Titanium-Pillared Clay: Preparation Optimization, Characterization, and Artificial Neural Network Modeling
Abstract
:1. Introduction
2. Materials and Methods
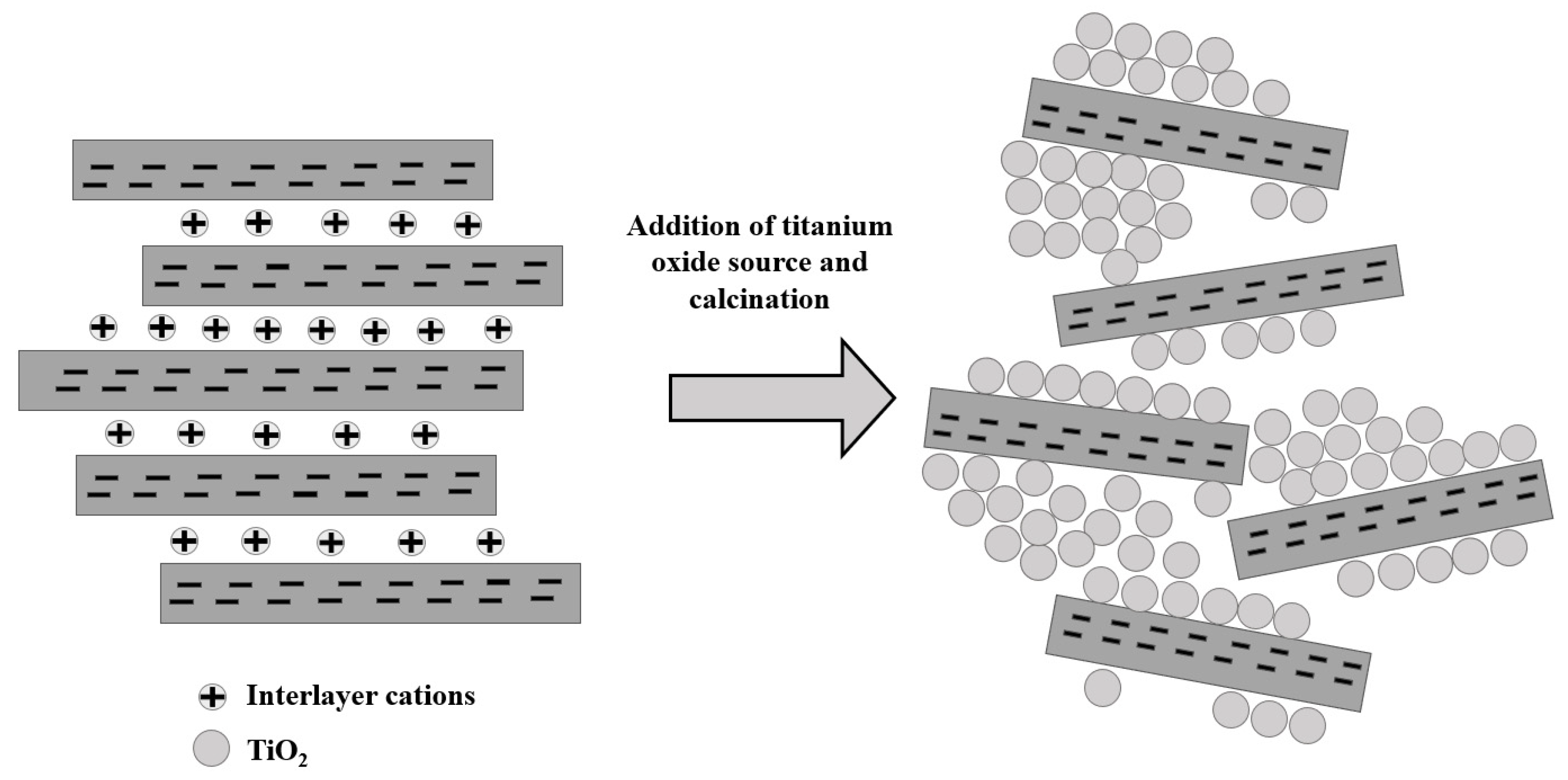
2.1. Preparation of Pillared Clay
2.2. Characterization Methods
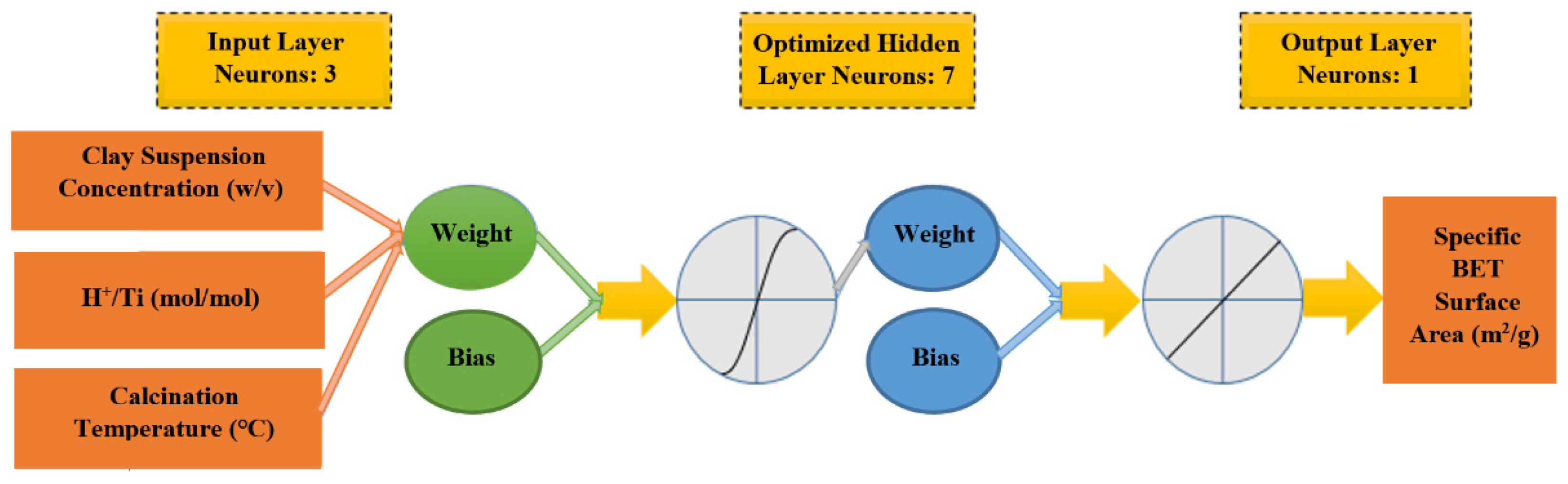
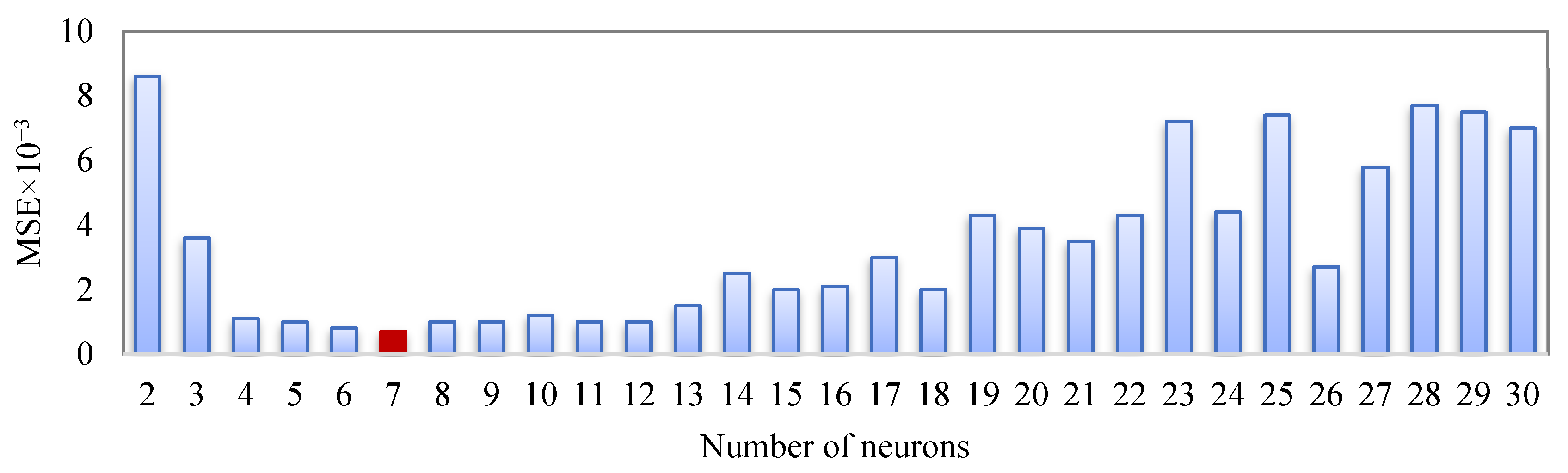
3. Artificial Neural Network Modeling
4. Results and Discussion
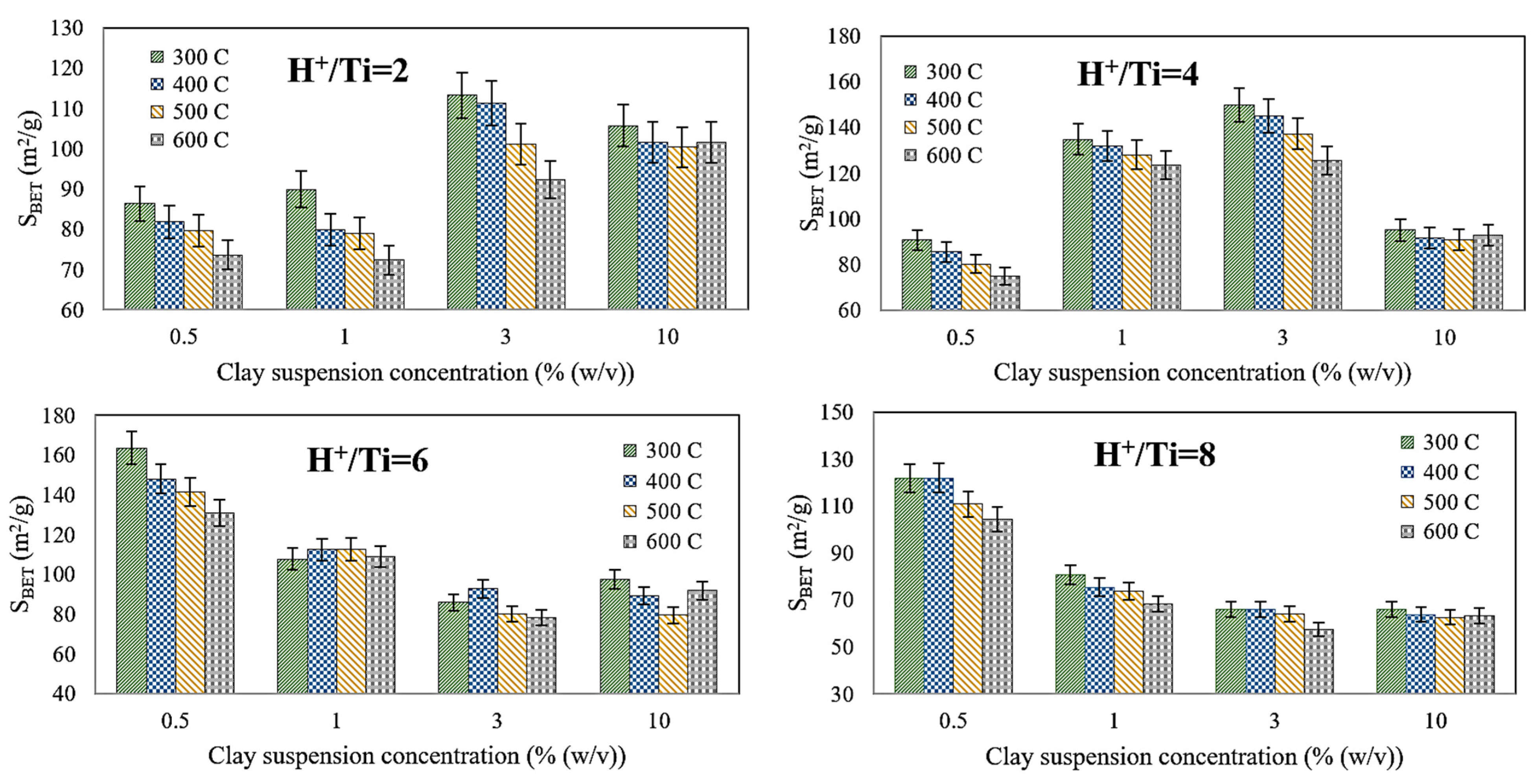
4.1. Effects of Operating Parameters and Optimization
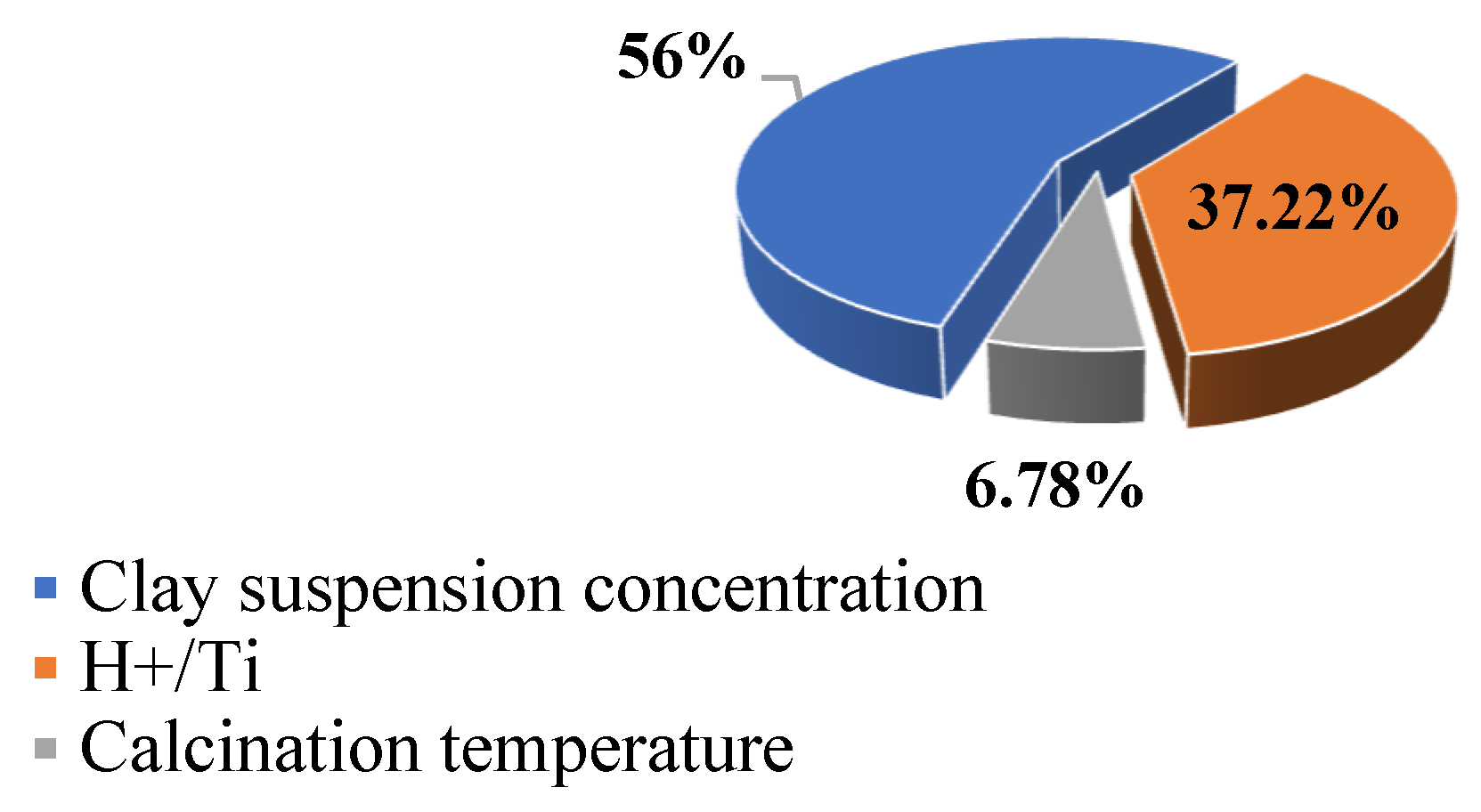
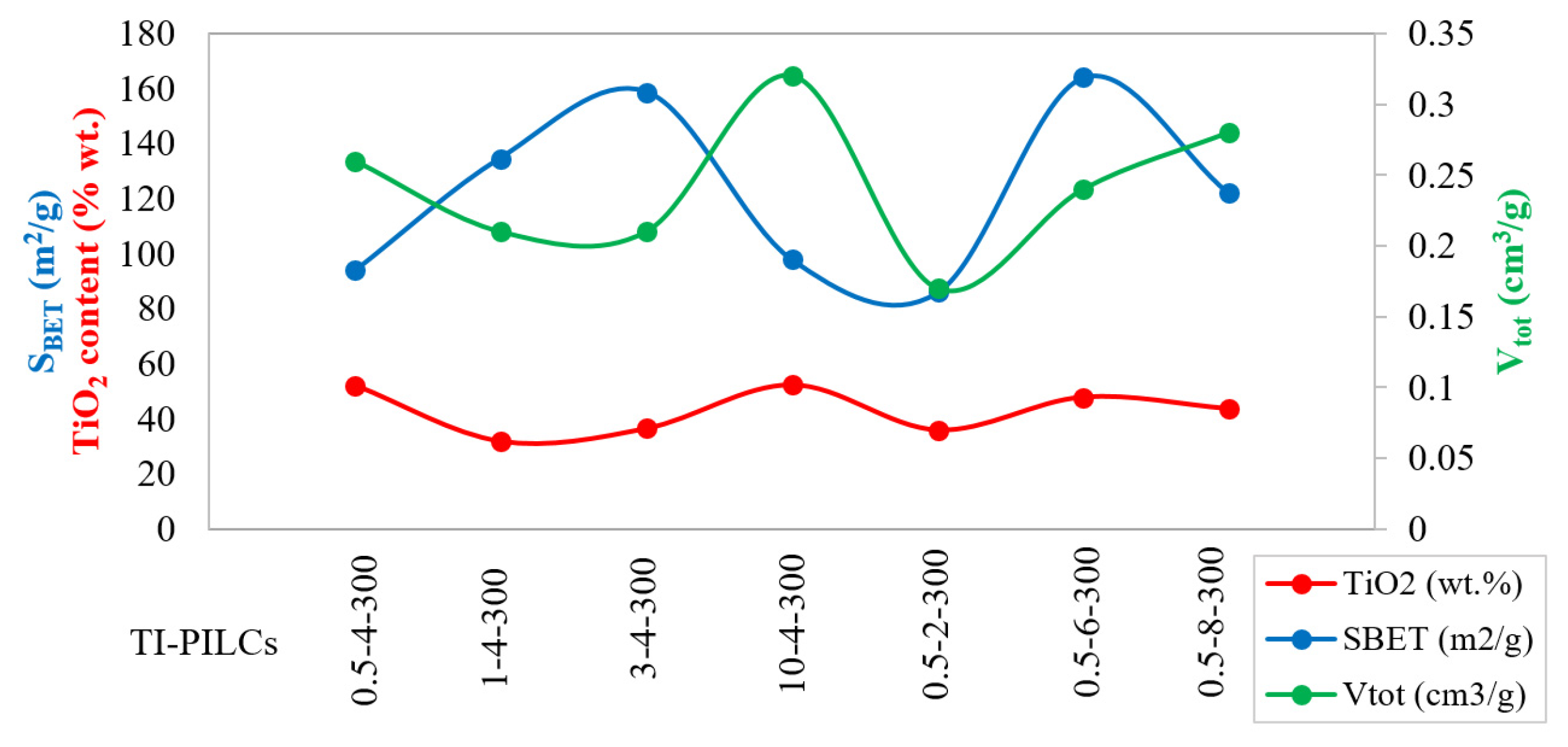
4.2. Relative Contribution of Operating Parameters
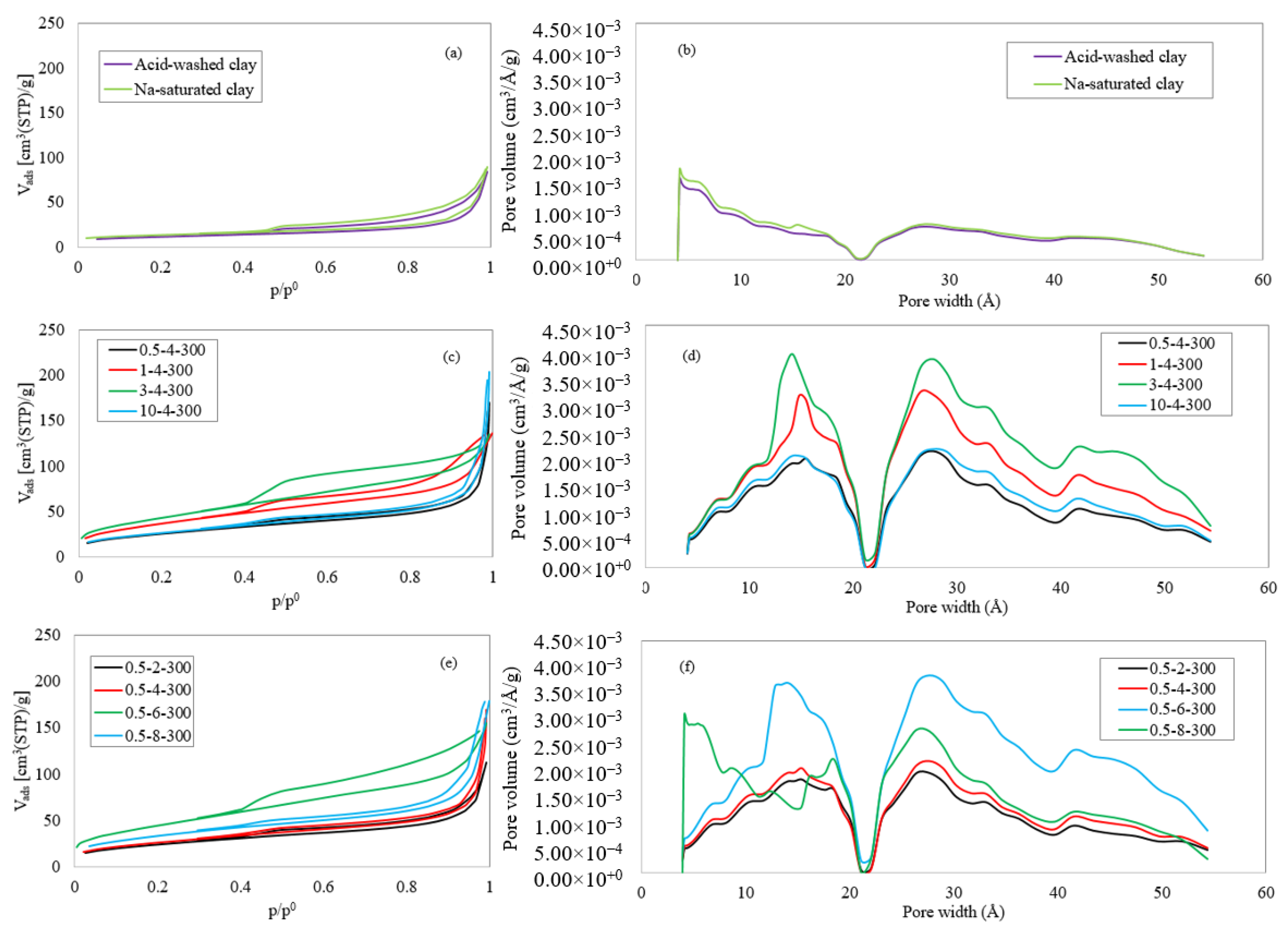
4.3. Porosity Analysis
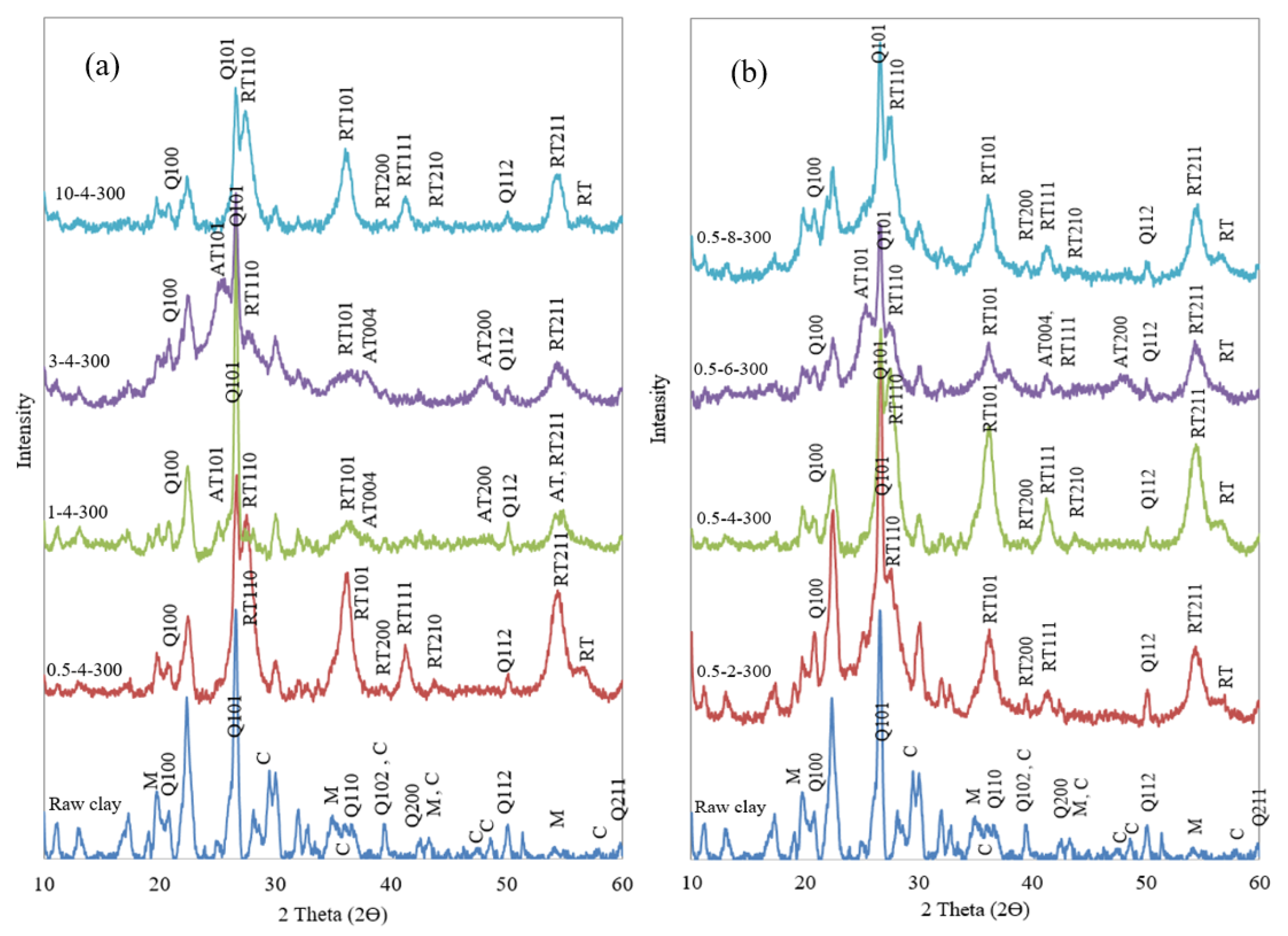
4.4. XRD and XRF Analysis
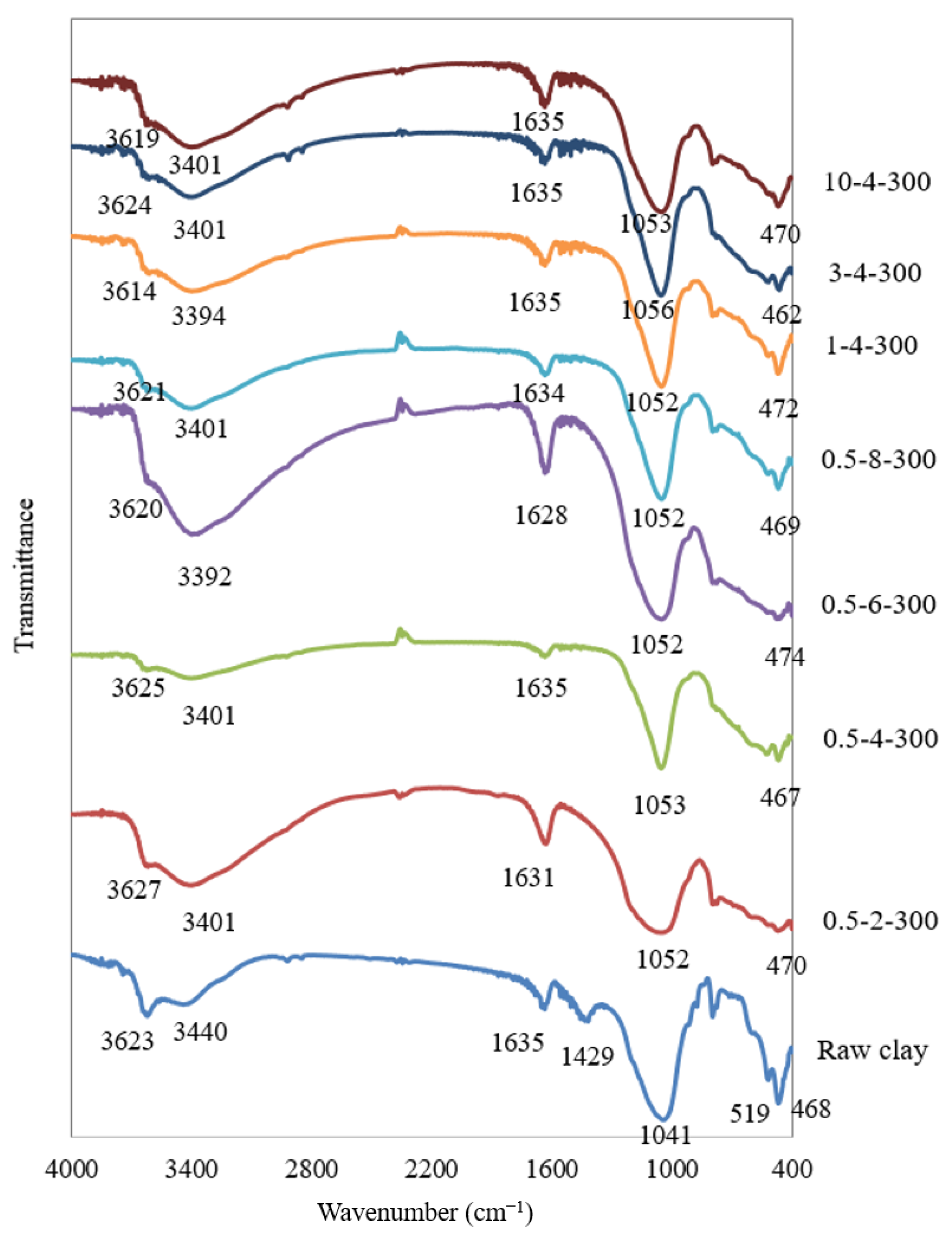
4.5. FTIR Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1–18. [Google Scholar]
- Tomul, F. The effect of ultrasonic treatment on iron–chromium pillared bentonite synthesis and catalytic wet peroxide oxidation of phenol. Appl. Clay Sci. 2016, 120, 121–134. [Google Scholar] [CrossRef]
- Taher, T.; Mohadi, R.; Lesbani, A. Effect of Ti4+/clay ratio on the properties of titanium pillared bentonite and its application for Cr (VI) removal. Rasayan J. Chem. 2018, 11, 1244–1254. [Google Scholar] [CrossRef]
- Najafi, H.; Farajfaed, S.; Zolgharnian, S.; Mosavi Mirak, S.H.; Asasian-Kolur, N.; Sharifian, S. A comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Saf. Environ. Prot. 2021, 147, 8–36. [Google Scholar] [CrossRef]
- Chauhan, M.; Saini, V.K.; Suthar, S. Ti-pillared montmorillonite clay for adsorptive removal of amoxicillin, imipramine, diclofenac-sodium, and paracetamol from water. J. Hazard. Mater. 2020, 399, 122832. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Manjaiah, K.M.; Datta, S.C.; Yadav, R.K.; Sarkar, B. Inorganically modified clay minerals: Preparation, characterization, and arsenic adsorption in contaminated water and soil. Appl. Clay Sci. 2017, 147, 1–10. [Google Scholar] [CrossRef]
