A Sequenced Study of Improved Dielectric Properties of Carbon Nanotubes and Metal Oxide-Reinforced Polymer Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Preparation of CNT/PVA Composites
2.2.1. Purification and Oxidation of SWCNTs
2.2.2. Preparation of Metal Oxides (MOs)
2.2.3. Composite Formation (PVA/MO/SWCNTs)
2.2.4. Solution-Casting Method
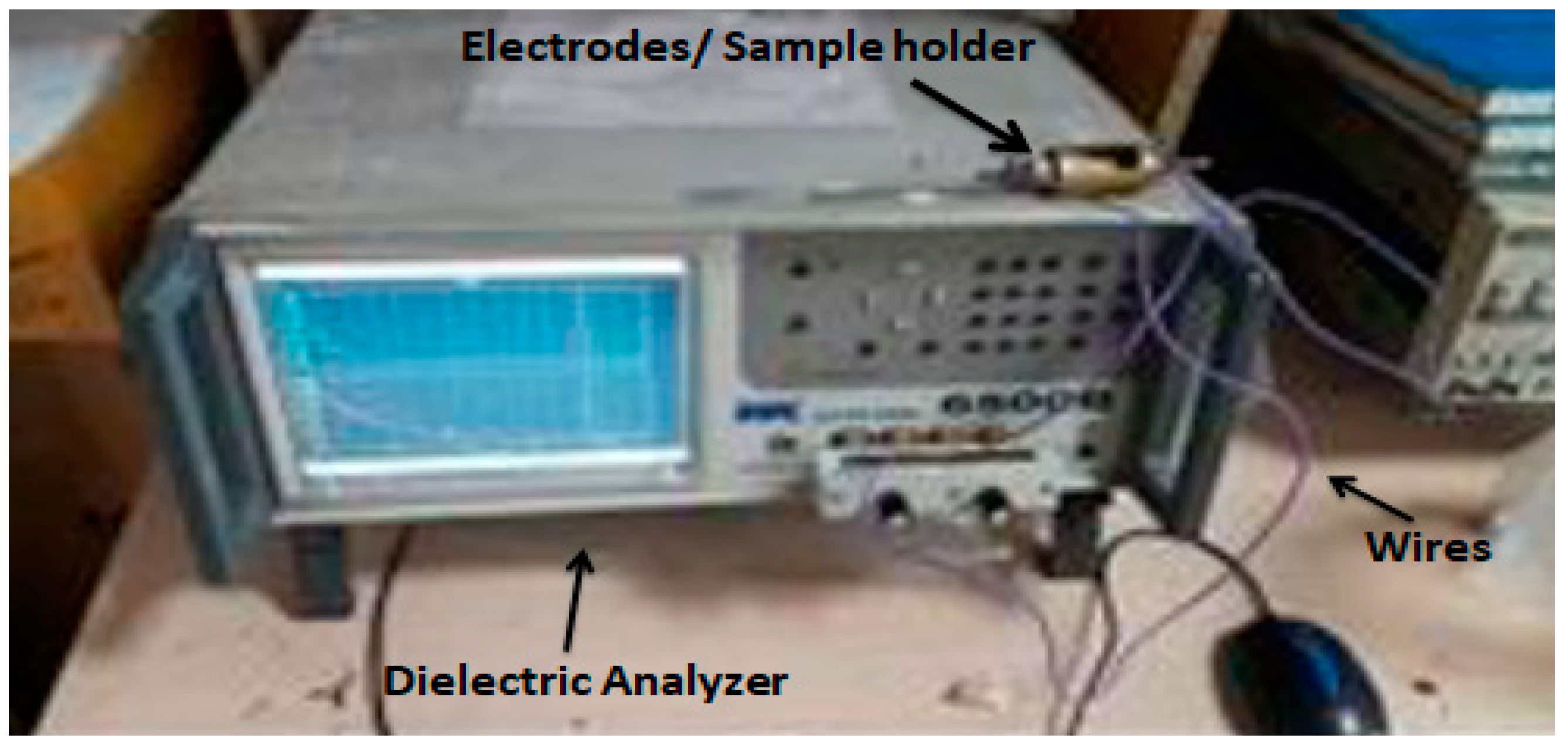
2.3. Characterization
3. Results and Discussion
3.1. Conformational Analysis
3.2. Physical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, S.; Verestek, W.; Zeizinger, H.; Schmauder, S. Characterization of Cure Behavior in Epoxy Using Molecular Dynamics Simulation Compared with Dielectric Analysis and DSC. Polymers 2021, 13, 3085. [Google Scholar] [CrossRef] [PubMed]
- Faiza, F.; Khattak, A.; Butt, S.; Imran, K.; Ulasyar, A.; Ali, A.; Khan, Z.; Mahmood, A.; Ullah, N.; Alahmadi, A.; et al. Investigation of Hydrothermally Stressed Silicone Rubber/Silica Micro and Nanocomposite for the Coating High Voltage Insulation Applications. Materials 2021, 14, 3567. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.; Amin, M.; Iqbal, M.; Abbas, N. Life estimation and analysis of dielectric strength, hydrocarbon backbone and oxidation of high voltage multi stressed EPDM composites. Mater. Res. Express 2018, 5, 025003. [Google Scholar] [CrossRef]
- Azevedo, J.V.; Dorp, E.R.V.; Hausnerova, B.; Möginger, B. The Effects of Chain-Extending Cross-Linkers on the Mechanical and Thermal Properties of Poly(butylene adipate terephthalate)/Poly(lactic acid) Blown Films. Polymers 2021, 13, 3092. [Google Scholar] [CrossRef]
- Elbayoumy, E.; El-Ghamaz, N.A.; Mohamed, F.S.; Diab, M.A.; Nakano, T. Dielectric Permittivity, AC Electrical Conductivity and Conduction Mechanism of High Crosslinked-Vinyl Polymers and Their Pd(OAc)2 Composites. Polymers 2021, 13, 3005. [Google Scholar] [CrossRef]
- Barber, P.; Balasubramanian, S.; Anguchamy, Y.; Gong, S.; Wibowo, A.; Gao, H.; Ploehn, H.J.; Zur Loye, H.-C. Polymer Composite and Nanocomposite Dielectric Materials for Pulse Power Energy Storage. Materials 2009, 2, 1697–1733. [Google Scholar] [CrossRef]
- Işık, B.; Kurtoğlu, A.E.; Gürdağ, G.; Keçeli, G. Radioactive cesium ion removal from wastewater using polymer metal oxide composites. J. Hazard. Mater. 2020, 403, 123652. [Google Scholar] [CrossRef]
- Arbatti, M.; Shan, X.; Cheng, Z.-Y. Ceramic–Polymer Composites with High Dielectric Constant. Adv. Mater. 2007, 19, 1369–1372. [Google Scholar] [CrossRef]
