Effect of Psyllium Husk Addition on the Structural and Physical Properties of Biodegradable Thermoplastic Starch Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Films
2.3. Microstructural Analysis
2.3.1. Stereoscopic Microscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Atomic Force Microscopy (AFM)
2.4. Surface Characterization
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Water Contact Angle Measurements (WCA)
2.4.3. Water Sorption Isotherms
2.5. Barrier Properties
2.5.1. Water Vapor Permeability (WVP)
2.5.2. UV-Vis Barrier Properties (Opacity and Light Transmittance)
2.6. Tensile Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microstructural Analysis
3.2. Surface Characterization
3.2.1. Fourier Transform Infrared (FTIR) Spectroscopy
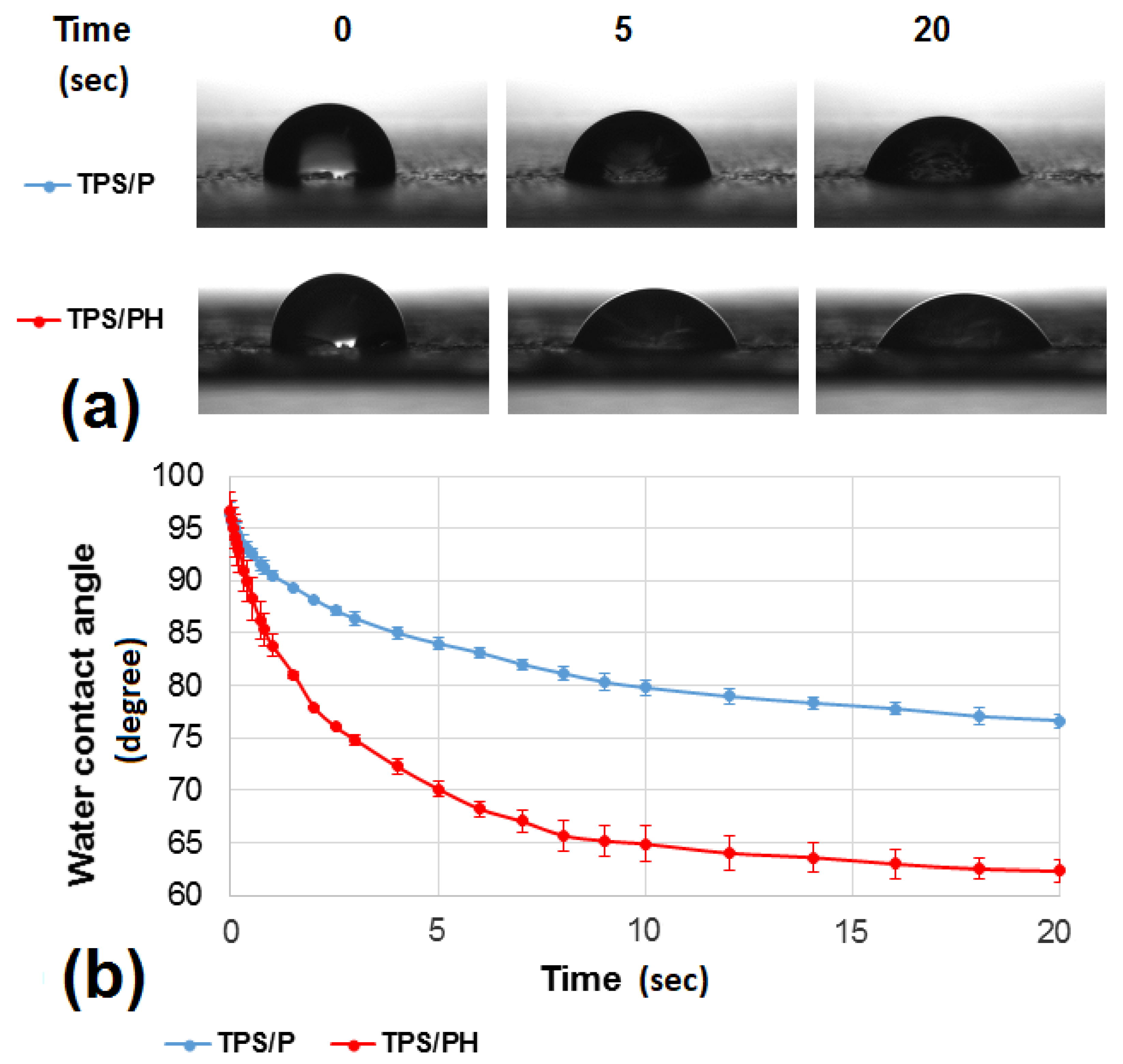
3.2.2. Contact Angle Measurements
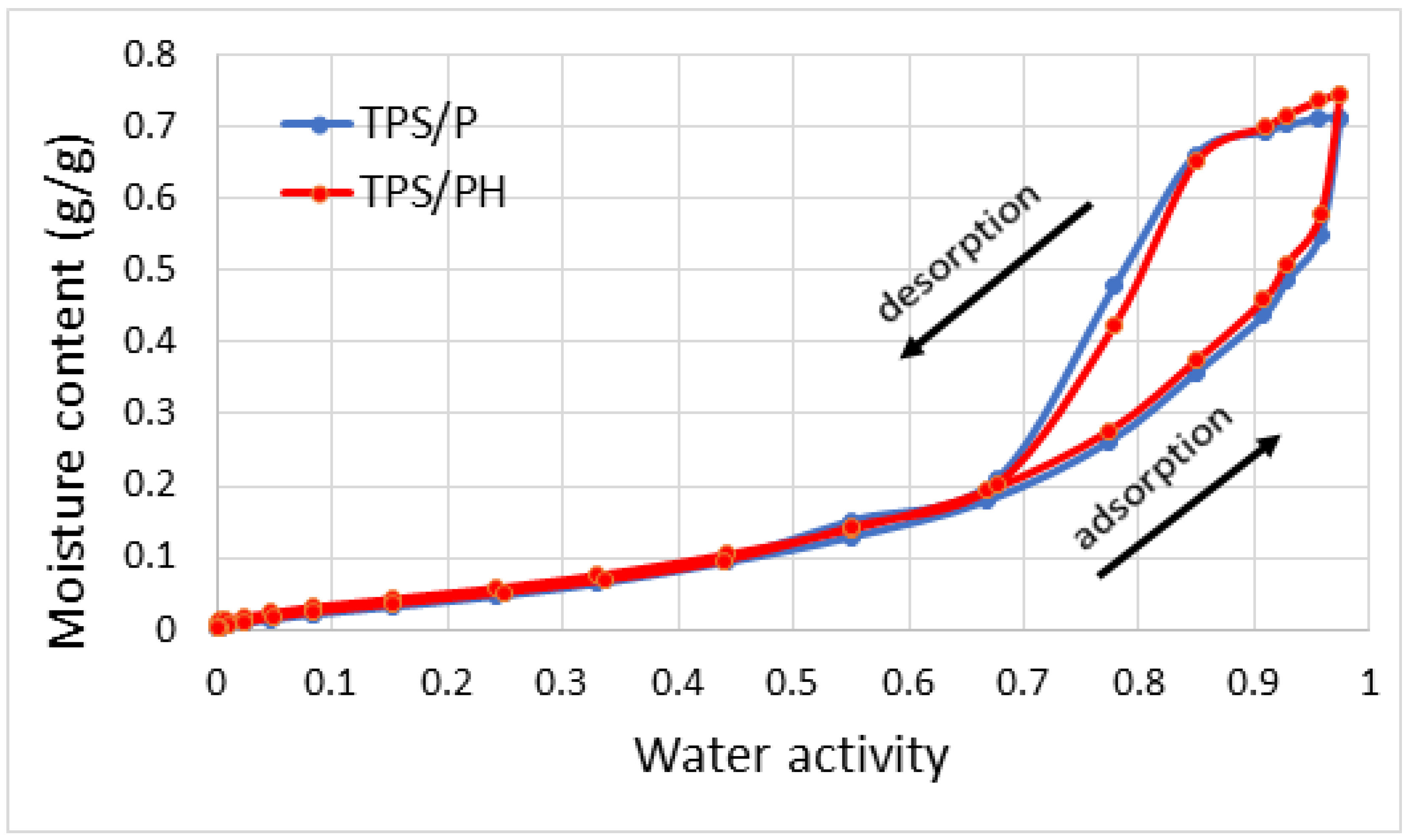
3.2.3. Water Sorption Isotherms
3.3. Barrier Properties
3.3.1. Water Vapor Permeability (WVP)
3.3.2. UV-Vis Barrier Properties (Opacity and Light Transmittance)
3.4. Tensile Properties
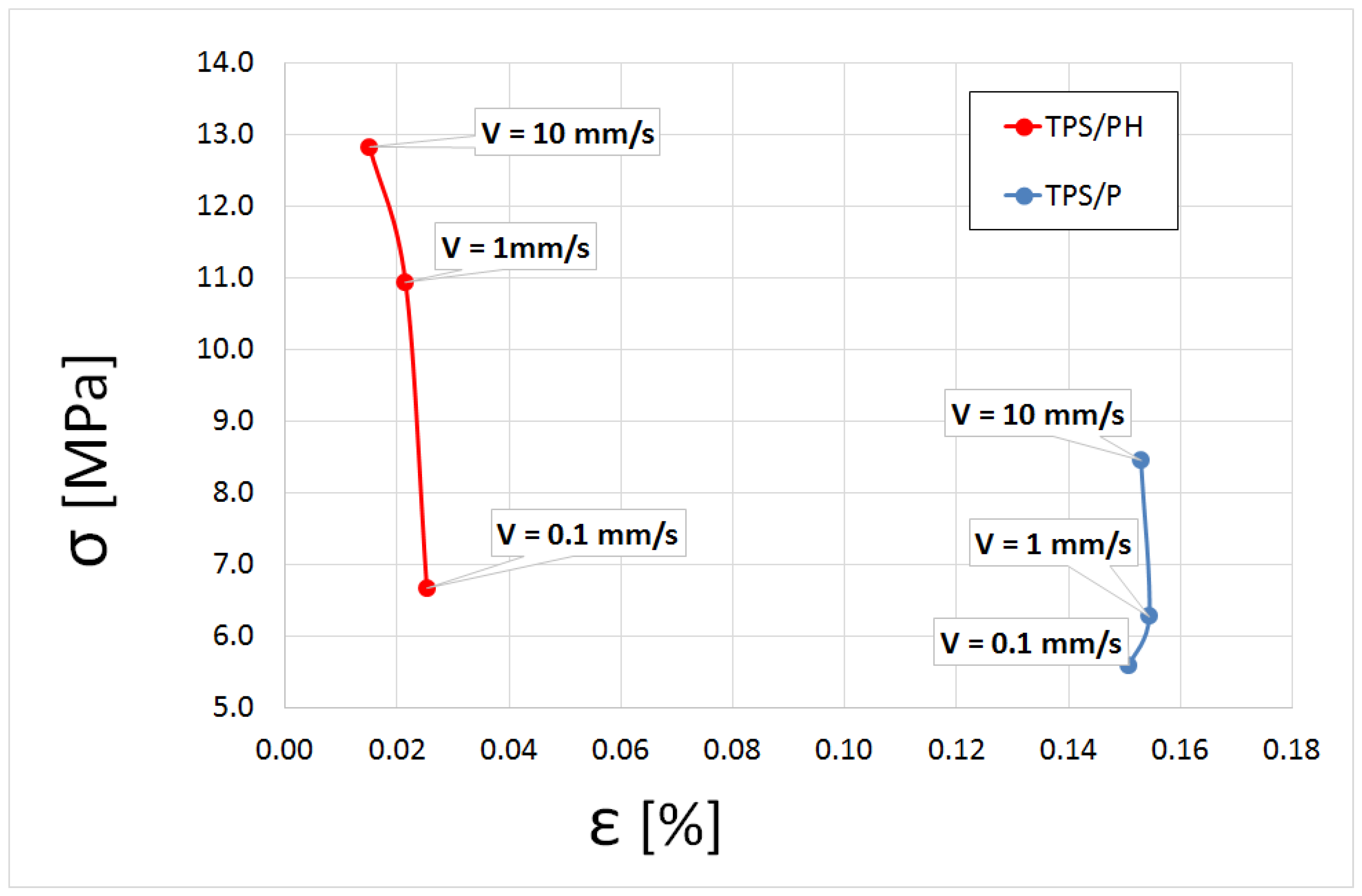
3.4.1. Tensile Tests Analysis
3.4.2. Fracture Surface Analyses (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nomenclature and Greek Symbols | |
| Eav | average adsorption energy (∆E/RT) |
| ∆E | difference between energy of adsorption (Ea) and condensation (Ec) of water vapor |
| R | universal gas constant (J·mol−1·K−1) |
| T | temperature (K) |
| D | surface fractal dimension |
| A | effective area of films (m2) |
| ∆m | the weight change of the permeation vessel (g) |
| t | time (s) |
| O600 | transparency (AU/mm) |
| T600 | transmittance at 600 nm (%) |
| Fmax | maximum force (N) |
| Ra | arithmetical mean deviation of the roughness profile (nm) |
| Rq | root-mean-square deviation of the roughness profile (nm) |
| Rp | maximum peak height of the roughness profile (nm) |
| Rv | maximum valley depth of the roughness profile (nm) |
| σf | failure stress (MPa) |
| εf | failure strain (%) |
| V | deformation velocities (mm/s) |
| δOH | OH bending vibrations |
| H-bonds | hydrogen bonding |
| Abbreviations | |
| TPS/P | pure thermoplastic starch film |
| TPS/PH | thermoplastic starch film + psyllium husk |
| PH | psyllium husk |
| UV | ultraviolet |
