The Role of Prussian Blue-Thallium and Potassium Similarities and Differences in Crystal Structures of Selected Cyanido Complexes of W, Fe and Mo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedures
2.2. Crystallography
3. Results
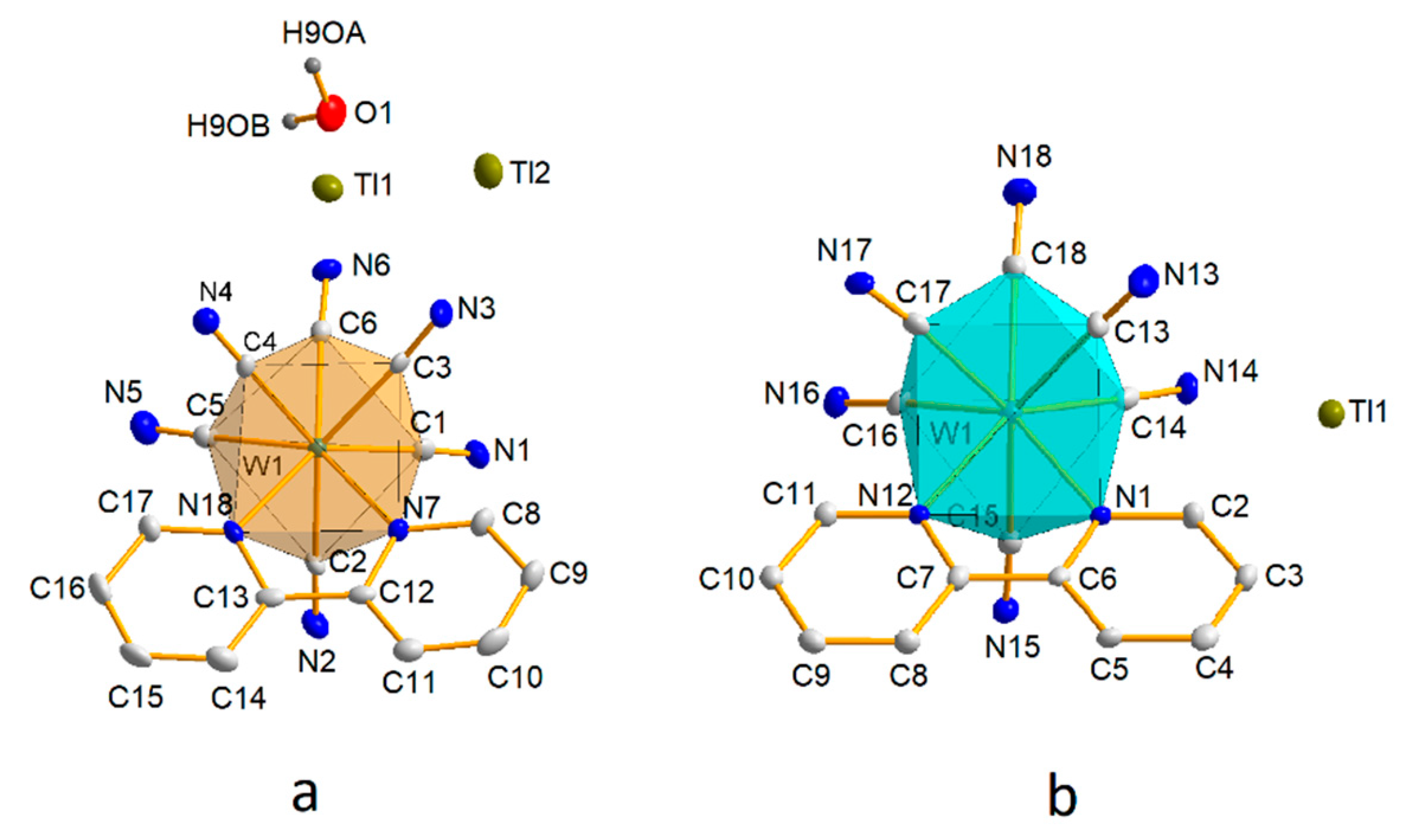
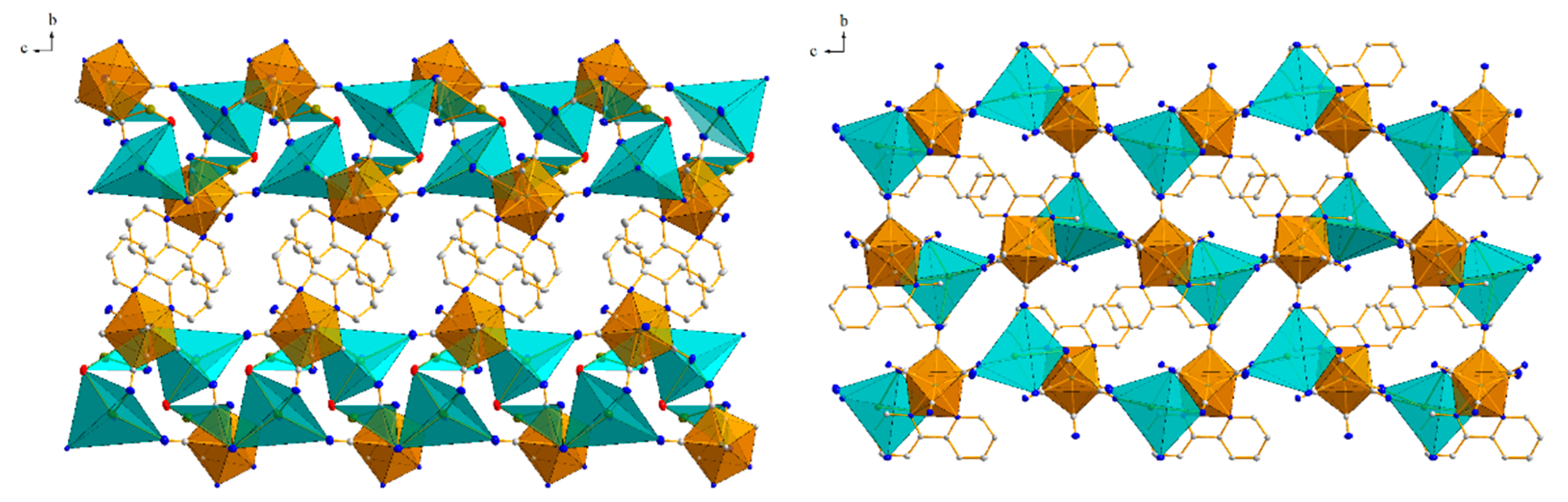
3.1. Crystallographic Studies
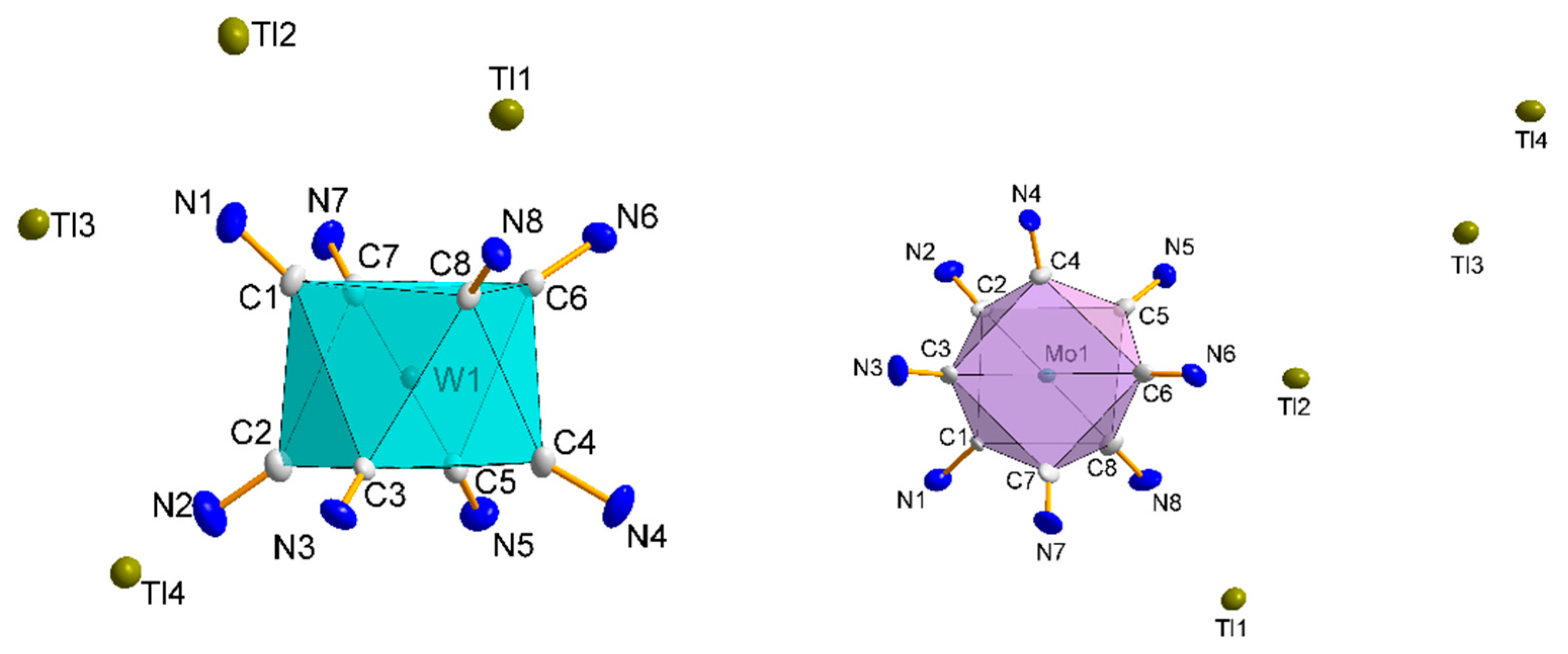
3.1.1. Structures of [WIV(CN)6(bpy)]2− (1) and [WV(CN)6(bpy)]− (2) Anions
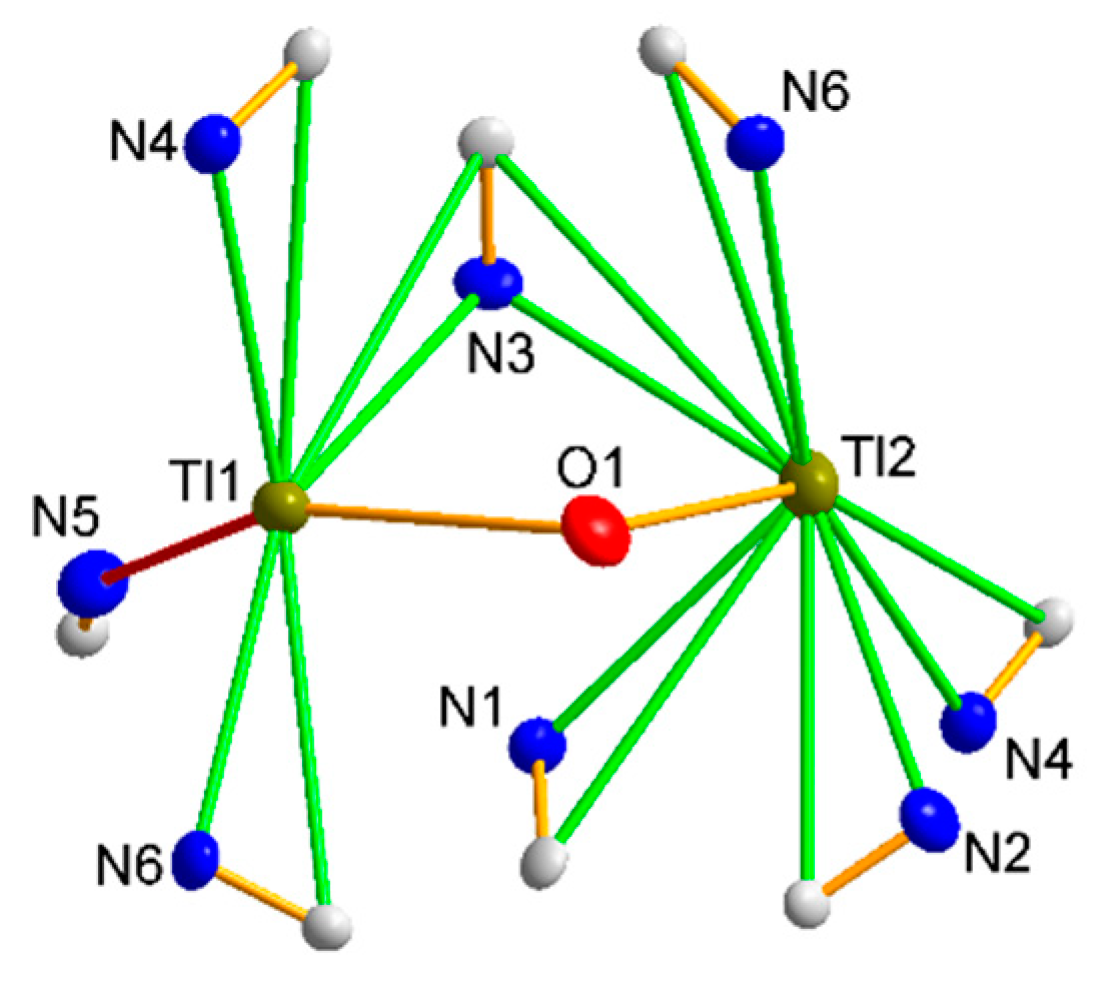
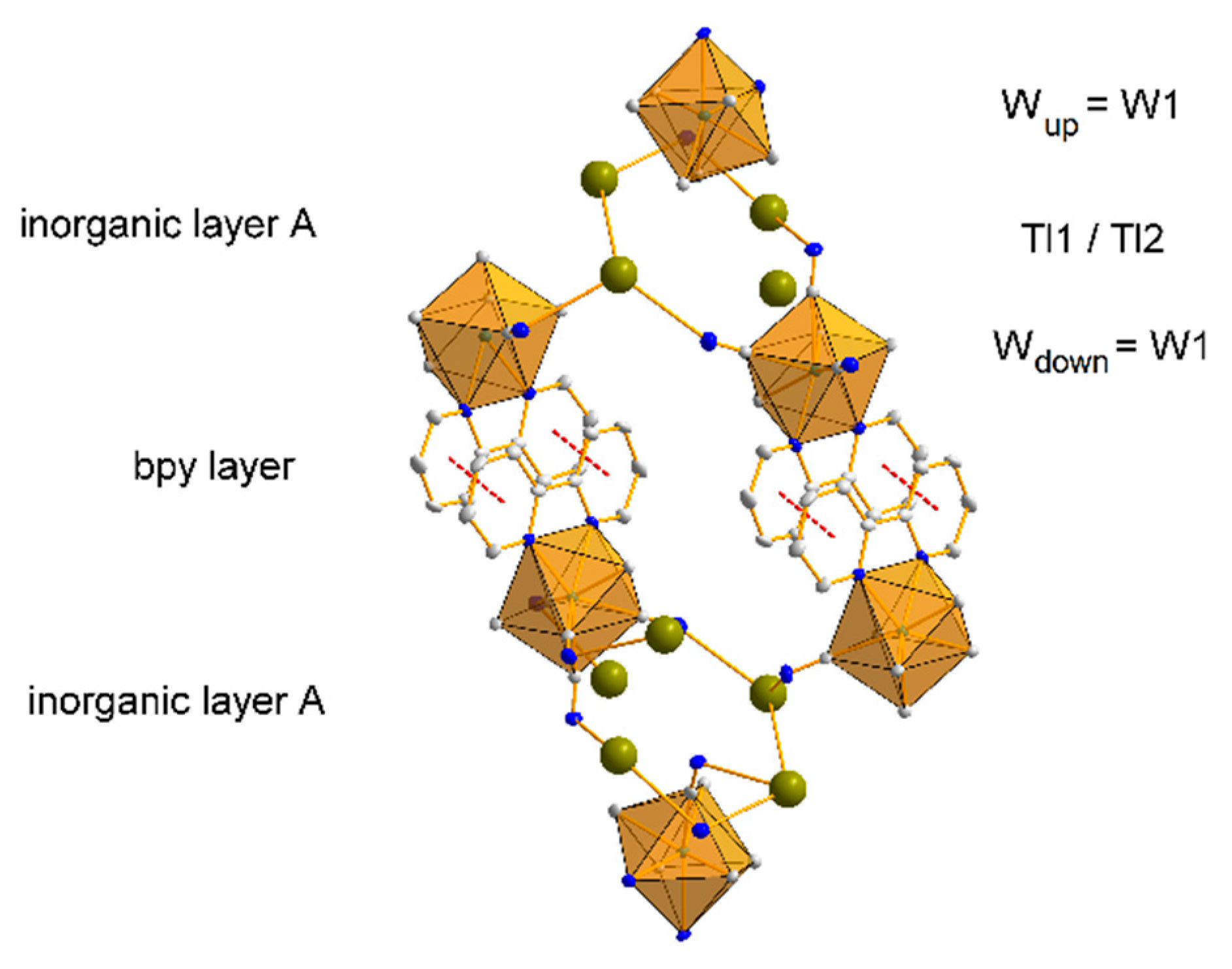
3.1.2. Structure 1
3.1.3. Structure 2
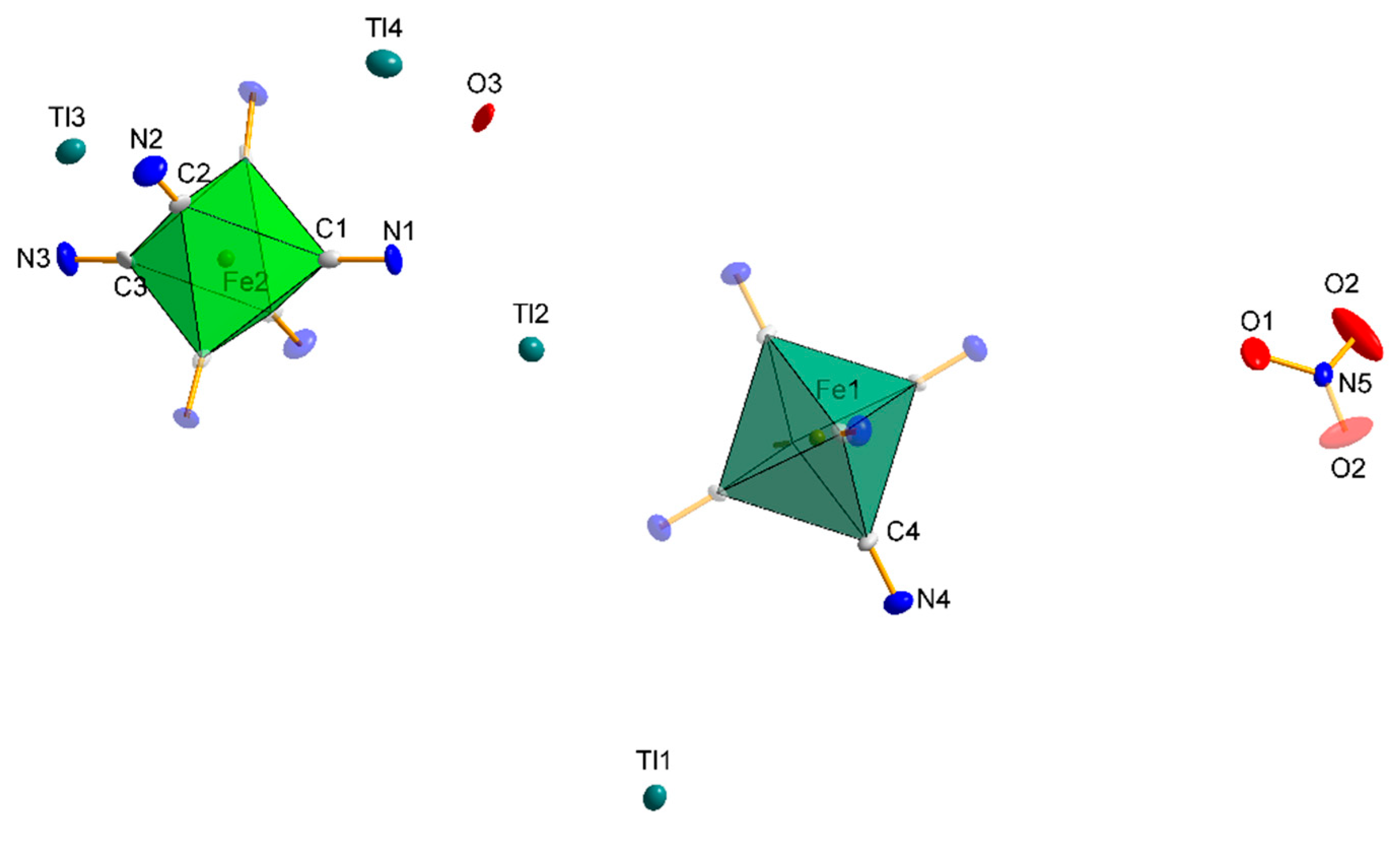
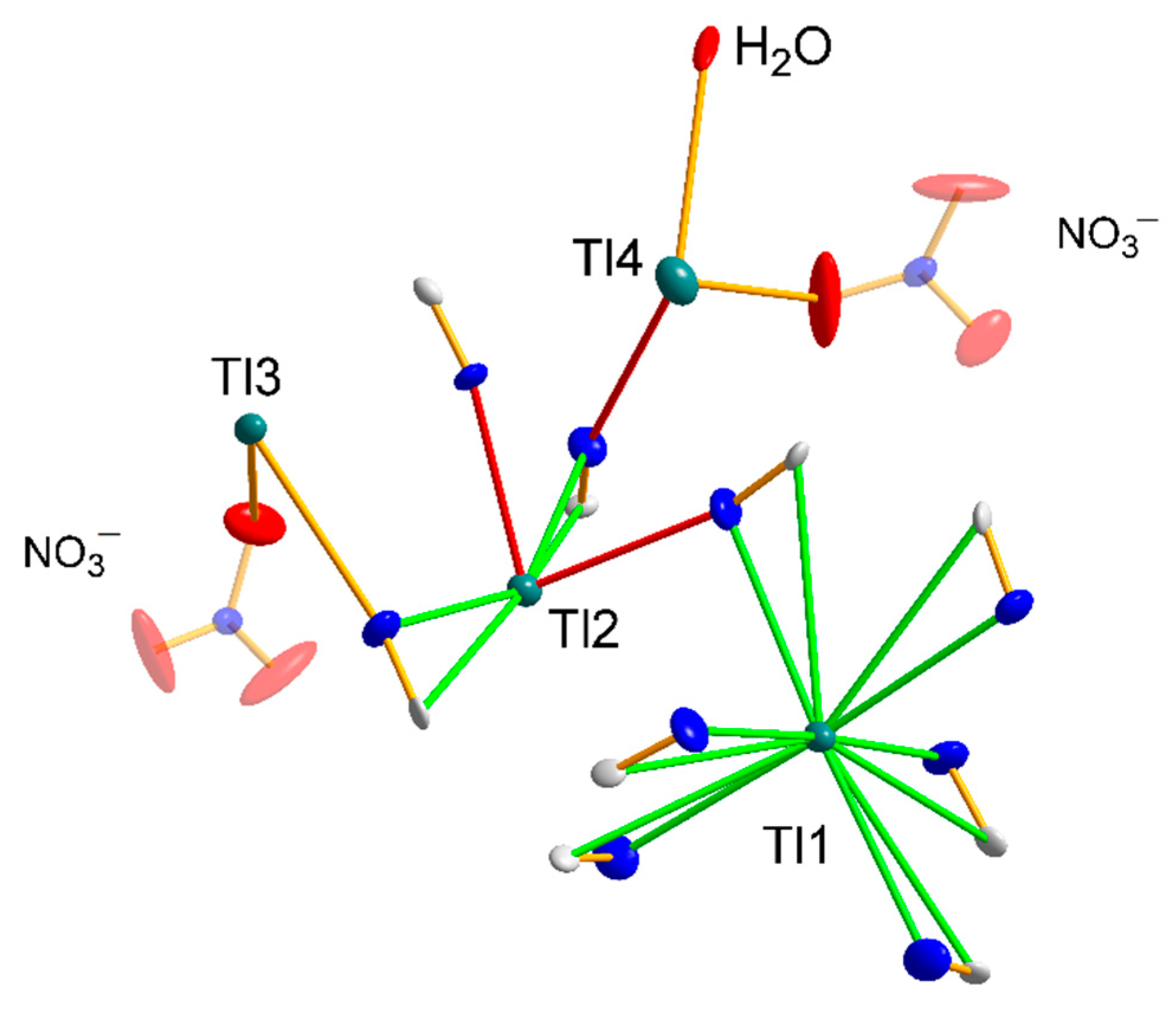
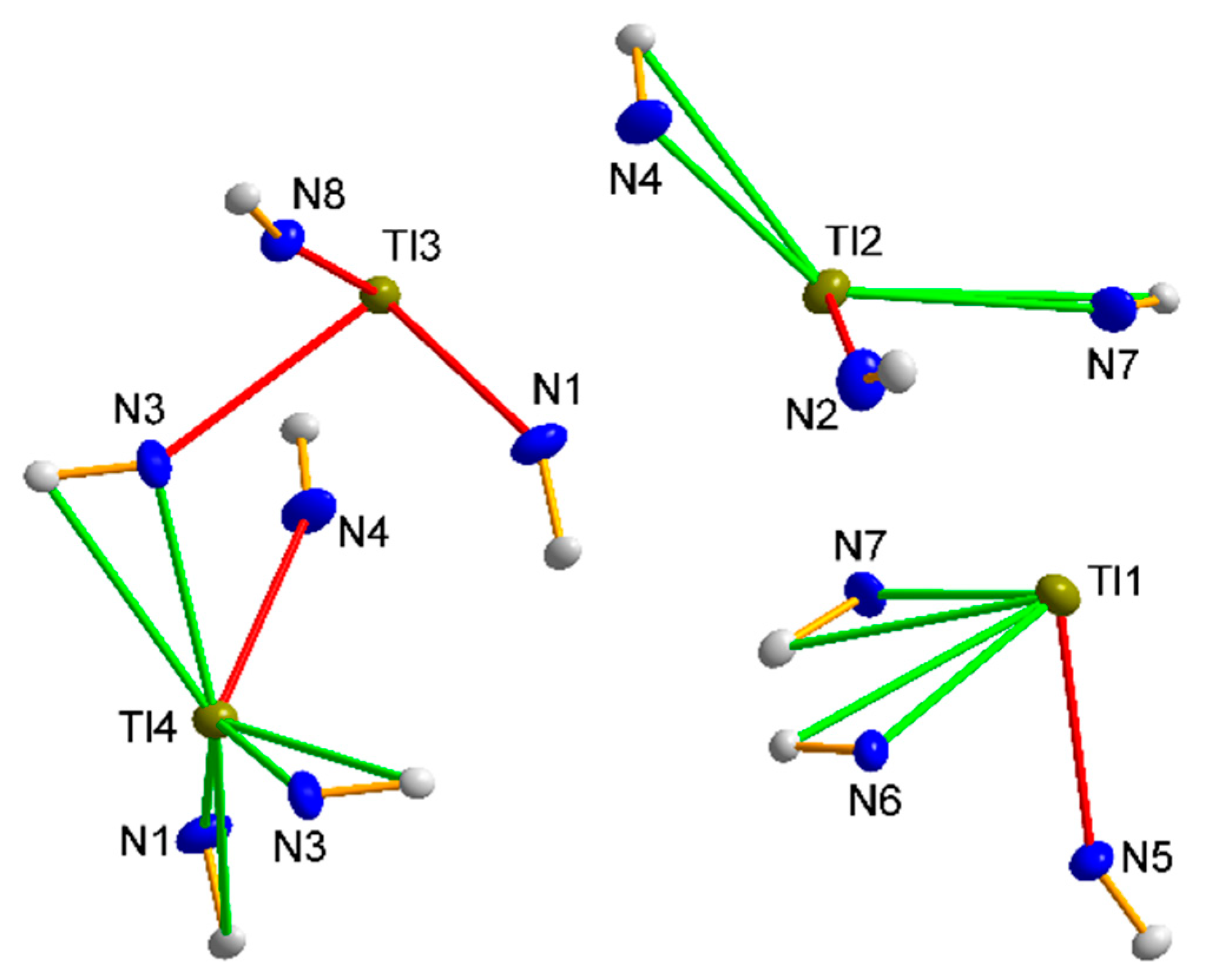
3.1.4. Structure 3
3.1.5. Structures 4 and 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mullins, L.J.; Moore, R.D. The movement of thallium ions in muscle. J. Gen. Physiol. 1960, 43, 759–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, P.J.; Hammond, P.B. The uptake of thallium by rabbit erythrocytes. J. Pharmacol. Exp. Ther. 1964, 145, 215–221. [Google Scholar] [PubMed]
- Gehring, P.J.; Hammond, P.B. The interrelationship between thallium and potassium in animals. J. Pharmacol. Exp. Ther. 1967, 155, 187–201. [Google Scholar] [PubMed]
- Kamerbeek, H.H.; Rauws, A.G.; Ten Ham, M.; Van Heijst, A.N.P. Prussian blue in therapy of thallotoxicosis: An experimental and clinical investigation. Acta Med. Scand. 1971, 189, 321–324. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Fedoroff, M.; Garnier, E.; Gravereau, P. Zinc and nickel ferrocyanides: Preparation, composition and structure. Talanta 1984, 31, 1133–1147. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Fedoroff, M.; Garnier, E. Preparation, composition and structure of some nickel and zinc ferrocyanides: Experimental results. Talanta 1989, 36, 749–759. [Google Scholar] [CrossRef]
