Correlation of Cell Proliferation with Surface Properties of Polymer-like Carbon Films of Different Thicknesses Prepared by a Radio-Frequency Plasma CVD Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLC Film Deposition Conditions
2.2. Spectroscopic Ellipsometer Analysis
2.3. Structure Analysis
2.4. Cell Culture Testing
2.5. Statistical Analysis
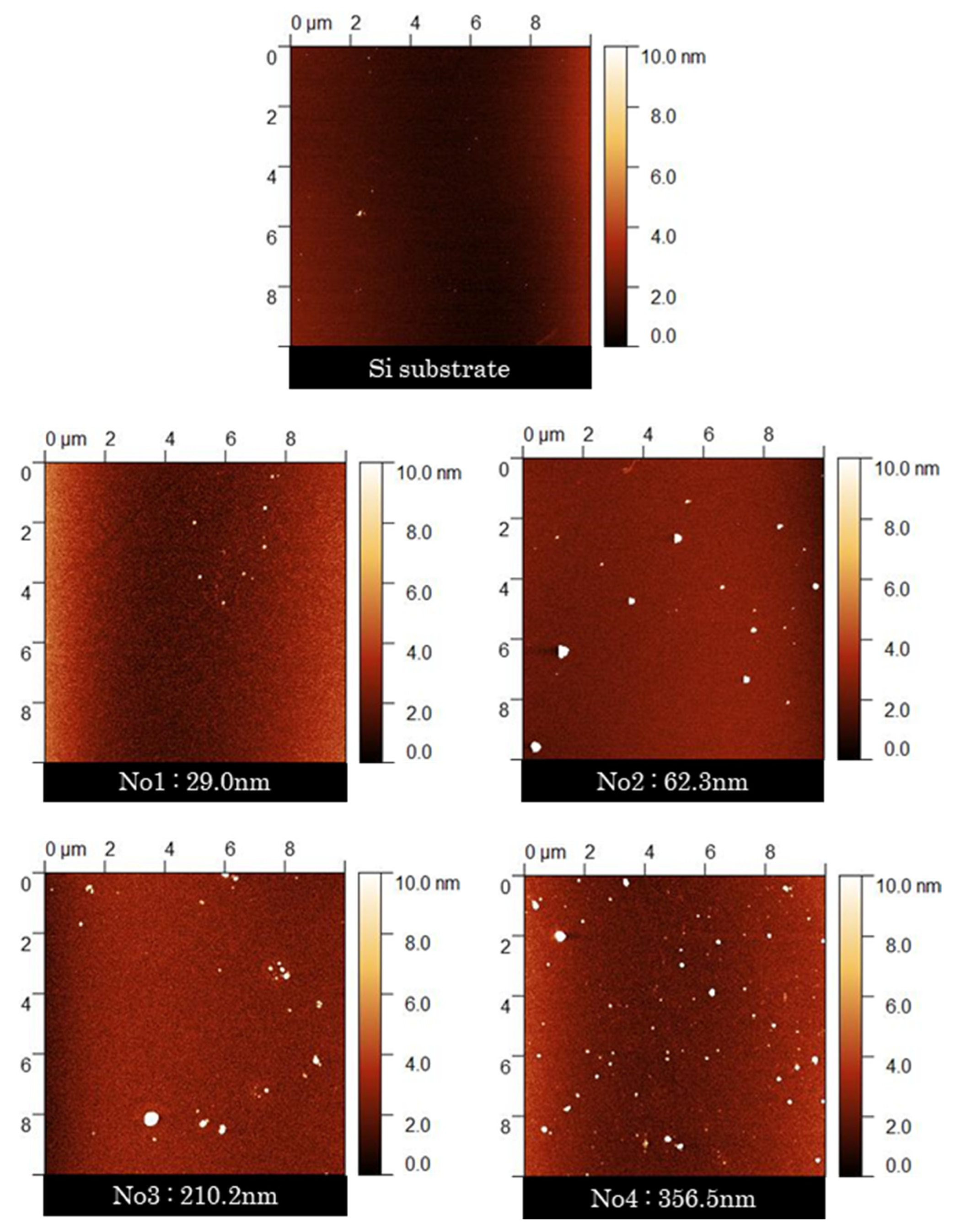
2.6. Surface Analysis
3. Results and Discussion
3.1. Classification of DLC Types Based on n–k Plots
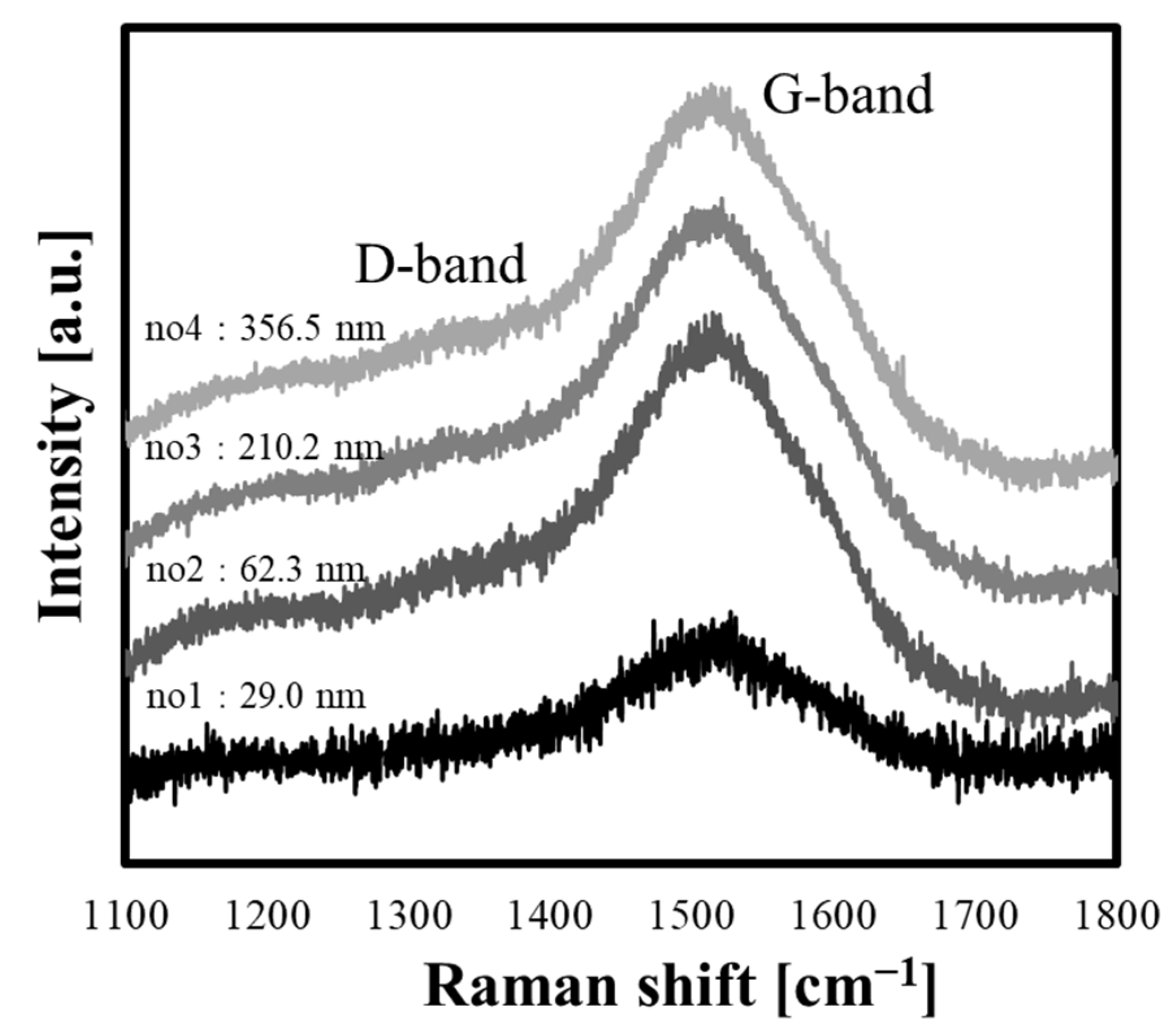
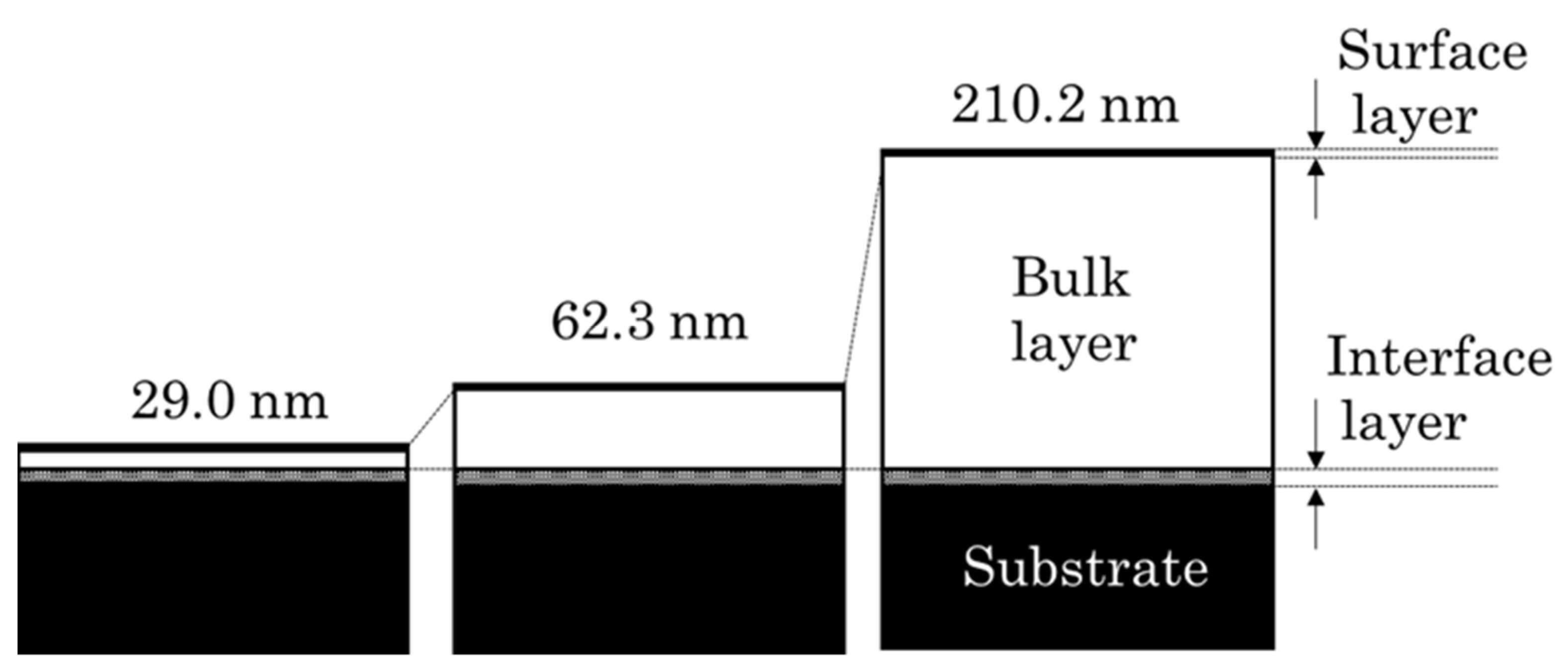
3.2. Structural Changes with Growth of the PLC Films
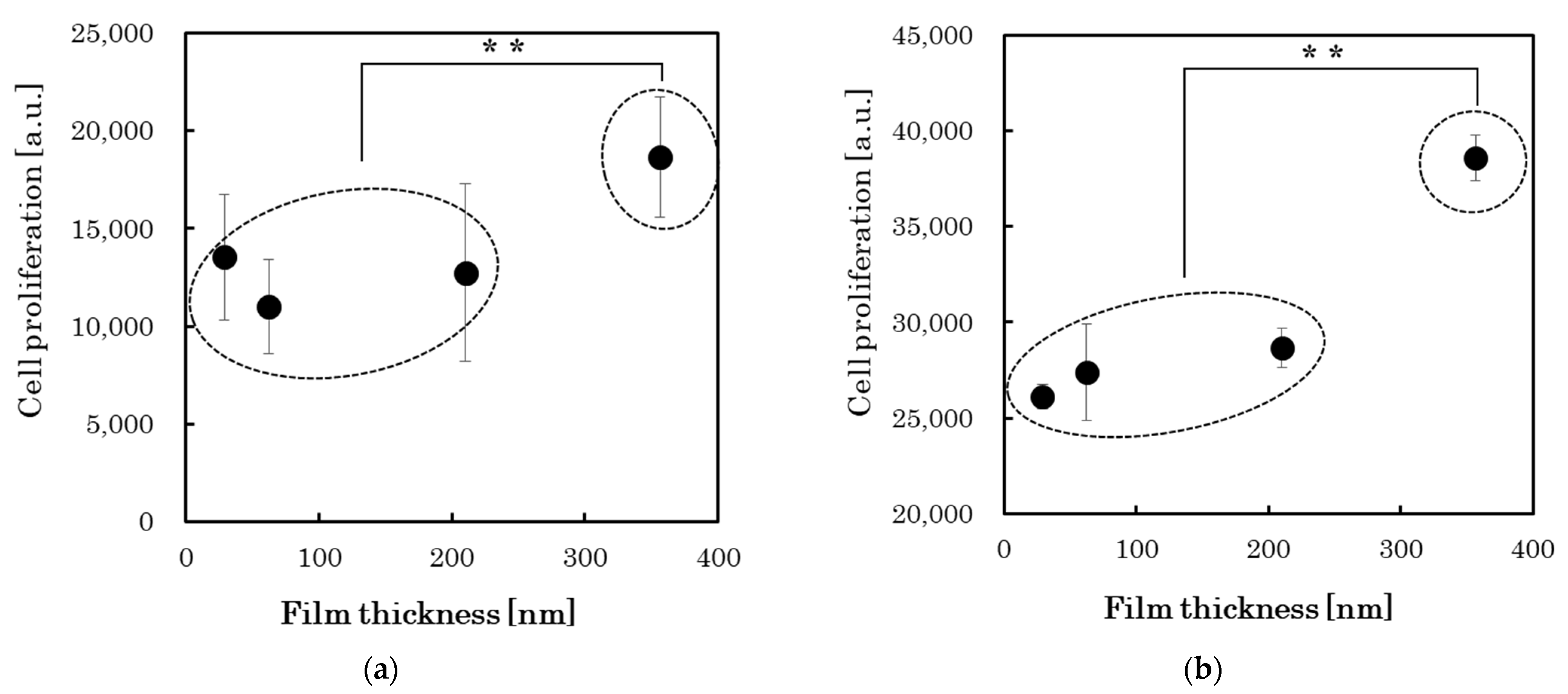
3.3. Cell Proliferation Changes with Growth of the PLC Films
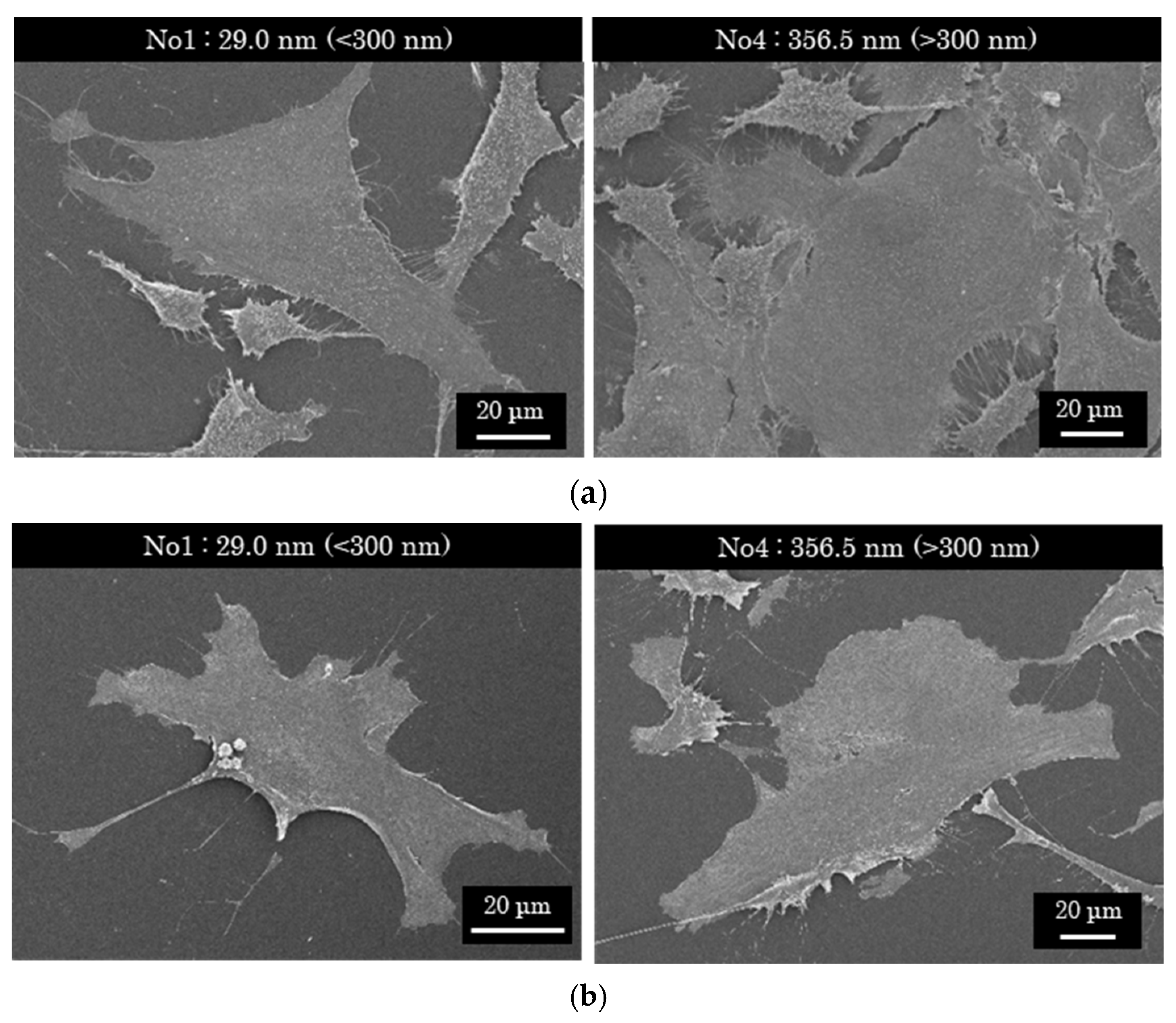
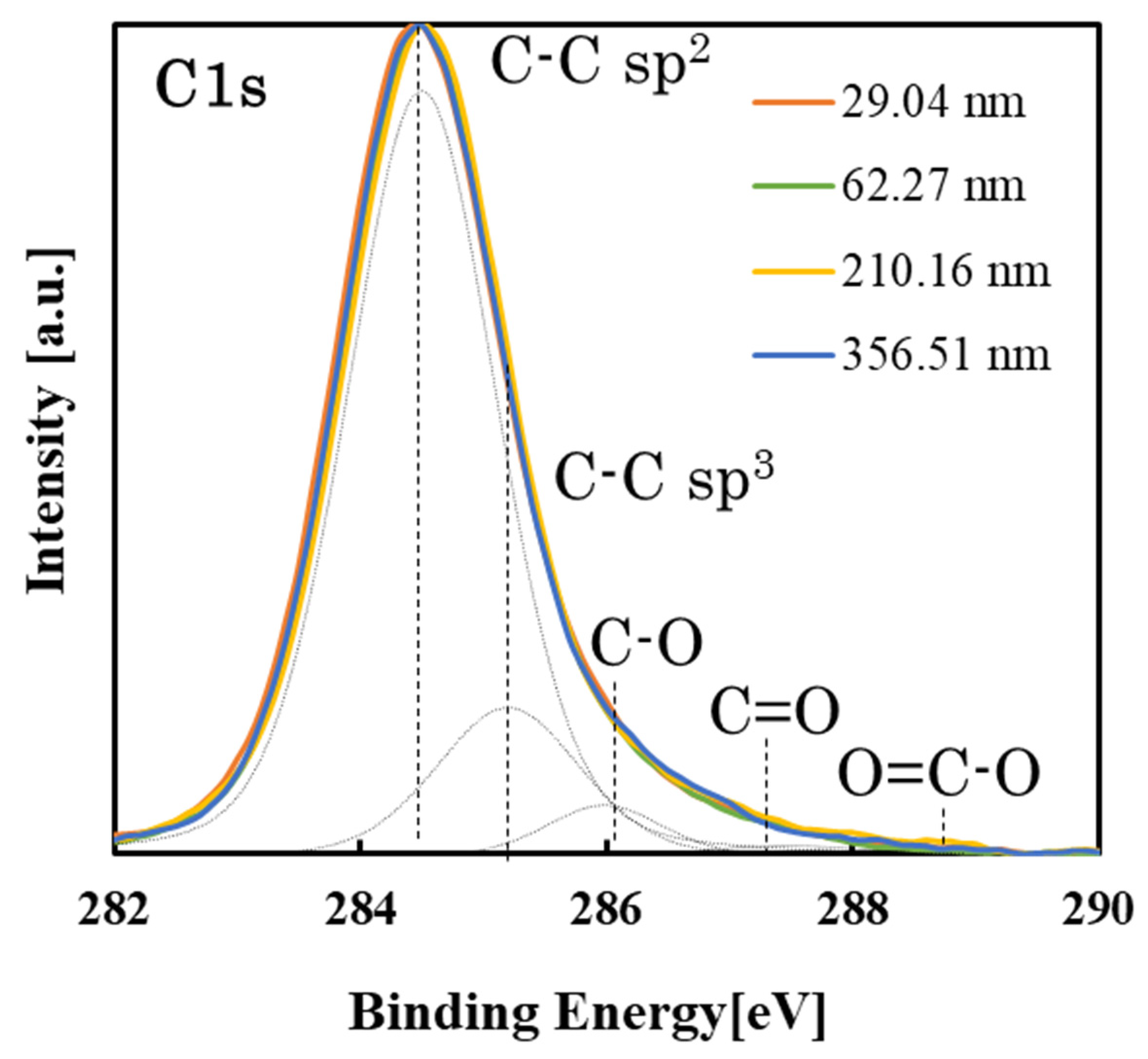
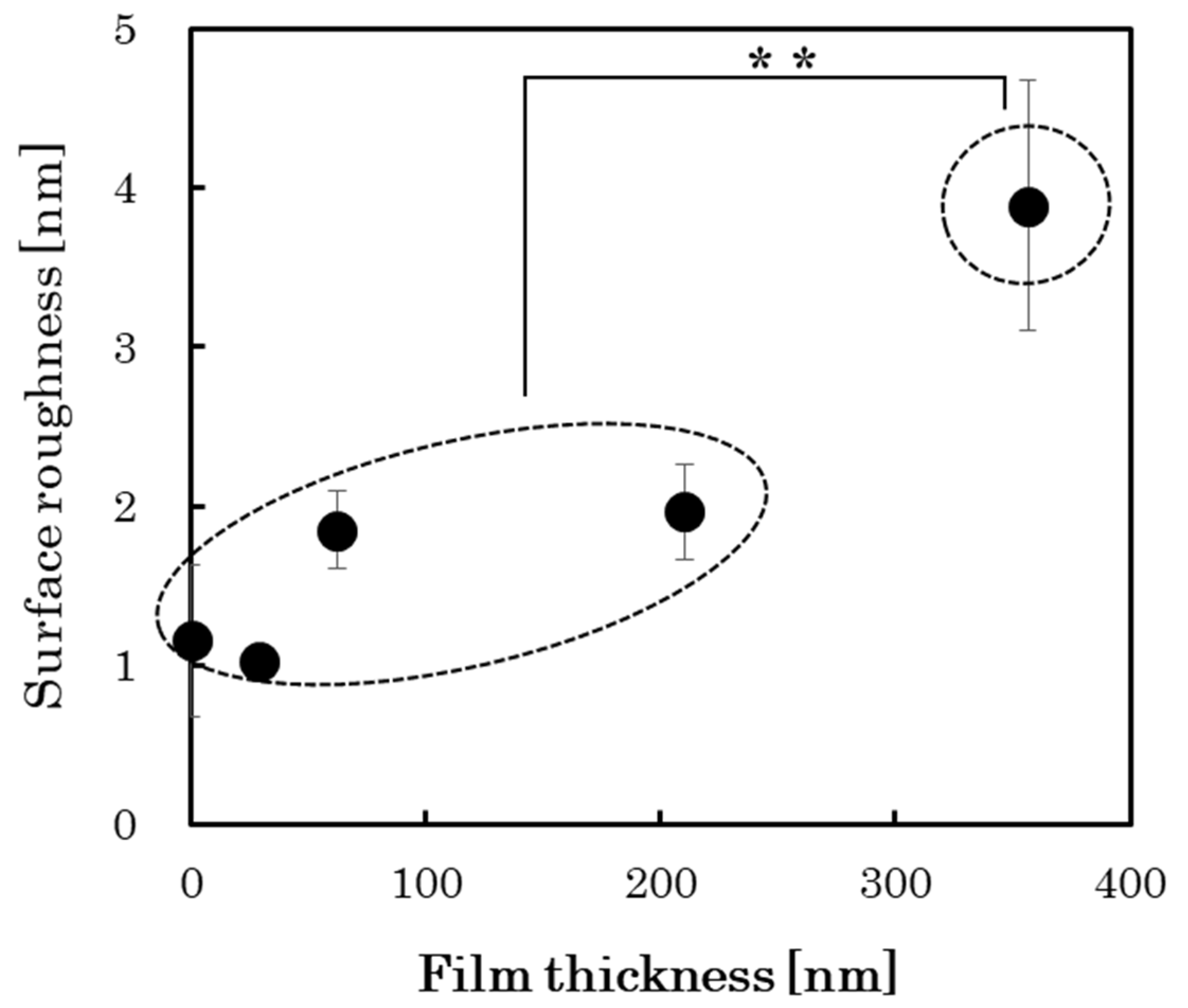
3.4. Changes in Surface Properties with the Growth of PLC Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, J. Diamond-Like Amorphous Carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Savvides, N.; Bell, T.J. Hardness and Elastic Modulus of Diamond and Diamond-Like Carbon Films. Thin Solid Films 1993, 228, 289–292. [Google Scholar] [CrossRef]
