Biting Innovations of Mosquito-Based Biomaterials and Medical Devices
Abstract
:1. Introduction
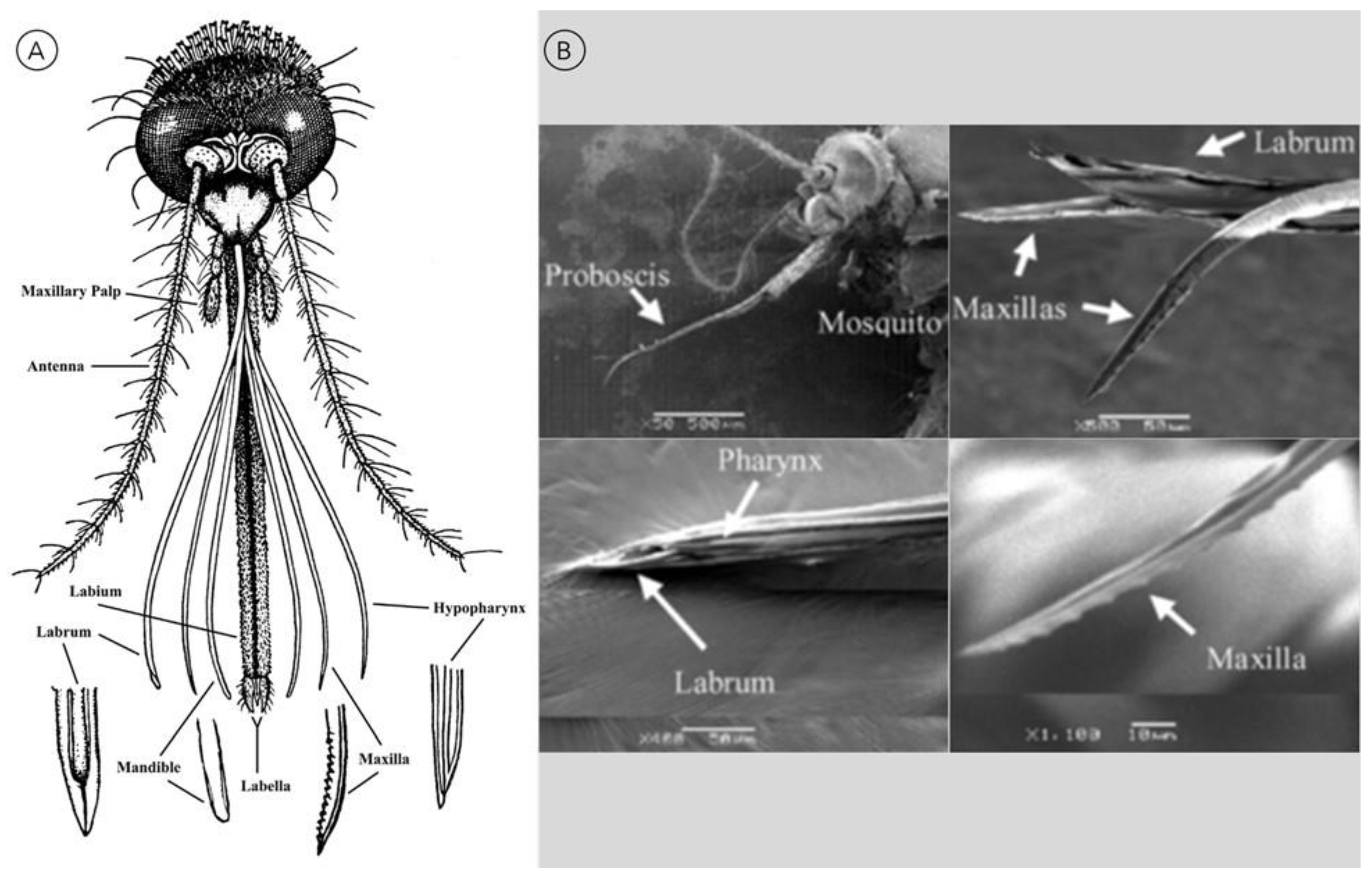
2. Mimicking the Mosquito Biting Process
2.1. Proboscis-Inspired Microneedles
2.1.1. Microneedle Material Composition
2.1.2. Microneedle Dimensions, Shapes, and Configurations and Associated Performance
2.2. Proboscis-Inspired Microelectrode Guide
2.3. Mosquito-Derived Polymers with Anticoagulant Properties
3. Mimicking Mosquito Visual, Motor, and Olfactory Functions
3.1. Mosquito-Derived Elastic Resilin-like Proteins
3.2. Mosquito Eye-Inspired Superhydrophobic Coating
3.3. Mosquito-Inspired Biosensor for Disease Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, N.H.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.B.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.P.; et al. Climate and Urbanization Drive Mosquito Preference for Humans. Curr. Biol. 2020, 30, 3570–3579.e6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vector-Borne Diseases. 2 March 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 10 June 2022).