- Ayrault, S.; Loos-Neskovic, C.; Fedoroff, M.; Garnier, E. Copper hexacyanoferrates: Preparation, composition and structure. Talanta 1994, 41, 1435–1452. [Google Scholar] [CrossRef]
- Ayrault, S.; Loos-Neskovic, C.; Fedoroff, M.; Garnier, E.; Jones, D.J. Compositions and structures of copper hexacyanoferrates (II) and (III): Experimental results. Talanta 1995, 42, 1581–1593. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Fedoroff, M. Fixation mechanisms of cesium on nickel and zinc ferrocyanides. Solvent Extr. Ion Exch. 1989, 7, 131–158. [Google Scholar] [CrossRef]
- Ayrault, S.; Jimenez, B.; Garnier, E.; Fedoroff, M.; Jones, D.; Loos-Neskovic, C. Sorption mechanisms of cesium on CuII2FeII(CN)6 and CuII3[FeIII(CN)6]2 Hexacyanoferrates and their relation to the crystalline structure. J. Solid State Chem. 1998, 141, 475–485. [Google Scholar] [CrossRef]
- Akhbari, K.; Morsali, A. Thallium (I) supramolecular compounds: Structural and properties consideration. Coord. Chem. Rev. 2010, 254, 1977–2006. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong closed-shell interactions in inorganic chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.M.; Gillespie, P.A.; Gause, C.D. Thallium (I) bis (mercaptoimidazolyl) borates. Polyhedron 2004, 23, 617–622. [Google Scholar] [CrossRef]
