Hydrothermal Synthesis of Binder-Free Metallic NiCo2O4 Nano-Needles Supported on Carbon Cloth as an Advanced Electrode for Supercapacitor Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Initial Treatment of CC
2.2. Typical Fabrication Mechanism
2.3. Assembly of the Positive Electrode for SC
2.4. Physical Analysis and Characterization
2.5. Electrochemical Characterization
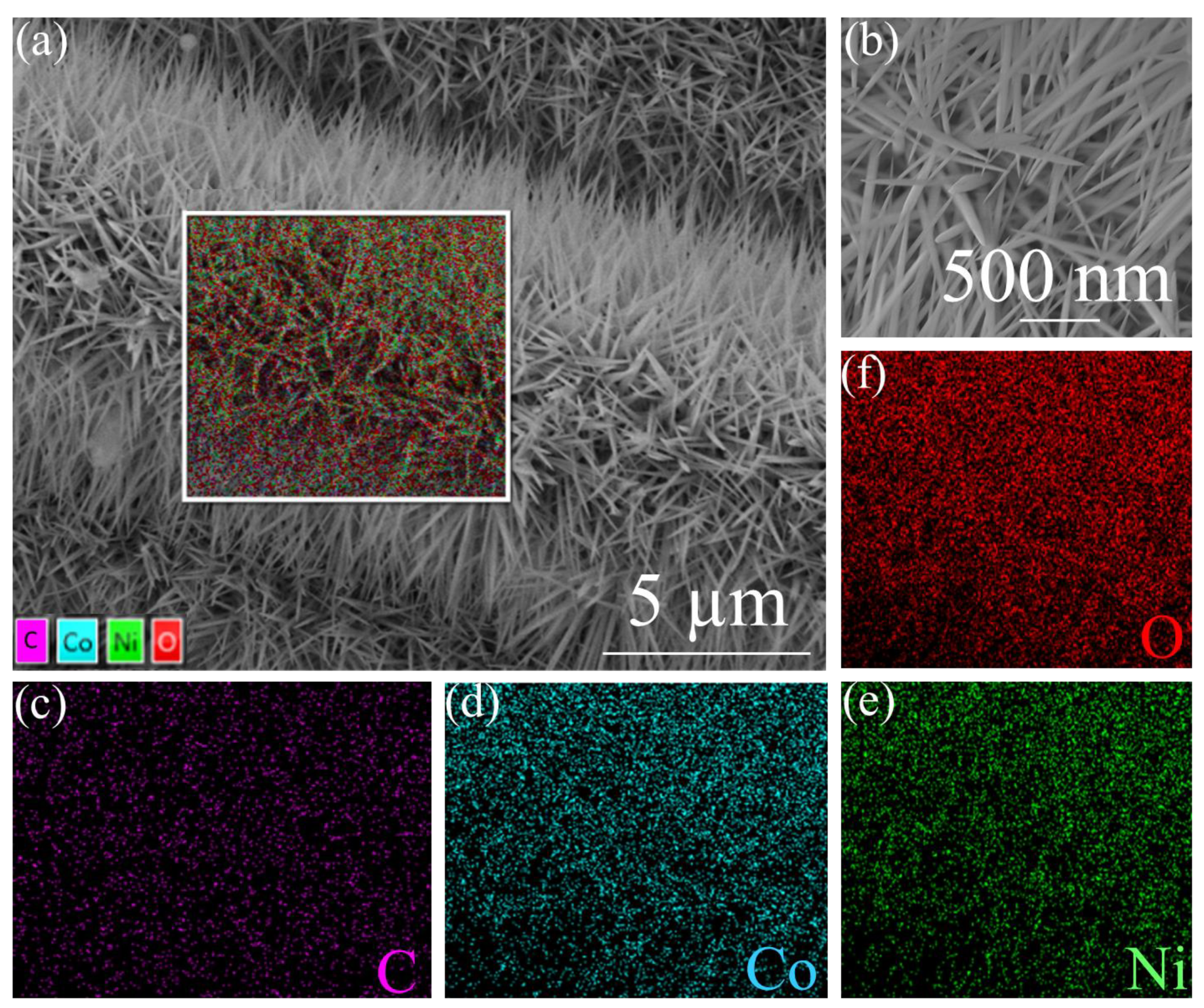
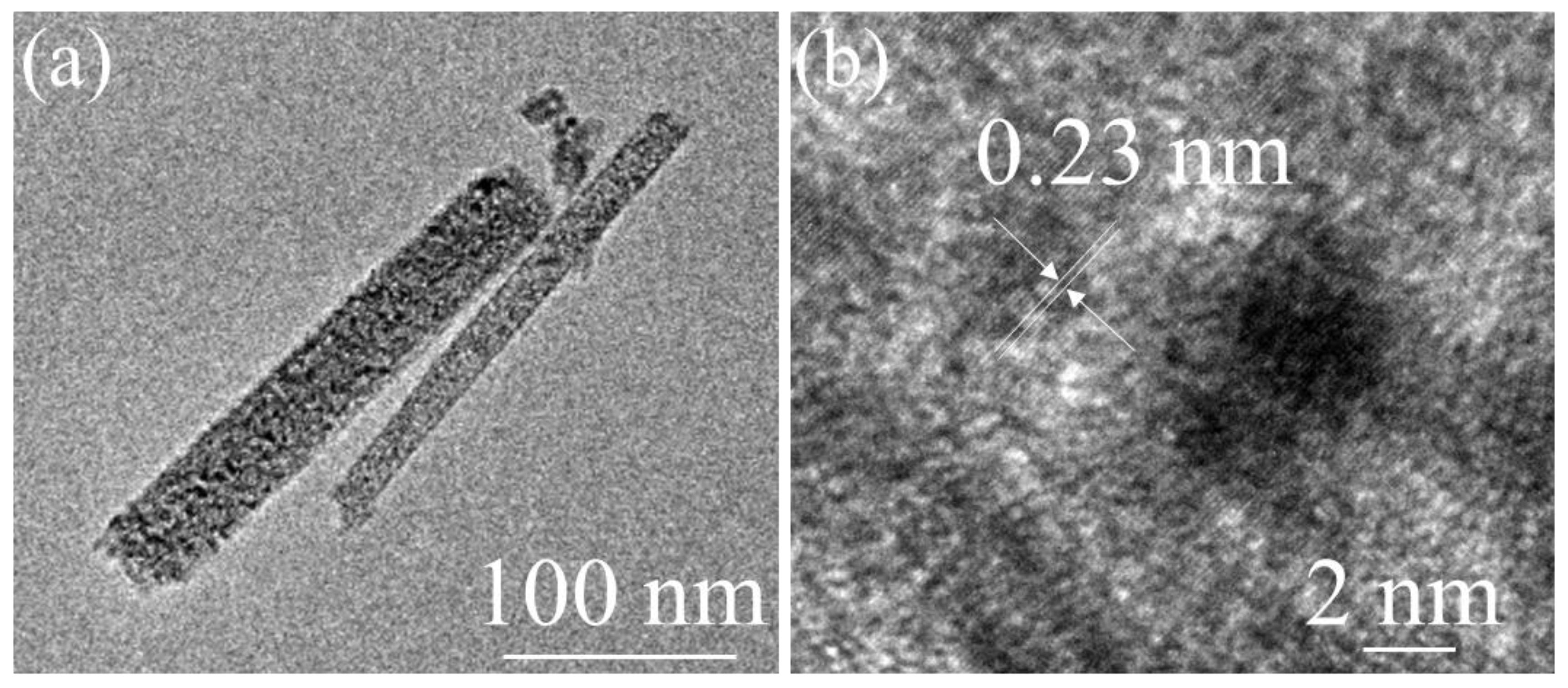
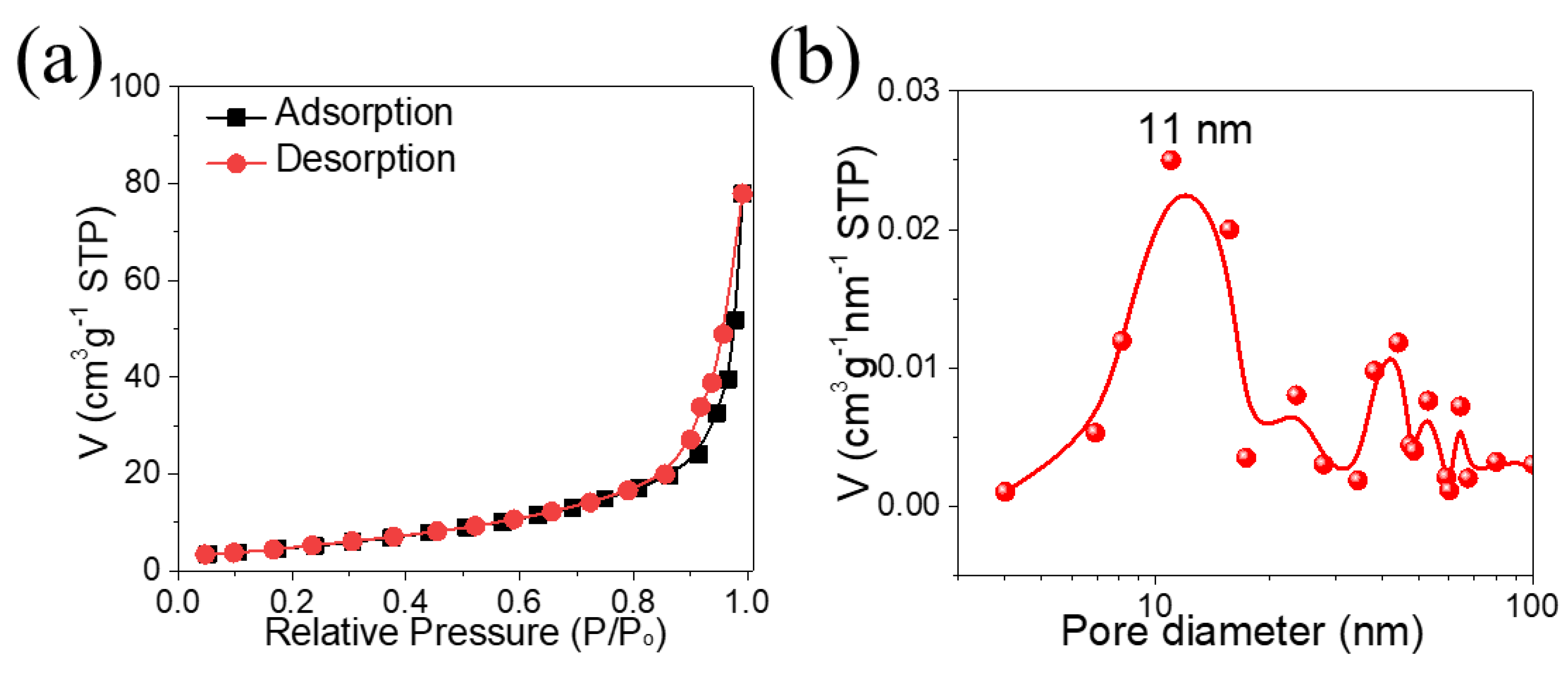
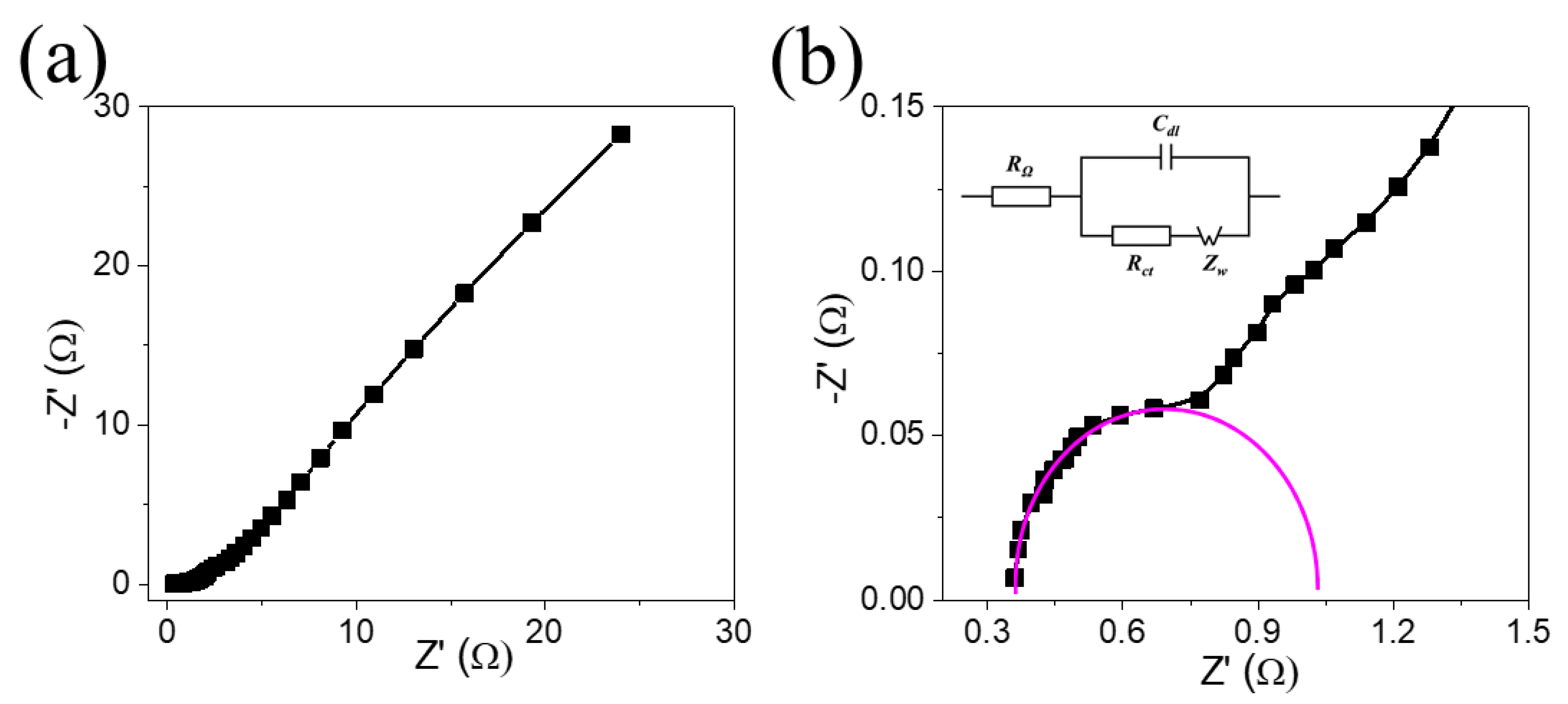
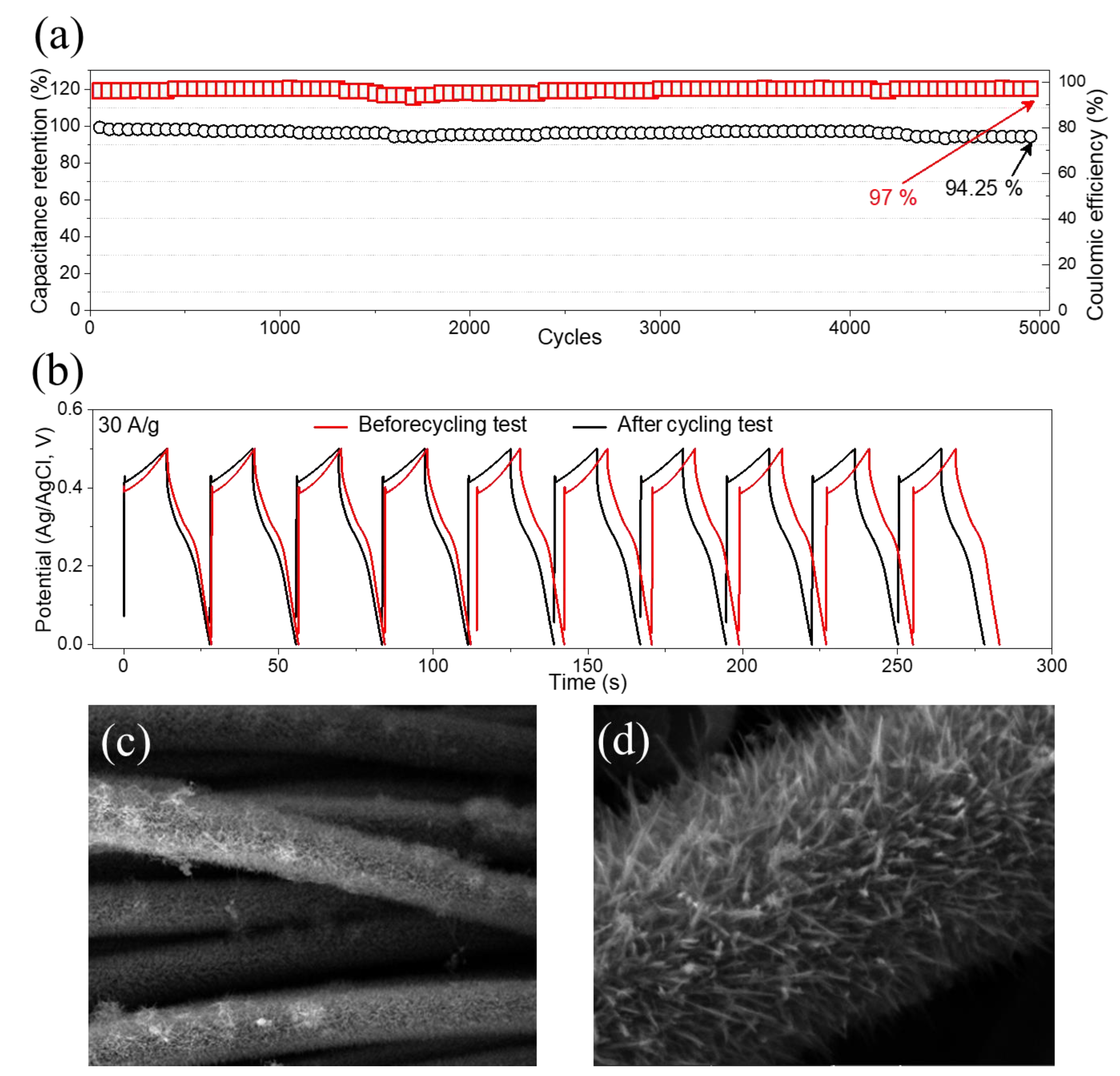
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Balaji, T.E.; Tanaya Das, H.; Maiyalagan, T. Recent trends in Bimetallic Oxides and their composites as electrode materials for Supercapacitor applications. ChemElectroChem 2021, 8, 1723–1746. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, M.Z.; Ansari, M.O. Manganese oxide as an effective electrode material for energy storage: A review. Environ. Chem. Lett. 2022, 20, 283–309. [Google Scholar] [CrossRef]
- Wang, W.; Guo, S.; Penchev, M.; Ruiz, I.; Bozhilov, K.N.; Yan, D.; Ozkan, M.; Ozkan, C.S. Three dimensional few layer graphene and carbon nanotube foam architectures for high fidelity supercapacitors. Nano Energy 2013, 2, 294–303. [Google Scholar] [CrossRef]
