Thermal Annealing Effects of V2O5 Thin Film as an Ionic Storage Layer for Electrochromic Application
Abstract
:1. Introduction
2. Experiments
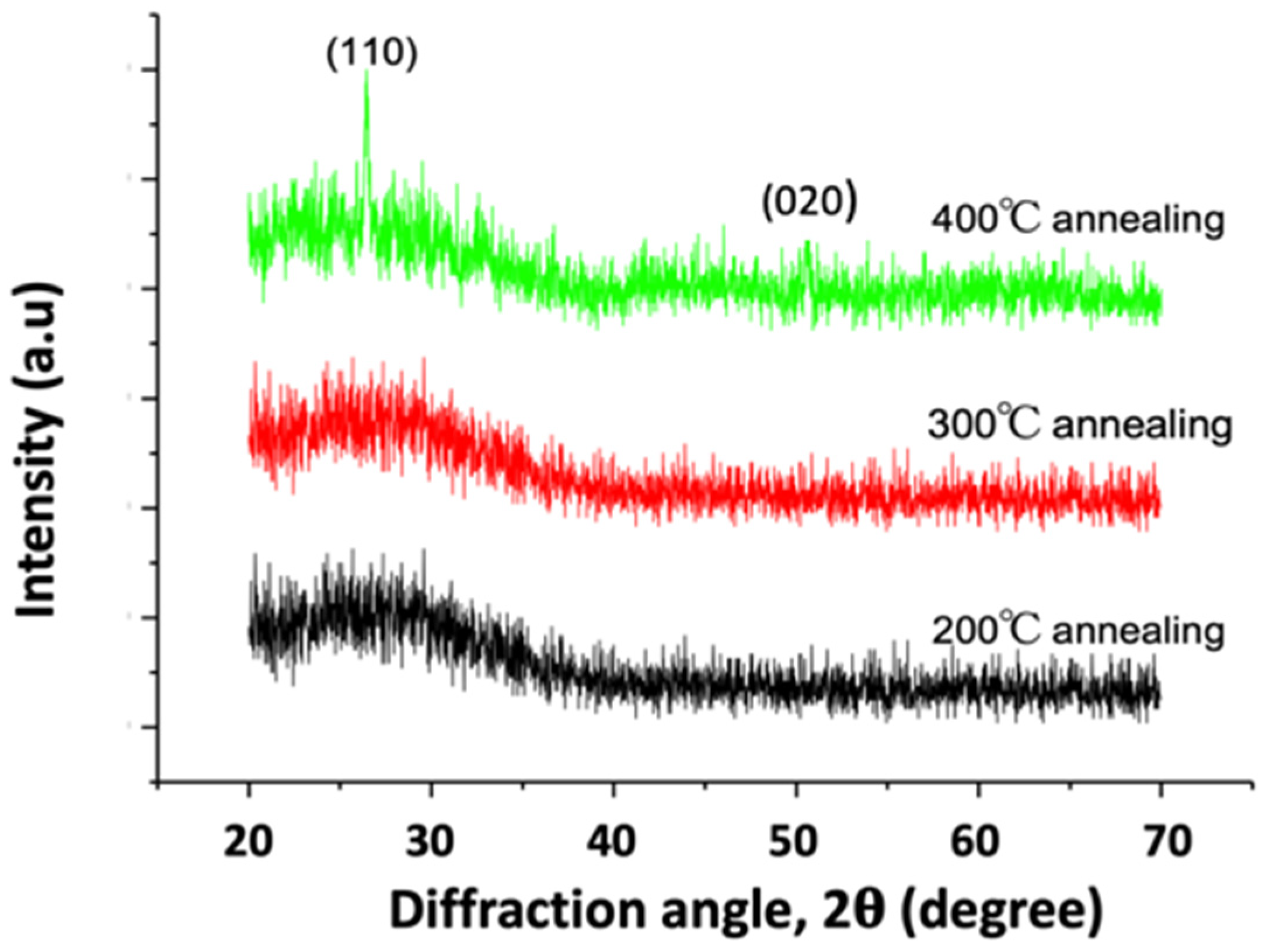
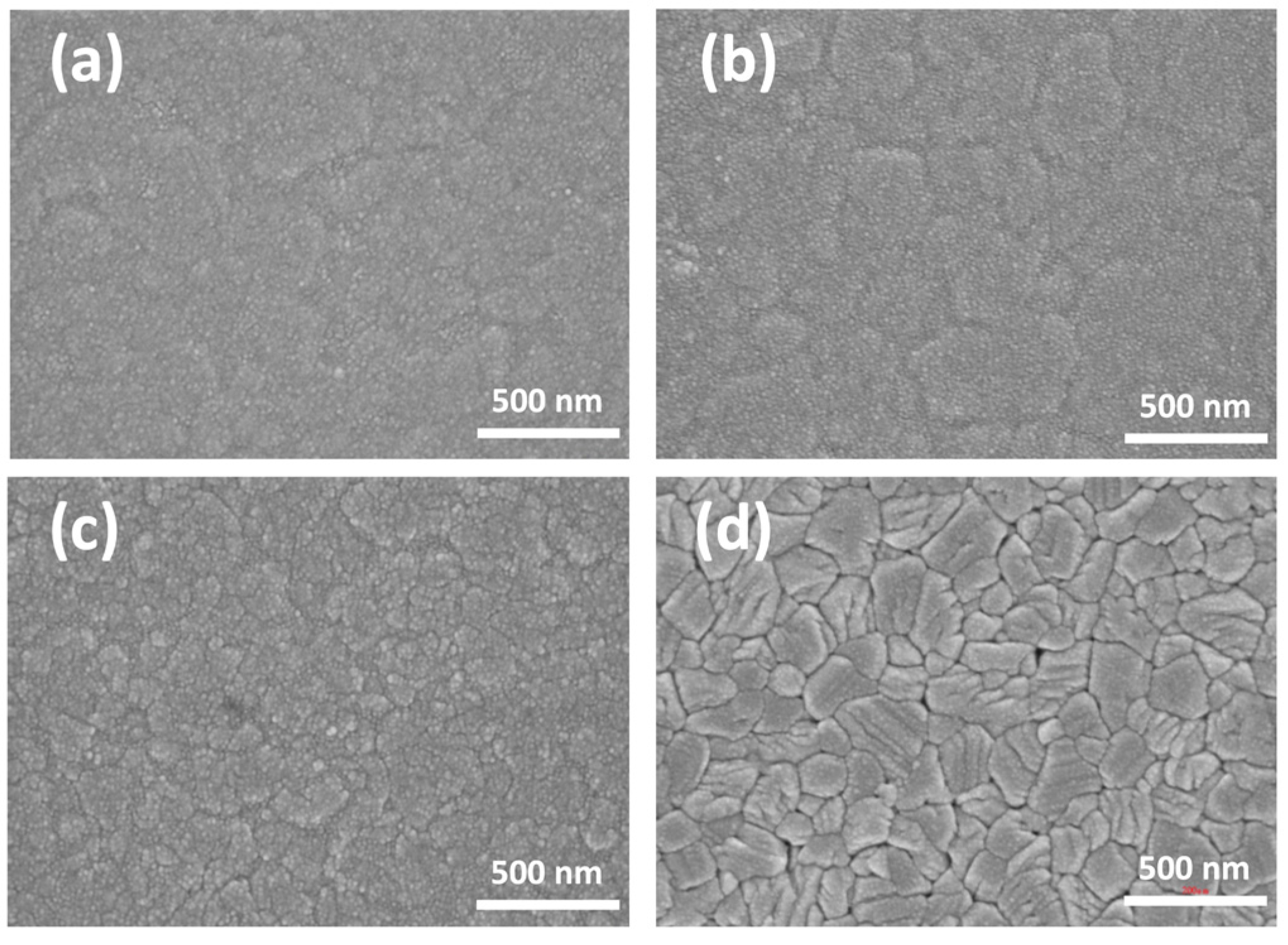
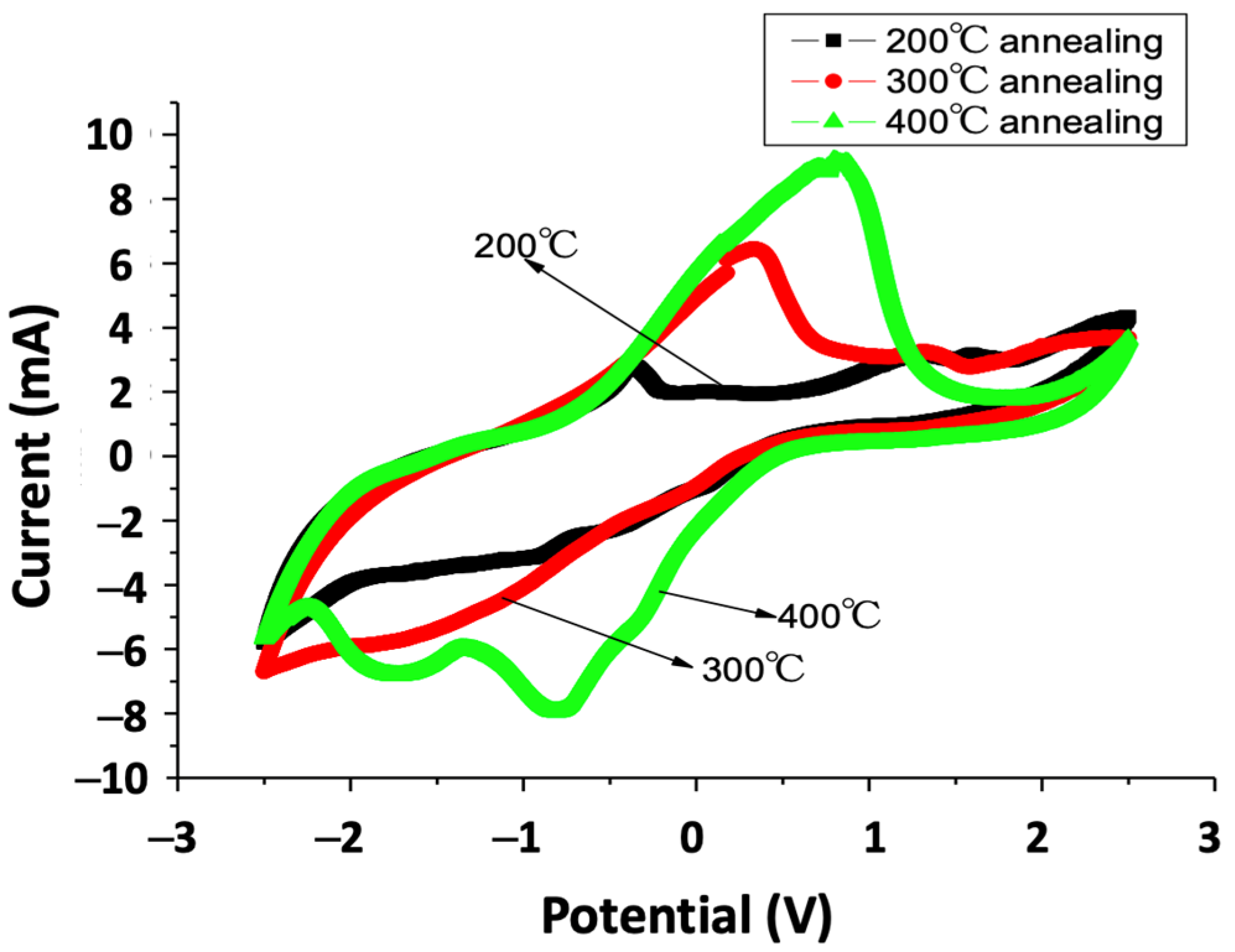
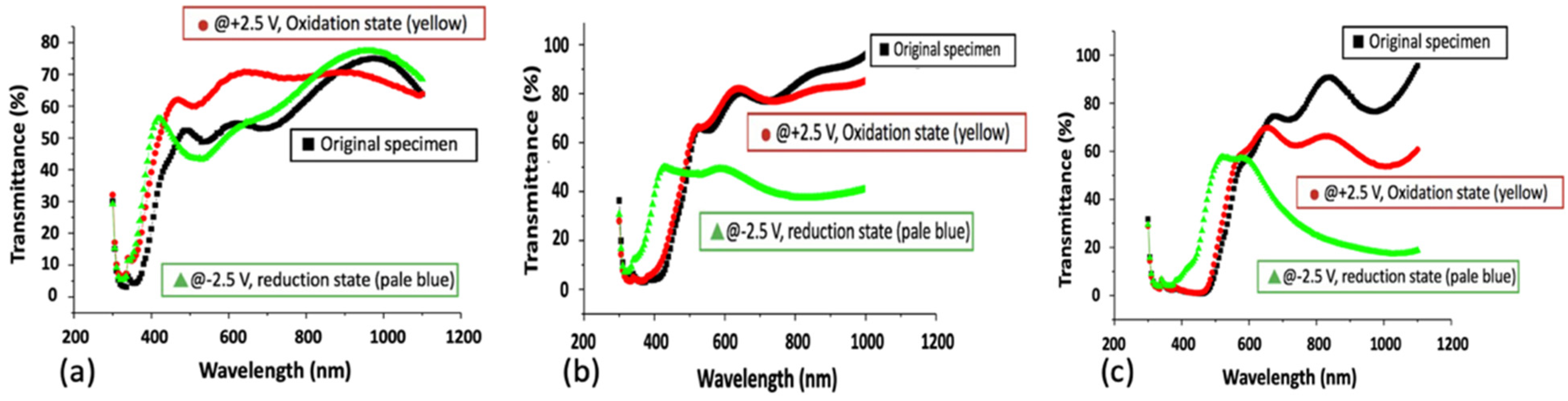
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granqvist, C.G.; Green, S.; Niklasson, G.A.; Mlyuka, N.R.; von Kræmer, S.; Georén, P. Advances in chromogenic materials and devices. Thin Solid Film. 2010, 518, 3046–3053. [Google Scholar] [CrossRef]
- Wang, Y.; Runnerstrom, E.L.; Milliron, D.J. Switchable Materials for Smart Windows. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Shao, J.Y.; Tian, H.M.; Li, X.M. Protective integrated transparent conductive film with high mechanical stability and uniform electric-field distribution. Nanotechnology 2019, 30, 185303. [Google Scholar] [CrossRef] [PubMed]
- Vernardou, D.; Psifis, K.; Louloudakis, D.; Papadimitropoulos, G.; Davazoglou, D.; Katsarakis, N.; Koudoumas, E. Low Pressure CVD of Electrochromic WO3 at 400 °C. J. Electrochem. Soc. 2015, 162, H579. [Google Scholar] [CrossRef]
- Vernardou, D. State-of-the-art of chemically grown vanadium pentoxide nanostructures with enhanced electrochemical properties. Adv. Mater. Lett. 2013, 4, 798–810. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Vernardou, D.; Koudoumas, E.; Tsoukalas, D.; Raptis, Y.S. Oxygen and temperature effects on the electrochemical and electrochromic properties of rf-sputtered V2O5 thin films. Electrochim. Acta 2017, 232, 54–63. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Ou, B.; Zhao, S.; Wang, Z. Some important issues of the commercial production of 1-D nano-PANI. Polymers 2019, 11, 681. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.; Koo, B.; Ahn, H. Fe doping effect of vanadium oxide films for enhanced switching electrochromic performances. Ceram. Int. 2019, 45, 7137–7142. [Google Scholar] [CrossRef]
