The Oxygen Reduction Performance of Pt Supported on the Hybrid of Porous Carbon Nanofibers and Carbon Black
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PCNF
2.3. Preparation of Electrocatalyst Pt/(PCNF + CB)
2.4. Characterization of Samples
2.5. Electrochemical Performance of Pt/(PCNF + CB)
3. Results and Discussion
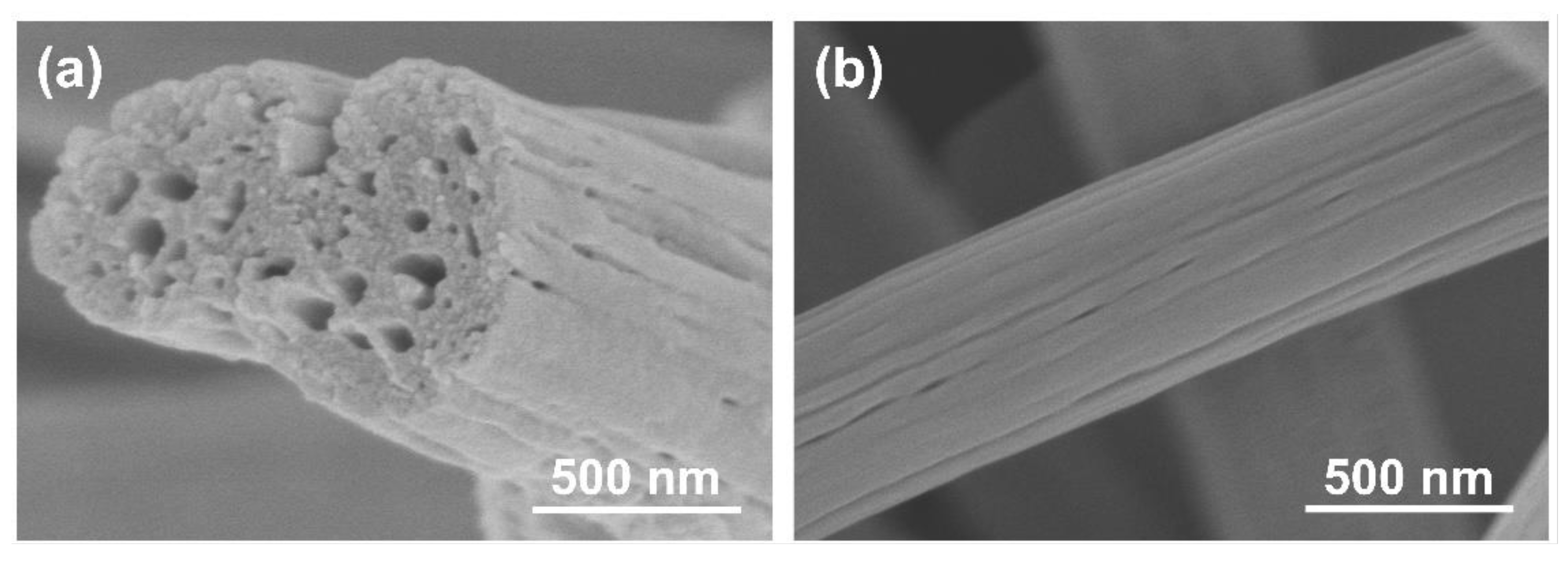
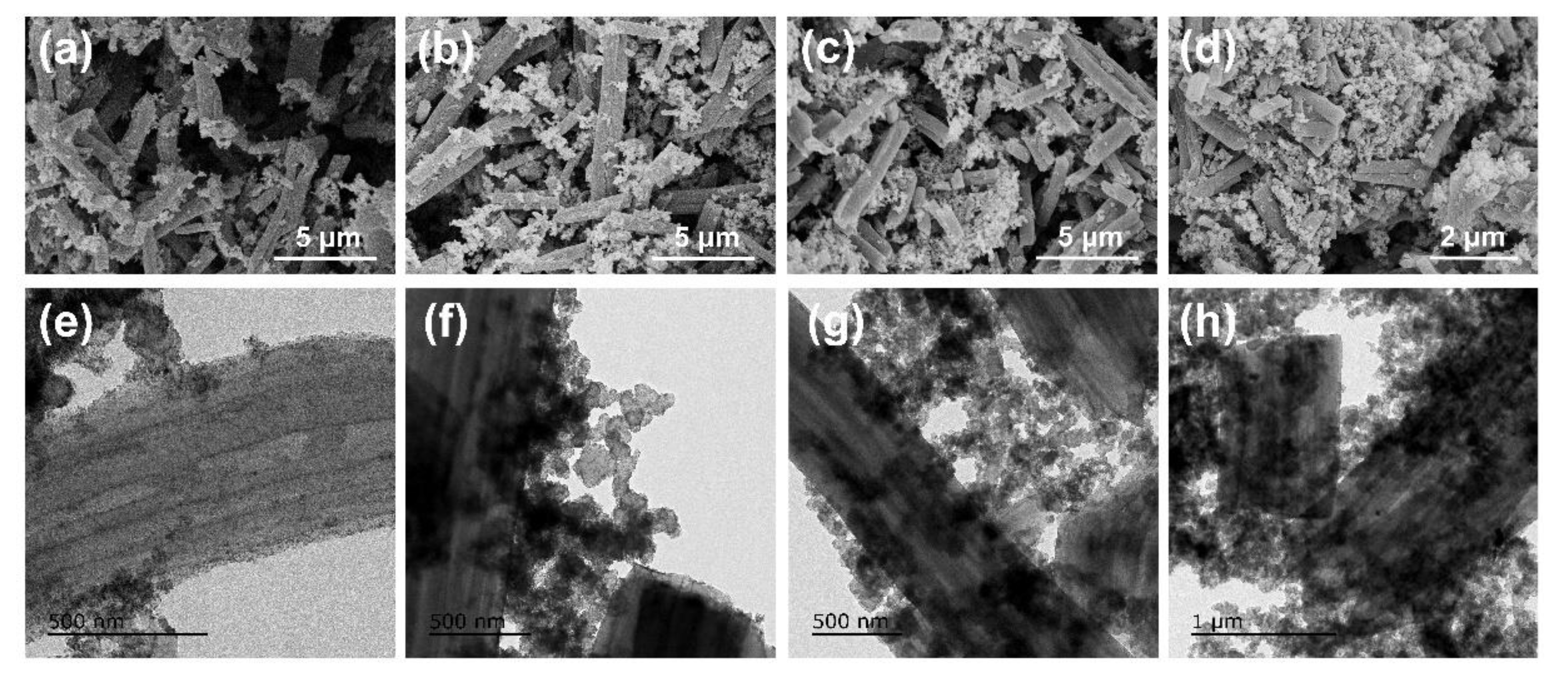
3.1. Morphology of PCNF and Pt/(PCNF + CB)
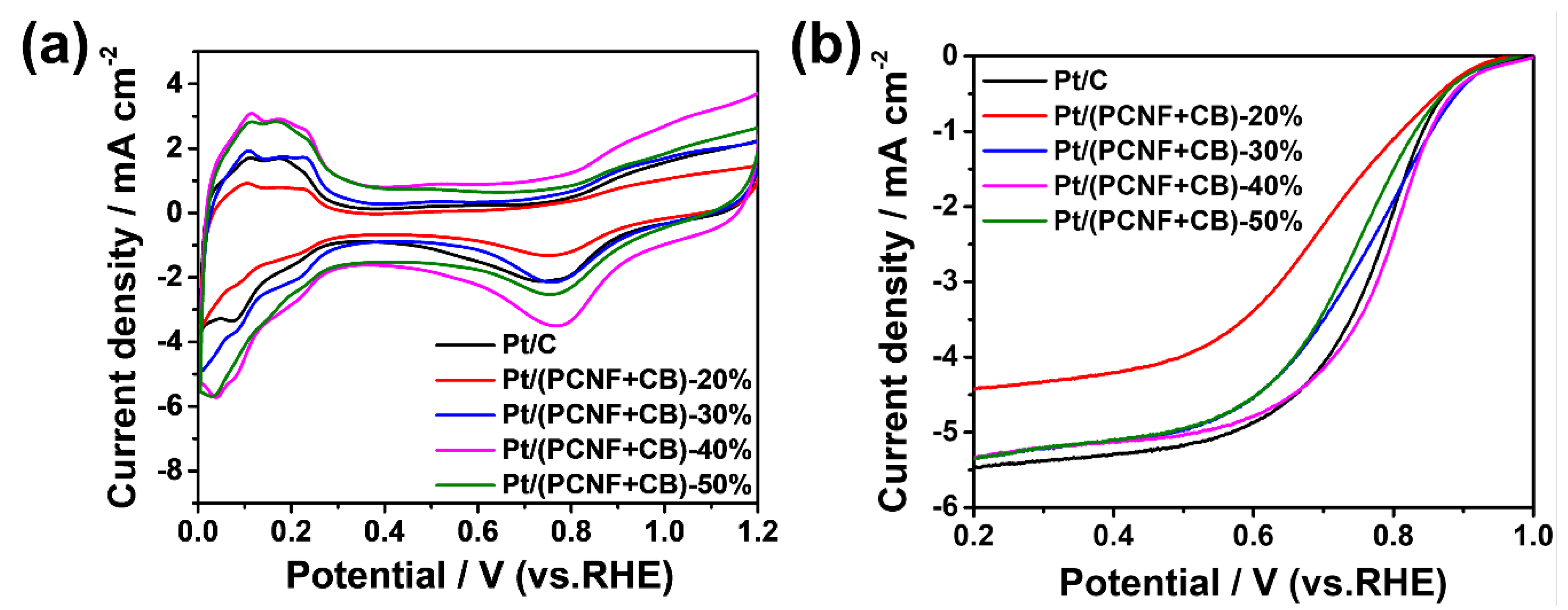
3.2. Electrocatalytic Activity of Pt/(PCNF + CB) toward ORR
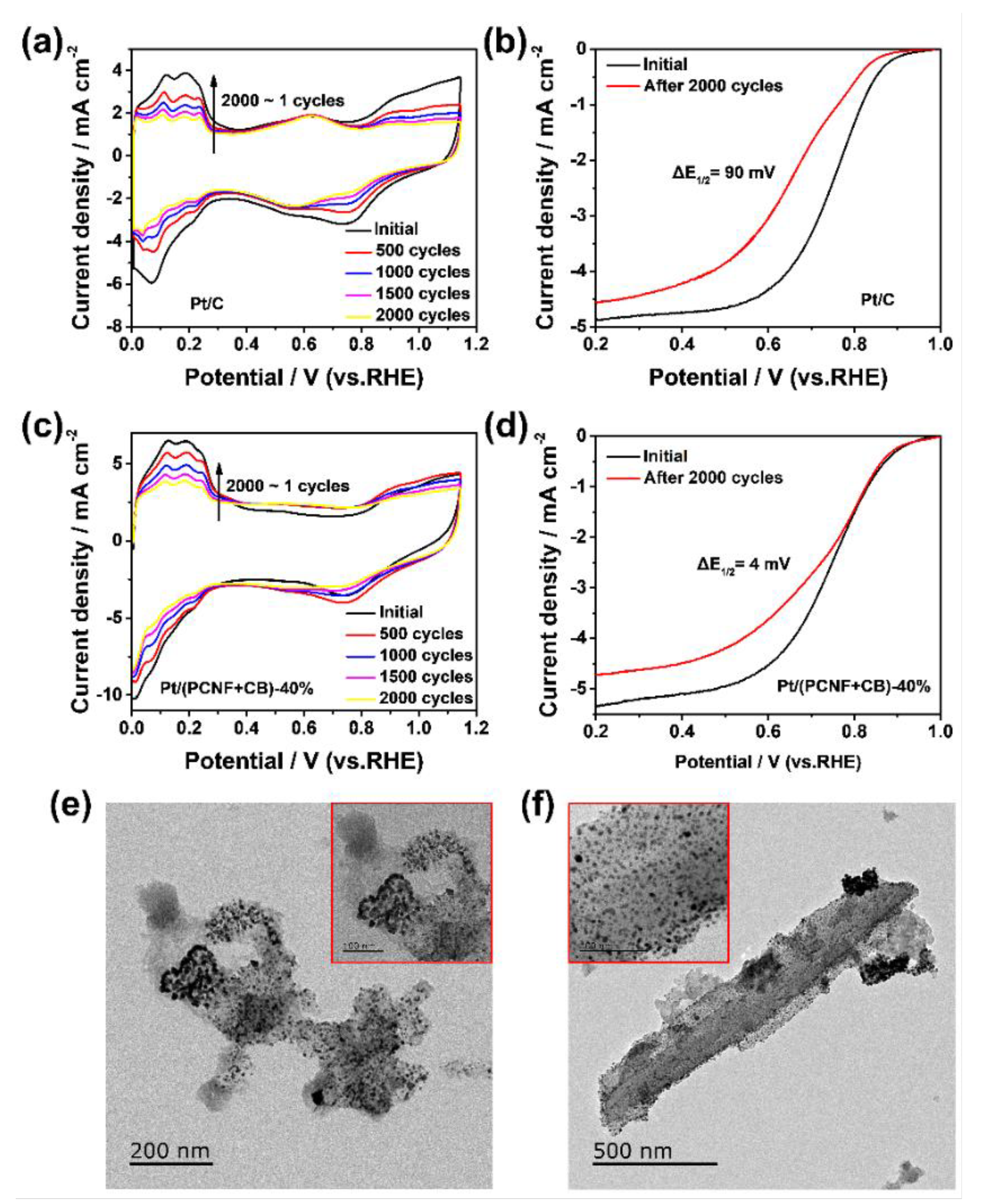
3.3. Durability of Pt/(PCNF + CB)
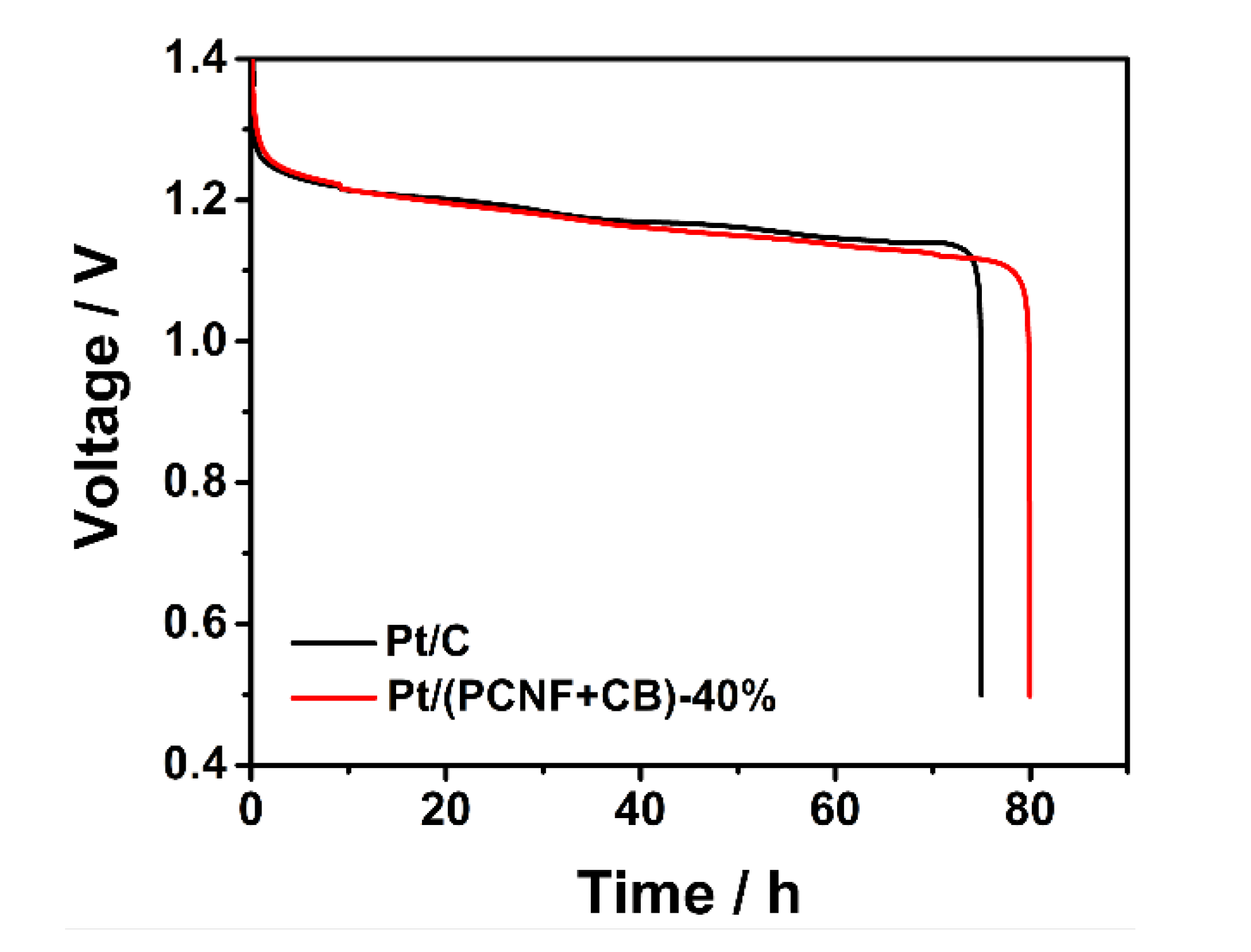
3.4. Discharge Behavior of Zinc–Air Battery with Pt/(PCNF + CB) as ORR Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandon, N.P.; Skinner, S.; Steele, B. Recent Advances in Materials for Fuel Cells. Annu. Rev. Mater. Res. 2003, 33, 183–213. [Google Scholar] [CrossRef]
- Ren, Z.; Tan, Z.; Xiang, S.; Chen, S.; Shen, S.; Zhang, J. Progress in non-noble metal oxygen reduction electrocatalysts based on MOFs. Sci. Sin. Technol. 2021, 52, 375–388. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.T.; Ortiz-Herrera, J.C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D.I. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Markovic, N.M.; Ross, P.N. New Electrocatalysts for Fuel Cells from Model Surfaces to Commercial Catalysts. Cattech 2000, 4, 110–126. [Google Scholar] [CrossRef]
- Norskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Shao, M.; Peles, A.; Shoemaker, K. Electrocatalysis on platinum nanoparticles: Particle size effect on oxygen reduction reaction activity. Nano Lett. 2011, 11, 3714–3719. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Geng, D.; Lin, L.-Y.; Meng, X. Insight into the correlation of Pt–support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 2020, 8, 9420–9446. [Google Scholar] [CrossRef]
- Li, Y.; Somorjai, G.A. Nanoscale Advances in Catalysis and Energy Applications. Nano Lett. 2010, 10, 2289–2295. [Google Scholar] [CrossRef] [Green Version]
- Yoo, E.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced Electrocatalytic Activity of Pt Subnanoclusters on Graphene Nanosheet Surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Rabis, A.; Rodriguez, P.; Schmidt, T.J. Electrocatalysis for Polymer Electrolyte Fuel Cells: Recent Achievements and Future Challenges. ACS Catal. 2012, 2, 864–890. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.; Markovic, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Comparative study on the oxygen reduction reaction electrocatalytic activities of iron phthalocyanines supported on reduced graphene oxide, mesoporous carbon vesicle, and ordered mesoporous carbon. J. Power Sources 2014, 264, 114–122. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Y.; Liu, A.; Zhang, Z.; Lv, Q.; Liu, B. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, N.; Zheng, J.; Zheng, J.P. Preparation and catalytic performance of Pt supported on Nafion® functionalized carbon nanotubes. J. Electroanal. Chem. 2019, 854, 113508. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O. Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms. Catalysts 2021, 11, 335. [Google Scholar] [CrossRef]
- Karuppanan, K.K.; Raghu, A.V.; Panthalingal, M.K.; Pullithadathil, B. Tailored Hollow Core/Mesoporous Shell Carbon Nanofibers as Highly Efficient and Durable Cathode Catalyst Supports for Polymer Electrolyte Fuel Cells. ChemElectroChem 2019, 6, 2029–2042. [Google Scholar] [CrossRef]
- Hao, Y.; Hu, F.; Chen, Y.; Wang, Y.; Xue, J.; Yang, S.; Peng, S. Recent Progress of Electrospun Nanofibers for Zinc–Air Batteries. Adv. Fiber Mater. 2021, 4, 185–202. [Google Scholar] [CrossRef]
- Liu, R.; Hou, L.; Yue, G.; Li, H.; Zhang, J.; Liu, J.; Miao, B.; Wang, N.; Bai, J.; Cui, Z.; et al. Progress of Fabrication and Applications of Electrospun Hierarchically Porous Nanofibers. Adv. Fiber Mater. 2022. [Google Scholar] [CrossRef]