- Chandrasegaran, K.; Lahondère, C.; Escobar, L.E.; Vinauger, C. Linking Mosquito Ecology, Traits, Behavior, and Disease Transmission. Trends Parasitol. 2020, 36, 393–403. [Google Scholar] [CrossRef]
- Konopka, J.K.; Task, D.; Afify, A.; Raji, J.; Deibel, K.; Maguire, S.; Lawrence, R.; Potter, C.J. Olfaction in Anopheles mosquitoes. Chem. Senses 2021, 46, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Sudo, T.; Niimi, M.; Tao, L.; Sun, B.; Kambayashi, J.; Watanabe, H.; Luo, E.; Matsuoka, H. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood 2008, 111, 2007–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, E.; Tokumasu, F.; Marinotti, O.; Villeval, J.L.; Ribeiro, J.M.C.; Francischetti, I.M.B. Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin α2β1, and von Willebrand factor. J. Biol. Chem. 2007, 282, 26928–26938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, E.; Tokumasu, F.; Mizurini, D.M.; McPhie, P.; Narum, D.L.; Ribeiro, J.M.C.; Monteiro, R.Q.; Francischetti, I.M.B. Aegyptin displays high-affinity for the von Willebrand factor binding site (RGQOGVMGF) in collagen and inhibits carotid thrombus formation in vivo. FEBS J. 2010, 277, 413–427. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Kim, S.J.; Kim, S. A novel anticoagulant protein with antithrombotic properties from the mosquito Culex pipiens pallens. Int. J. Biol. Macromol. 2016, 93, 156–166. [Google Scholar] [CrossRef]
- Ha, Y.R.; Oh, S.R.; Seo, E.S.; Kim, B.H.; Lee, D.K.; Lee, S.J. Detection of heparin in the salivary gland and midgut of Aedes togoi. Korean J. Parasitol. 2014, 52, 183–188. [Google Scholar] [CrossRef]
- Hayashi, H.; Kyushiki, H.; Nagano, K.; Sudo, T.; Matsuoka, H.; Yoshida, S. Anopheline anti-platelet protein from a malaria vector mosquito has anti-thrombotic effects in vivo without compromising hemostasis. Thromb. Res. 2012, 129, 169–175. [Google Scholar] [CrossRef]
- Isawa, H.; Yuda, M.; Orito, Y.; Chinzei, Y. A mosquito salivary protein inhibits activation of the plasma contact system by binding to factor XII and high molecular weight kininogen. J. Biol. Chem. 2002, 277, 27651–27658. [Google Scholar] [CrossRef] [Green Version]
- Ramasubramanian, M.K.; Barham, O.M.; Swaminathan, V. Mechanics of a mosquito bite with applications to microneedle design. Bioinspir. Biomim. 2008, 3, 046001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoffstall, A.J.; Srinivasan, S.; Willis, M.; Stiller, A.M.; Ecker, M.; Voit, W.E.; Pancrazio, J.J.; Capadona, J.R. A Mosquito Inspired Strategy to Implant Microprobes into the Brain. Sci. Rep. 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michels, J.; Appel, E.; Gorb, S.N. Functional diversity of resilin in Arthropoda. Beilstein J. Nanotechnol. 2016, 7, 1241–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The dry-style antifogging properties of mosquito compound eyes and artificial analogues prepared by soft lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Zwiebel, L.J.; Takken, W. Olfactory regulation of mosquito–host interactions. Insect Biochem. Mol. Biol. 2004, 34, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Tong, Z.; Jia, X.; Kiick, K.L. Resilin-like polypeptide hydrogels engineered for versatile biological function. Soft Matter 2013, 9, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, X.; Wang, R.; Long, F.; Zhao, P.; Liu, L. A mosquito-eye-like superhydrophobic coating with super robustness against abrasion. Mater. Des. 2021, 203, 109552. [Google Scholar] [CrossRef]