- Maharramov, A.A.; Ramazanov, M.A.; Di Palma, L.; Shirinova, H.A.; Hajiyeva, F.V. Influence of Magnetite Nanoparticles on the Dielectric Properties of Metal Oxide/Polymer Nano-composites Based on Polypropylene. Russ. Phys. J. 2018, 60, 1572–1576. [Google Scholar] [CrossRef]
- Yao, X.; Kou, X.; Qiu, J.; Moloney, M.G. Generation Mechanism of Negative Dielectric Properties of Metallic Oxide Crystals/Polyaniline Composites. J. Phys. Chem. C 2016, 120, 4937–4944. [Google Scholar] [CrossRef]
- Kim, P.; Jones, S.C.; Hotchkiss, P.J.; Haddock, J.N.; Kippelen, B.; Marder, S.R.; Perry, J.W. Phosphonic Acid-Modified Barium Titanate Polymer Nanocomposites with High Permittivity and Dielectric Strength. Adv. Mater. 2007, 19, 1001–1005. [Google Scholar] [CrossRef]
- Demirezen, S.; Eroğlu, A.; Azizian-Kalandaragh, Y.; Altındal, Ş. Electric and dielectric parameters in Au/n-Si (MS) capacitors with metal oxide-polymer interlayer as function of frequency and voltage. J. Mater. Sci. Mater. Electron. 2020, 31, 15589–15598. [Google Scholar] [CrossRef]
- Ushakov, N.M.; Kosobudsky, I.D. About the features of electric conductivity models for polymer composite nano-materials based on Cu (Cu2O)-LDPE. Semiconductors 2020, 54, 1692–1694. [Google Scholar] [CrossRef]
- Munkaila, S.; Bentley, J.; Schimmel, K.; Ahamad, T.; Alshehri, S.M.; Bastakoti, B.P. Polymer directed synthesis of NiO nanoflowers to remove pollutant from wastewater. J. Mol. Liq. 2021, 324, 114676. [Google Scholar] [CrossRef]
- Ezzat, H.A.; Hegazy, M.A.; Nada, N.A.; Osman, O.; Ibrahim, M.A. Development of natural polymer/metal oxide nanocomposite reinforced with graphene oxide for optoelectronic applications. NRIAG J. Astron. Geophys. 2020, 10, 10–22. [Google Scholar] [CrossRef]
- Rehman, M.N.U.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Maqbool, A.; Riaz, M.; Manzoor, S.; Ashiq, M.N.; Iqbal, F. Facile synthesis and characterization of conducting polymer-metal oxide based core-shell PANI-Pr2O–NiO–Co3O4 nanocomposite: As electrode material for supercapacitor. Ceram. Int. 2021, 47, 18497–18509. [Google Scholar] [CrossRef]
- Srivastava, M.; Surana, K.; Singh, P.K.; Singh, R.C. Nickel Oxide embedded with Polymer Electrolyte as Efficient Hole Transport Material for Perovskite Solar Cell. Eng. Sci. 2021, 17, 216–223. [Google Scholar] [CrossRef]
- Rani, P.; Ahamed, B.; Deshmukh, K. Dielectric and electromagnetic interference shielding properties of zeolite 13X and carbon black nanoparticles based PVDF nanocomposites. J. Appl. Polym. Sci. 2021, 138, 50107. [Google Scholar] [CrossRef]