- Li, J.J.; Guo, Q.F.; Lu, Y.; Nie, G.M. Polyindole vertical nanowire array based electrochromic-supercapacitor difunctional device for energy storage and utilization. Eur. Polym. J. 2019, 113, 29–35. [Google Scholar] [CrossRef]
- Li, H.; McRae, L.; Firby, C.J.; Elezzabi, A.Y. Rechargeable aqueous electrochromic batteries utilizing Ti-substituted tungsten molybdenum oxide based Zn2+ ion intercalation cathodes. Adv. Mater. 2019, 31, 1807065. [Google Scholar] [CrossRef]
- Karaca, G.Y.; Eren, E.; Cogal, G.C.; Uygun, E.; Oksuz, L.; Oksuz, A.U. Enhanced electrochromic characteristics induced by Au/PEDOT/Pt microtubes in WO3 based electrochromic devices. Opt. Mater. 2019, 88, 472–478. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.L.; Xia, X.H.; Gu, C.D.; Zhao, Z.J.; Tu, J.P. Enhanced electro- chromic performance of macroporous WO3 films formed by anodic oxidation of DC-sputtered tungsten layers. Electrochim. Acta 2010, 55, 6953. [Google Scholar] [CrossRef]
- Psifis, K.; Louloudakis, D.; Vernardou, D.; Spanakis, E.; Papadimitropoulos, G.; Davazoglou, D.; Katsarakis, N.; Koudoumas, E. Effect of O2 flow rate on the electrochromic response of WO3 grown by LPCVD. Phys. Status Solidi C 2015, 212, 1011–1015. [Google Scholar] [CrossRef]
- Patil, P.R.; Patil, P.S. Preparation of mixed oxide MoO3-WO3 thin films by spray pyrolysis technique and their characterization. Thin Solid Film. 2001, 382, 13–22. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, J.; Huang, X.H.; Wang, X.L.; Zhang, W.K.; Huang, H. Multicolor and fast electrochromism of nanoporous NiO/poly (3,4-ethylenedioxythiophene) composite thin film. Electrochem. Commun. 2009, 11, 702–705. [Google Scholar] [CrossRef]