- Ozeki, K.; Nagashima, I.; Ohgoe, Y.; Hirakuri, K.K.; Mukaibayashi, H.; Masuzawa, T. Gas Barrier Properties of Diamond-Like Carbon Films Coated on PTFE. Appl. Surf. Sci. 2009, 255, 7286–7290. [Google Scholar] [CrossRef]
- Huang, G.F.; Lingping, Z.; Weiqing, H.; Lihua, Z.; Shaolu, L.; Deyi, L. The Mechanical Performance and Anti-Corrosion Behavior of Diamond-Like Carbon Film. Diam. Relat. Mater. 2003, 12, 1406–1410. [Google Scholar] [CrossRef]
- Grill, A. Diamond-Like Carbon Coatings as Biocompatible Materials—An Overview. Diam. Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Dearnaley, G.; Arps, J.H. Biomedical Applications of Diamond-Like Carbon (DLC) Coatings: A Review. Surf. Coat. Technol. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Nakamori, H.; Kogo, Y.; Sakurai, M.; Ohtake, N.; Saitoh, H. Correlation between Optical Properties and Hardness of Diamond-Like Carbon Films. J. Solid Mech. Mater. Eng. 2013, 7, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Kanasugi, K.; Ohgoe, Y.; Hiratsuka, M.; Nakamori, H.; Homma, A.; Hirakuri, K. Classification of DLC Films for Cell Proliferation Based on Optical Constants. Diam. Relat. Mater. 2021, 113, 108266. [Google Scholar] [CrossRef]
- Fan, Y.W.; Cui, F.Z.; Hou, S.P.; Xu, Q.Y.; Chen, L.N.; Lee, I.S. Culture of neural cells on silicon wafers with nano-scale surface topograph. J. Neurosci. Methods 2002, 120, 17–23. [Google Scholar] [CrossRef]
- Ban, M.; Tobe, S.; Takeuchi, L. Effects of diamond-like carbon thin film and wrinkle microstructure on cell proliferation. Diam. Relat. Mater. 2018, 90, 194–201. [Google Scholar] [CrossRef]
- Harigai, T.; Yasuoka, Y.; Nitta, N.; Furuta, H.; Hatta, A. X-Ray Reflectivity Analysis on Initial Stage of Diamond-Like Carbon Film Deposition on Si Substrate by RF Plasma CVD and on Removal of the Sub-Surface Layer by Oxygen Plasma Etching. Diam. Relat. Mater. 2013, 38, 36–40. [Google Scholar] [CrossRef]