- Georgescu, A.-M.; Nardou, F.; Zichil, V.; Nistor, I.D. Adsorption of lead(II) ions from aqueous solutions onto Cr-pillared clays. Appl. Clay Sci. 2018, 152, 44–50. [Google Scholar] [CrossRef]
- Mnasri Ghnimi, S.; Frini-Srasra, N. A comparison of single and mixed pillared clays for zinc and chromium cations removal. Appl. Clay Sci. 2018, 158, 150–157. [Google Scholar] [CrossRef]
- Ortiz-Martínez, K.; Reddy, P.; Cabrera-Lafaurie, W.A.; Román, F.R.; Hernández-Maldonado, A.J. Single and multi-component adsorptive removal of bisphenol A and 2,4-dichlorophenol from aqueous solutions with transition metal modified inorganic–organic pillared clay composites: Effect of pH and presence of humic acid. J. Hazard. Mater. 2016, 312, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yan, X.; Komarneni, S. CO2 Adsorption by Several Types of Pillared Montmorillonite Clays. Appl. Petrochem. Res. 2018, 8, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, M.; Saini, V.K.; Suthar, S. Removal of pharmaceuticals and personal care products (PPCPs) from water by adsorption on aluminum pillared clay. J. Porous Mater. 2020, 27, 383–393. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, Q.; Zheng, C.; Cheng, S.; Kang, T.; Dai, H. Effect of ceria loading on Zr-pillared clay catalysts for selective catalytic reduction of NO with NH3. New J. Chem. 2019, 43, 10850–10858. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J.; Ntho, T. Synthesis and characterization of an efficient and stable Al/Fe pillared clay catalyst for the catalytic wet air oxidation of phenol. RSC Adv. 2018, 8, 30115–30124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos Silva, A.; Seitovna Kalmakhanova, M.; Kabykenovna Massalimova, B.; Juliana, G.S.; Diaz de Tuesta, J.L.; Gomes, H.T. Wet Peroxide Oxidation of Paracetamol Using Acid Activated and Fe/Co-Pillared Clay Catalysts Prepared from Natural Clays. Catalysts 2019, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Chen, Z.; Li, J.; Zuo, S.; Yang, P. Mesoporous silica-pillared clays supported nanosized Co3O4-CeO2 for catalytic combustion of toluene. Appl. Surf. Sci. 2018, 459, 32–39. [Google Scholar] [CrossRef]