- Mahmood, A.; Zhao, B.; Javed, M.S.; He, D.; Cheong, W.C.; Han, D.; Niu, L. Unprecedented Duel Role of Polyaniline for Enhanced Pseudocapacitance of Cobalt-iron Layered Double Hydroxide. Macromol. Rapid Commun. 2022, 43, 2100905. [Google Scholar] [CrossRef]
- Javed, M.S.; Shaheen, N.; Hussain, S.; Li, J.; Shah, S.S.A.; Abbas, Y.; Ahmad, M.A.; Raza, R.; Mai, W. An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra. J. Mater. Chem. A 2019, 7, 946–957. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 5386–5393. [Google Scholar] [CrossRef]
- Wang, X.; Yan, C.; Sumboja, A.; Lee, P.S. High performance porous nickel cobalt oxide nanowires for asymmetric supercapacitor. Nano Energy 2014, 3, 119–126. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Lin, J.; Zhang, X.; Xiong, S. Polymer-assisted synthesis of a 3D hierarchical porous network-like spinel NiCo2O4 framework towards high-performance electrochemical capacitors. J. Mater. Chem. A 2013, 1, 11145–11151. [Google Scholar] [CrossRef]
- Ansari, S.A.; Parveen, N.; Al-Othoum, M.A.S.; Ansari, M.O. Effect of Washing on the Electrochemical Performance of a Three-Dimensional Current Collector for Energy Storage Applications. Nanomaterials 2021, 11, 1596. [Google Scholar] [CrossRef]