- Shirakura, A.; Nakaya, M.; Koga, Y.; Kodama, H.; Hasebe, T.; Suzuki, T. Diamond-Like Carbon Films for PET Bottles and Medical Applications. Thin Solid Films 2006, 494, 84–91. [Google Scholar] [CrossRef]
- Ohgoe, Y.; Hirakuri, K.K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC Films in Terms of Biological Response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]
- Liao, T.T.; Zhang, T.F.; Li, S.S.; Deng, Q.Y.; Wu, B.J.; Zhang, Y.Z.; Zhou, Y.J.; Guo, Y.B.; Leng, Y.X.; Huang, N. Biological Responses of Diamond-Like Carbon (DLC) Films with Different Structures in Biomedical Application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 751–759. [Google Scholar] [CrossRef]
- Chai, F.; Mathis, N.; Blanchemain, N.; Meunier, C.; Hildebrand, H.F. Osteoblast interaction with DLC-coated Si substrates. Acta Biomater. 2008, 4, 1369–1381. [Google Scholar] [CrossRef]
- Anita, N.; Bibhuti, B.M.; Abikesh, P.K.M.; Abiresh, P.K.M. Multiple comparison test by Tukey’s honestly significant difference (HSD): Do the confident level control type I error. Int. J. Appl. Math. Stat. 2021, 6, 59–65. [Google Scholar] [CrossRef]