- Marcos, F.C.F.; Assaf, J.M.; Assaf, E.M. CuFe and CuCo supported on pillared clay as catalysts for CO2 hydrogenation into value-added products in one-step. Mol. Catal. 2018, 458, 297–306. [Google Scholar] [CrossRef]
- del Castillo, H.L.; Gil, A.; Grange, P. Selective catalytic reduction of NO by NH3 on titanium pillared montmorillonite. Catal. Lett. 1996, 36, 237–239. [Google Scholar] [CrossRef]
- Martin del Campo, E.; Romero, R.; Roa, G.; Peralta-Reyes, E.; Espino-Valencia, J.; Natividad, R. Photo-Fenton oxidation of phenolic compounds catalyzed by iron-PILC. Fuel 2014, 138, 149–155. [Google Scholar] [CrossRef]
- Vinod, V.P.; Anirudhan, T.S. Sorption of tannic acid on zirconium pillared clay. J. Chem. Technol. Biotechnol. 2002, 77, 92–101. [Google Scholar] [CrossRef]
- Song, Y.; Wang, S.; Yang, L.-Y.; Di Yu Wang, Y.-G.; Ouyang, X.-K. Facile fabrication of core–shell/bead-like ethylenediamine-functionalized Al-pillared montmorillonite/calcium alginate for As(V) ion adsorption. Int. J. Biol. Macromol. 2019, 131, 971–979. [Google Scholar] [CrossRef]
- Sahel, K.; Bouhent, M.; Belkhadem, F.; Ferchichi, M.; Dappozze, F.; Guillard, C.; Figueras, F. Photocatalytic degradation of anionic and cationic dyes over TiO2 P25, and Ti-pillared clays and Ag-doped Ti-pillared clays. Appl. Clay Sci. 2014, 95, 205–210. [Google Scholar] [CrossRef]
- Shen, B.; Chen, J.; Yue, S. Removal of elemental mercury by titanium pillared clay impregnated with potassium iodine. Microporous Mesoporous Mater. 2015, 203, 216–223. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zuo, S.; Yu, Y. Preparation and photocatalytic activity of silver and TiO2 nanoparticles/montmorillonite composites. Appl. Clay Sci. 2007, 37, 275–280. [Google Scholar] [CrossRef]
- Bernier, A.; Admaiai, L.F.; Grange, P. Synthesis and characterization of titanium pillared clays Influence of the temperature of preparation. Appl. Catal. 1991, 77, 269–281. [Google Scholar] [CrossRef]