| WVP | water vapor permeability (g·m−1 s−1 Pa−1) |
| RH | relative humidity (%) |
| WCA | water contact angle (deg) |
| SSA | specific surface area (m2/g) |
| WVTR | water vapor transmission (g/s) |
| AX | arabinoxylans |
References
- Tóth, A.; Halász, K. Characterization of edible biocomposite films directly prepared from psyllium seed husk and husk flour. Food Packag. Shelf Life 2019, 20, 100299. [Google Scholar] [CrossRef]
- EU Directive 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment (Text with EEA Relevance) PE/11/2019/REV/. Available online: http://data.europa.eu/eli/dir/2019/904/oj (accessed on 2 March 2022).
- Single-Use Plastics: New EU Rules to Reduce Marine Litter. Available online: https://ec.europa.eu/commission/presscorner/detail/en/MEMO_18_3909 (accessed on 19 May 2022).
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Llamazares, S.R. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 3, 136–177. [Google Scholar] [CrossRef]
- Temesgen, S.; Rennert, M.; Tesfaye, T.; Nase, M. Review on Spinning of Biopolymer Fibers from Starch. Polymers 2021, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2019, 3, 8–18. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Thermoplastic starch blown films with improved mechanical and barrier properties. Int. J. Biol. Macromol. 2021, 188, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Avérous, L.; Boquillon, N. Biocomposites based on plasticized starch: Thermal and mechanical behaviors. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Tosif, M.; Najda, A.; Bains, A.; Zawiślak, G.; Maj, G.; Chawla, P. Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective. Polymers 2021, 13, 2588. [Google Scholar] [CrossRef]
- Ren, J.; Dang, K.M.; Pollet, E.; Avérous, L. Preparation and Characterization of Thermoplastic Potato Starch/Halloysite Nano-Biocomposites: Effect of Plasticizer Nature and Nanoclay Content. Polymers 2018, 10, 808. [Google Scholar] [CrossRef] [Green Version]
- Girijappa, Y.G.T.; Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019, 6, 226. [Google Scholar] [CrossRef]