- Darren, L.W.; Anselm, T.K.; Mark, A.A.; Madison, B.H.; Megan, M.K.; Elizabeth, I.N. Computerized Measurement of Contact Angles; Galvanotechnik 10/2010; Galvanotechnik: Heilbronn, Germany, 2010. [Google Scholar]
- Nitta, Y.; Okamoto, K.; Nakatani, T.; Hoshi, H.; Homma, A.; Tatsumi, E.; Taenaka, Y. Diamond-Like Carbon Thin Film with Controlled Zeta Potential for Medical Material Application. Diam. Relat. Mater. 2008, 17, 1972–1976. [Google Scholar] [CrossRef]
- Zhou, X.; Suzuki, T.; Nakajima, H.; Komatsu, K.; Kanda, K.; Ito, H.; Saitoh, H. Structural Analysis of Amorphous Carbon Films by Spectroscopic Ellipsometry, RBS/ERDA, and NEXAFS. Appl. Phys. Lett. 2017, 110, 201902. [Google Scholar] [CrossRef] [Green Version]
- Park, C.K.; Chang, S.M.; Uhm, H.S.; Seo, S.H.; Park, J.S. XPS and XRR Studies on Microstructures and Interfaces of DLC Films Deposited by FCVA Method. Thin Solid Films 2002, 420–421, 235–240. [Google Scholar] [CrossRef]
- Kanasugi, K.; Ohgoe, Y.; Hirakuri, K.K.; Fukui, Y. Cytocompatibility of Modified a-C:H Film Deposited on Complicated Polymeric Medical Apparatus. J. Appl. Phys. 2009, 105, 094702. [Google Scholar] [CrossRef]
