Facile Surface Depolymerization Promotes the Welding of Hard Epoxy Vitrimer
Abstract
:1. Introduction
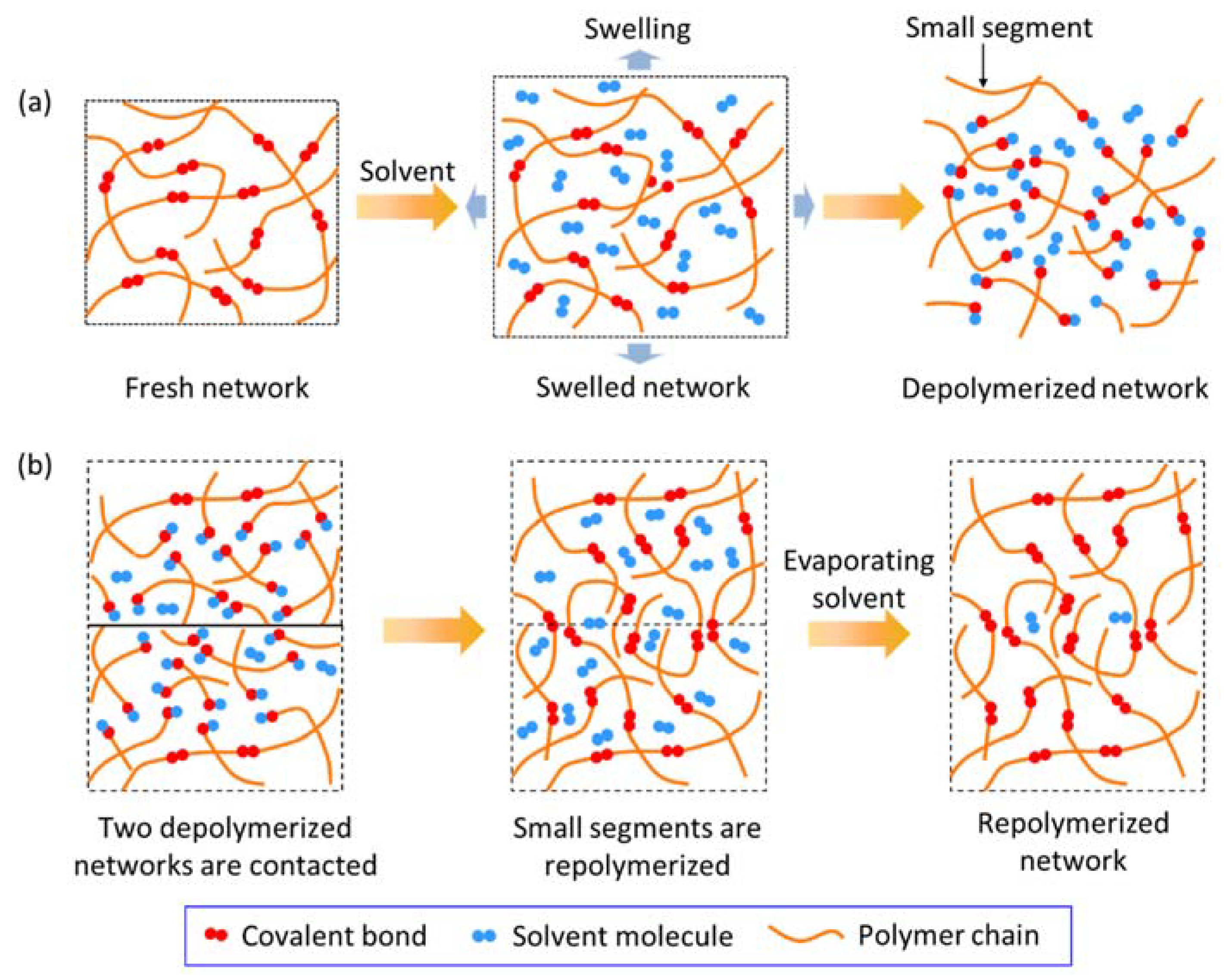
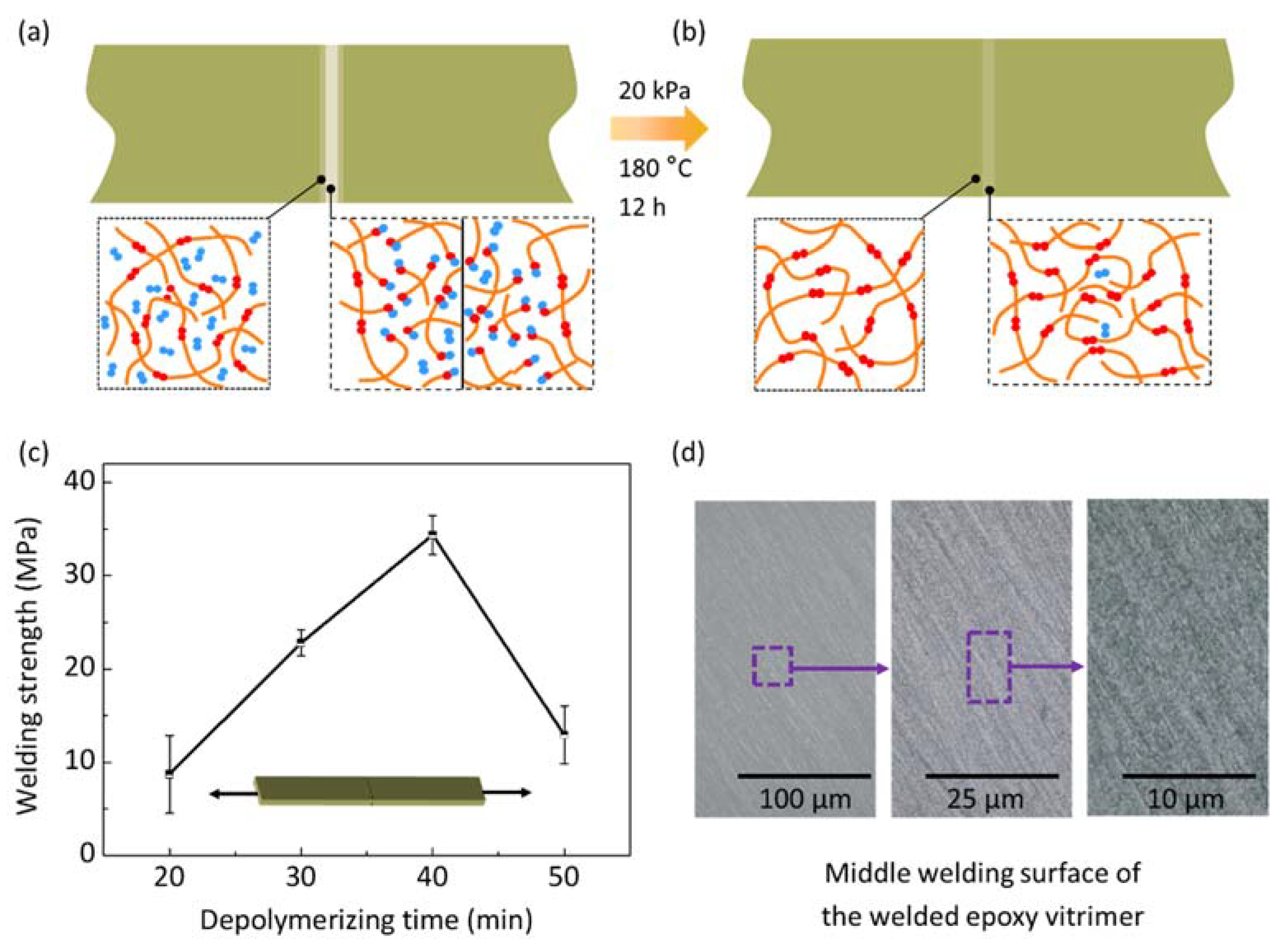
2. Depolymerizing and Welding Mechanism
3. Experimental
3.1. Materials
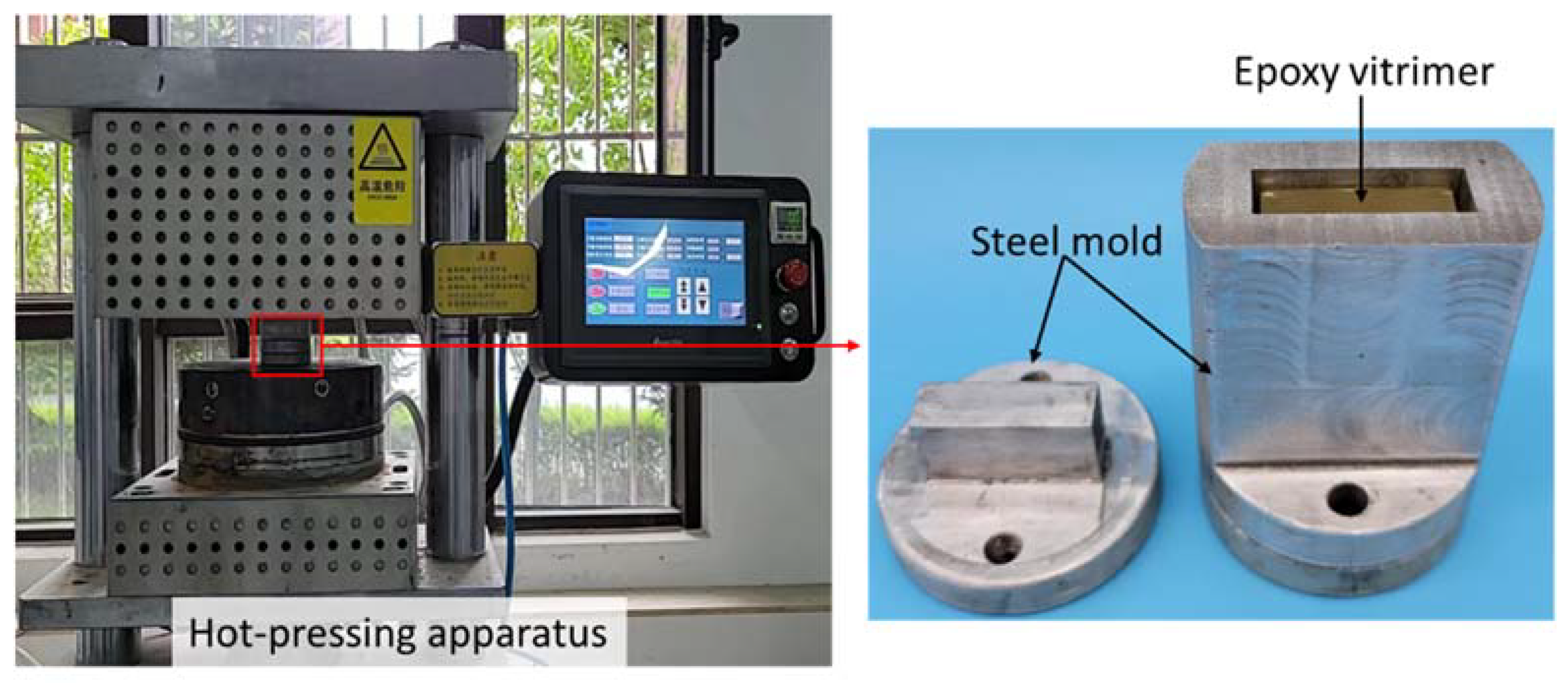
3.2. Preparation of Hard Epoxy Vitrimer
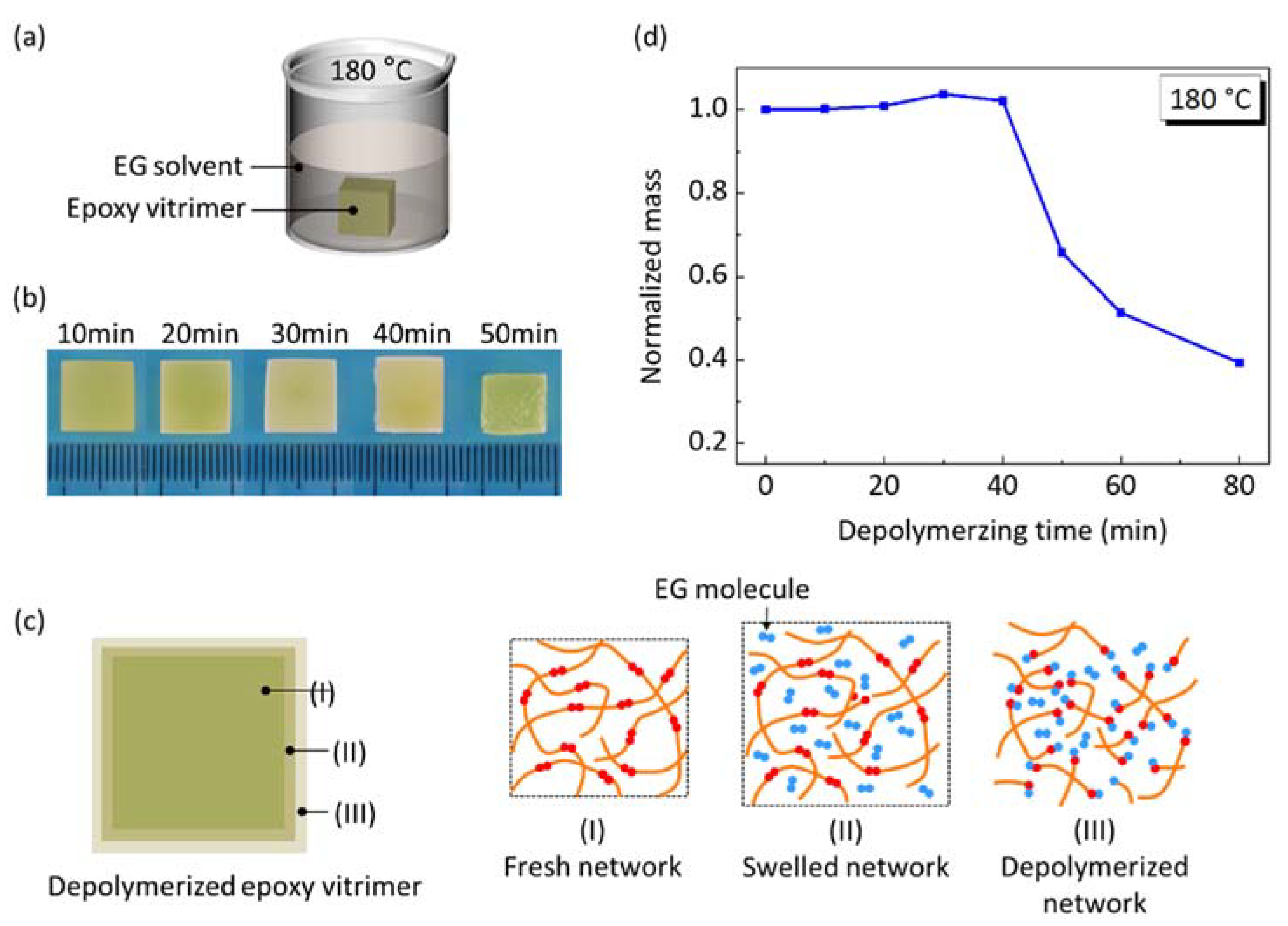
3.3. Dissolving Experiment
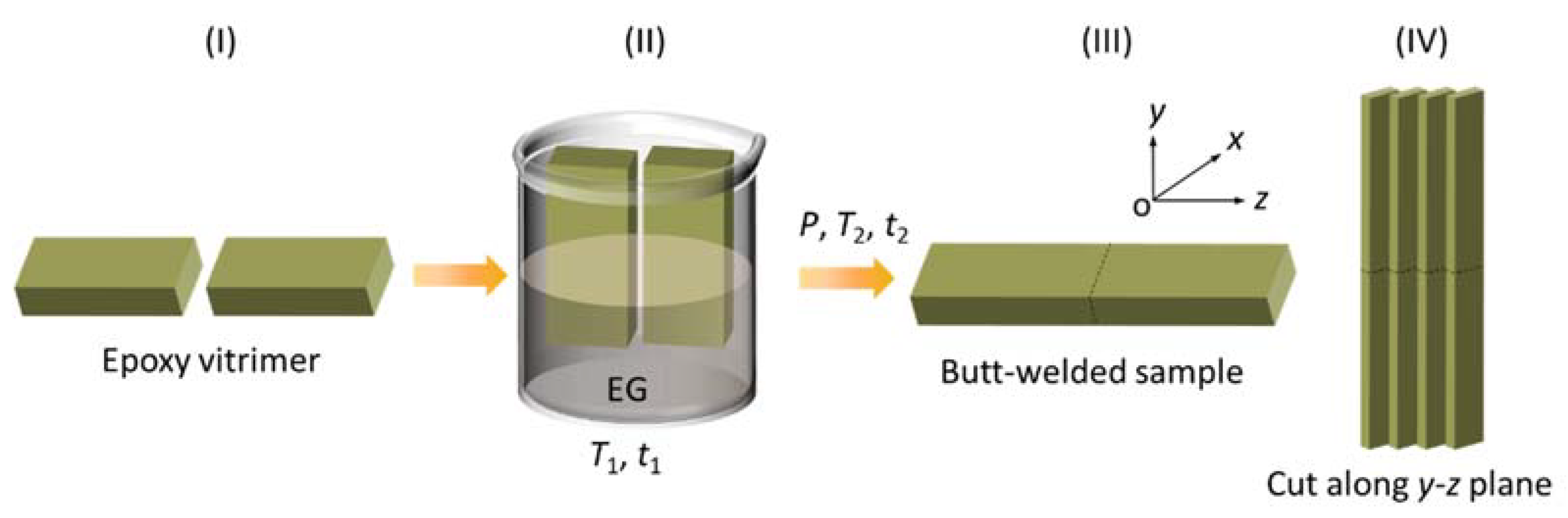
3.4. Welding Experiments
3.5. Characterization
4. Results and Discussions
4.1. The Influence of Depolymerizing Time
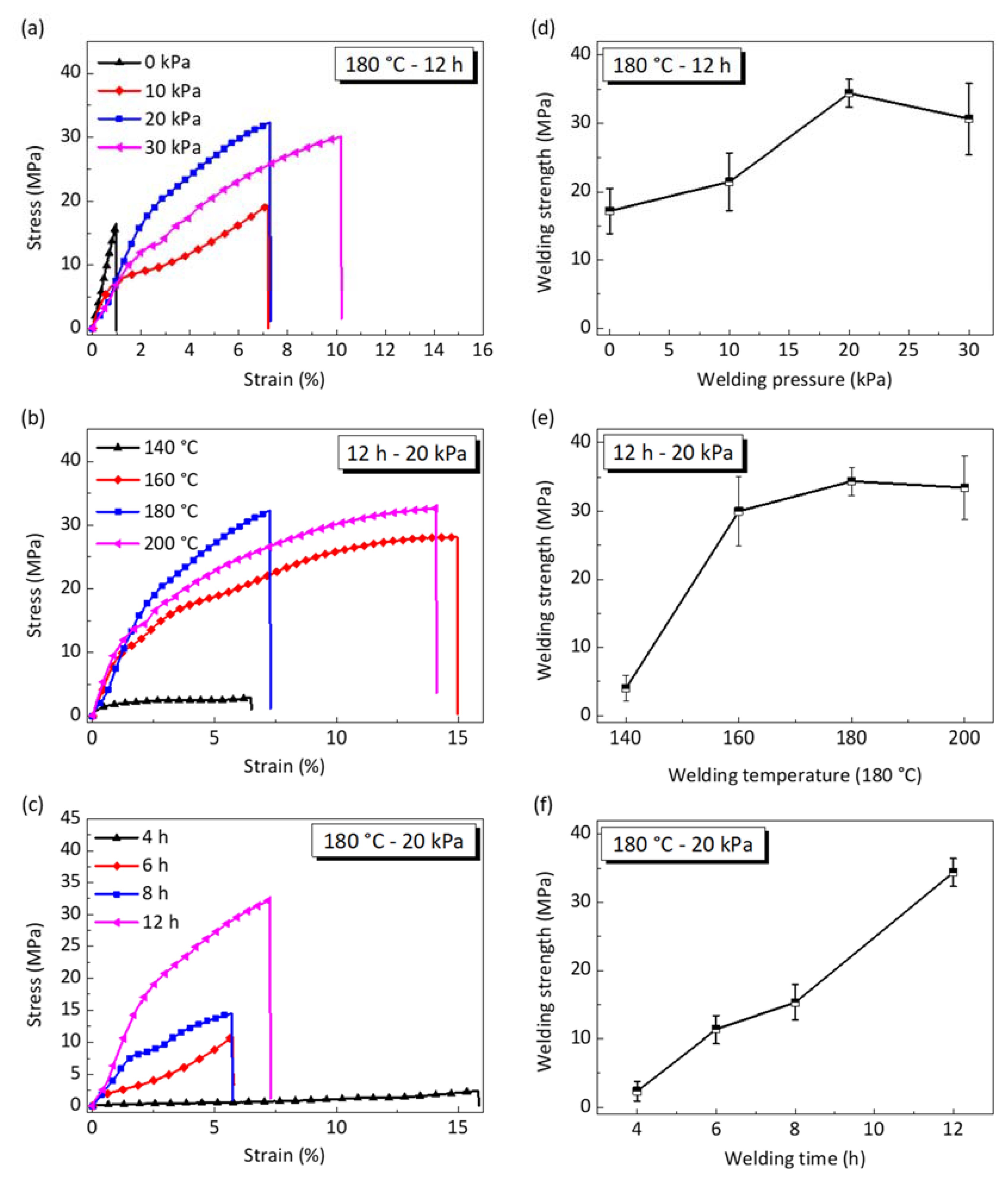
4.2. The Influences of Welding Conditions
4.3. Comparison to Welding with no Surface Depolymerization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. The Mechanical and Thermal Properties of Hard Epoxy Vitrimer

| Tg/°C | Tensile Strength/MPa | Elastic Modulus/MPa | Elongation at Break/% | T5%/°C | T10%/°C | Tmax1/°C | Tmax2/°C |
|---|---|---|---|---|---|---|---|