- Yamada, T.; Sugiura, H.; Mimura, H.; Kamiya, K.; Osaki, T.; Takeuchi, S. Highly sensitive VOC detectors using insect olfactory receptors reconstituted into lipid bilayers. Sci. Adv. 2021, 7, 2013–2026. [Google Scholar] [CrossRef]
- Tiuca, I.-D. Biochemistry and Stereochemistry of Anticoagulants. In Anticoagulation Therapy; IntechOpen: Rijeka, Croatia, 2016; Chapter 1; ISBN 978-953-51-2667-6. [Google Scholar]
- Suzuki, M.; Sawa, T.; Takahashi, T.; Aoyagi, S. Fabrication of Microneedle Mimicking Mosquito Proboscis Using Nanoscale 3D Laser Lithography System. Int. J. Autom. Technol. 2015, 9, 655–661. [Google Scholar] [CrossRef]
- Potter, C.J. Stop the biting: Targeting a mosquito’s sense of smell. Cell 2014, 156, 878–881. [Google Scholar] [CrossRef] [Green Version]
- Takken, W.; Verhulst, N.O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013, 58, 433–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasubramanian, M.K.; Agarwala, R. Biomimetic Mosquito-Like Microneedles. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2016; pp. 322–331. ISBN 978-94-017-9780-1. [Google Scholar]
- Choo, Y.M.; Buss, G.K.; Tan, K.; Leal, W.S. Multitasking roles of mosquito labrum in oviposition and blood feeding. Front. Physiol. 2015, 6, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurera, D.; Bhushan, B.; Kumar, N. Lessons from mosquitoes’ painless piercing. J. Mech. Behav. Biomed. Mater. 2018, 84, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.A.; Walker, E.D. Mosquitoes (culicidae). In Medical and Veterinary Entomology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–325. ISBN 9780128140437. [Google Scholar]
- Izumi, H.; Suzuki, M.; Aoyagi, S.; Kanzaki, T. Realistic imitation of mosquito’s proboscis: Electrochemically etched sharp and jagged needles and their cooperative inserting motion. Sens. Actuators A Phys. 2011, 165, 115–123. [Google Scholar] [CrossRef]
- Izumi, H.; Yajima, T.; Aoyagi, S.; Tagawa, N.; Arai, Y.; Hirata, M.; Yorifuji, S. Combined harpoonlike jagged microneedles imitating Mosquito’s proboscis and its insertion experiment with vibration. IEEJ Trans. Electr. Electron. Eng. 2008, 3, 425–431. [Google Scholar] [CrossRef]
- Aoyagi, S.; Izumi, H.; Fukuda, M. Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito’s proboscis. Sens. Actuators A Phys. 2008, 143, 20–28. [Google Scholar] [CrossRef]
- Izumi, H.; Aoyagi, S. Novel fabrication method for long silicon microneedles with three-dimensional sharp tips and complicated shank shapes by isotropic dry etching. IEEJ Trans. Electr. Electron. Eng. 2007, 2, 328–334. [Google Scholar] [CrossRef]
- Li, A.D.R.; Putra, K.B.; Chen, L.; Montgomery, J.S.; Shih, A. Mosquito proboscis-inspired needle insertion to reduce tissue deformation and organ displacement. Sci. Rep. 2020, 10, 12248. [Google Scholar] [CrossRef]
- Hara, Y.; Yamada, M.; Tatsukawa, C.; Takahashi, T.; Suzuki, M.; Aoyagi, S. Laser fabrication of jagged-shaped stainless steel microneedle imitating mosquito’s maxilla. Int. J. Autom. Technol. 2016, 10, 958–964. [Google Scholar] [CrossRef]
- Suzuki, M.; Sawa, T.; Terada, Y.; Takahashi, T.; Aoyagi, S. Fabrication of microneedles precisely imitating mosquito’s proboscis by nanoscale tree dimensional laser lithography and its characterization. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems, TRANSDUCERS 2015, Anchorage, AK, USA, 21–25 June 2015; pp. 121–124. [Google Scholar]
- Oka, K.; Aoyagi, S.; Arai, Y.; Isono, Y.; Hashiguchi, G.; Fujita, H. Fabrication of a micro needle for a trace blood test. Sens. Actuators A Phys. 2002, 97–98, 478–485. [Google Scholar] [CrossRef]