- Sharma, R.P.; Bala, R.; Sharma, R.; Raczyńska, J.; Rychlewska, U. Coordination network: Synthesis, characterization, crystal structure and packing of thallium m-nitrobenzenesulfonate, Tl (m-NO2C6H4SO3). J. Mol. Struct. 2005, 738, 247–252. [Google Scholar] [CrossRef]
- Mahjoub, A.R.; Morsali, A.; Bagherzadeh, H. Syntheses and characterization of thallium (I) complexes with 3-nitrophenoxide [Tl(3-np)], 4-nitrobenzoate [Tl(4-nb)] and 2,4-dinitrophenoxide [Tl(2,4-dnp)]: X-ray crystal structures of [Tl(3-np)]n and Tl(2,4-dnp)(two new polymeric compounds). Polyhedron 2002, 21, 2555–2560. [Google Scholar] [CrossRef]
- Wiesbrock, F.; Schmidbaur, H. Complexity of coordinative bonding in thallium (I) anthranilates and salicylates. J. Am. Chem. Soc. 2003, 125, 3622–3630. [Google Scholar] [CrossRef]
- Janiak, C.; Hoffmann, R.J. Thallium (I)-thallium (I) and indium (I)-indium (I) interactions: From the molecular to the solid state. Am. Chem. Soc. 1990, 112, 5924–5946. [Google Scholar] [CrossRef]
- Janiak, C.; Hoffmann, R. TII-TII-Wechselwirkung in Molekülen–eine MO-Analyse. Angew. Chem. 1989, 101, 1706–1708. [Google Scholar] [CrossRef]
- Budzelaar, P.H.M.; Boersma, J. Metal-Metal interactions in indium (I) and thallium (I) cyclopentadienyls. Recl. Trav. Chim. Pays-Bas 1990, 109, 187–189. [Google Scholar] [CrossRef]
- Schwerdtfeger, P. Metal-metal bonds in thallium (I)-thallium (I) compounds: Fact or fiction? Inorg. Chem. 1991, 30, 1660–1663. [Google Scholar] [CrossRef]
- Pyykk, P.; Straka, M.; Tamm, T. Calculations on indium and thallium cyclopentadienyls. Metal–metal interactions and possible new species. Phys. Chem. Chem. Phys. 1999, 1, 3441–3444. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Samotus, A. A novel cyano complex of tungsten (IV) with 2,2′-bipyridyl. Transit. Met. Chem. 1988, 13, 69–71. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Fawcett, J.; Russell, D.R. Synthesis and X-ray crystal structures of Cs2[W(bpy)(CN)6]·2H2O and (AsPh4)2[W(bpy)(CN)6]·3.5H2O. Transit. Met. Chem. 2004, 29, 56–60. [Google Scholar] [CrossRef]
- Hodorowicz, M.; Szklarzewicz, J.; Jurowska, A. The versatility of lithium cation coordination modes in salts with [W(CN)6(bpy)]2− anions. CrystEngComm 2020, 22, 3991–3998. [Google Scholar] [CrossRef]
- Hodorowicz, M.; Jurowska, A.; Szklarzewicz, J. Unusual structural changes going from Li+ to Cs+ in [W(CN)6(bpy)]2− ion salts: The Na+ case. CrystEngComm 2021, 23, 4301–4311. [Google Scholar] [CrossRef]
- Hodorowicz, M.; Jurowska, A.; Szklarzewicz, J. X-ray crystal structures of K+ and Rb+ salts of [W(CN)6(bpy)]2− ion. The unusual cation–anion interactions and structure changes going from Li+ to Cs+ salts. CrystEngComm 2021, 23, 1207–1217. [Google Scholar] [CrossRef]
- Hodorowicz, M.; Szklarzewicz, J.; Jurowska, A.; Mikuriya, M.; Mitsuhashi, R.; Yoshioka, D. Anion–cation versus weak intermolecular interactions in the structures of Et4N+, Pr4N+, and Bu4N+ cation salts with the [W(CN)6(bpy)]2– anion. J. Struct. Chem. 2021, 62, 905–917. [Google Scholar] [CrossRef]
- Hodorowicz, M.; Jurowska, A.; Szklarzewicz, J. Structures of alkali metal salts with [W(CN)6(bpy)]− ion. Comparative studies to W (IV) analogues. Polyhedron 2021, 207, 115369. [Google Scholar] [CrossRef]
- Leipoldt, J.G.; Bok, L.D.C.; Cilliers, P.J.Z. The preparation of potassium octacyanotungstate (IV) dihydrate. Anorg. Allg. Chem. 1974, 407, 350–352. [Google Scholar] [CrossRef]
