Eye-Visible Oxygen Sensing via In-Situ Synthesizing Blue-Emitting Cu(I) Cluster in Red-Emitting COF: Characterization and Performance
Abstract
:1. Introduction
2. Experimental Details
2.1. Reagents and Apparatus
2.2. Synthesis of Br-COF and Cl-COF
2.3. Synthesis of CuPBr-COF and CuPCl-COF
2.4. Synthesis of Reference Compounds CuPBr and CuPCl
2.5. Oxygen-Sensing Operation
3. Results and Discussion
3.1. Characterization on CuPX and CuPX-COF, X = Br or Cl
3.1.1. Single Crystal Structure of CuPBr
3.1.2. Electronic Structure of CuPBr
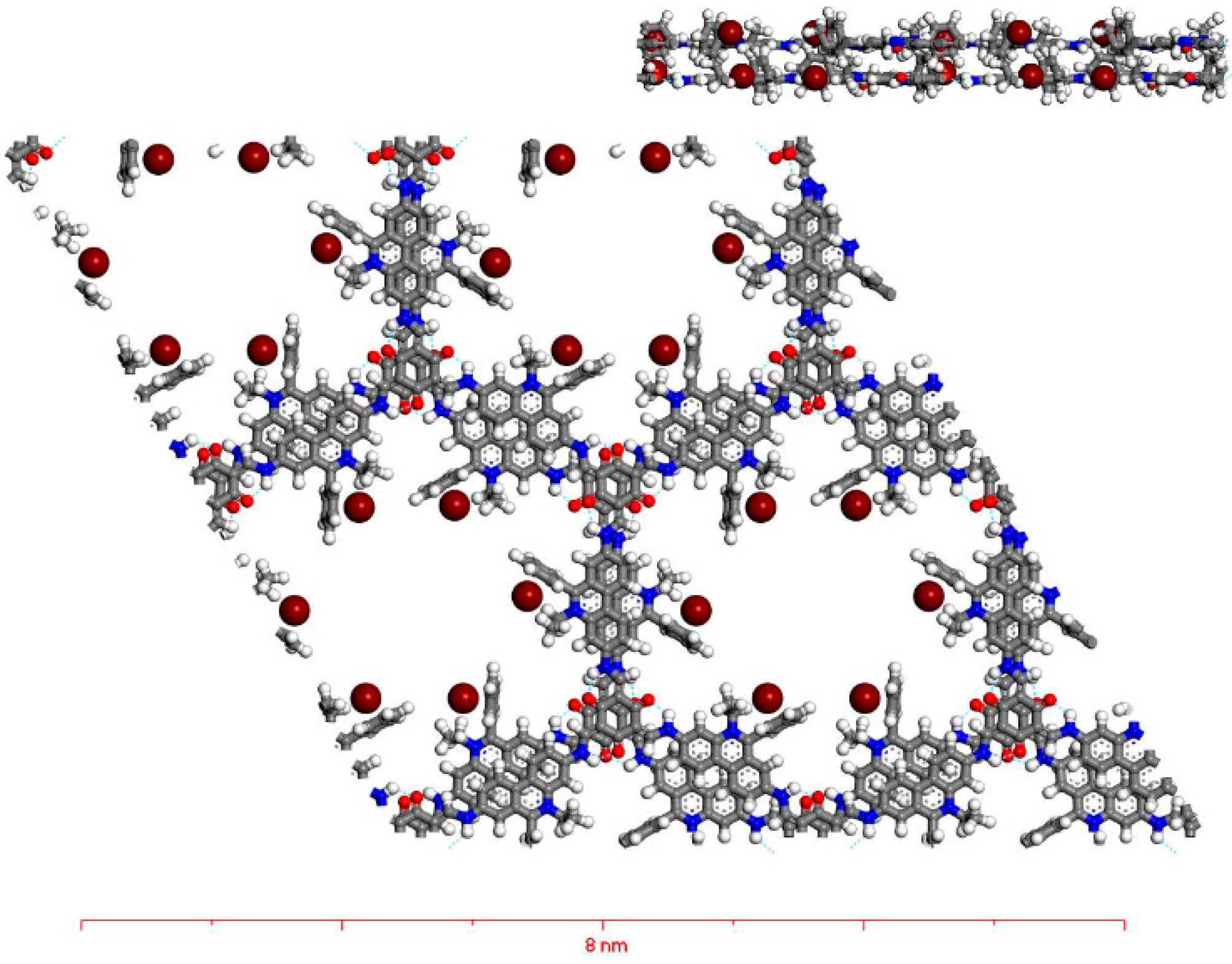
3.1.3. Simulated Structure of X-COF
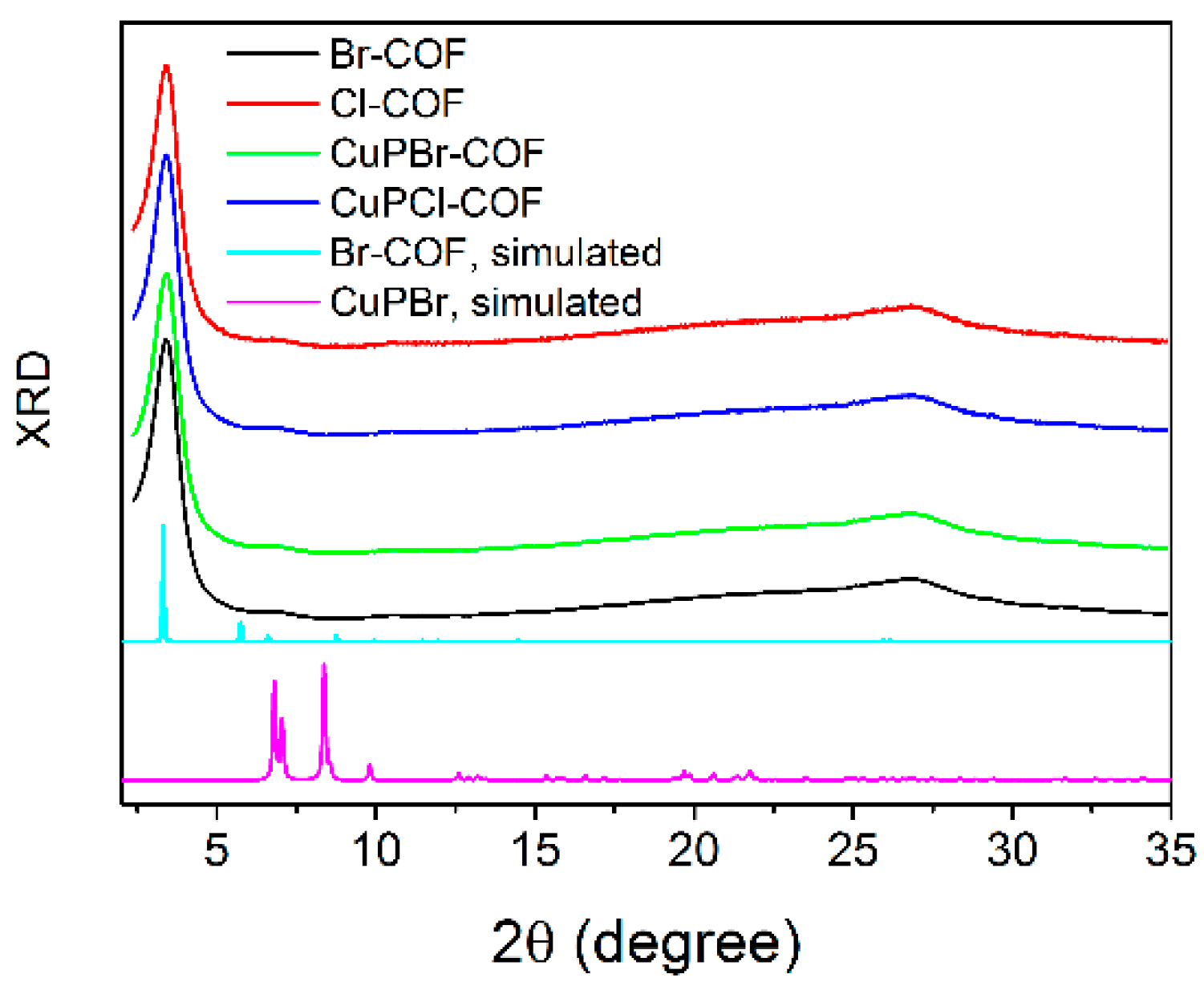
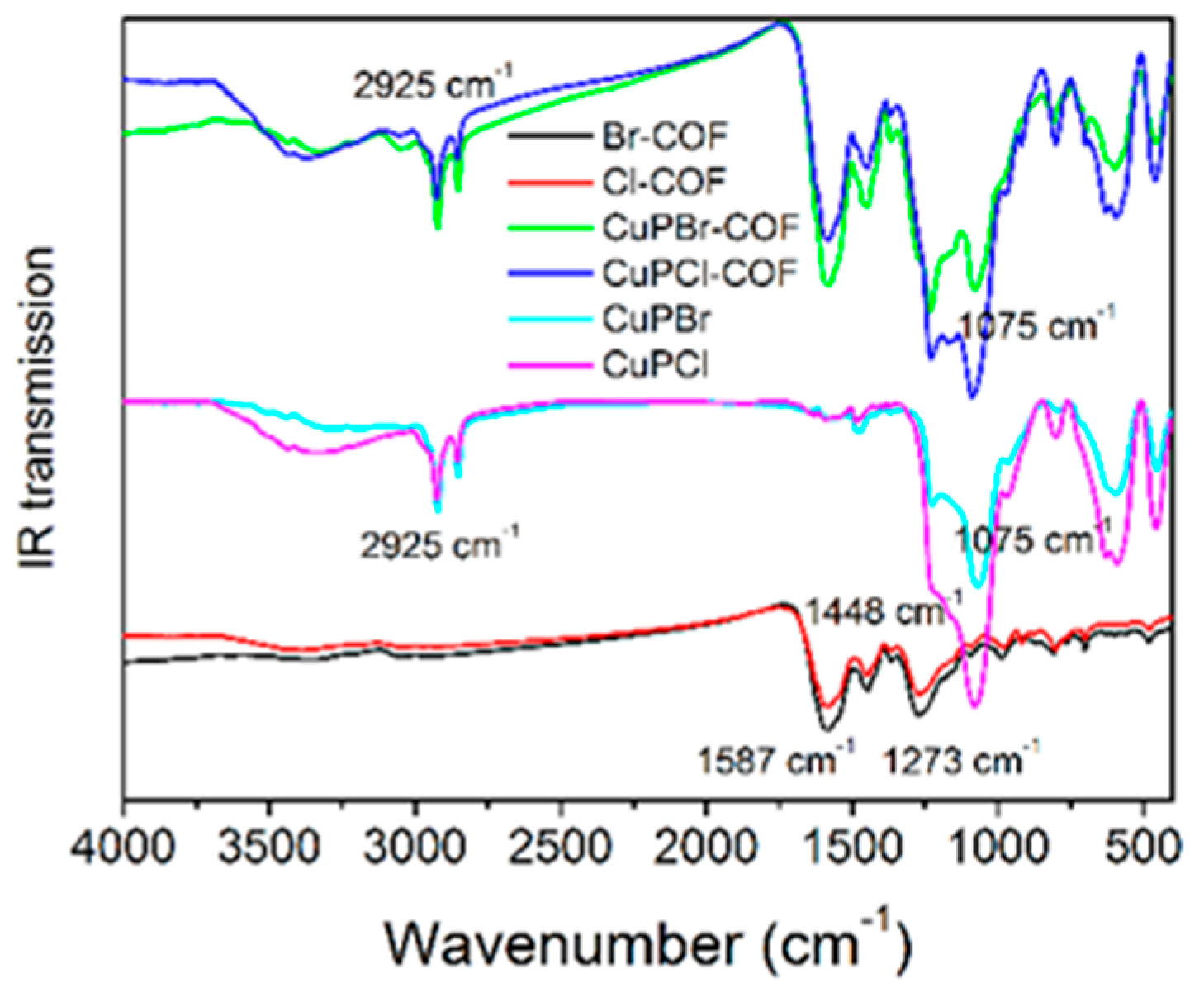
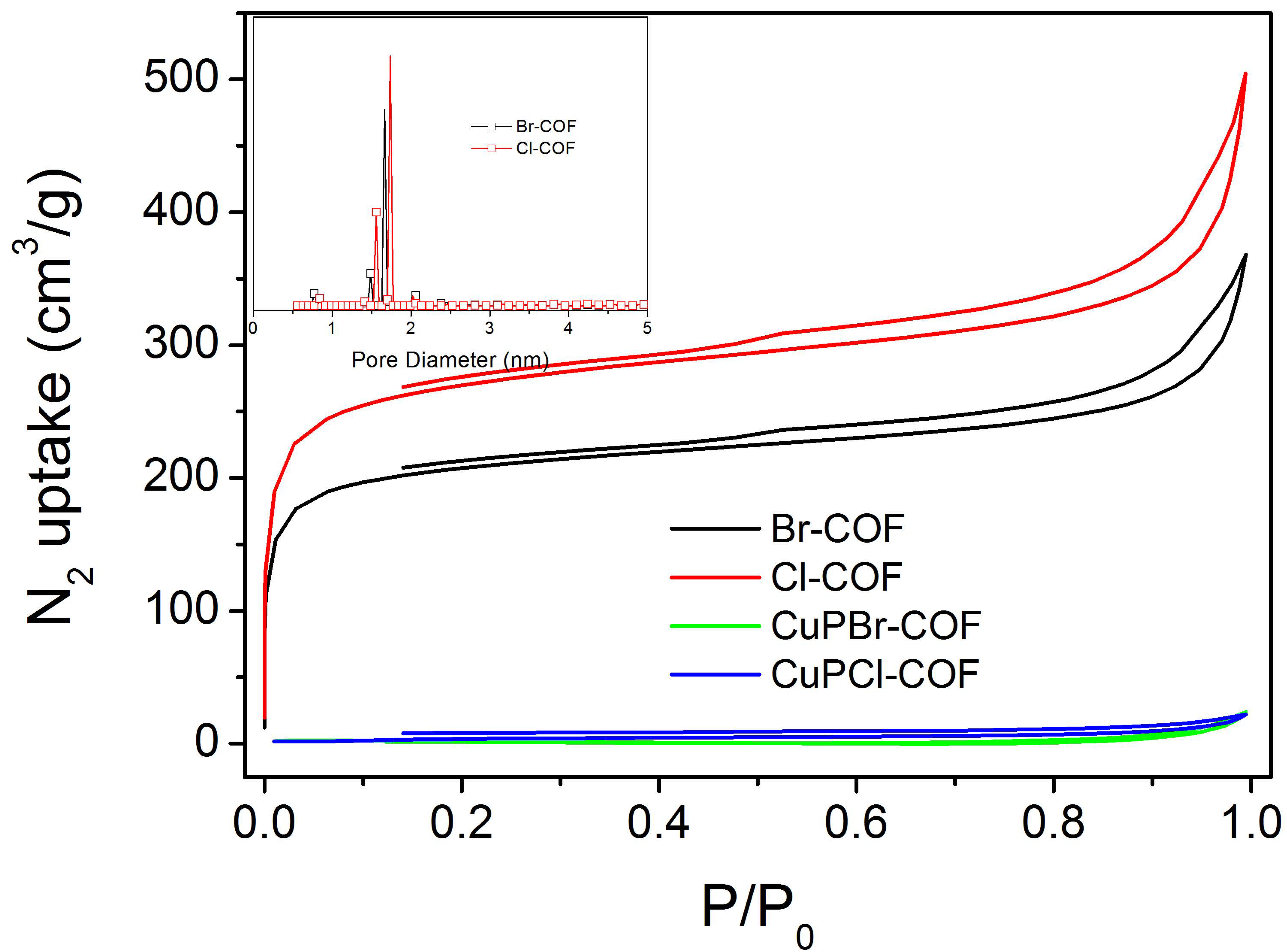
3.1.4. XRD Analysis, SEM Morphology, IR Spectra and Microporous Structure
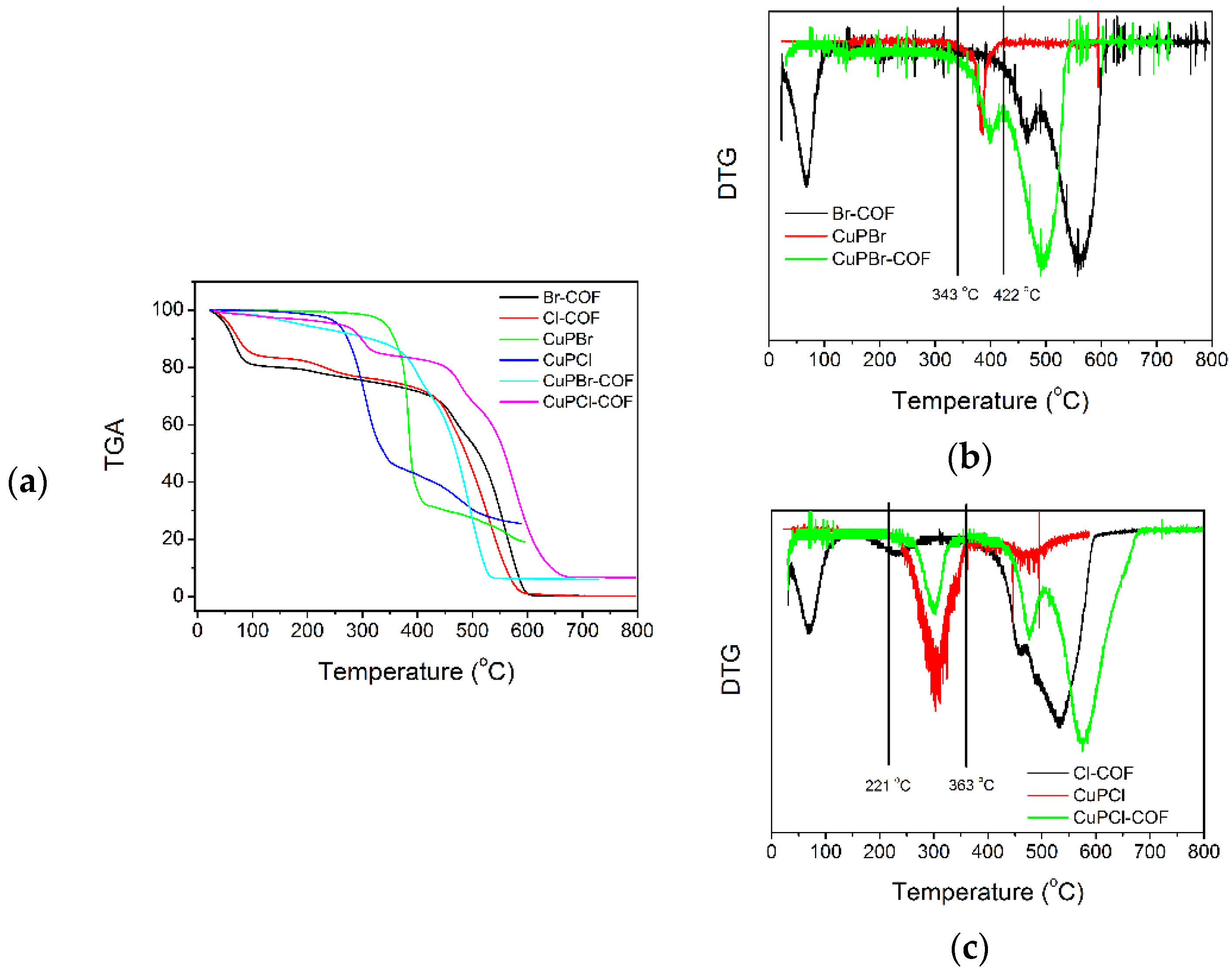
3.1.5. Doping Level Determined by Elemental Data and Thermal Analysis
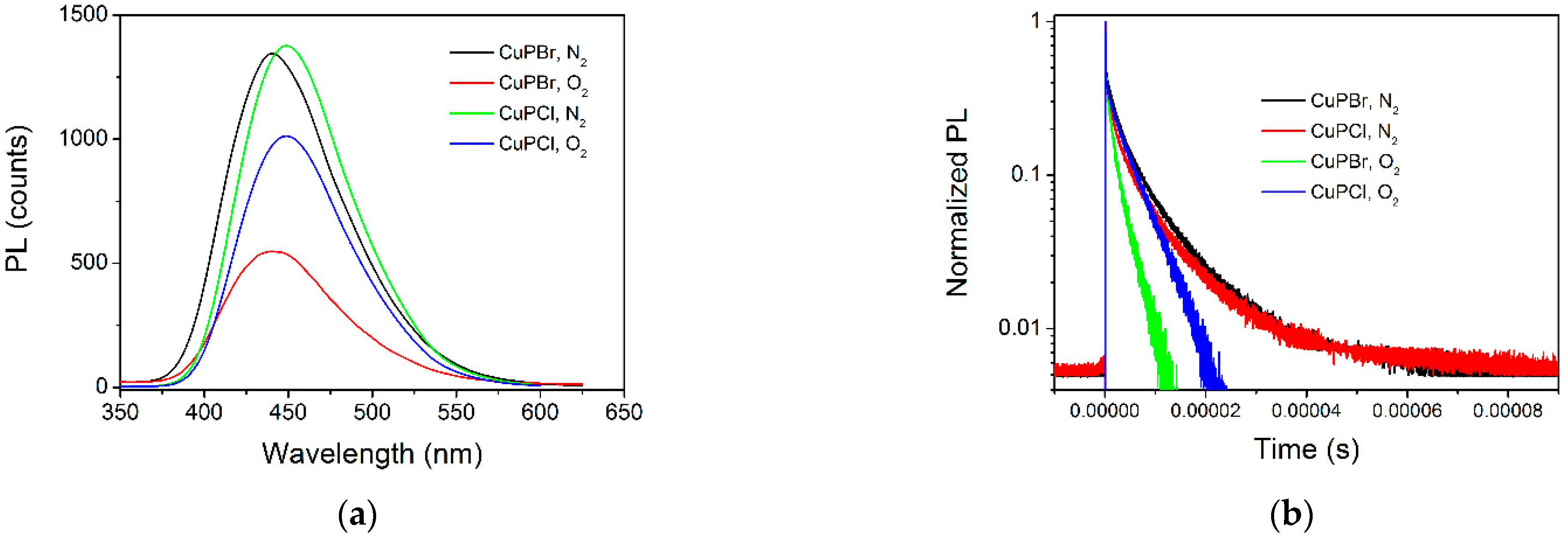