- Lenau, T.A.; Hesselberg, T.; Drakidis, A.; Silva, P.; Gomes, S. Mosquito inspired medical needles. In Bioinspiration, Biomimetics, and Bioreplication; SPIE: Bellingham, WA, USA, 2017; Volume 10162, p. 1016208. [Google Scholar]
- Chaudhri, B.P.; Ceyssens, F.; De Moor, P.; Van Hoof, C.; Puers, R. A high aspect ratio SU-8 fabrication technique for hollow microneedles for transdermal drug delivery and blood extraction. J. Micromech. Microeng. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Oki, A.; Takai, M.; Ogawa, H.; Takamura, Y.; Fukasawa, T.; Kikuchi, J.; Ito, Y.; Ichiki, T.; Horiike, Y. Healthcare chip for checking health condition from analysis of trace blood collected by painless needle. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2003, 42, 3722–3727. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tsuchiya, K. Development and fluidic simulation of microneedles for painless pathological interfacing with living systems. J. Appl. Phys. 2008, 103, 114701. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Nakanishi, N.; Nakamachi, E. Development of blood extraction system for health monitoring system. In BioMEMS and Nanotechnology; SPIE: Bellingham, WA, USA, 2004; Volume 5275, p. 257. [Google Scholar]
- Tsuchiya, K.; Isobata, K.; Sato, M.; Uetsuji, Y.; Nakamachi, E.; Kajiwara, K.; Kimura, M. Design of painless microneedle for blood extraction system. In BioMEMS and Nanotechnology III; SPIE: Bellingham, WA, USA, 2007; Volume 6799, p. 67990Q. [Google Scholar]
- Yoshida, Y.; Takei, T. Fabrication of a microneedle using human hair. Jpn. J. Appl. Phys. 2009, 48, 0980071–0980072. [Google Scholar] [CrossRef]
- Kosoglu, M.A.; Hood, R.L.; Chen, Y.; Xu, Y.; Rylander, M.N.; Rylander, C.G. Fiber optic microneedles for transdermal light delivery: Ex vivo porcine skin penetration experiments. J. Biomech. Eng. 2010, 132, 091014. [Google Scholar] [CrossRef]
- Roxhed, N.; Gasser, T.C.; Griss, P.; Holzapfel, G.A.; Stemme, G. Penetration-Enhanced Ultrasharp Microneedles and Prediction on Skin Interaction for Efficient Transdermal Drug Delivery. J. Microelectromech. Syst. 2007, 16, 1429–1440. [Google Scholar] [CrossRef]
- Aoyagi, S.; Izumi, H.; Isono, Y.; Fukuda, M.; Ogawa, H. Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle. Sens. Actuators A Phys. 2007, 139, 293–302. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tanaka, T.; Takaoki, Y.; Izumi, H.; Takahashi, T.; Suzuki, M.; Aoyagi, S. Fabrication of Metallic Microneedle by Electroplating and Sharpening of it by Electrochemical Etching. IEEJ Trans. Sens. Micromach. 2011, 131, 373–380. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef]
- Pappalardo, F.; Maj, G.; Scandroglio, A.; Sampietro, F.; Zangrillo, A.; Koster, A. Bioline® heparin-coated ECMO with bivalirudin anticoagulation in a patient with acute heparin-induced thrombocytopenia: The immune reaction appeared to continue unabated. Perfusion 2009, 24, 135–137. [Google Scholar] [CrossRef]