- Guo, N.; DiBenedetto, S.A.; Tewari, P.; Lanagan, M.T.; Ratner, M.A.; Marks, T.J. Nanoparticle, size, shape, and interfacial effects on leakage current density, permittivity, and breakdown strength of metal oxide-polyolefin nanocomposites: Experiment and theory. Chem. Mater. 2010, 22, 1567–1578. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Formation and Corrosion Behavior of Nickel/Alumina Nanocomposites. Solid State Phenom. 2020, 299, 100–106. [Google Scholar] [CrossRef]
- Yu, L.; Ranjan, V.; Nardelli, M.B.; Bernholc, J. First-principles investigations of the dielectric properties of polypropylene/metal-oxide interfaces. Phys. Rev. B 2009, 80, 165432. [Google Scholar] [CrossRef]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.; Jasiuk, I. Experimental trends in polymer nanocomposites—A review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Flahaut, E.; Peigney, A.; Laurent, C.; Marliere, C.; Chastel, F.; Rousset, A. Carbon nanotube–metal–oxide nanocomposites: Microstructure, electrical conductivity and mechanical properties. Acta Mater. 2000, 48, 3803–3812. [Google Scholar] [CrossRef]
- Peigney, A.; Flahaut, E.; Laurent, C.; Chastel, F.; Rousset, A. Aligned carbon nanotubes in ceramic-matrix nanocomposites prepared by high-temperature extrusion. Chem. Phys. Lett. 2002, 352, 20–25. [Google Scholar] [CrossRef]
- Li, Z.; Fredin, L.A.; Tewari, P.; DiBenedetto, S.A.; Lanagan, M.T.; Ratner, M.A.; Marks, T.J. In Situ Catalytic Encapsulation of Core-Shell Nanoparticles Having Variable Shell Thickness: Dielectric and Energy Storage Properties of High-Permittivity Metal Oxide Nanocomposites. Chem. Mater. 2010, 22, 5154–5164. [Google Scholar] [CrossRef]
- Giustino, F.; Pasquarello, A. Theory of atomic-scale dielectric permittivity at insulator interfaces. Phys. Rev. B 2005, 71, 144104. [Google Scholar] [CrossRef]
- Halima, N.B. Poly (vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Ciobotaru, C.C.; Damian, C.M.; Iovu, H. Single-wall carbon nanotubes purification and oxidation. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2013, 75, 55–66. [Google Scholar]
- Duraisamy, N.; Numan, A.; Fatin, S.O.; Ramesh, K.; Ramesh, S. Facile sonochemical synthesis of nanostructured NiO with different particle sizes and its electrochemical properties for supercapacitor application. J. Colloid Interface Sci. 2016, 471, 136–144. [Google Scholar] [CrossRef]
- Qammar, M.; Malik, Z.; Malik, F.; Baig, T.; Chaudhary, A.J. Antibacterial activity of Mg1-xNixO (x= 0.5) nano-solid solution; experimental and computational approach. J. Mol. Struct. 2019, 1179, 347–353. [Google Scholar] [CrossRef]
- Zhu, J.; Li, D.; Chen, H.; Yang, X.; Lu, L.; Wang, X. Highly dispersed CuO nanoparticles prepared by a novel quick-precipitation method. Mater. Lett. 2004, 58, 3324–3327. [Google Scholar] [CrossRef]