| 77.2 | 63.0 ± 3.4 | 1192.9 ± 34.5 | 8.6 ± 2.4 | 346 | 382 | 420 | 582 |
References
- De Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide cross-links to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagiotopoulos, C.; Porfyris, A.; Korres, D.; Vouyiouka, S. Solid-state polymerization as a vitrimerization tool starting from available thermoplastics: The effect of eeaction temperature. Materials 2020, 14, 9. [Google Scholar] [CrossRef]
- Alabiso, W.; Schlögl, S. The impact of vitrimers on the industry of the future: Chemistry, properties and sustainable forward-looking applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: A molecular platform for designing functions beyond chemical recycling and self-healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable crosslinks in polymeric materials: Resolving the intersection of thermoplastics and thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, B.; Zhang, J. Recent development of repairable, malleable and recyclable thermosetting polymers through dynamic transesterification. Polymer 2020, 194, 122392. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, H.; Hart, K.R.; Wu, Y.; Hsu, A.J.; Coppola, A.M.; Kim, T.A.; Yang, K.; Sottos, N.R.; White, S.R.; et al. Malleable and recyclable poly(urea-urethane) thermosets bearing hindered urea bonds. Adv. Mater. 2016, 28, 7646–7651. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers: The effect of transesterification reactions on the network structure. Polymers 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Zheng, L.J.; Zeng, J.B. Dynamic crosslinking: An efficient approach to fabricate epoxy vitrimer. Materials 2021, 14, 919. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Wang, H.; Huang, X.; Huang, G.; Wu, J. Weldable, malleable and programmable epoxy vitrimers with high mechanical properties and water insensitivity. Chem. Eng. J. 2019, 368, 61–70. [Google Scholar] [CrossRef]