- Lu, C.W.; Malaga, K.A.; Chou, K.L.; Chestek, C.A.; Patil, P.G. High density microelectrode recording predicts span of therapeutic tissue activation volumes in subthalamic deep brain stimulation for Parkinson disease. Brain Stimul. 2020, 13, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möttönen, T.; Katisko, J.; Haapasalo, J.; Tähtinen, T.; Kiekara, T.; Kähärä, V.; Peltola, J.; Öhman, J.; Lehtimäki, K. Defining the anterior nucleus of the thalamus (ANT) as a deep brain stimulation target in refractory epilepsy: Delineation using 3 T MRI and intraoperative microelectrode recording. NeuroImage Clin. 2015, 7, 823–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; De Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.C.; Neuenschwander, P.F.; Chou, S.-F. Engineering Approaches to Prevent Blood Clotting from Medical Implants. Arch. Biomed. Eng. Biotechnol. 2019, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Small, M.; Faglie, A.; Craig, A.J.; Pieper, M.; Narcisse, V.E.F.; Neuenschwander, P.F.; Chou, S.-F. Nanostructure-Enabled and Macromolecule-Grafted Surfaces for Biomedical Applications. Micromachines 2018, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Sci. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Dabiri, A.E.; Kassab, G.S. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- Stark, K.R.; James, A.A. A Factor Xa-Directed Anticoagulant from the Salivary Glands of the Yellow Fever Mosquito Aedes aegypti. Exp. Parasitol. 1995, 81, 321–331. [Google Scholar] [CrossRef]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef]
- Sun, N.; Ye, Z.; Hao, T.; Zheng, S.; Sun, Y.; Zhang, Y.; Zhang, L. Inhibition of Arterial Thrombus Formation by Blocking Exposed Collagen Surface Using LWWNSYY-Poly(l-Glutamic Acid) Nanoconjugate. Langmuir 2021, 37, 6792–6799. [Google Scholar] [CrossRef] [PubMed]
- Baroletti, S.A.; Goldhaber, S.Z. Heparin-Induced Thrombocytopenia. Circulation 2006, 114, e355–e356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Y.; Jia, B.; Ye, M.; Shen, H.; Chen, W.; Zhang, H. Application of Heparin/Collagen-REDV Selective Active Interface on ePTFE Films to Enhance Endothelialization and Anticoagulation. Artif. Organs 2018, 42, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Savage, B.; Almus-Jacobs, F.; Ruggeri, Z.M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 1998, 94, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, R.; Usama, S.M.; Babiker, H.M.; Usama, S.M. Physiology, Coagulation Pathways; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Klijn, C.J.M.; Kappelle, L.J.; Tulleken, C.A.F.; van Gijn, J. Symptomatic Carotid Artery Occlusion. Stroke 1997, 28, 2084–2093. [Google Scholar] [CrossRef]
- WEIS-FOGH, T. A Rubber-Like Protein in Insect Cuticle. J. Exp. Biol. 1960, 37, 889–907. [Google Scholar] [CrossRef]
- Bennet-Clark, H.C.; Lucey, E.C. The jump of the flea: A study of the energetics and a model of the mechanism. J. Exp. Biol. 1967, 47, 59–67. [Google Scholar] [CrossRef]
- Bennet-Clark, H.C. Resonators in insect sound production: How insects produce loud pure-tone songs. J. Exp. Biol. 1999, 202, 3347–3357. [Google Scholar] [CrossRef]
- Behura, S.K.; Haugen, M.; Flannery, E.; Sarro, J.; Tessier, C.R.; Severson, D.W.; Duman-Scheel, M. Comparative genomic analysis of drosophila melanogaster and vector mosquito developmental genes. PLoS ONE 2011, 6, e21504. [Google Scholar] [CrossRef] [Green Version]
- Lerch, S.; Zuber, R.; Gehring, N.; Wang, Y.; Eckel, B.; Klass, K.-D.; Lehmann, F.-O.; Moussian, B. Resilin matrix distribution, variability and function in Drosophila. BMC Biol. 2020, 18, 195. [Google Scholar] [CrossRef]