- Lyu, L.; Seong, K.-D.; Kim, J.M.; Zhang, W.; Jin, X.; Kim, D.K.; Jeon, Y.; Kang, J.; Piao, Y. CNT/high mass loading MnO2/graphene-grafted carbon cloth electrodes for high-energy asymmetric supercapacitors. Nano-Micro Lett. 2019, 11, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Ye, J.; Lu, H.; Wang, G.; Lv, J.; Ning, G. Flexible all-solid-state supercapacitors based on boron and nitrogen-doped carbon network anchored on carbon fiber cloth. Chem. Eng. J. 2021, 410, 128365. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, A.; Zhang, X.; Mu, J.; Che, H.; Wang, Y.; Wang, T.-T.; Zhang, Z.; Kou, Z. Fundamentals, advances and challenges of transition metal compounds-based supercapacitors. Chem. Eng. J. 2021, 412, 128611. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Xi, Y.; Lang, Q.; Guo, D.; Hu, C. Faradic redox active material of Cu 7 S 4 nanowires with a high conductance for flexible solid state supercapacitors. Nanoscale 2015, 7, 13610–13618. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, J.; Zhou, W.; Xu, J.; Wong, C.-P. High-performance flexible and self-healable quasi-solid-state zinc-ion hybrid supercapacitor based on borax-crosslinked polyvinyl alcohol/nanocellulose hydrogel electrolyte. J. Mater. Chem. A 2019, 7, 26524–26532. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, S.; Shi, W. Co3S4 nanoneedles decorated on NiCo2O4 nanosheets for high-performance asymmetric supercapacitors. Mater. Lett. 2018, 214, 194–197. [Google Scholar] [CrossRef]