- Mouratis, K.; Tudose, V.; Romanitan, C.; Pachiu, C.; Tutunaru, O.; Suchea, M.; Couris, S.; Vernardou, D.; Emmanouel, K. Electrochromic Performance of V2O5 Thin Films Grown by Spray Pyrolysis. Materials 2020, 13, 3859. [Google Scholar] [CrossRef]
- Yonghong, Y.; Jiayu, Z.; Peifu, G.; Xu, L.; Jinfa, T. Electrochromism of titanium oxide thin films. Thin Solid Film. 1997, 298, 197–199. [Google Scholar] [CrossRef]
- Dinh, N.N.; Oanh, N.T.T.; Long, P.D.; Bernard, M.C.; Hugot-Le Goff, A. Electro-chromic properties of TiO2 anatase thin films prepared by a dipping sol-gel method. Thin Solid Film. 2003, 423, 70–76. [Google Scholar] [CrossRef]
- Deb, S.K. A novel electrophotographic system. Appl. Opt. 1969, 8, 192–195. [Google Scholar] [CrossRef]
- Avendano, E.; Berggren, L.; Niklasson, G.A.; Granqvist, C.G.; Azens, A. Electrochromic materials and devices: Brief survey and new data on optical absorption in tungsten oxide and nickel oxide films. Thin Solid Films 2006, 496, 30–36. [Google Scholar] [CrossRef]
- Meda, L.; Breitkopf, R.C.; Haas, T.E.; Kirss, R.U. Investigation of electrochromic properties of nanocrystalline tungsten oxide thin film. Thin Solid Film. 2002, 402, 126–130. [Google Scholar] [CrossRef]
- Hamelmann, F.; Gesheva, K.; Ivanov, T.; Szekeres, A.M.; Brashev, M.A.; Heinzmann, U. Optical and electrochromic characterization of multilayered mixed metal oxide thin films. J. Optoelectron. Adv. Mater. 2005, 7, 393–396. [Google Scholar]
- Lin, Y.S.; Tsai, C.W.; Chen, P.W. Electrochromic properties of V2O5−z thin films sputtered on to flexible PET/ITO substrates. Solid State Ion. 2008, 179, 290–297. [Google Scholar] [CrossRef]