- Koc, Y.; De Mello, A.J.; McHale, G.; Newton, M.I.; Roach, P.; Shirtcliffe, N.J. Nano-scale superhydrophobicity: Suppression of protein adsorption and promotion of flow-induced detachment. Lab Chip 2008, 8, 582–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltin, B.D.; Matsumura, Y.; Reid, A.; Windmill, J.F.; Gorb, S.N.; Jackson, J.C. Material stiffness variation in mosquito antennae. J. R. Soc. Interface 2019, 16, 20190049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cator, L.J.; Arthur, B.J.; Harrington, L.C.; Hoy, R.R. Harmonic convergence in the love songs of the dengue vector mosquito. Science 2009, 323, 1077–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarwar, M. Typical Flies: Natural History, Lifestyle and Diversity of Diptera. In Life Cycle and Development of Diptera; IntechOpen: Rijeka, Croatia, 2020; Chapter 1; ISBN 978-1-83880-226-4. [Google Scholar]
- Saltin, B.D.; Matsumura, Y.; Reid, A.; Windmill, J.F.; Gorb, S.N.; Jackson, J.C. Resilin Distribution and Sexual Dimorphism in the Midge Antenna and Their Influence on Frequency Sensitivity. Insects 2020, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- McGann, C.L.; Levenson, E.A.; Kiick, K.L. Resilin-based hybrid hydrogels for cardiovascular tissue engineering. Macromol. Chem. Phys. 2013, 214, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Gill, E.E.; Liu, J.C. Enzymatic Cross-Linking of Resilin-Based Proteins for Vascular Tissue Engineering Applications. Biomacromolecules 2016, 17, 2530–2539. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [Green Version]
- Kryuchkov, M.; Bilousov, O.; Lehmann, J.; Fiebig, M.; Katanaev, V.L. Reverse and forward engineering of Drosophila corneal nanocoatings. Nature 2020, 585, 383–389. [Google Scholar] [CrossRef]
- Takken, W.; Knols, B.G.J. Odor-Mediated Behavior of Afrotropical Malaria Mosquitoes. Annu. Rev. Entomol. 1999, 44, 131–157. [Google Scholar] [CrossRef]
- Mayavan, S.; Dutta, N.K.; Choudhury, N.R.; Kim, M.; Elvin, C.M.; Hill, A.J. Self-organization, interfacial interaction and photophysical properties of gold nanoparticle complexes derived from resilin-mimetic fluorescent protein rec1-resilin. Biomaterials 2011, 32, 2786–2796. [Google Scholar] [CrossRef]
- Lyons, R.E.; Lesieur, E.; Kim, M.; Wong, D.C.C.; Huson, M.G.; Nairn, K.M.; Brownlee, A.G.; Pearson, R.D.; Elvin, C.M. Design and facile production of recombinant resilin-like polypeptides: Gene construction and a rapid protein purification method. Protein Eng. Des. Sel. 2007, 20, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Dutta, N.K.; Choudhury, N.R.; Elvin, C.M.; Lyons, R.E.; Knott, R.; Hill, A.J. An16-resilin: An advanced multi-stimuli-responsive resilin-mimetic protein polymer. Acta Biomater. 2014, 10, 4768–4777. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Dutta, N.K.; Dutta, A.K.; Choudhury, N.R. Resilin-mimetics as a smart biomaterial platform for biomedical applications. Nat. Commun. 2021, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Renner, J.N.; Cherry, K.M.; Su, R.S.C.; Liu, J.C. Characterization of resilin-based materials for tissue engineering applications. Biomacromolecules 2012, 13, 3678–3685. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Su, R.S.C.; Galas, R.J.; Lin, C.Y.; Liu, J.C. Redox-Responsive Resilin-Like Hydrogels for Tissue Engineering and Drug Delivery Applications. Macromol. Biosci. 2019, 19, e1900122. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Chapter 1 redox state and redox environment in biology. In Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles; Forman, H.J., Fukuto, J., Torres, M., Eds.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Oliveira, S.M.; Song, W.; Alves, N.M.; Mano, J.F. Chemical modification of bioinspired superhydrophobic polystyrene surfaces to control cell attachment/proliferation. Soft Matter 2011, 7, 8932–8941. [Google Scholar] [CrossRef]