- Caro, M.A.; Aarva, A.; Deringer, V.L.; Csányi, G.; Laurila, T. Reactivity of Amorphous Carbon Surfaces: Rationalizing the Role of Structural Motifs in Functionalization Using Machine Learning. Chem. Mater. 2018, 30, 7446–7455. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]

| Sample No. | Deposition Method | Precursor | Power [W] | Gas Pressure [Pa] | Deposition Time [min] |
|---|---|---|---|---|---|
| 1 | 13.56 MHz RF-PECVD | CH4 (37 sccm) | 200 | 50 | 0.8 |
| 2 | 2.2 | ||||
| 3 | 7.7 | ||||
| 4 | 13.2 |
| Cell | Fibroblast (NIH-3T3) | Osteoblast (MC-3T3) |
|---|---|---|
| Seeding density | 1.0 × 104 cells/cm2 | 1.0 × 104 cells/cm2 |
| Medium | D-MEM/F-12 | MEM-α |
| CO2 concentration | 5.0% | 5.0% |
| Temperature | 37.0 °C | 37.0 °C |
| Incubation time | 72 h | 72 h |
| pH | 6.8–7.2 | 6.8–7.2 |
| Sample No. | Film Thickness [nm] | n | k | χ2 | Type |
|---|---|---|---|---|---|
| λ = 550 nm | |||||
| 1 | 29.0 ± 0.2 | 1.714 | 0.012 | 0.25 | Polymer-like carbon |
| 2 | 62.3 ± 0.4 | 1.763 | 0.014 | 0.87 | |