- He, D.; Jiang, Y.; Lv, H.; Pan, M.; Mu, S. Nitrogen-doped reduced graphene oxide supports for noble metal catalysts with greatly enhanced activity and stability. Appl. Catal. B Environ. 2013, 132–133, 379–388. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Yang, S.; Li, G.; Qiao, J. Highly active and stable platinum catalyst supported on porous carbon nanofibers for improved performance of PEMFC. Electrochim. Acta 2015, 177, 181–189. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kwon, Y.; Kim, T.-W.; Kim, K.; Kim, H.J. Enhanced electrochemical oxygen reduction reaction performance with Pt nanocluster catalysts supported on microporous graphene-like 3D carbon. J. Electroanal. Chem. 2019, 838, 89–93. [Google Scholar] [CrossRef]
- Zhang, W.; Sherrell, P.; Minett, A.I.; Razal, J.M.; Chen, J. Carbon nanotube architectures as catalyst supports for proton exchange membrane fuel cells. Energy Environ. Sci. 2010, 3, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Kar, K.K. Hierarchically structured catalyst layer for the oxygen reduction reaction fabricated by electrodeposition of platinum on carbon nanotube coated carbon fiber. RSC Adv. 2015, 5, 66518–66527. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Size-Dependent Activity of Palladium Nanoparticles for Oxygen Electroreduction in Alkaline Solutions. J. Electrochem. Soc. 2009, 156, B643–B649. [Google Scholar] [CrossRef]