3.2. Photophysical Parameters of CuPX under N2 and O2: Quantum Yield and Lifetime
3.3. Oxygen-Sensing Performance of CuPX-COF
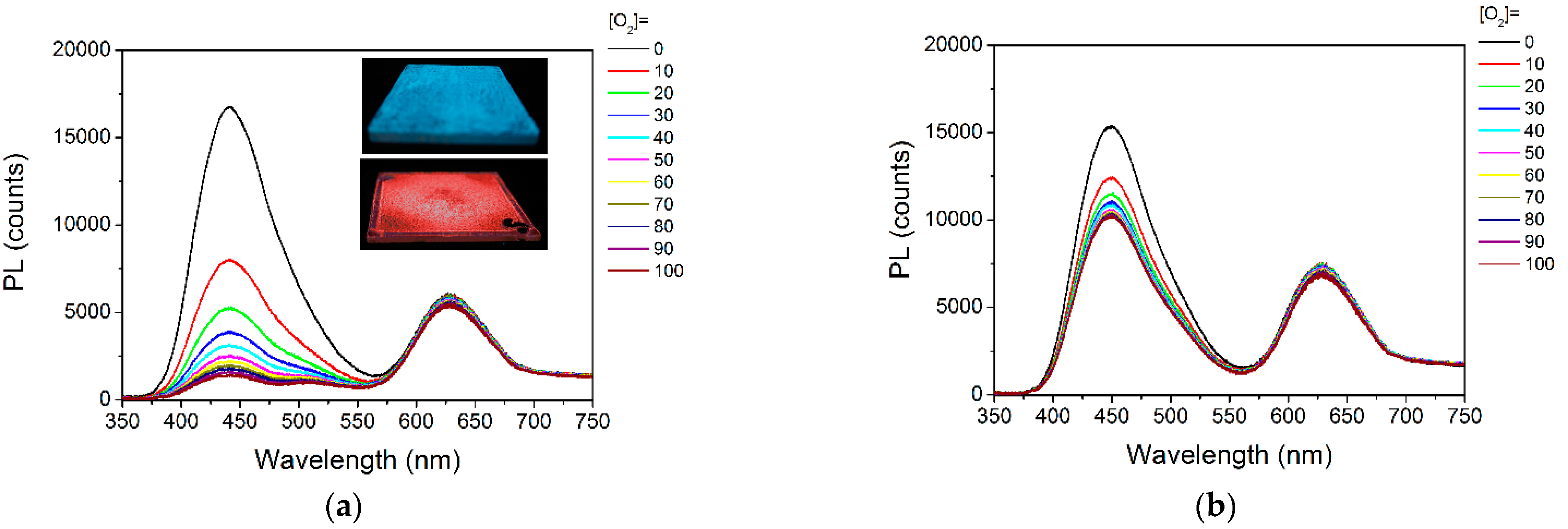
3.3.1. Emission Spectra under Various O2 levels
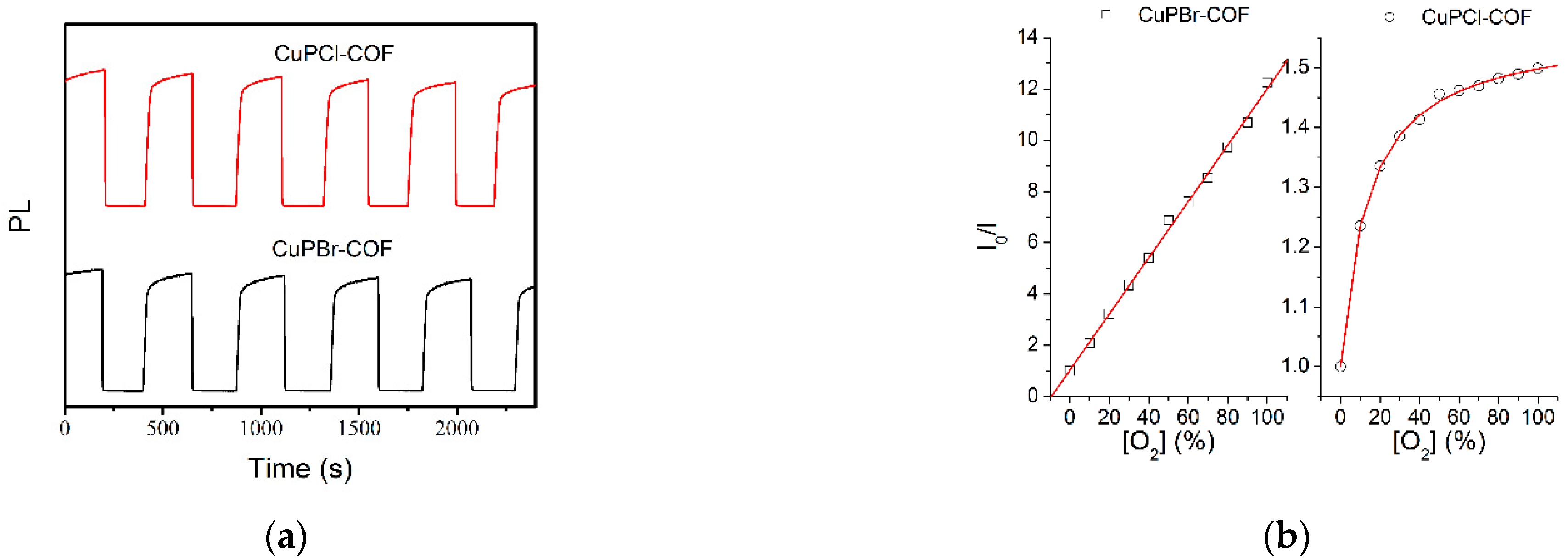
3.3.2. Response and Recovery
3.3.3. Calibration Curve
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816. [Google Scholar] [CrossRef] [PubMed]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Levendorf, M.P.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 2011, 332, 228. [Google Scholar] [CrossRef] [PubMed]
- Doonan, C.J.; Tranchemontagne, D.J.; Glover, T.G.; Hunt, J.R.; Yaghi, O.M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2010, 2, 235. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An azine-linked covalent organic framework. J. Am. Chem. Soc. 2013, 135, 17310. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. A Pyrene-Based, Fluorescent Three-Dimensional Covalent Organic Framework. Acc. Chem. Res. 2015, 48, 3053. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, 6238. [Google Scholar] [CrossRef]
- Colson, J.W.; Dichtel, W.R. Rationally synthesized two-dimensional polymers. Nat. Chem. 2013, 5, 453–465. [Google Scholar] [CrossRef]
- Nagai, A.; Guo, Z.; Feng, X.; Jin, S.; Chen, X.; Ding, X.; Jiang, D. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011, 2, 536. [Google Scholar] [CrossRef]
- Lei, B.; Li, B.; Zhang, H.; Lu, S.; Zheng, Z.; Li, W.; Wang, Y. Mesostructured silica chemically doped with RuII as a superior optical oxygen sensor. Adv. Funct. Mater. 2006, 16, 1883. [Google Scholar] [CrossRef]
- Lei, B.F.; Li, B.; Zhang, H.R.; Zhang, L.M.; Li, W.L. Synthesis, characterization, and oxygen sensing properties of functionalized mesoporous SBA-15 and MCM-41 with a covalently linked ruthenium (II) complex. J. Phys. Chem. C 2007, 111, 11291. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ma, H.; Zhang, L.; Zheng, Y. Rapid and facile ratiometric detection of an anthrax biomarker by regulating energy transfer process in bio-metal-organic framework. Biosens. Bioelectron. 2016, 85, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Ma, H.; Zhang, L.; Zhang, W. An RGH–MOF as a naked eye colorimetric fluorescent sensor for picric acid recognition. J. Mater. Chem. C 2017, 5, 4661. [Google Scholar] [CrossRef]
- Buyukcakir, O.; Je, S.; Choi, D.; Talapaneni, S.; Seo, Y.; Jung, Y.; Polychronopoulou, K.; Coskun, A. Porous cationic polymers: The impact of counteranions and charges on CO2 capture and conversion. Chem. Commun. 2016, 52, 934. [Google Scholar] [CrossRef] [PubMed]
- van Humbeck, J.F.; Aubrey, M.L.; Alsbaiee, A.; Ameloot, R.; Coates, G.W.; Dichtel, W.R.; Long, J.R. Tetraarylborate polymer networks as single-ion conducting solid electrolytes. Chem. Sci. 2015, 6, 5499. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wu, Y.; Zhang, Y.; Zhang, W.; Fu, Y.; Huang, W.; Yan, T.; Ma, H. Tuning proton dissociation energy in proton carrier doped 2D covalent organic frameworks for anhydrous proton conduction at elevated temperature. J. Mater. Chem. A 2020, 8, 13702. [Google Scholar] [CrossRef]
- Naughton, K.L.; Boedicker, J.Q. Simulations to Aid in the Design of Microbes for Synthesis of Metallic Nanomaterials. ACS Synth. Biol. 2021, 10, 3475. [Google Scholar] [CrossRef]
- Wallesch, M.; Nieger, M.; Volz, D.; Brase, S. Copper(I) complexes of 8-(diphenylphosphanyl-oxy)-quinoline: Photophysics, structures and reactivity. Inorg. Chem. Commun. 2017, 86, 232. [Google Scholar] [CrossRef] [Green Version]
- Eggleston, M.K.; McMillin, D.R.; Koenig, K.S.; Pallenberg, A.J. Steric effects in the ground and excited states of Cu(NN)2+ systems. Inorg. Chem. 1997, 36, 172. [Google Scholar] [CrossRef]
- Yang, L.; Feng, J.K.; Ren, A.M.; Zhang, M.; Ma, Y.G.; Liu, X.D. Structures, electronic states and electroluminescent properties of a series of CuI complexes. Eur. J. Inorg. Chem. 2005, 10, 1867. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B. Room-temperature pure blue-emitting phosphorescent multinuclear Cu(I)-based emitters. J. Electrochem. Soc. 2009, 156, J174. [Google Scholar] [CrossRef]
- Wu, X.; Song, L.; Li, B.; Liu, Y. Synthesis, characterization, and oxygen sensing properties of Ru(II) complex covalently grafted to mesoporous MCM-41. J. Lumin. 2010, 130, 374. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Liu, Y.; Zhang, L.; Zuo, Q.; Shi, L.; Su, Z. Highly sensitive oxygen sensors based on Cu(I) complex–polystyrene composite nanofibrous membranes prepared by electrospinning. Chem. Commun. 2009, 39, 5868. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.T.; Karpishin, T.B. Oxygen sensing by photoluminescence quenching of a heteroleptic ž/ž. Sens. Actuators B 1999, 61, 222. [Google Scholar]