- Valverde, J.L.; Sánchez, P.; Dorado, F.; Molina, C.B.; Romero, A. Influence of the synthesis conditions on the preparation of titanium-pillared clays using hydrolyzed titanium ethoxide as the pillaring agent. Microporous Mesoporous Mater. 2002, 54, 155–165. [Google Scholar] [CrossRef]
- Basoglu, F.T. Effect of titanium source on structural properties and acidity of Ti-pillared bentonite. Chem. Pap. 2016, 70, 933–945. [Google Scholar] [CrossRef]
- Del Castillo, H.L.; Gil, A.; Grange, P. Influence of the nature of titanium alkoxide and of the acid of hydrolysis in the preparation of titanium-pillared montmorillonites. J. Phys. Chem. Solids 1997, 58, 1053–1062. [Google Scholar] [CrossRef]
- Yoda, S.; Sakurai, Y.; Endo, A.; Miyata, T.; Yanagishita, H.; Otake, K.; Tsuchiya, T. Synthesis of titania-pillared montmorillonite via intercalation of titanium alkoxide dissolved in supercritical carbon dioxide. J. Mater. Chem. 2004, 14, 2763–2767. [Google Scholar] [CrossRef]
- Ishii, R.; Ooi, K. Reaction in supercritical CO2; an intercalation of 4-phenylazoaniline between layers of montmorillonite pillared with tetramethylammonium ions. Chem. Commun. 1998, 16, 1705–1706. [Google Scholar] [CrossRef]
- Manova, E.; Aranda, P.; Martín-Luengo, M.A.; Letaïef, S.; Ruiz-Hitzky, E. New titania-clay nanostructured porous materials. Microporous Mesoporous Mater. 2010, 131, 252–260. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Liang, T.; Sun, Q.; Wang, H. Photocatalytic degradation of Orange G using sepiolite-TiO2 nanocomposites: Optimization of physicochemical parameters and kinetics studies. Chem. Eng. Sci. 2018, 183, 231–239. [Google Scholar] [CrossRef]
- Pichat, P.; Khalaf, H.; Tabet, D.; Houari, M.; Saidi, M. Ti-montmorillonite as photocatalyst to remove 4-chlorophenol in water and methanol in air. Environ. Chem. Lett. 2005, 2, 191–194. [Google Scholar] [CrossRef]
- Gordan, M.; Abdul Razak, H.; Ismail, Z.; Ghaedi, K. Recent Developments in Damage Identification of Structures Using Data Mining. Lat. Am. J. Solids Struct. 2017, 14, 2373–2401. [Google Scholar] [CrossRef]