- Yang, J.; Tang, K.; Qin, G.; Chen, Y.; Peng, L.; Wan, X.; Xiao, H.; Xia, Q. Hydrogen bonding energy determined by molecular dynamics simulation and correlation to properties of thermoplastic starch films. Carbohydr. Polym. 2017, 166, 256–263. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gołacki, K.; Stropek, Z.; Kołodziej, P.; Gładyszewska, B.; Zaremba, M.; Rejak, A. Effect of additives on strength characteristics of a biodegradable starch film. Przem. Chem. 2014, 93, 728–731. [Google Scholar]
- Khan, B.; Niazi, M.B.K.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material-A Review. J. Food Process Eng. 2016, 40, e12447. [Google Scholar] [CrossRef]
- Stropek, Z.; Gołacki, K.; Kołodziej, P.; Gładyszewska, B.; Samociuk, W.; Rejak, A. Effect of polyvinyl alcohol and keratin on stress relaxation course in thermoplastic starch. Przem. Chem. 2014, 93, 364–367. [Google Scholar]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Grzesiakowska, A.; Kuchta-Gładysz, M.; Kawecka, A.; Grzebieniarz, W.; Nowak, N. The Functional and Application Possibilities of Starch/Chitosan Polymer Composites Modified by Graphene Oxide. Int. J. Mol. Sci. 2022, 23, 5956. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, R.; Kalbasi-Ashtari, A.; Oromiehie, A.; Yarmand, M.-S.; Jahandideh, F. Development and characterization of a novel biodegradable edible film obtained from psyllium seed (Plantago ovata Forsk). J. Food Eng. 2012, 109, 745–751. [Google Scholar] [CrossRef]
- Patil, B.S.; Mastiholimath, V.S.; Kulkarni, A.R. Development and evaluation of psyllium seed husk polysaccharide based wound dressing films. Orient. Pharm. Exp. Med. 2011, 11, 123–129. [Google Scholar] [CrossRef]
- Hussain, M.A.; Muhammad, G.; Jantan, I.; Bukhari, S.N.A. Psyllium Arabinoxylan: A Versatile Biomaterial for Potential Medicinal and Pharmaceutical Applications. Polym. Rev. 2015, 56, 1–30. [Google Scholar] [CrossRef]
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity Homogeneity and Structure Affect Water Vapor Permeability of Model Edible Films. J. Food Sci. 1993, 58, 426–429. [Google Scholar] [CrossRef]
- Fischer, M.H.; Yu, N.; Gray, G.R.; Ralph, J.; Anderson, L.; Marlett, J.A. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr. Res. 2004, 339, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Sukhija, S.; Singh, S.; Riar, C.S. Analyzing the effect of whey protein concentrate and psyllium husk on various characteristics of biodegradable film from lotus (Nelumbo nucifera) rhizome starch. Food Hydrocoll. 