- Yarar Kaplan, B.; Haghmoradi, N.; Biçer, E.; Merino, C.; Alkan Gürsel, S. High performance electrocatalysts supported on graphene based hybrids for polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 23221–23230. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Ahn, C.; You, D.J.; Pak, C.; Hur, S.H.; Kim, J.; Joo, S.H. Ordered mesoporous carbon–carbon nanotube nanocomposites as highly conductive and durable cathode catalyst supports for polymer electrolyte fuel cells. J. Mater. Chem. A 2013, 1, 1270–1283. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Meng, M.; Wang, L.; Wu, A.; Tian, C.; Zhao, L.; Fu, H. Small-sized tungsten nitride anchoring into a 3D CNT-rGO framework as a superior bifunctional catalyst for the methanol oxidation and oxygen reduction reactions. Nano Res. 2015, 9, 329–343. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Y.; Wang, Z.; Lei, Y.; Wang, B.; Wu, N.; Han, C.; Xie, S.; Gou, Y. Three-dimensional (3D) interconnected networks fabricated via in-situ growth of N-doped graphene/carbon nanotubes on Co-containing carbon nanofibers for enhanced oxygen reduction. Nano Res. 2015, 9, 317–328. [Google Scholar] [CrossRef]
- Mu, X.; Xu, Z.; Ma, Y.; Xie, Y.; Mi, H.; Ma, J. Graphene-carbon nanofiber hybrid supported Pt nanoparticles with enhanced catalytic performance for methanol oxidation and oxygen reduction. Electrochim. Acta 2017, 253, 171–177. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Palafox-Segoviano, J.A.; Pérez-Díaz, P.J.; Medina-Ramírez, A. Synthesis of supported Pt nanoparticles by sonication for ORR: Effect of the graphene oxide-carbon composite. Int. J. Hydrogen Energy 2021, 46, 26027–26039. [Google Scholar] [CrossRef]
- Yarar Kaplan, B.; Haghmoradi, N.; Jamil, E.; Merino, C.; Alkan Gürsel, S. Platinum nanoparticles decorated carbon nanofiber hybrids as highly active electrocatalysts for polymer electrolyte membrane fuel cells. Int. J. Energy Res. 2020, 44, 10251–10261. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Rahman, M.M.; Inaba, K.; Batnyagt, G.; Saikawa, M.; Kato, Y.; Awata, R.; Delgertsetsega, B.; Kaneta, Y.; Higashi, K.; Uruga, T.; et al. Synthesis of catalysts with fine platinum particles supported by high-surface-area activated carbons and optimization of their catalytic activities for polymer electrolyte fuel cells. RSC Adv. 2021, 11, 20601–20611. [Google Scholar] [CrossRef] [PubMed]

| Electrocatalysts | ECSA (m2/gPt) | Half-Wave Potential (V) | Onset Potential (V) | Limiting Current Density (mA cm−2) |