- Tang, P.; Zhang, W.; Wang, Y.; Zhang, B.; Wang, H.; Lin, C.; Zhang, L. Effect of Superhydrophobic Surface of Titanium on Staphylococcus aureus Adhesion. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.C.; Song, W.; Blanco-Fernandez, B.; Alvarez-Lorenzo, C.; Mano, J.F. Synthesis of temperature-responsive Dextran-MA/PNIPAAm particles for controlled drug delivery using superhydrophobic surfaces. Pharm. Res. 2011, 28, 1294–1305. [Google Scholar] [CrossRef] [Green Version]
- Brammer, J.D. The ultrastructure of the compound eye of a mosquito Aedes aegypti L. J. Exp. Zool. 1970, 175, 181–195. [Google Scholar] [CrossRef]
- Raji, J.I.; DeGennaro, M. Genetic analysis of mosquito detection of humans. Curr. Opin. Insect Sci. 2017, 20, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Leal, W.S. Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc. Natl. Acad. Sci. USA 2009, 106, 18803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raji, J.I.; Melo, N.; Castillo, J.S.; Gonzalez, S.; Saldana, V.; Stensmyr, M.C.; DeGennaro, M. Aedes aegypti Mosquitoes Detect Acidic Volatiles Found in Human Odor Using the IR8a Pathway. Curr. Biol. 2019, 29, 1253–1262.e7. [Google Scholar] [CrossRef] [Green Version]
- Bohbot, J.D.; Durand, N.F.; Vinyard, B.T.; Dickens, J.C.; Loudon, C.; Lei, H. Functional development of the octenol response in Aedes aegypti. Front. Physiol. 2013, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, R.; Dong, L.; Zhang, S.; Deng, C.; Liu, T.; Wang, J.; Shen, X. Investigation of volatile biomarkers in liver cancer blood using solid-phase microextraction and gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1181–1186. [Google Scholar] [CrossRef]
- Garner, C.E.; Smith, S.; de Lacy Costello, B.; White, P.; Spencer, R.; Probert, C.S.J.; Ratcliffem, N.M. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007, 21, 1675–1688. [Google Scholar] [CrossRef] [Green Version]
- Guernion, N.; Ratcliffe, N.M.; Spencer-Phillips, P.T.N.; Howe, R.A. Identifying bacteria in human urine: Current practice and the potential for rapid, near-patient diagnosis by sensing volatile organic compounds. Clin. Chem. Lab. Med. 2001, 39, 893–906. [Google Scholar] [CrossRef]
- Thomas, A.; Riazanskaia, S.; Cheung, W.; Xu, Y.; Goodacre, R.; Thomas, C.L.P.; Baguneid, M.; Bayat, A. Novel noninvasive identification of biomarkers by analytical profiling of chronic wounds using volatile organic compounds. Wound Repair Regen. 2010, 18, 391–400. [Google Scholar] [CrossRef]

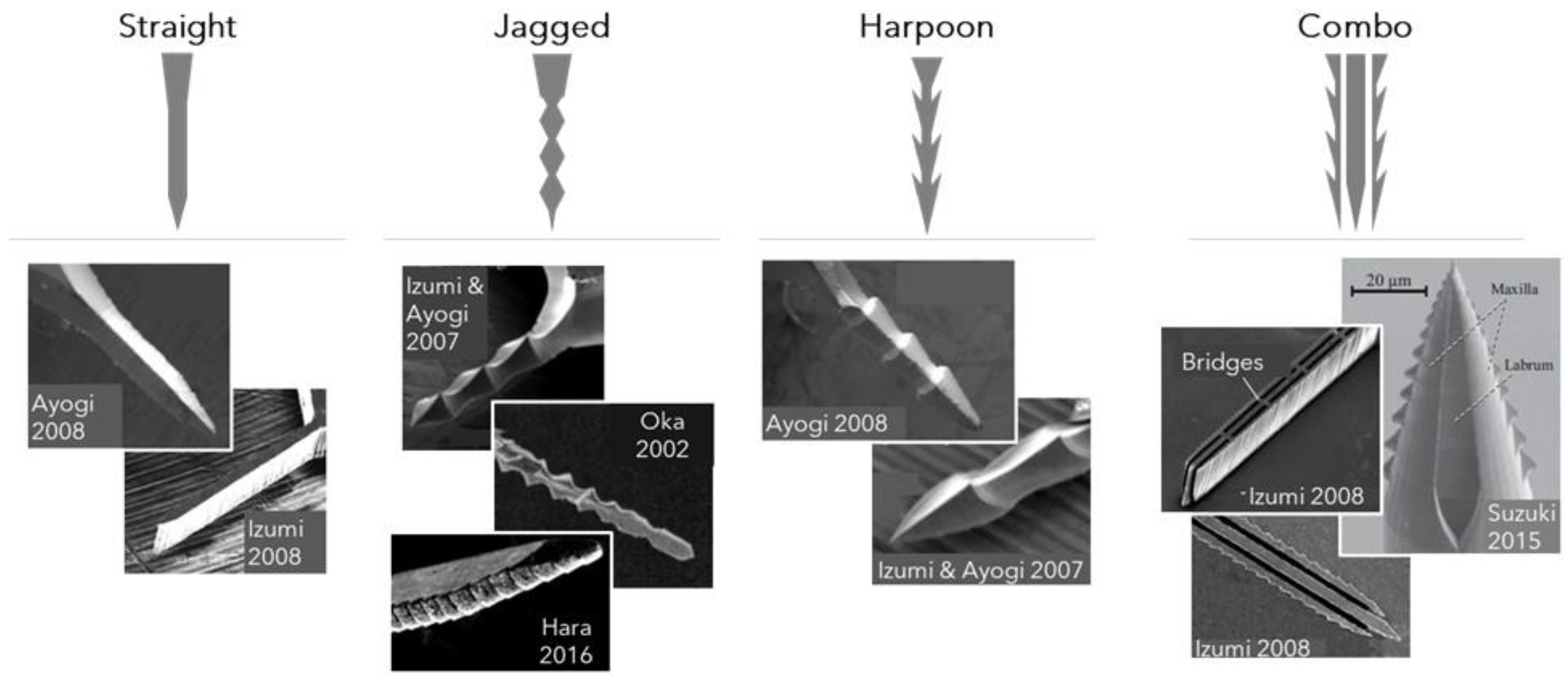



| Microneedle Type | Mimicked Part | Material | Dimensions | Reference |
|---|---|---|---|---|
| Hollow | Labrum | SU-8 | Dinner = 100 μm, H = 1540 μm, Twall = 15 μm | [37] |
| Hollow | Labrum | Stainless steel, 2-methacryloyloxyethyl phosphorylcholine internal coating | Dinner = 50 μm, Douter = 90 μm, tip ∡ = 10o | [38] |
| Hollow | Labium | Titanium | Dinner = 25 μm, Douter = 50 μm or 60 μm, L = 3.8/4 mm | [39,40,41] |
| Hollow | Labium | Human hair | Dinner ≥ 10 μm, Douter = 80 μm, L = 1.1 mm | [42] |
| Hollow, fiber optic | Labium | Silica | Sharp Tip: Dinflection = 2 − 8 μm, Dbase = 73 − 125 μm, L = 3 mm Flat Tip: Douter = Dbase = 125 − 139 μm, Taper ∡1 = 0 − 0.9°, Taper ∡2 = 3.2 − 10.7°, L = 3 mm | [43] |
| Hollow, jagged | Labrum; maxillae | Silicon dioxide, poly-Si coating | L = 1 mm, Twall = 1.6 μm | [35] |
| Solid, straight, 3D sharp tips | Labrum | Silicon or polylactic acid | L = 1 mm, W = 150 μm, Tip ∡ = 18o | [28] |