2016, 60, 128–137. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Physical, Mechanical, Morphological, and Barrier Properties of Elephant Foot Yam Starch, Whey Protein Concentrate and psyllium Husk Based Composite Biodegradable Films. Polym. Compos. 2018, 39, E407–E415. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Development and characterization of biodegradable films from whey protein concentrate, psyllium husk and oxidized, crosslinked, dual-modified lotus rhizome starch composite. J. Sci. Food Agric. 2019, 99, 3398–3409. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Shi, Q. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol. 2020, 153, 892–901. [Google Scholar] [CrossRef]
- Karel, M. Water activity and food preservation. In Physical Principles of Food Preservation—Principles of Food Science—Part 2; Karel, M., Fennema, O.R., Lund, D.B., Eds.; Marcel Dekker: New York, NY, USA, 1975; pp. 237–263. [Google Scholar]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Lukowska, M.; Szerement, J. Determination of Energetic and Geometric Properties of Plant Roots Specific Surface from Adsorption/Desorption Ishoterm. Am. J. Plant Sci. 2013, 4, 1554–1561. [Google Scholar] [CrossRef] [Green Version]
- Sokołowska, Z.; Hajnos, M.; Borówko, M.; Sokołowski, S. Adsorption of Nitrogen on Thermally Treated Peat Soils: The Role of Energetic and Geometric Heterogeneity. J. Colloid Interface Sci. 1999, 219, 1–10. [Google Scholar] [CrossRef]
- Souza, A.; Benze, R.; Ferrão, E.; Ditchfield, C.; Coelho, A.; Tadini, C. Cassava starch biodegradable films: Influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT 2011, 46, 110–117. [Google Scholar] [CrossRef]
- ASTM. Standard test methods for water vapor transmission of materials. Standards designations: E96/E96M-16. In Annual Book of ASTM Standards; American Society for Testing and Material: Philadelphia, PA, USA, 2016. [Google Scholar]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, C.; Zhai, X.; Zhang, R.; Zhang, S.; Sun, C.; Wang, W.; Hou, H. Effect of lipids with different physical state on the physicochemical properties of starch/gelatin edible films prepared by extrusion blowing. Int. J. Biol. Macromol. 2021, 185, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gao, S.; Wang, W.; Hou, H.; Lim, L.-T. Low temperature extrusion blown ε-polylysine hydrochloride-loaded starch/gelatin edible antimicrobial films. Carbohydr. Polym. 2021, 278, 118990. [Google Scholar] [CrossRef]