- Ottaviano, L.; Pennisi, A.; Simone, F.; Salvi, A.M. RF sputtered electrochromic V2O5 films. Opt. Mater. 2004, 27, 307–313. [Google Scholar] [CrossRef]
- Loia, M.R.; Mourab, E.A.; Westphala, T.M.; Balbonia, R.D.C.; Gündelc, A.; Floresc, W.H.; Pereirad, M.B.; Santose, M.J.L.; Santose, J.F.L.; Pawlickaf, A.; et al. Impact of Zr precursor on the electrochemical properties of V2O5 sol-gel films. J. Electroanal. Chem. 2019, 839, 67–74. [Google Scholar] [CrossRef]
- Benmoussa, M.; Outzourhit, A.; Bennouna, A.; Ameziane, E.L. Electrochromism in sputtered V2O5 thin films: Structural and optical studies. Thin Solid Film. 2002, 405, 11–16. [Google Scholar] [CrossRef]
- Semenenko, D.A.; Kozmenkova, A.Y.; Itkis, D.M.; Goodilin, E.A.; Kulova, T.L.; Skundin, A.M.; Tretyakov, Y.D. Growth of thin vanadia nanobelts with improved lithium storage capacity in hydrothermally aged vanadia gels. Cryst Eng Comm 2012, 14, 1561–1567. [Google Scholar] [CrossRef]
- Cogan, S.F.; Nguyen, N.M.; Perrotti, S.J.; Rauh, R.D. Optical properties of electrochromic vanadium pentoxide. J. Appl. Phys. 1989, 66, 1333–1337. [Google Scholar] [CrossRef]
- Dickens, P.G.; Reynolds, G.J. Transport and equilibrium properties of some oxide insertion compound. Solid State Ion. 1981, 5, 331–334. [Google Scholar] [CrossRef]
- Pan, A.; Zhang, J.G.; Nie, Z.; Cao, G.; Arey, B.W.; Li, G.; Liang, S.Q.; Liu, J. Facile synthesized nanorod structured vanadium pentoxide for high-rate lithium batteries. J. Mater. Chem. 2010, 20, 9193–9199. [Google Scholar] [CrossRef]
