cementitious Materials Using Isothermal Conduction Calorimetry. ASTM International: West Conshohocken, PA, USA, 2017.
- Dong, Z.; Torbati-Sarraf, H.; Hussein, H.Z.; Poursaee, A. Harmonic Analysis On the Effect of Potential Perturbations and Electrodes Arrangements On the Electrochemical Impedance (EIS) Measurement of Cementitious Material. Constr. Build. Mater. 2021, 273, 121701. [Google Scholar] [CrossRef]
- Tambara, L.U.D.; Cheriaf, M.; Rocha, J.C.; Palomo, A.; Fernández-Jiménez, A. Effect of Alkalis Content On Calcium Sulfoaluminate (CSA) Cement Hydration. Cem. Concr. Res. 2020, 128, 105953. [Google Scholar] [CrossRef]
- Madej, D.; Kruk, A. Monitoring Hydration of Sr-doped Calcium Zirconium Aluminate (Ca, Sr)7ZrAl6O18 Cement Via Electrochemical Impedance Spectroscopy (EIS) and Supported Techniques. Constr. Build. Mater. 2019, 206, 307–320. [Google Scholar] [CrossRef]
- Hay, R.; Li, L.; Celik, K. Shrinkage, Hydration, and Strength Development of Limestone Calcined Clay Cement (LC3) with Different Sulfation Levels. Cem. Concr. Compos. 2022, 127, 104403. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Wu, Y.; Zhou, Y.; Tang, S. Hydration, Shrinkage, Pore Structure and Fractal Dimension of Silica Fume Modified Low Heat Portland Cement-Based Materials. Constr. Build. Mater. 2021, 272, 121952. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Evaluation of the Corrosion Resistance of Phosphate Coatings Deposited on the Surface of the Carbon Steel Used for Carabiners Manufacturing. Appl. Sci. 2020, 10, 2753. [Google Scholar] [CrossRef]
- Gu, P.; Xie, P.; Beaudoin, J.J.; Brousseau, R.A.C. Impedance Spectroscopy (I): A New Equivalent Circuit Model for Hydrated Portland Cement Paste. Cem. Concr. Res. 1992, 22, 833–840. [Google Scholar] [CrossRef]
- Tang, S.W.; Cai, X.H.; He, Z.; Zhou, W.; Shao, H.Y.; Li, Z.J.; Wu, T.; Chen, E. The Review of Early Hydration of Cement-Based Materials by Electrical Methods. Constr. Build. Mater. 2017, 146, 15–29. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, J.; Wang, Y.; Fang, G.; Liu, Y.; Xing, F. Evolutionary Trace for Early Hydration of Cement Paste Using Electrical Resistivity Method. Constr. Build. Mater. 2016, 119, 16–20. [Google Scholar] [CrossRef]
- Liao, Y.; Wei, X. Relationship between Chemical Shrinkage and Electrical Resistivity for Cement Pastes at Early Age. J. Mater. Civ. Eng. 2014, 26, 384–387. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Tao, Z.; Wang, K. Piezoresistive Properties of Cement-Based Sensors: Review and Perspective. Constr. Build. Mater. 2019, 203, 146–163. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, P.; Yan, C. Role of Layered Double Hydroxides in Setting, Hydration Degree, Microstructure and Compressive Strength of Cement Paste. Appl. Clay Sci. 2018, 158, 123–131. [Google Scholar] [CrossRef]
- He, H.; Zhu, Y.; Zhou, A. Electrochemical Impedance Spectroscopy (EIS) Used to Evaluate Influence of Different External Pressures, Curing Ages and Self-Healing Environments On the Self-Healing Behavior of Engineered Cementitious Composites (ECC). Constr. Build. Mater. 2018, 188, 153–160. [Google Scholar] [CrossRef]
- Sanish, K.B.; Neithalath, N.; Santhanam, M. Monitoring the Evolution of Material Structure in Cement Pastes and Concretes Using Electrical Property Measurements. Constr. Build. Mater. 2013, 49, 288–297. [Google Scholar] [CrossRef]
- Tang, S.W.; Cai, X.H.; He, Z.; Zhou, W.; Shao, H.Y.; Li, Z.J.; Wu, T.; Chen, E. The Review of Pore Structure Evaluation in Cementitious Materials by Electrical Methods. Constr. Build. Mater. 2016, 117, 273–284. [Google Scholar] [CrossRef]
- Klaver, J.; Hemes, S.; Houben, M.; Desbois, G.; Radi, Z.; Urai, J.L. The Connectivity of Pore Space in Mudstones: Insights From High-Pressure Wood’s Metal Injection, BIB-SEM Imaging, and Mercury Intrusion Porosimetry. Geofluids 2015, 15, 577–591. [Google Scholar] [CrossRef]
- Rajabipour, F.; Weiss, J. Electrical Conductivity of Drying Cement Paste. Mater. Struct. 2007, 40, 1143–1160. [Google Scholar] [CrossRef]





| SiO2 | CaO | Fe2O3 | Al2O3 | Na2O | K2O | MgO | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|
| 14.16 | 49.6 | 2.69 | 21.65 | 0.13 | 0.25 | 0.96 | 9.54 | 1.02 |
| BCSA0.35 | BCSA0.45 | BCSA0.50 | |
|---|---|---|---|
| Porosity | 38.01 | 44.94 | 48.45 |
| Tortuosity | 88.61 | 63.82 | 51.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, L.; Wang, M.; Wang, Z.; Li, Z.; Peng, B.; Li, J. Nondestructive Monitoring Hydration of Belite Calcium Sulfoaluminate Cement by EIS Measurement. Materials 2022, 15, 4433. https://doi.org/10.3390/ma15134433
Chi L, Wang M, Wang Z, Li Z, Peng B, Li J. Nondestructive Monitoring Hydration of Belite Calcium Sulfoaluminate Cement by EIS Measurement. Materials. 2022; 15(13):4433. https://doi.org/10.3390/ma15134433
Chicago/Turabian StyleChi, Lin, Mian Wang, Zhuolin Wang, Zhenming Li, Bin Peng, and Junjie Li. 2022. "Nondestructive Monitoring Hydration of Belite Calcium Sulfoaluminate Cement by EIS Measurement" Materials 15, no. 13: 4433. https://doi.org/10.3390/ma15134433
APA StyleChi, L., Wang, M., Wang, Z., Li, Z., Peng, B., & Li, J. (2022). Nondestructive Monitoring Hydration of Belite Calcium Sulfoaluminate Cement by EIS Measurement. Materials, 15(13), 4433. https://doi.org/10.3390/ma15134433