- Huntrakul, K.; Yoksan, R.; Sane, A.; Harnkarnsujarit, N. Effects of pea protein on properties of cassava starch edible films produced by blown-film extrusion for oil packaging. Food Packag. Shelf Life 2020, 24, 100480. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Jia, R.; Dai, Y.; Dong, H.; Hou, H.; Guo, Q. High performance extrusion blown starch/polyvinyl alcohol/clay nanocomposite films. Food Hydrocoll. 2018, 79, 534–543. [Google Scholar] [CrossRef]
- Acosta, S.; Jiménez, A.; Cháfer, M.; González-Martínez, C.; Chiralt, A. Physical properties and stability of starch-gelatin based films as affected by the addition of esters of fatty acids. Food Hydrocoll. 2015, 49, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.J.; Álvarez, V. Bionanocomposite films developed from corn starch and natural and modified nano-clays with or without added blueberry extract. Food Hydrocoll. 2018, 77, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Kwaśniewska, A.; Świetlicki, M.; Prószyński, A.; Gładyszewski, G. The Quantitative Nanomechanical Mapping of Starch/Kaolin Film Surfaces by Peak Force AFM. Polymers 2021, 13, 244. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccharides with use of IR spectra deconvolution. Biopolymers 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Mujtaba, M.; Koç, B.; Salaberria, A.M.; Ilk, S.; Duman, D.C.; Akyüz, L.; Cakmak, Y.S.; Kaya, M.; Khawar, K.M.; Labidi, J.; et al. Production of novel chia-mucilage nanocomposite films with starch nanocrystals; An inclusive biological and physicochemical perspective. Int. J. Biol. Macromol. 2019, 133, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Krystyjan, M.; Khachatryan, G.; Ciesielski, W.; Buksa, K.; Sikora, M. Preparation and characteristics of mechanical and functional properties of starch/Plantago psyllium seeds mucilage films. Starch 2017, 69, 1700014. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Donald, A.M. The influence of amylose on starch granule structure. Int. J. Biol. Macromol. 1995, 17, 315–321. [Google Scholar] [CrossRef]