- Roudsari, G.M.; Mohanty, A.K.; Misra, M. Green approaches to engineer tough biobased epoxies: A review. ACS Sustain. Chem. Eng. 2017, 5, 9528–9541. [Google Scholar] [CrossRef]
- Zhao, W.; An, L.; Wang, S. Recyclable high-performance epoxy-anhydride resins with DMP-30 as the catalyst of transesterification reactions. Polymers 2021, 13, 296. [Google Scholar] [CrossRef]
- Yu, Y.; Storti, G.; Morbidelli, M. Kinetics of ring-opening polymerization ofl, l-lactide. Ind. Eng. Chem. Res. 2011, 50, 7927–7940. [Google Scholar] [CrossRef]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Reprocessing and recycling of thermosetting polymers based on bond exchange reactions. RSC Adv. 2014, 4, 10108–10117. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Li, H.; Jabour, J.; Yang, H.; Dunn, M.L.; Wang, T.J.; Qi, H.J. Interfacial welding of dynamic covalent network polymers. J. Mech. Phys. Solids 2016, 94, 1–17. [Google Scholar] [CrossRef] [Green Version]
- An, L.; Shi, Q.; Jin, C.; Zhao, W.; Wang, T.J. Chain diffusion based framework for modeling the welding of vitrimers. J. Mech. Phys. Solids 2022, 164, 104883. [Google Scholar] [CrossRef]