|---|---|---|---|---|
| Pt/(PCNF + CB)-20% | 27 | 0.703 | 0.928 | 4.43 |
| Pt/(PCNF + CB)-30% | 54 | 0.756 | 0.945 | 5.34 |
| Pt/(PCNF + CB)-40% | 73 | 0.799 | 0.963 | 5.34 |
| Pt/(PCNF + CB)-50% | 66 | 0.740 | 0.942 | 5.36 |
| Pt/C | 72 | 0.774 | 0.944 | 5.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Zhang, J.; Yang, S.; Jin, J.; Wang, B.; Li, G. The Oxygen Reduction Performance of Pt Supported on the Hybrid of Porous Carbon Nanofibers and Carbon Black. Materials 2022, 15, 4560. https://doi.org/10.3390/ma15134560
Zhou T, Zhang J, Yang S, Jin J, Wang B, Li G. The Oxygen Reduction Performance of Pt Supported on the Hybrid of Porous Carbon Nanofibers and Carbon Black. Materials. 2022; 15(13):4560. https://doi.org/10.3390/ma15134560
Chicago/Turabian StyleZhou, Tongyu, Jingjing Zhang, Shenglin Yang, Junhong Jin, Biao Wang, and Guang Li. 2022. "The Oxygen Reduction Performance of Pt Supported on the Hybrid of Porous Carbon Nanofibers and Carbon Black" Materials 15, no. 13: 4560. https://doi.org/10.3390/ma15134560
APA StyleZhou, T., Zhang, J., Yang, S., Jin, J., Wang, B., & Li, G. (2022). The Oxygen Reduction Performance of Pt Supported on the Hybrid of Porous Carbon Nanofibers and Carbon Black. Materials, 15(13), 4560. https://doi.org/10.3390/ma15134560