- Leipoldt, J.G.; Bok, L.D.C.; Cilliers, P.J. The preparation of potassium octacyanomolybdate (IV) dihydrate. Z. Anorg. Allg. Chem. 1974, 409, 343–344. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis PRO; Rigaku Oxford Diffraction: Yarnton, UK, 2015. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.G.; Polidori, G. Crystal structure determination and refinement viaSIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putz, H.; Brandenburg, K. Diamond Crystal and Molecular Structure Visualization; Crystal Impact: Bonn, Germany, 2022. [Google Scholar]
- Szklarzewicz, J.; Podgajny, R.; Lewiński, K.; Sieklucka, B. Basket weave-like 2-D coordination polymer generated by the self-assembly of [Mn(H2O)6]2+ and geometrically anisotropic [W(CN)6bpy]2− precursors. CrystEngComm 2002, 4, 199–201. [Google Scholar] [CrossRef]
- Alcock, N.W.; Samotus, A.; Szklarzewicz, J. Charge-transfer interactions between bipyridinium ions and octacyano-molybdates and-tungstates. Dalton Trans. 1993, 885–889. [Google Scholar] [CrossRef]
- Samotus, A.; Szklarzewicz, J. Photochemistry of transition metal octacyanides and related compounds. Past, present and future. Coord. Chem. Rev. 1993, 125, 63–74. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Batsanov, S. Van der Waals radii of elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Contakes, S.M.; Rauchfuss, T.B. Alkali metal-templated assembly of the tetrahedral cyanometallate cages [M⊂Mo4(μ-CN)6(CO)12]5−(M= Li, Na). Chem. Commun. 2001, 553–554. [Google Scholar] [CrossRef]