- Javed, M.S.; Aslam, M.K.; Asim, S.; Batool, S.; Idrees, M.; Hussain, S.; Shah, S.S.A.; Saleem, M.; Mai, W.; Hu, C.J.E.A. High-performance flexible hybrid-supercapacitor enabled by pairing binder-free ultrathin Ni–Co–O nanosheets and metal-organic framework derived N-doped carbon nanosheets. Electrochim. Acta 2020, 349, 136384. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol–gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Liao, M.; Hu, X.; Fang, X. Electrical transport properties of large, individual NiCo2O4 nanoplates. Adv. Funct. Mater. 2012, 22, 998–1004. [Google Scholar] [CrossRef]
- Liu, Q.; Hong, X.; Zhang, X.; Wang, W.; Guo, W.; Liu, X.; Ye, M. Hierarchically structured Co9S8@NiCo2O4 nanobrushes for high-performance flexible asymmetric supercapacitors. Chem. Eng. J. 2019, 356, 985–993. [Google Scholar] [CrossRef]
- Zhou, W.; Kong, D.; Jia, X.; Ding, C.; Cheng, C.; Wen, G. NiCo2O4 nanosheet supported hierarchical core–shell arrays for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 6310–6315. [Google Scholar] [CrossRef]
- Zou, R.; Xu, K.; Wang, T.; He, G.; Liu, Q.; Liu, X.; Zhang, Z.; Hu, J. Chain-like NiCo2O4 nanowires with different exposed reactive planes for high-performance supercapacitors. J. Mater. Chem. A 2013, 1, 8560–8566. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Cheah, Y.; Teh, P.; Wang, J.; Wee, G.; Ko, Y.; Wong, C.; Srinivasan, M. Electrospun porous NiCo2O4 nanotubes as advanced electrodes for electrochemical capacitors. Chem. Eur. J. 2013, 19, 5892–5898. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Lin, J.; Pang, G.; Zhang, L.; Lian, L.; Zhang, X. Template-engaged synthesis of uniform mesoporous hollow NiCo 2 O 4 sub-microspheres towards high-performance electrochemical capacitors. RSC Adv. 2013, 3, 18573–18578. [Google Scholar] [CrossRef]
- Fang, J.; Kang, C.; Fu, L.; Li, S.; Liu, Q. Fabrication of hollow bamboo-shaped NiCo2O4 with controllable shell morphologies for high performance hybrid supercapacitors. J. Alloys Compd. 2020, 849, 156317. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, D.; Gu, L.; Liu, B.; Guo, F.; Ren, Y.; Hao, S. Support-induced morphology and content tailored NiCo2O4 nanostructures on temperature-dependent carbon nanofibers with enhanced pseudocapacitive performance. Electrochim. Acta 2018, 286, 1–13. [Google Scholar] [CrossRef]
- Mateen, A.; Javed, M.S.; Khan, S.; Saleem, A.; Majeed, M.K.; Khan, A.J.; Tahir, M.F.; Ahmad, M.A.; Assiri, M.A.; Peng, K.-Q. Metal-organic framework-derived walnut-like hierarchical Co-O-nanosheets as an advanced binder-free electrode material for flexible supercapacitor. J. Energy Storage 2022, 49, 104150. [Google Scholar] [CrossRef]
