The Influence of Carbon Sources on the Microstructures of In Situ-Synthesized TiC in Al Melts
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
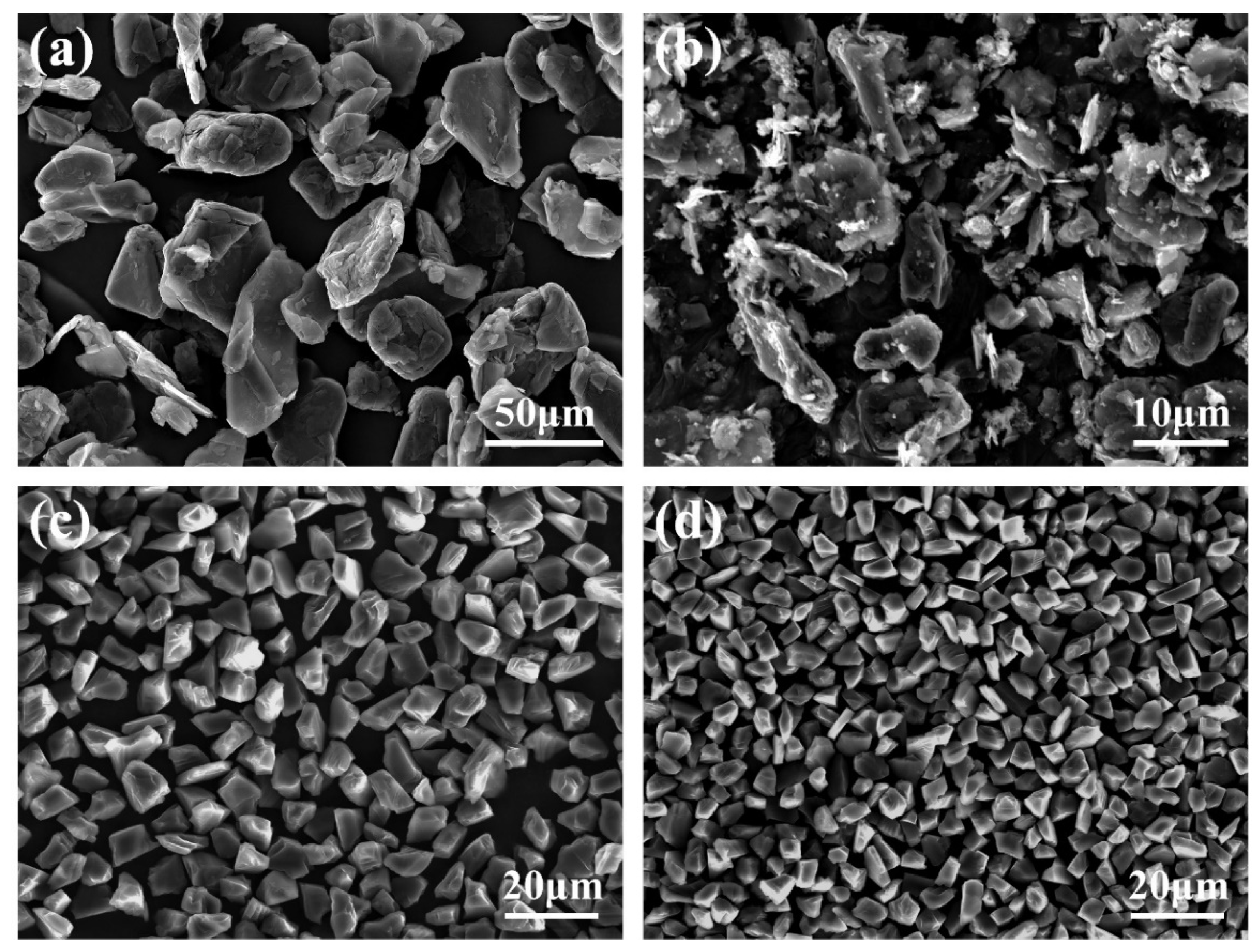
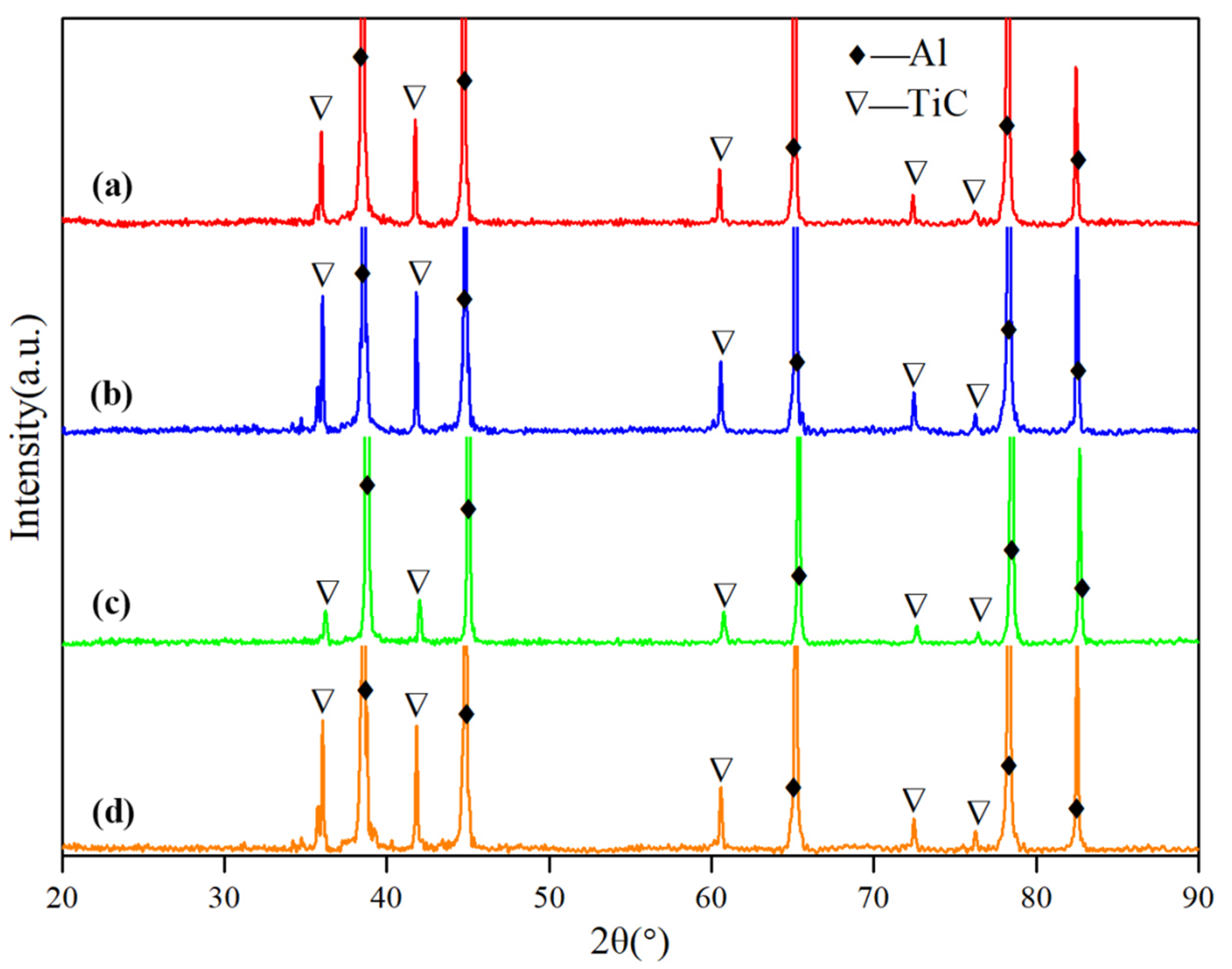