- Davar, F.; Fereshteh, Z.; Salavati-Niasari, M. Nanoparticles Ni and NiO: Synthesis, characterization and magnetic properties. J. Alloys Compd. 2009, 476, 797–801. [Google Scholar] [CrossRef]
- Kouklin, N.; Tzolov, M.; Straus, D.; Yin, A.; Xu, J.M. Infrared absorption properties of carbon nanotubes synthesized by chemical vapor deposition. Appl. Phys. Lett. 2004, 85, 4463. [Google Scholar] [CrossRef]
- Heitz, T.; Drevillon, B.; Godet, C.; Bouree, J.E. Quantitative study of C—H bonding in polymerlike amorphous carbon films using in situ infrared ellipsometry. Phys. Rev. B 1998, 58, 13957. [Google Scholar] [CrossRef]
- Barua, S.; Chattopadhyay, P.; Phukan, M.M.; Konwar, B.K.; Karak, N. Hyperbranched epoxy/MWCNT-CuO-nystatin nanocomposite as a high performance, biocompatible, anti-microbial material. Mater. Res. Express 2014, 1, 045402. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Sun, F.; Xiao, R.; Guo, Y.; Song, J. Liquid crystal microphase separation of cellulose nanocrystals in wet-spun PVA composite fibers. RSC Adv. 2014, 4, 30784–30789. [Google Scholar] [CrossRef]
- Abdullah, O.; Aziz, S.B.; Omer, K.; Salih, Y.M. Reducing the optical band gap of polyvinyl alcohol (PVA) based nanocomposite. J. Mater. Sci. Mater. Electron. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Popovics, S. A numerical approach to the complete stress-strain curve of concrete. Cem. Concr. Res. 1973, 3, 583–599. [Google Scholar] [CrossRef]
- Hsu, T.T.; Slate, F.O.; Sturman, G.M.; Winter, G. Microcracking of plain concrete and the shape of the stress-strain curve. ACI J. Proc. 1963, 60, 209–224. [Google Scholar]
- Gómez, I.; Otazo, E.; Hernández, H.; Rubio, E.; Varela, J.; Ramirez-Cardona, M.; Barajas, I.; Gordillo, A. Thermal degradation study of PVA derivative with pendant phenylthionecarbamate groups by DSC/TGA and GC/MS. Polym. Degrad. Stab. 2015, 112, 132–136. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, M.; Chen, F.; Yang, J. Visible light photocatalytic activity of TiO2/D-PVA for MO degradation. Appl. Catal. B Environ. 2009, 90, 249–254. [Google Scholar] [CrossRef]
- Mustafa, E.; Afia, R.S.A.; Nouini, O.; Tamus, Z. Implementation of Non-Destructive Electrical Condition Monitoring Techniques on Low-Voltage Nuclear Cables: I. Irradiation Aging of EPR/CSPE Cables. Energies 2021, 14, 5139. [Google Scholar] [CrossRef]
- Mustafa, E.; Afia, R.S.A.; Tamus, Z.A. Application of Non-Destructive Condition Monitoring Techniques on Irradiated Low Voltage Unshielded Nuclear Power Cables. IEEE Access 2020, 8, 166024–166033. [Google Scholar] [CrossRef]