- Phat, D.T.; Thao, P.M.; Van Nghia, N.; Son, L.T.; Thu, T.V.; Lan, N.T.; Quyen, N.Q.; Van Ky, N.; Van Nguyen, T. Morphology controlled synthesis of battery-type NiCo2O4 supported on nickel foam for high performance hybrid supercapacitors. J. Energy Storage 2021, 33, 102030. [Google Scholar] [CrossRef]
- Yan, D.; Wang, W.; Luo, X.; Chen, C.; Zeng, Y.; Zhu, Z. NiCo2O4 with oxygen vacancies as better performance electrode material for supercapacitor. Chem. Eng. J. 2018, 334, 864–872. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Song, X.; Shen, H.; Kong, X.; Xu, H. Low-cost 3D porous sea-hedgehog-like NiCo2O4/C as anode for Li-ion battery. Nanotechnology 2020, 31, 415704. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mi, R.; Li, S.; Guo, P.; Mei, J.; Liu, H.; Lau, W.-M.; Liu, L.-M. Hierarchical three-dimensional NiCo2O4 nanoneedle arrays supported on Ni foam for high-performance supercapacitors. RSC Adv. 2015, 5, 25304–25311. [Google Scholar] [CrossRef]
- Khalid, S.; Cao, C.; Ahmad, A.; Wang, L.; Tanveer, M.; Aslam, I.; Tahir, M.; Idrees, F.; Zhu, Y. Microwave assisted synthesis of mesoporous NiCo2O4 nanosheets as electrode material for advanced flexible supercapacitors. RSC Adv. 2015, 5, 33146–33154. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Rajeshkhanna, G.; Justin, P.; Rao, G.R. Synthesis of mesoporous NiCo2O4–rGO by a solvothermal method for charge storage applications. RSC Adv. 2015, 5, 66657–66666. [Google Scholar] [CrossRef]
- Xiong, W.; Gao, Y.; Wu, X.; Hu, X.; Lan, D.; Chen, Y.; Pu, X.; Zeng, Y.; Su, J.; Zhu, Z. Composite of Macroporous Carbon with Honeycomb-Like Structure from Mollusc Shell and NiCo2O4 Nanowires for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 19416–19423. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W.J.A.m. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Qi, T.; Xia, D.; Wan, H. Facilely synthesized porous NiCo2O4 flowerlike nanostructure for high-rate supercapacitors. J. Power Sources 2014, 248, 28–36. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Zhang, M.; Meng, A.; Li, Q. Direct Growth of Ultrathin NiCo2O4/NiO Nanosheets on SiC Nanowires as a Free-Standing Advanced Electrode for High-Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 3598–3608. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Yang, J.; Tang, H.; Ye, Z.; Zeng, Y.; Lu, J. Three-Dimensional NiCo2O4@MnMoO4 Core–Shell Nanoarrays for High-Performance Asymmetric Supercapacitors. Langmuir 2017, 33, 10446–10454. [Google Scholar] [CrossRef]