- Rui, X.; Lu, Z.; Yin, Z.; Sim, D.H.; Xiao, N.; Lim, T.M.; Hng, H.H.; Zhang, H.; Yan, Q. Oriented molecular attachments through sol–gel chemistry for synthesis of ultrathin hydrated vanadium pentoxide nanosheets and their applications. Small 2013, 9, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Margoni, M.M.; Mathuri, S.; Ramamurthi, K.; Babu, R.R.; Ganesh, V.; Sethuraman, K. Hydrothermally grown nano and microstructured V2O5 thin films for electrochromic application. Appl. Surf. Sci. 2018, 449, 193–202. [Google Scholar] [CrossRef]
- Zhu, C.; Shu, J.; Wu, X.; Li, P.; Li, X. Electrospun V2O5 micro/nanorods as cathode materials for lithium ion battery. J. Electroanal. Chem. 2015, 759, 184–189. [Google Scholar] [CrossRef]
- Iida, Y.; Kaneko, Y.; Kanno, Y. Fabrication of pulsed-laser deposited V2O5 thin films for electrochromic devices. J. Mater. Process. Technol. 2008, 197, 261–267. [Google Scholar] [CrossRef]
- Kalu, E.E.; Nwoga, T.T.; Srinivasan, V.; Weidner, J.W. Cyclic voltammetric studies of the effects of time and temperature on the capacitance of electrochemically deposited nickel hydroxide. J. Power Sources 2001, 92, 163–167. [Google Scholar] [CrossRef]
- Abd-Alghafour, N.M.; Ahmed, N.M.; Hassan, Z.; Almessiere, M.A. Hydrothermal synthesis and structural properties of V2O5 nanoflowers at low temperature. J. Phys. Conf. Ser. 2018, 1083, 012036. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Liu, K.; Hu, M. Vanadium pentoxide hierarchical structure networks for high performance ethanol gas sensor with dual working temperature characteristics. Sens. Actuators B Chem. 2014, 190, 141–148. [Google Scholar] [CrossRef]
- Glynn, C.; Creedon, D.; Geaney, H.; O’Connell, J.; Holmes, J.D.; O’Dwyer, C. Optimizing vanadium pentoxide thin films and multilayers from dip-coated nanofluid precursors. ACS Appl. Mater. Inter. 2014, 6, 2031–2038. [Google Scholar] [CrossRef]