- Lourdin, D.; Della Valle, G.; Colonna, P. Influence of amylose content on starch films and foams. Carbohydr. Polym. 1995, 27, 261–270. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S.; Prodpran, T.; Nuthong, P. Effect of Psyllium (Plantago ovata Forks) Husk on Characteristics, Rheological and Textural Properties of Threadfin Bream Surimi Gel. Foods 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, J.; Yu, T.; Zhang, B. Versatile nonfluorinated superhydrophobic coating with self-cleaning, anti-fouling, anti-corrosion and mechanical stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128701. [Google Scholar] [CrossRef]
- Li, X.W.; Wang, H.X.; Shi, T.; Zhang, C.W.; Jiang, X.N.; Zhou, X.G.; Li, C. Efficient preparation and anticorrosion mechanism of superhydrophobic 7075 aviation aluminum alloy. Rare Met. Mater. Eng. 2022, 51, 6–10. [Google Scholar]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; HadiNezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef]
- Białopiotrowicz, T. Wettability of starch gel films. Food Hydrocoll. 2003, 17, 141–147. [Google Scholar] [CrossRef]
- Wiącek, A.E. Effect of surface modification on starch biopolymer wettability. Food Hydrocoll. 2015, 48, 228–237. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact angle measurement on porous substrates: Effect of liquid absorption and drop size. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Gambaryan-Roisman, T. Liquids on porous layers: Wetting, imbibition and transport processes. Curr. Opin. Colloid Interface Sci. 2014, 19, 320–335. [Google Scholar] [CrossRef]
- Espín, L.; Kumar, S. Droplet spreading and absorption on rough, permeable substrates. J. Fluid Mech. 2015, 784, 465–486. [Google Scholar] [CrossRef]
- Susana, L.; Campaci, F.; Santomaso, A.C. Wettability of mineral and metallic powders: Applicability and limitations of sessile drop method and Washburn’s technique. Powder Technol. 2012, 226, 68–77. [Google Scholar] [CrossRef]
- André, V.; Zosel, A.D. Dynamic wetting on porous and non porous substrates. Influence of surface tension, viscosity and porosity. Ber. Bunsenges. Phys. Chem. 1994, 98, 429–434. [Google Scholar] [CrossRef]