- Jasso-Salcedo, A.B.; Hoppe, S.; Pla, F.; Escobar-Barrios, V.A.; Camargo, M.; Meimaroglou, D. Modeling and optimization of a photocatalytic process: Degradation of endocrine disruptor compounds by Ag/ZnO. Chem. Eng. Res. Des. 2017, 128, 174–191. [Google Scholar] [CrossRef]
- Sharifian, S.; Madadkhani, M.; Rahimi, M.; Mir, M.; Baghban, A. QSPR based ANFIS model for predicting standard molar chemical exergy of organic materials. Pet. Sci. Technol. 2019, 37, 2174–2181. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Cho, D.R.; Kim, S.Y.; Jermy, B.R.; Park, D.W. Vanadia-doped titania-pillared montmorillonite clay for the selective catalytic oxidation of H2S. Catal. Commun. 2008, 9, 2040–2043. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lee, C.-Y.; Yeng, M.-Y.; Chiu, H.-T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Kitayama, Y.; Kodama, T.; Abe, M.; Shimotsuma, H.; Matsuda, Y. Synthesis of Titania Pillared Saponite in Aqueous Solution of Acetic Acid. J. Porous Mater. 1998, 5, 121–126. [Google Scholar] [CrossRef]
- Bouras, O.; Bollinger, J.-C.; Baudu, M.; Khalaf, H. Adsorption of diuron and its degradation products from aqueous solution by surfactant-modified pillared clays. Appl. Clay Sci. 2007, 37, 240–250. [Google Scholar] [CrossRef]
- Rezala, H.; Khalaf, H.; Valverde, J.L.; Romero, A.; Molinari, A.; Maldotti, A. Photocatalysis with Ti-pillared clays for the oxofunctionalization of alkylaromatics by O2. Appl. Catal. A Gen. 2009, 352, 234–242. [Google Scholar] [CrossRef]
- Damardji, B.; Khalaf, H.; Duclaux, L.; David, B. Preparation of TiO2-pillared montmorillonite as photocatalyst Part I. Microwave calcination, characterisation, and adsorption of a textile azo dye. Appl. Clay Sci. 2009, 44, 201–205. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Kuśtrowski, P.; Węgrzyn, A.; Gil, B.; Kowalczyk, A.; Dudek, B.; Dziembaj, R.; Michalik, M. Comparison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process. Appl. Clay Sci. 2011, 53, 164–173. [Google Scholar] [CrossRef]