- Shi, L.F.; Li, B.; Yue, S.M.; Fan, D. Synthesis, photophysical and oxygen-sensing properties of a novel bluish-green emission Cu(I) complex. Sens. Actuators B 2009, 137, 386. [Google Scholar] [CrossRef]
- Shi, L.F.; Li, B. A Series of Cu I Complexes Containing 1,10-Phenanthroline Derivative Ligands: Synthesis, Characterization, Photophysical, and Oxygen-Sensing Properties. Eur. J. Inorg. Chem. 2009, 2009, 2294–2302. [Google Scholar] [CrossRef]




| System | I0/I100 | Ksv (O2%−1) | Tres (s) | Trec (s) | Linearity | Citation |
|---|---|---|---|---|---|---|
| CuPBr-COF | 12.25 | 0.110 | 13 | 38 | linear | this |
| CuPCL-COF | 1.50 | 0.112/0.00001 | 13 | 40 | no | this |
| [Cu(POP)(PTZ)]BF4-MCM-41 | 11.16 | 0.500/0.002 | 4 | 78 | no | [24] |
| [Cu(dpephos)(pip)]BF4-SBA-15 | 7.44 | 2.043/0.002 | 3 | 178 | no | [25] |
| [Cu(dpephos)(epip)]BF4-SBA-15 | 5.68 | 1.508/0.002 | 4 | 158 | no | [25] |
| [Cu(dpephos)(nip)]BF4-SBA-15 | 5.51 | 1.816/0.001 | 3 | 119 | no | [25] |
| [Cu(dpephos)(enip)]BF4-MCM-41 | 5.95 | 0.843/0.008 | 8 | 39 | no | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Lin, L.; Chen, W.; Liu, L. Eye-Visible Oxygen Sensing via In-Situ Synthesizing Blue-Emitting Cu(I) Cluster in Red-Emitting COF: Characterization and Performance. Materials 2022, 15, 4525. https://doi.org/10.3390/ma15134525
Zhu P, Lin L, Chen W, Liu L. Eye-Visible Oxygen Sensing via In-Situ Synthesizing Blue-Emitting Cu(I) Cluster in Red-Emitting COF: Characterization and Performance. Materials. 2022; 15(13):4525. https://doi.org/10.3390/ma15134525
Chicago/Turabian StyleZhu, Peibin, Lixiong Lin, Wen Chen, and Liang Liu. 2022. "Eye-Visible Oxygen Sensing via In-Situ Synthesizing Blue-Emitting Cu(I) Cluster in Red-Emitting COF: Characterization and Performance" Materials 15, no. 13: 4525. https://doi.org/10.3390/ma15134525