- Letellier, P.; Mayaffre, A.; Turmine, M. Drop size effect on contact angle explained by nonextensive thermodynamics. Young’s equation revisited. J. Colloid Interface Sci. 2007, 314, 604–614. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, Z.; Li, T.; Xu, X.; Chen, X.; Guo, X. Pore structure characteristics and evaluation of lacustrine mixed fine-grained sedimentary rocks: A case study of the Lucaogou Formation in the Malang Sag, Santanghu Basin, Western China. J. Pet. Sci. Eng. 2021, 201, 108545. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Cheviron, P.; Gouanvé, F.; Espuche, E. Preparation, characterization and barrier properties of silver/montmorillonite/starch nanocomposite films. J. Membr. Sci. 2016, 497, 162–171. [Google Scholar] [CrossRef]
- Ayadi, F.; Dole, P. Stoichiometric interpretation of thermoplastic starch water sorption and relation to mechanical behavior. Carbohydr. Polym. 2011, 84, 872–880. [Google Scholar] [CrossRef]
- Caurie, M. Hysteresis phenomenon in foods. Int. J. Food Sci. Technol. 2007, 42, 45–49. [Google Scholar] [CrossRef]
- Liu, K.; Ostadhassan, M.; Jang, H.W.; Zakharova, N.V.; Shokouhimehr, M. Comparison of fractal dimensions from nitrogen adsorption data in shale via different models. RSC Adv. 2021, 11, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Sadeghi, E.; Mohammadi, R.; Rouhi, M.; Taghizadeh, M.; Shirgardoun, M.H.; Kariminejad, M. The physicochemical and structural properties of psyllium gum/modified starch composite edible film. J. Food Process. Preserv. 2018, 42, e13715. [Google Scholar] [CrossRef]
- Stading, M.; Rindlav-Westling, Å.; Gatenholm, P. Humidity-induced structural transitions in amylose and amylopectin films. Carbohydr. Polym. 2001, 45, 209–217. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, T.; Jiang, S.; Lin, L.; Lu, J. Effects of psyllium husk powder on the gel properties of silver carp (Hypophthalmichthys molitrix) surimi. J. Food Process. Preserv. 2022, 46, e16452. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Daudt, R.; Avena-Bustillos, R.; Williams, T.; Wood, D.; Külkamp-Guerreiro, I.; Marczak, L.; McHugh, T. Comparative study on properties of edible films based on pinhão (Araucaria angustifolia) starch and flour. Food Hydrocoll. 2016, 60, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Chinma, C.E.; Ariahu, C.C.; Abu, J.O. Development and characterization of cassava starch and soy protein concentrate based edible films. Int. J. Food Sci. Technol. 2011, 47, 383–389. [Google Scholar] [CrossRef]