- Chen, D.; Du, G.; Zhu, Q.; Zhou, F. Synthesis and characterization of TiO2 pillared montmorillonites: Application for methylene blue degradation. J. Colloid Interface Sci. 2013, 409, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liu, H.; He, H.; Yang, C. Oxidative desulfurization of dibenzothiophene using molybdenum catalyst supported on Ti-pillared montmorillonite and separation of sulfones by filtration. Fuel 2018, 234, 1229–1237. [Google Scholar] [CrossRef]
- Vellayan, K.; González, B.; Trujillano, R.; Vicente, M.A.; Gil, A. Pd supported on Cu-doped Ti-pillared montmorillonite as catalyst for the Ullmann coupling reaction. Appl. Clay Sci. 2018, 160, 126–131. [Google Scholar] [CrossRef]
- Garson, G.D. Interpreting Neural-Network Connection Weights. AI Expert 1991, 6, 42–46. [Google Scholar]
- Goh, A.T.C. Back-propagation neural networks for modeling complex systems. Artif. Intell. Eng. 1995, 9, 143–151. [Google Scholar] [CrossRef]
- Montaño, J.J.; Palmer, A. Numeric sensitivity analysis applied to feedforward neural networks. Neural Comput. Appl. 2003, 12, 119–125. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Farhadian Azizi, K.; Bagheri-Mohagheghi, M.-M. Transition from anatase to rutile phase in titanium dioxide (TiO2) nanoparticles synthesized by complexing sol–gel process: Effect of kind of complexing agent and calcinating temperature. J. Sol-Gel Sci. Technol. 2013, 65, 329–335. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef] [Green Version]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is Anatase a Better Photocatalyst Than Rutile?—Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoye, I.P.; Obi, C. Synthesis and characterization of titanium pillared bentonite clay mineral. Int. Arch. Appl. Sci. Technol. 2011, 2, 84–89. [Google Scholar]





| Titanium Source * | Raw Clay SBET (m2/g) | Ti-PILC SBET (m2/g) | Ti-PILC Vtot (cm3/g) | SBET Enhancement after Pillaring (%) | Ref. |
|---|---|---|---|---|---|
| TC | 70 | 185–360 | - | 414 | [24] |