- Contakes, S.M.; Rauchfuss, T.B. {K⊂[Mo6(μ-CN)9(CO)18]}8−: A Trigonal-Prismatic Cyanometalate Cage. Angew. Chem. Int. Ed. 2000, 39, 1984–1986. [Google Scholar] [CrossRef]
- Kuhlman, M.L.; Rauchfuss, T.B. Structural Chemistry of “Defect” Cyanometalate Boxes:{Cs⊂[CpCo(CN)3]4[Cp*Ru]3} and {M⊂[Cp*Rh(CN)3]4[Cp*Ru]3}(M= NH4, Cs). J. Am. Chem. Soc. 2003, 125, 10084–10092. [Google Scholar] [CrossRef]
- Hu, J.X.; Barbour, L.J.; Gokel, G.W. Cation-π Interactions Between Alkali Metal Cations and Neutral Double Bonds. Collect. Czech. Chem. Commun. 2004, 69, 1050–1062. [Google Scholar] [CrossRef]
- Hu, J.; Gokel, G.W. Solution complexation between potassium iodide and lariat ethers having pi-donor sidearms. Chem. Commun. 2003, 2536–2537. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W. The aromatic sidechains of amino acids as neutral donor groups for alkali metal cations. Chem. Commun. 2003, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Barbour, J.L.J.; Gokel, G.W. Solid-State Evidence for Pi-Complexation of Sodium and Potassium Cations by Carbon−Carbon Triple Bonds. J. Am. Chem. Soc. 2001, 123, 9486–9487. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.J.; Spiccia, L.; Moubaraki, B.; Murray, K.S.; Hockless, D.C.R.; Rae, A.D.; Willis, A.C. Structure and magnetism of heptanuclear complexes formed on encapsulation of hexacyanoferrate (II) with the Mn (II) and Ni (II) complexes of 1, 4-bis (2-pyridylmethyl)-1, 4, 7-triazacyclononane. Inorg. Chem. 2002, 41, 2489–2495. [Google Scholar] [CrossRef]