| Solid, straight | Labrum | Silicon, parylene coating * | L = no limit; tip ∡ = 30o or 60o | [31] |
| Solid, straight | Labrum | Silicon, parylene coating * | L = 1.0 mm, W = 60 μm, T = 100 μm | [29] |
| Solid, straight, biodegradable | Labrum | Polylactic acid | W = 120 − 230 µm, T = 60 − 115 µm, Tip ∡ = 15 − 75° | [30] |
| Solid, jagged | Maxillae | Silicon, parylene coating * | L = 1.0 mm, W = 60 μm, T = 100 μm, Pteeth = 10 μm | [29] |
| Solid, jagged | Maxillae | Stainless steel | L = 2.2 mm, W = 70 µm, Tip ∡ = 15o, Pteeth = 20 µm, DPteeth = 7 µm | [33] |
| Solid, jagged or harpoon, long, 3D sharp tips | Maxillae | Silicon, parylene coating * | L = no limit; Tip ∡ = 30o or 60o | [31] |
| Solid, jagged, biodegradable | Maxillae | Polylactic acid | Tip ∡ = 30o | [30] |
| Solid, hooked, biodegradable | Maxillae | Polylactic acid | Tip ∡ = 30o | [30] |
| Combination, 2-part (alternatively moving halves), jagged, hollow, holes in walls | Labrum; maxillae | IP-S | Dinner = 50 μm, Douter = 100 μm, L = 1 − 2 mm | [34] |
| Combination, 3 needles (1 central straight, 2 outer harpoon-like jagged), fixed (with bridges) or free (with no bridges) | Labrum; maxillae | Silicon, parylene coating * | Central needle: L = 1.0 mm, T = 100 μm, W = 30 μm Outer needles: L = 1.0 mm, T = 100 μm, W = 15 μm | [29] |
| Combination, 3 needles (1 central straight and hollow, 2 outer jagged) | Labrum; maxillae | IP-S; IP-DipTM | Central needle (cone shape): Dbase = 30 μm, Hbase = 100 μm, Tip Dinner = 20 μm, Tip Douter = 30 μm Outer needle (solid cylinder): Dinner = 40 μm, Douter = 50 μm; 14 graded serrated projections (W = 0.6 − 2.0 μm, H= 0.8 − 6.0 μm, L = 1.0 -8.0 μm) All needles: Ltotal = 2 mm, Inter-needle gap = 10 μm | [21] |
| Biomolecule | Mosquito Type | Vector-Borne Human Disease | Mechanism of Action | Reference |
|---|---|---|---|---|
| Anopheline Antiplatelet Protein (AAPP) | Anopheles stephensi | Malaria, Lymphatic Filariasis | Binds collagen; inhibits interaction with glycoprotein VI and integrin 21 | [2,10] |
| Aegyptin | Aedes aegypti | Yellow Fever, Chikungunya, Zika Fever, Dengue Fever | Binds to collagen preventing its interaction with von Willebrand factor, integrin a2b1, and glycoprotein VI; inhibits factor Xa | [6,7,57] |
| CCP Protein | Culex pipiens pallens | Japanese Encephalitis, Lymphatic Filariasis West Nile Virus | Inhibits enzymatic activity of thrombin and factor Xa; may inhibit interaction between coagulation factors and platelet receptors | [22,8] |
| Hamadarin | Anopheles stephensi | Malaria, Lymphatic Filariasis | Inhibits activation of plasma contact system by binding to factor XII and high-molecular-weight kininogen | [2,11] |
| Heparin | Aedes togoi | Japanese Encephalitis, Filariasis, Yellow Fever | Inhibits thrombin and factor Xa by activating antithrombin | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixon, A.R.; Vondra, I. Biting Innovations of Mosquito-Based Biomaterials and Medical Devices. Materials 2022, 15, 4587. https://doi.org/10.3390/ma15134587
Dixon AR, Vondra I. Biting Innovations of Mosquito-Based Biomaterials and Medical Devices. Materials. 2022; 15(13):4587. https://doi.org/10.3390/ma15134587
Chicago/Turabian StyleDixon, Angela R., and Isabelle Vondra. 2022. "Biting Innovations of Mosquito-Based Biomaterials and Medical Devices" Materials 15, no. 13: 4587. https://doi.org/10.3390/ma15134587
APA StyleDixon, A. R., & Vondra, I. (2022). Biting Innovations of Mosquito-Based Biomaterials and Medical Devices. Materials, 15(13), 4587. https://doi.org/10.3390/ma15134587