| TIP | 152 | 298–404 | - | 166 | [38] |
| TC | 91 | 280 | - | 208 | [39] |
| TIP | 85 | 250–283 | 0.18–0.23 | 231 | [40] |
| TIP | 90 | 151 | - | 68 | [41] |
| TC | 77 | 212 | 0.22 | 175 | [42] |
| TB | 10 | 136 | 0.16 | 1235 | [43] |
| TE | 117 | 208 | - | 78 | [21] |
| TIP | 77 | 149 | 0.14 | 92 | [22] |
| TIP | 57 | 191–373 | 0.22–0.32 | 557 | [26] |
| TE | 74 | 230–334 | - | 211–351 | [27] |
| TNP | 190–306 | - | 157–313 | ||
| TIP | 146–273 | - | 97–269 | ||
| TB | 124–265 | - | 68–258 | ||
| TIP | 104 | 105 | 0.13 | 1 | [30] |
| TIP | 23 | 66 | 0.10 | 187 | |
| TB | 149 | 157 | 0.23 | 5 | [44] |
| TC | 49 | 329 | 0.14 | 571 | [45] |
| TC | 65 | 216 | - | 232 | [5] |
| TIP | 44 | 164 | 0.13–0.45 | 273 | This paper |
| Number of Neurons in the Hidden Layer | Weights and Biases between Input and Hidden Layers | Weights and Biases between Hidden and Output Layers | ||||
|---|---|---|---|---|---|---|
| Normalized Input Variables | Bias | Normalized Output SBET | Bias | |||
| Clay Suspension Concentration | H+/Ti | Calcination Temperature | ||||
| 1 | 1.6329 | −2.1759 | 0.1419 | −3.1969 | 1.7711 | 1.0061 |
| 2 | −2.4581 | 0.0579 | −0.4061 | 0.4805 | 3.1642 | |
| 3 | 0.9654 | 1.2839 | −0.0339 | 0.9716 | 5.9584 | |
| 4 | −6.8028 | −2.7467 | 0.1051 | −4.8613 | 2.2528 | |
| 5 | −0.5566 | 2.5503 | −0.9633 | 0.8387 | 0.0390 | |
| 6 | −2.3382 | 2.6971 | −0.0558 | −1.5031 | −3.1359 | |
| 7 | −5.7059 | 0.1926 | −0.1625 | −7.0700 | 3.3928 | |
| Sample | SBET (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | dmic. (Å) | dmes. (Å) | davg. (Å) | |
|---|---|---|---|---|---|---|---|---|
| Acid-washed Bentonite | 40 | 0.13 | 0.02 | 0.13 | 13.8 | 36.7 | 130.3 | |
| Na-saturated Bentonite | 44 | 0.14 | 0.02 | 0.13 | 12.6 | 37.2 | 125.4 | |
| Ti-PILC | 0.5-2-300 ** | 86 | 0.17 | 0.03 | 0.16 | 12.6 | 34.2 | 80.9 |
| 0.5-4-300 | 94 | 0.26 | 0.04 | 0.24 | 12.6 | 34.0 | 112.2 | |
| 0.5-6-300 | 164 | 0.24 | 0.06 | 0.22 | 10.0 | 34.3 | 58.4 | |
| 0.5-8-300 | 122 | 0.28 | 0.05 | 0.25 | 12.9 | 34.0 | 91.0 | |