| 3 | 210.2 ± 1.1 | 1.803 | 0.030 | 2.35 | |
| 4 | 356.5 ± 3.4 | 1.830 | 0.031 | 5.52 | |
| Sample No. | D-Peak Position [cm−1] | G-Peak Position [cm−1] | ID/IG Ratio |
|---|---|---|---|
| 1 | 1337.06 ± 5.38 | 1515.77 ± 0.85 | 0.14 |
| 2 | 1328.68 ± 3.54 | 1521.66 ± 0.21 | 0.44 |
| 3 | 1336.72 ± 2.78 | 1523.71 ± 0.18 | 0.48 |
| 4 | 1334.38 ± 3.39 | 1524.85 ± 0.18 | 0.44 |
| Sample No. | Cell Proliferation (n = 3) | Compositional Bond Intensity of C1s Peaks | Pure Water Contact Angle [deg] | Surface Roughness RMS [nm] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fibroblast (NIH-3T3) | Osteoblast (MC-3T3) | C-Csp2 | C-Csp3 | C-O | C=O | O=C-O | Ave. (n = 10) | Ave. (n = 5) | |
| 1 | 13,546 ± 2930 | 26,116 ± 640 | 0.94 | 0.13 | 0.05 | 0.01 | 0.00 | 84.2 ± 0.8 | 1.03 ± 0.05 |
| 2 | 11,004 ± 2434 | 27,406 ± 2525 | 0.93 | 0.21 | 0.07 | 0.01 | 0.00 | 81.0 ± 0.7 | 1.85 ± 0.24 |
| 3 | 12,729 ± 4577 | 28,672 ± 1028 | 0.91 | 0.25 | 0.06 | 0.02 | 0.00 | 78.6 ± 0.8 | 1.96 ± 0.30 |
| 4 | 18,667 ± 3011 | 38,585 ± 1176 | 0.92 | 0.18 | 0.06 | 0.01 | 0.00 | 76.6 ± 0.8 | 3.89 ± 0.79 |