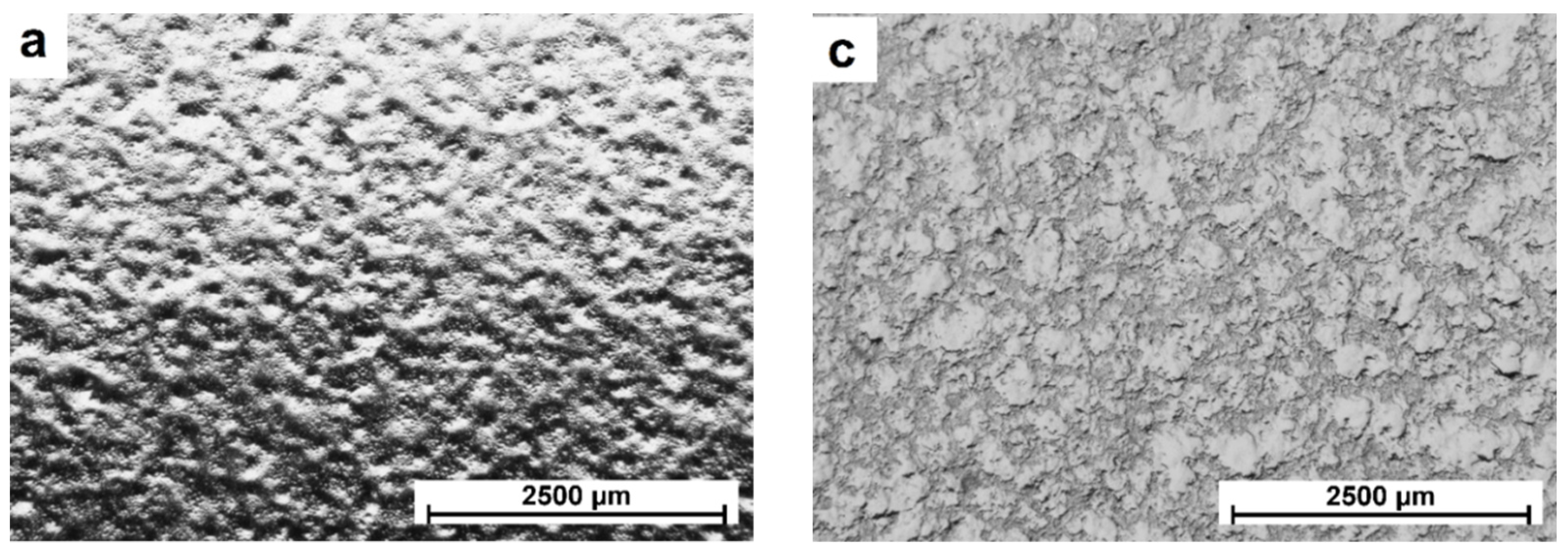
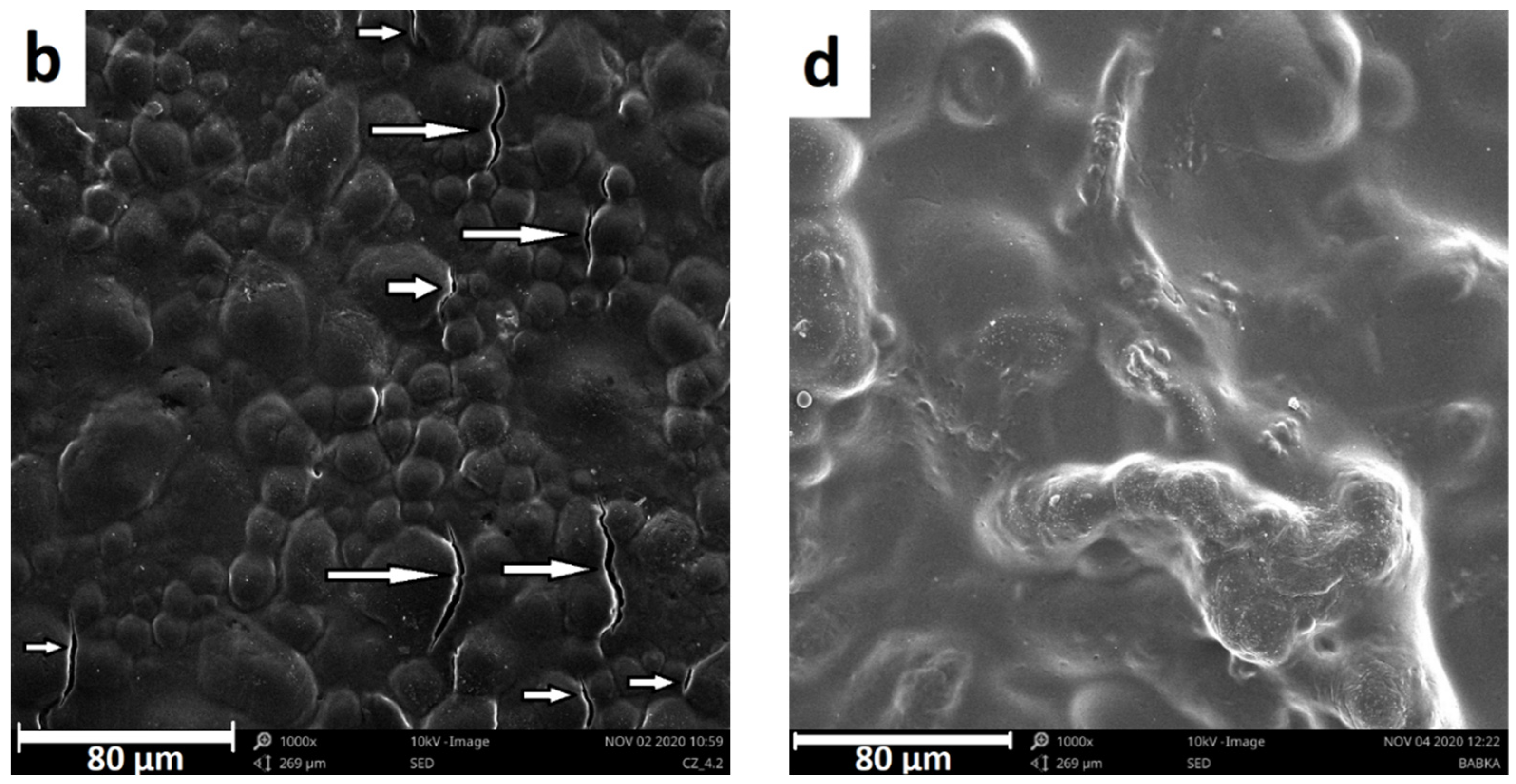
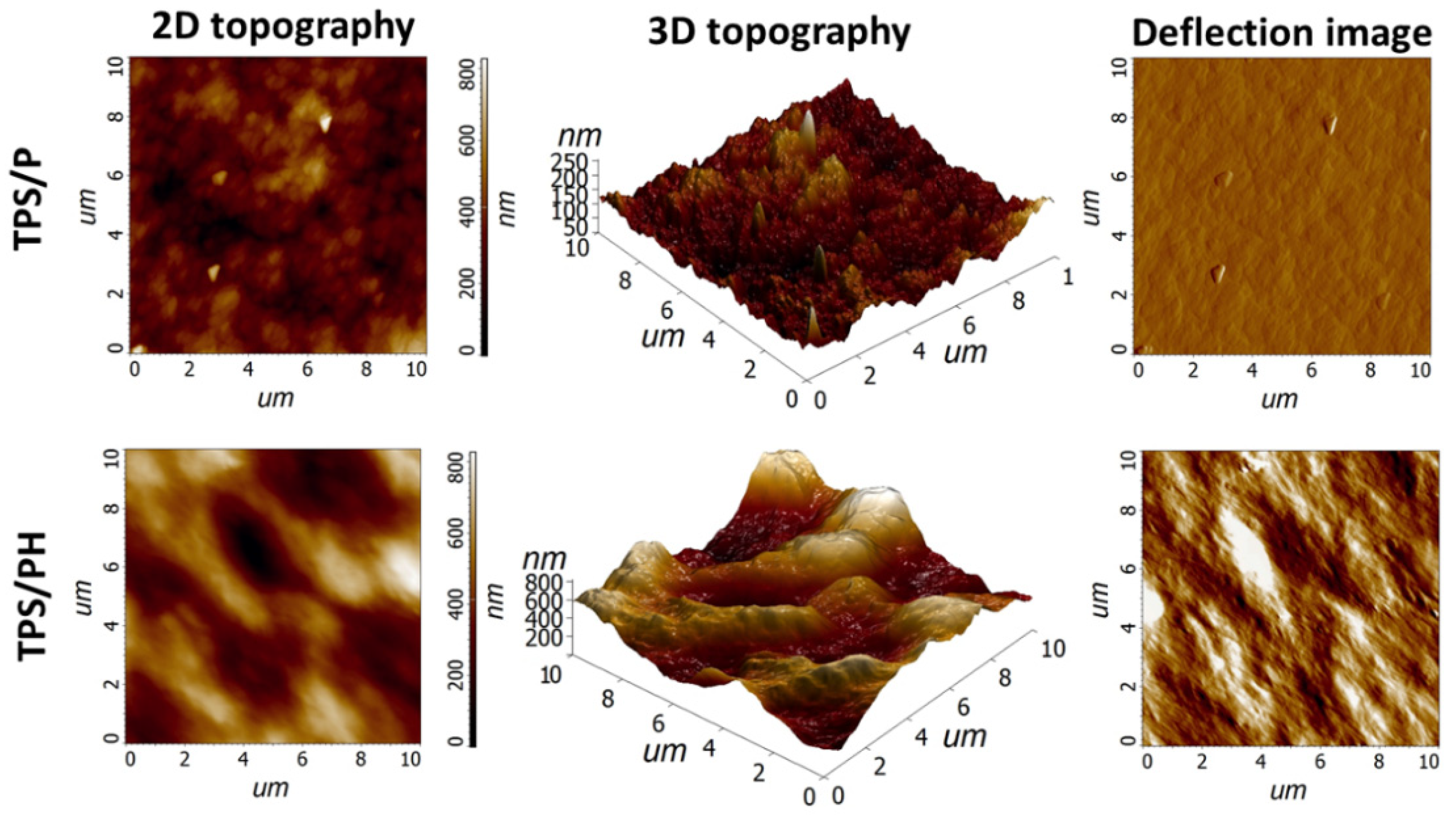
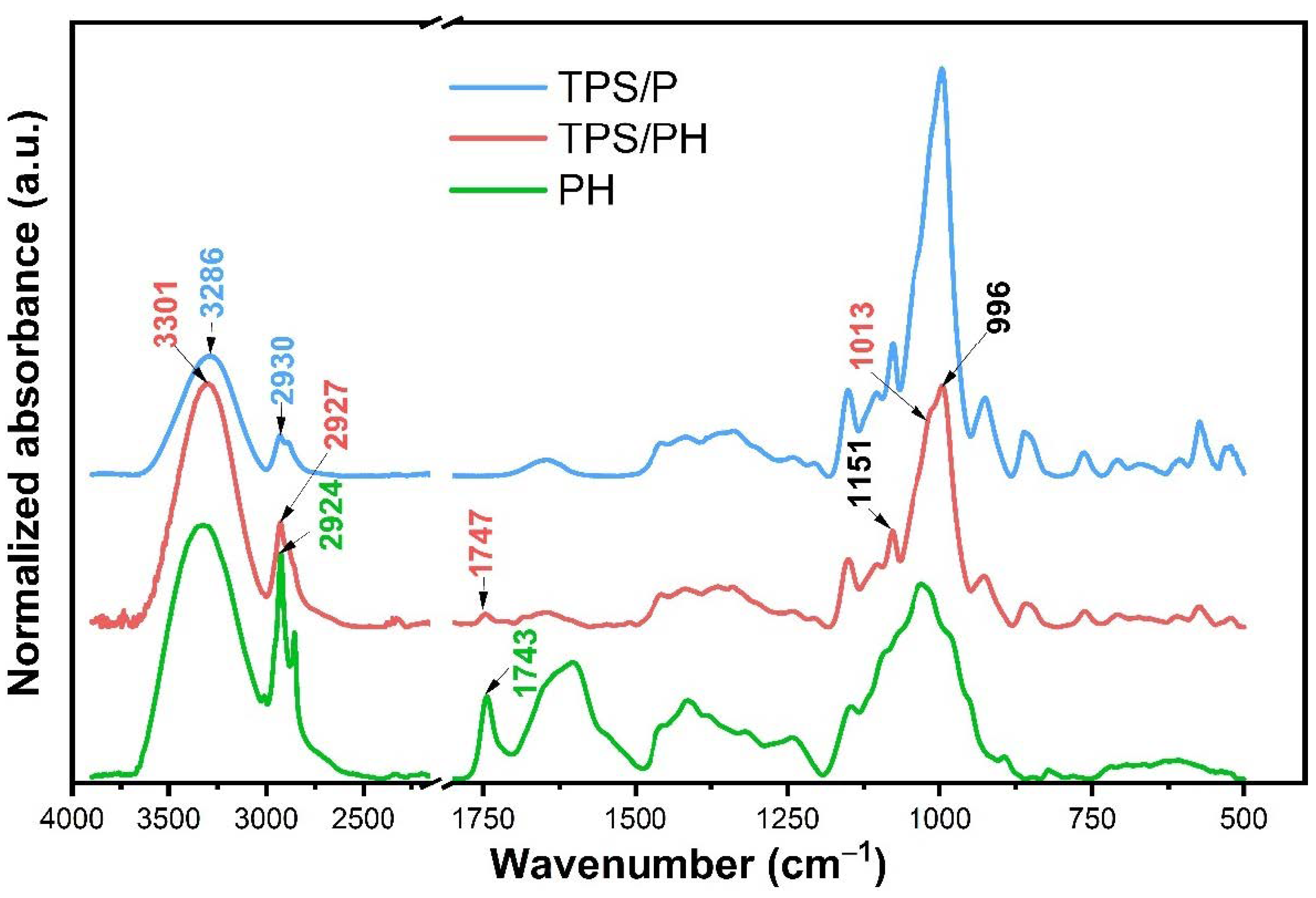


| Sample | Thickness (mm) | Ra (nm) | Rq (nm) | Rp (nm) | Rv (nm) |
|---|---|---|---|---|---|
| TPS/P | 0.09 ± 0.01 | 22.15 ± 4.40 a | 28.23 ± 5.90 a | 82.91 ± 32.42 a | 60.23 ± 15.54 a |
| TPS/PH | 0.16 ± 0.02 | 119.99 ± 30.48 b | 145.09 ± 34.23 b | 291.32 ± 92.33 b | 271.65 ± 86.25 b |
| TPS/P | TPS/PH | |||||
|---|---|---|---|---|---|---|
| Time (s): | 0 | 5 | 20 | 0 | 5 | 20 |
| Drop volume (mm3) | 1.99 (±0.06) | 1.94 (±0.08) | 1.90 (±0.10) | 1.97 (±0.06) | 1.93 (±0.07) | 1.90 (±0.08) |
| Contact surface (mm2) | 2.75 (±0.07) | 3.58 (±0.27) | 3.97 (±0.27) | 2.87 (±0.19) | 4.24 (±0.20) | 4.86 (±0.25) |
| Sample | WVP (g m−1 s−1 Pa−1) | Tr280 (%) | Tr600 (%) | Opacity (AU/mm) |
|---|---|---|---|---|
| TPS/P | 3.38 × 10−10 ± 8.57 × 10−12 a | 69.39 ± 2.74 a | 94.62 ± 0.98 a | 0.28 ± 0.05 a |
| TPS/PH | 4.55 × 10−10 ± 1.74 × 10−12 b | 23.87 ± 1.59 b | 78.97 ± 1.29 b | 0.65 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beer-Lech, K.; Skic, A.; Skic, K.; Stropek, Z.; Arczewska, M. Effect of Psyllium Husk Addition on the Structural and Physical Properties of Biodegradable Thermoplastic Starch Film. Materials 2022, 15, 4459. https://doi.org/10.3390/ma15134459
Beer-Lech K, Skic A, Skic K, Stropek Z, Arczewska M. Effect of Psyllium Husk Addition on the Structural and Physical Properties of Biodegradable Thermoplastic Starch Film. Materials. 2022; 15(13):4459. https://doi.org/10.3390/ma15134459
Chicago/Turabian StyleBeer-Lech, Karolina, Anna Skic, Kamil Skic, Zbigniew Stropek, and Marta Arczewska. 2022. "Effect of Psyllium Husk Addition on the Structural and Physical Properties of Biodegradable Thermoplastic Starch Film" Materials 15, no. 13: 4459. https://doi.org/10.3390/ma15134459
APA StyleBeer-Lech, K., Skic, A., Skic, K., Stropek, Z., & Arczewska, M. (2022). Effect of Psyllium Husk Addition on the Structural and Physical Properties of Biodegradable Thermoplastic Starch Film. Materials, 15(13), 4459. https://doi.org/10.3390/ma15134459