- Mukherjee, C.; Hoeke, V.; Stammler, A.; Bogge, H.; Schnack, J.; Glaser, T. Switching from antiferromagnetic to ferromagnetic coupling in heptanuclear [Mt6Mc]n+ complexes by going from an achiral to a chiral triplesalen ligand. Dalton Trans. 2014, 43, 9690–9703. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Tong, Y.-X.; Chen, Z.-N.; Yu, K.-B.; Kang, B.-S. Cyano-bridged extended heteronuclear supramolecular architectures with hexacyanoferrates (II) as building blocks. J. Organomet. Chem. 2000, 598, 63–70. [Google Scholar] [CrossRef]
- Constant, G.; Daran, J.-C.; Jeannin, Y. Etude structurale du tetrachloroferrate (III) d’hexamethylisonitrilefer (II). J. Inorg. Nucl. Chem. 1973, 35, 4083–4091. [Google Scholar] [CrossRef]
- Parker, R.J.; Spiccia, L.; Batten, S.R.; Cashion, J.D.; Fallon, G.D. The encapsulation of ferrocyanide by copper (II) complexes of tripodal tetradentate ligands. Novel H-bonding networks incorporating heptanuclear and pentanuclear heterometallic assemblies. Inorg. Chem. 2001, 40, 4696–4704. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Figgis, B.N.; Gerloch, M.; Mason, R.; Nyholm, R.S. The crystallography and paramagnetic anisotropy of potassium ferricyanide. Proc. Math. Phys. Eng. Sci. 1969, 309, 91–118. [Google Scholar]



| π…π | Shift | ||
|---|---|---|---|
| 1 | |||
| Cg1…Cg2 [2 − X,−Y,1 − Z] | 3.993(4) | 1.840 | Cg1: N7-C8-C9-C10-C11-C12, Cg2: N18-C13-C14-C15-C16-C17 |
| Cg2…Cg1 [2 − X,−Y,1 − Z] | 3.994(4) | 2.097 | |
| 2 | |||
| Cg1…Cg1 [1 − X,−Y,1 − Z] | 4.1066(1) | 1.702 | Cg1: N1-C2-C3-C4-C5-C6 Cg2: N12-C7-C8-C9-C10-C11 |
| Cg1…Cg1 [2 − X,−Y,1 − Z] | 4.1662(1) | 2.362 | |
| D-H…A | d(D-H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| 1 | ||||
| C(16)-H(16)…N(2)#3 | 0.93 | 2.52 | 3.347(10) | 147.8 |
| C(11)-H(11)…N(4)#4 | 0.93 | 2.67 | 3.557(10) | 160.8 |