- Chabert, E.; Vial, J.; Cauchois, J.-P.; Mihaluta, M.; Tournilhac, F. Multiple welding of long fiber epoxy vitrimer composites. Soft Matter 2016, 12, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Hamel, C.M.; Kuang, X.; Chen, K.; Qi, H.J. Reaction-diffusion model for thermosetting polymer dissolution through exchange reactions assisted by small-molecule solvents. Macromolecules 2019, 52, 3636–3645. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.J.; Qi, H.J. Carbon fiber reinforced thermoset composite with near 100% recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106. [Google Scholar] [CrossRef]
- Mu, Q.; An, L.; Hu, Z.; Kuang, X. Fast and sustainable recycling of epoxy and composites using mixed solvents. Polym. Degrad. Stab. 2022, 199, 109895. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.J.; Qi, H.J. Solvent assisted pressure-free surface welding and reprocessing of malleable epoxy polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]
- Kuang, X.; Shi, Q.; Zhou, Y.; Zhao, Z.; Wang, T.J.; Qi, H.J. Dissolution of epoxy thermosets via mild alcoholysis: The mechanism and kinetics study. RSC Adv. 2018, 8, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ding, X.; Urban, M.W. Chemical and physical aspects of self-healing materials. Prog. Polym. Sci. 2015, 49, 34–59. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.; Liu, X.; Sheng, D.; Yang, Y. Epoxy-vitrimer composites based on exchangeable aromatic disulfide bonds: Reprocessibility, adhesive, multi-shape memory effect. Polymer 2020, 197, 122514. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ji, Y.; Wei, Y. Functional epoxy vitrimers and composites. Prog. Mater. Sci. 2020, 120, 100710. [Google Scholar] [CrossRef]

| Group | Depolymerizing Time t1 (min) | Depolymerizing Temperature T1 (°C) | Welding Pressure P (kPa) | Welding Temperature T2 (°C) | Welding Time t2 (h) |
|---|---|---|---|---|---|