| 1-4-300 | 135 | 0.21 | 0.05 | 0.19 | 11.6 | 34.3 | 62.0 | |
| 3-4-300 | 159 | 0.21 | 0.06 | 0.19 | 10.3 | 34.4 | 52.5 | |
| 10-4-300 | 98 | 0.32 | 0.04 | 0.30 | 12.7 | 34.1 | 129.3 | |
| Sample | Weight Percentage (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | MgO | TiO2 | |
| Raw Clay | 74.6 | 11.7 | 2.3 | 5.7 | 1.8 | 0.9 | 2.1 | 0.3 |
| 0.5-4-300 | 38.5 | 5.7 | 1.2 | 0.5 | 0.1 | 0.3 | 1.1 | 52.2 |
| 1-4-300 | 55.5 | 8.5 | 1.2 | 0.9 | 0.2 | 0.5 | 0.9 | 31.9 |
| 3-4-300 | 50.1 | 9.6 | 1.3 | 0.6 | 0.2 | 0.4 | 1.1 | 36.5 |
| 10-4-300 | 38.4 | 5.7 | 1.1 | 0.4 | 0.2 | 0.4 | 1.1 | 52.3 |
| 0.5-2-300 | 52.3 | 7.1 | 1.2 | 0.9 | 0.3 | 0.7 | 1.0 | 36.0 |
| 0.5-6-300 | 41.5 | 6.8 | 1.2 | 0.6 | 0.2 | 0.3 | 1.1 | 47.9 |
| 0.5-8-300 | 45.2 | 6.8 | 1.4 | 0.7 | 0.2 | 0.3 | 1.3 | 43.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosavi Mirak, S.H.; Sharifian, S.; Esmaeili Khalil Saraei, F.; Asasian-Kolur, N.; Haddadi, B.; Jordan, C.; Harasek, M. Titanium-Pillared Clay: Preparation Optimization, Characterization, and Artificial Neural Network Modeling. Materials 2022, 15, 4502. https://doi.org/10.3390/ma15134502
Mosavi Mirak SH, Sharifian S, Esmaeili Khalil Saraei F, Asasian-Kolur N, Haddadi B, Jordan C, Harasek M. Titanium-Pillared Clay: Preparation Optimization, Characterization, and Artificial Neural Network Modeling. Materials. 2022; 15(13):4502. https://doi.org/10.3390/ma15134502
Chicago/Turabian StyleMosavi Mirak, Seyed Heydar, Seyedmehdi Sharifian, Fatemeh Esmaeili Khalil Saraei, Neda Asasian-Kolur, Bahram Haddadi, Christian Jordan, and Michael Harasek. 2022. "Titanium-Pillared Clay: Preparation Optimization, Characterization, and Artificial Neural Network Modeling" Materials 15, no. 13: 4502. https://doi.org/10.3390/ma15134502
APA StyleMosavi Mirak, S. H., Sharifian, S., Esmaeili Khalil Saraei, F., Asasian-Kolur, N., Haddadi, B., Jordan, C., & Harasek, M. (2022). Titanium-Pillared Clay: Preparation Optimization, Characterization, and Artificial Neural Network Modeling. Materials, 15(13), 4502. https://doi.org/10.3390/ma15134502