- El-Mallah, H.M. AC electrical conductivity and dielectric properties of perovskite (Pb, Ca) TiO3 ceramic. Acta Phys. Pol.-Ser. A Gen. Phys. 2012, 122, 174. [Google Scholar] [CrossRef]
- Roy, A.S.; Gupta, S.; Sindhu, S.; Parveen, A. Ramamurthy, Dielectric properties of novel PVA/ZnO hybrid nanocomposite films. Compos. Part B Eng. 2013, 47, 314–319. [Google Scholar] [CrossRef]
- Radoń, A.; Włodarczyk, P.; Drygała, A.; Łukowiec, D. Electrical properties of epoxy nanocomposites containing Fe3O4 nanoparticles and Fe3O4 nanoparticles deposited on the surface of electrochemically exfoliated and oxidized graphite. Appl. Surf. Sci. 2018, 474, 66–77. [Google Scholar] [CrossRef]












| Sr. no | Sample Code | Concentration of Reagents Used (wt.%) | ||
|---|---|---|---|---|
| SWCNTs | NiO | CuO | ||
| 1 | CCP 1 | 0.01 | - | 2 |
| 2 | CCP 2 | 0.01 | - | 4 |
| 3 | CCP 3 | 0.01 | - | 6 |
| 4 | NCP 1 | 0.01 | 2 | - |
| 5 | NCP 2 | 0.01 | 4 | - |
| 6 | NCP 3 | 0.01 | 6 | - |
| Sr. No. | Sample Code | Bandgap (eV) |
|---|---|---|
| 1 | CCP 1 | 4.41 |
| 2 | CCP 2 | 4.36 |
| 3 | CCP 3 | 4.34 |
| 4 | NCP 1 | 4.48 |
| 5 | NCP 2 | 4.42 |
| 6 | NCP 3 | 4.41 |
| Sr. No. | Sample | E (MPa) | Sy (MPa) | UTS (MPa) | etotal | eelastic |
|---|---|---|---|---|---|---|
| 1 | CCP 1 | 62.5 | 3.2 | 20.5 | 1.12 | 1.02 |
| 2 | CCP 2 | 22.4 | 1.2 | 11.3 | 1.17 | 1.10 |
| 3 | CCP 3 | 57.7 | 4.3 | 13.02 | 0.51 | 0.41 |
| 4 | CNP 1 | 28.3 | 1.62 | 7.2 | 0.50 | 0.44 |
| 5 | CNP 2 | 31.3 | 3.9 | 14.9 | 0.74 | 0.63 |
| 6 | CNP 3 | 59.0 | 5.44 | 10.04 | 0.49 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faiza; Qammar, M.; Butt, S.U.; Malik, Z.; Alahamadi, A.A.; Khattak, A. A Sequenced Study of Improved Dielectric Properties of Carbon Nanotubes and Metal Oxide-Reinforced Polymer Composites. Materials 2022, 15, 4592. https://doi.org/10.3390/ma15134592
Faiza, Qammar M, Butt SU, Malik Z, Alahamadi AA, Khattak A. A Sequenced Study of Improved Dielectric Properties of Carbon Nanotubes and Metal Oxide-Reinforced Polymer Composites. Materials. 2022; 15(13):4592. https://doi.org/10.3390/ma15134592
Chicago/Turabian StyleFaiza, Memoona Qammar, Safi Ullah Butt, Zahida Malik, Ahmad Aziz Alahamadi, and Abraiz Khattak. 2022. "A Sequenced Study of Improved Dielectric Properties of Carbon Nanotubes and Metal Oxide-Reinforced Polymer Composites" Materials 15, no. 13: 4592. https://doi.org/10.3390/ma15134592
APA StyleFaiza, Qammar, M., Butt, S. U., Malik, Z., Alahamadi, A. A., & Khattak, A. (2022). A Sequenced Study of Improved Dielectric Properties of Carbon Nanotubes and Metal Oxide-Reinforced Polymer Composites. Materials, 15(13), 4592. https://doi.org/10.3390/ma15134592