- Lin, T.C.; Jheng, B.J.; Huang, W.C. Electrochromic properties of the vanadium pentoxide doped with nickel as an ionic storage layer. Energies 2021, 14, 2065. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Vernardou, D.; Koudoumas, E.; Tsoukalas, D.; Raptis, Y.S. Tungsten doping effect on V2O5 thin film electrochromic performance. Electrochim. Acta 2019, 321, 134743–134750. [Google Scholar] [CrossRef]

| Annealing Temperature | Without | 200 °C | 300 °C | 400 °C |
|---|---|---|---|---|
| ∆T(%) at 650 nm | 15% | 13% | 37% | 31% |
| Charge capacity (mC/cm2) | 54.7 | 52.6 | 69.7 | 97.9 |
| Coloration efficiency (cm2/C) | 3.6 | 3.9 | 8.9 | 6.3 |
| Annealing Temperature | Without | 200 °C | 300 °C | 400 °C |
|---|---|---|---|---|
| Original |  |  |  |  |
| Bleached |  |  |  |  |
| Colored |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-C.; Jheng, B.-J.; Yen, H.-M.; Huang, W.-C. Thermal Annealing Effects of V2O5 Thin Film as an Ionic Storage Layer for Electrochromic Application. Materials 2022, 15, 4598. https://doi.org/10.3390/ma15134598
Lin T-C, Jheng B-J, Yen H-M, Huang W-C. Thermal Annealing Effects of V2O5 Thin Film as an Ionic Storage Layer for Electrochromic Application. Materials. 2022; 15(13):4598. https://doi.org/10.3390/ma15134598
Chicago/Turabian StyleLin, Tien-Chai, Bai-Jhong Jheng, Hui-Min Yen, and Wen-Chang Huang. 2022. "Thermal Annealing Effects of V2O5 Thin Film as an Ionic Storage Layer for Electrochromic Application" Materials 15, no. 13: 4598. https://doi.org/10.3390/ma15134598
APA StyleLin, T.-C., Jheng, B.-J., Yen, H.-M., & Huang, W.-C. (2022). Thermal Annealing Effects of V2O5 Thin Film as an Ionic Storage Layer for Electrochromic Application. Materials, 15(13), 4598. https://doi.org/10.3390/ma15134598