| Group 1 | 20, 30, 40, 50 | 180 | 20 | 180 | 12 |
| Group 2 | 40 | 180 | 0, 10, 20, 30 | 180 | 12 |
| Group 3 | 40 | 180 | 20 | 140, 160, 180, 200 | 12 |
| Group 4 | 40 | 180 | 20 | 180 | 4, 6, 8, 10, 12 |
| Group 5 | / | / | 1000, 5000, 10,000, 20,000 | 180 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, L.; Zhao, W. Facile Surface Depolymerization Promotes the Welding of Hard Epoxy Vitrimer. Materials 2022, 15, 4488. https://doi.org/10.3390/ma15134488
An L, Zhao W. Facile Surface Depolymerization Promotes the Welding of Hard Epoxy Vitrimer. Materials. 2022; 15(13):4488. https://doi.org/10.3390/ma15134488
Chicago/Turabian StyleAn, Le, and Wenzhe Zhao. 2022. "Facile Surface Depolymerization Promotes the Welding of Hard Epoxy Vitrimer" Materials 15, no. 13: 4488. https://doi.org/10.3390/ma15134488
APA StyleAn, L., & Zhao, W. (2022). Facile Surface Depolymerization Promotes the Welding of Hard Epoxy Vitrimer. Materials, 15(13), 4488. https://doi.org/10.3390/ma15134488