- Huang, B.; Wang, H.; Liang, S.; Qin, H.; Li, Y.; Luo, Z.; Zhao, C.; Xie, L.; Chen, L. Two-dimensional porous cobalt–nickel tungstate thin sheets for high performance supercapattery. Energy Storage Mater. 2020, 32, 105–114. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Q.; Siyal, S.H.; Mateen, A.; Hassan, N.U.; Idrees, A.; Rehman, Z.U.; Din, E.M.T.E.; Bajaber, M.A.; Javed, M.S. Hydrothermal Synthesis of Binder-Free Metallic NiCo2O4 Nano-Needles Supported on Carbon Cloth as an Advanced Electrode for Supercapacitor Applications. Materials 2022, 15, 4499. https://doi.org/10.3390/ma15134499
Abbas Q, Siyal SH, Mateen A, Hassan NU, Idrees A, Rehman ZU, Din EMTE, Bajaber MA, Javed MS. Hydrothermal Synthesis of Binder-Free Metallic NiCo2O4 Nano-Needles Supported on Carbon Cloth as an Advanced Electrode for Supercapacitor Applications. Materials. 2022; 15(13):4499. https://doi.org/10.3390/ma15134499
Chicago/Turabian StyleAbbas, Qasim, Sajid Hussain Siyal, Abdul Mateen, Najam Ul Hassan, Asim Idrees, Zia Ur Rehman, ElSayed M. Tag El Din, Majed A. Bajaber, and Muhammad Sufyan Javed. 2022. "Hydrothermal Synthesis of Binder-Free Metallic NiCo2O4 Nano-Needles Supported on Carbon Cloth as an Advanced Electrode for Supercapacitor Applications" Materials 15, no. 13: 4499. https://doi.org/10.3390/ma15134499