| Si substrate | 7233 ± 443 | 25,651 ± 2605 | - | - | - | - | - | - | 1.16 ± 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanasugi, K.; Eguchi, H.; Ohgoe, Y.; Manome, Y.; Alanazi, A.; Hirakuri, K. Correlation of Cell Proliferation with Surface Properties of Polymer-like Carbon Films of Different Thicknesses Prepared by a Radio-Frequency Plasma CVD Process. Materials 2022, 15, 4466. https://doi.org/10.3390/ma15134466
Kanasugi K, Eguchi H, Ohgoe Y, Manome Y, Alanazi A, Hirakuri K. Correlation of Cell Proliferation with Surface Properties of Polymer-like Carbon Films of Different Thicknesses Prepared by a Radio-Frequency Plasma CVD Process. Materials. 2022; 15(13):4466. https://doi.org/10.3390/ma15134466
Chicago/Turabian StyleKanasugi, Kazuya, Hiroaki Eguchi, Yasuharu Ohgoe, Yoshinobu Manome, Ali Alanazi, and Kenji Hirakuri. 2022. "Correlation of Cell Proliferation with Surface Properties of Polymer-like Carbon Films of Different Thicknesses Prepared by a Radio-Frequency Plasma CVD Process" Materials 15, no. 13: 4466. https://doi.org/10.3390/ma15134466