| C(17)-H(17)…N(5) | 0.93 | 2.69 | 3.346(10) | 128.7 |
| C(14)-H(14)…N(4)#4 | 0.93 | 2.57 | 3.413(10) | 151.0 |
| O(1)-H(9OB)…N(3)#5 | 0.94(2) | 1.97(4) | 2.866(8) | 160(7) |
| #1 x + 1/2,−y + 1/2,z − 1/2 #2 x − 1/2,−y + 1/2,z + 1/2 #3 −x + 2,−y,−z #4 −x + 2,−y,−z + 1 #5 x − 1/2,−y + 1/2,z − 1/2 | ||||
| 2 | ||||
| C(5)-H(5)…N(17)#1 | 0.95 | 2.55 | 3.472(11) | 164.3 |
| C(8)-H(8)…N(17)#1 | 0.95 | 2.34 | 3.228(11) | 155.2 |
| C(2)-H(2)…N(16)#2 | 0.95 | 2.68 | 3.352(11) | 128.5 |
| #1 −x + 2,y + 1/2,−z + 3/2 #2 x,−y + 1/2,z + 1/2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodorowicz, M.; Szklarzewicz, J.; Jurowska, A. The Role of Prussian Blue-Thallium and Potassium Similarities and Differences in Crystal Structures of Selected Cyanido Complexes of W, Fe and Mo. Materials 2022, 15, 4586. https://doi.org/10.3390/ma15134586
Hodorowicz M, Szklarzewicz J, Jurowska A. The Role of Prussian Blue-Thallium and Potassium Similarities and Differences in Crystal Structures of Selected Cyanido Complexes of W, Fe and Mo. Materials. 2022; 15(13):4586. https://doi.org/10.3390/ma15134586
Chicago/Turabian StyleHodorowicz, Maciej, Janusz Szklarzewicz, and Anna Jurowska. 2022. "The Role of Prussian Blue-Thallium and Potassium Similarities and Differences in Crystal Structures of Selected Cyanido Complexes of W, Fe and Mo" Materials 15, no. 13: 4586. https://doi.org/10.3390/ma15134586
APA StyleHodorowicz, M., Szklarzewicz, J., & Jurowska, A. (2022). The Role of Prussian Blue-Thallium and Potassium Similarities and Differences in Crystal Structures of Selected Cyanido Complexes of W, Fe and Mo. Materials, 15(13), 4586. https://doi.org/10.3390/ma15134586