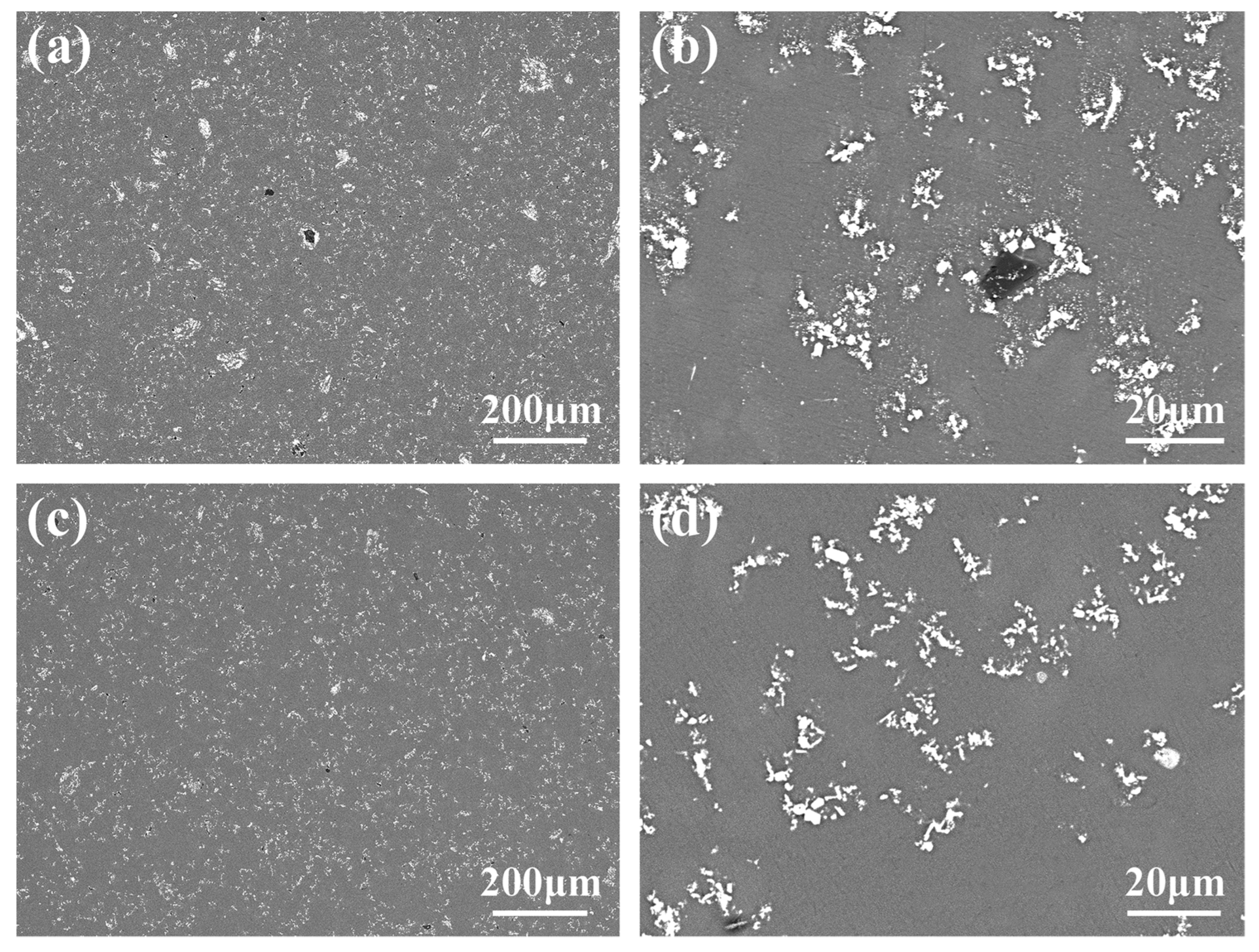
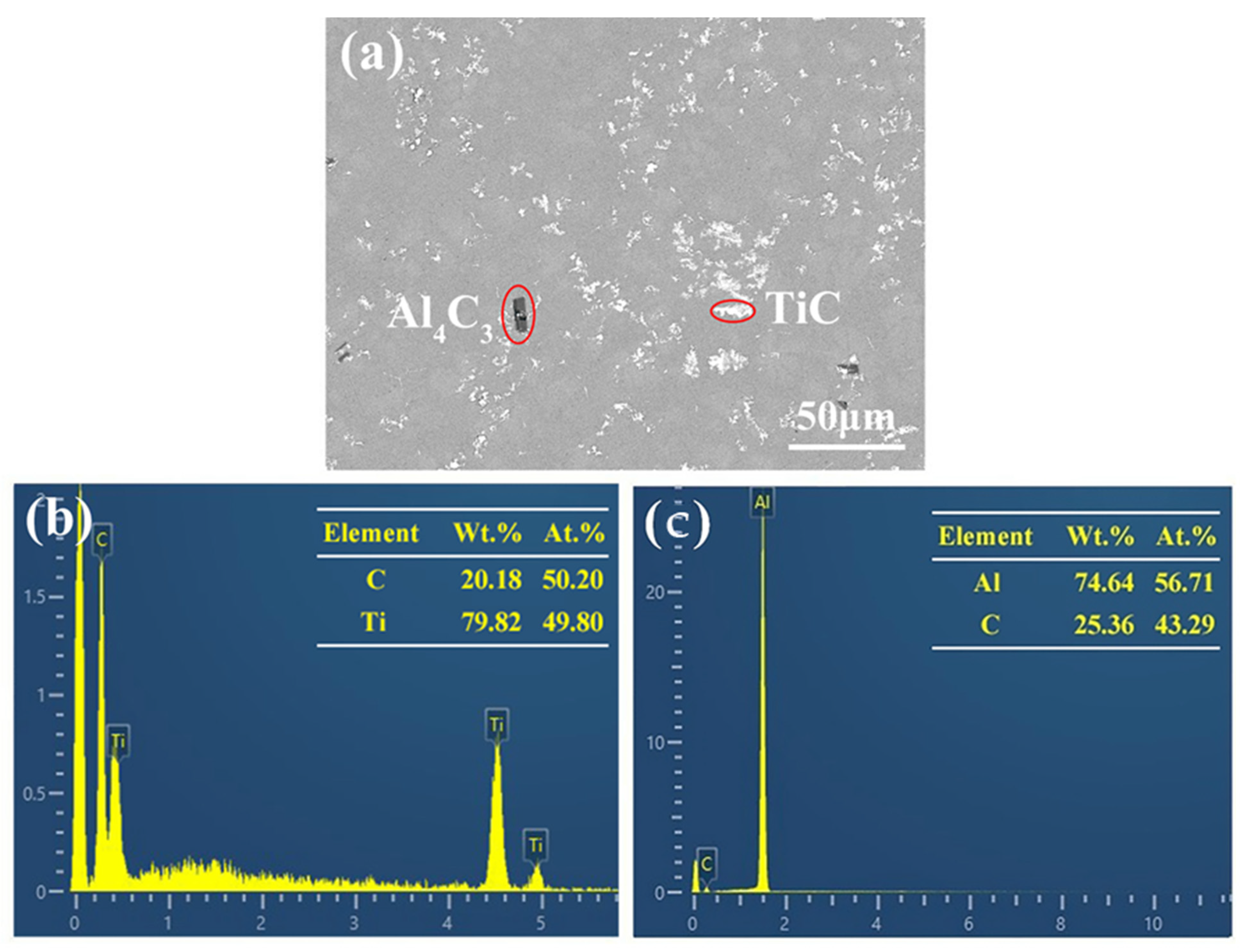
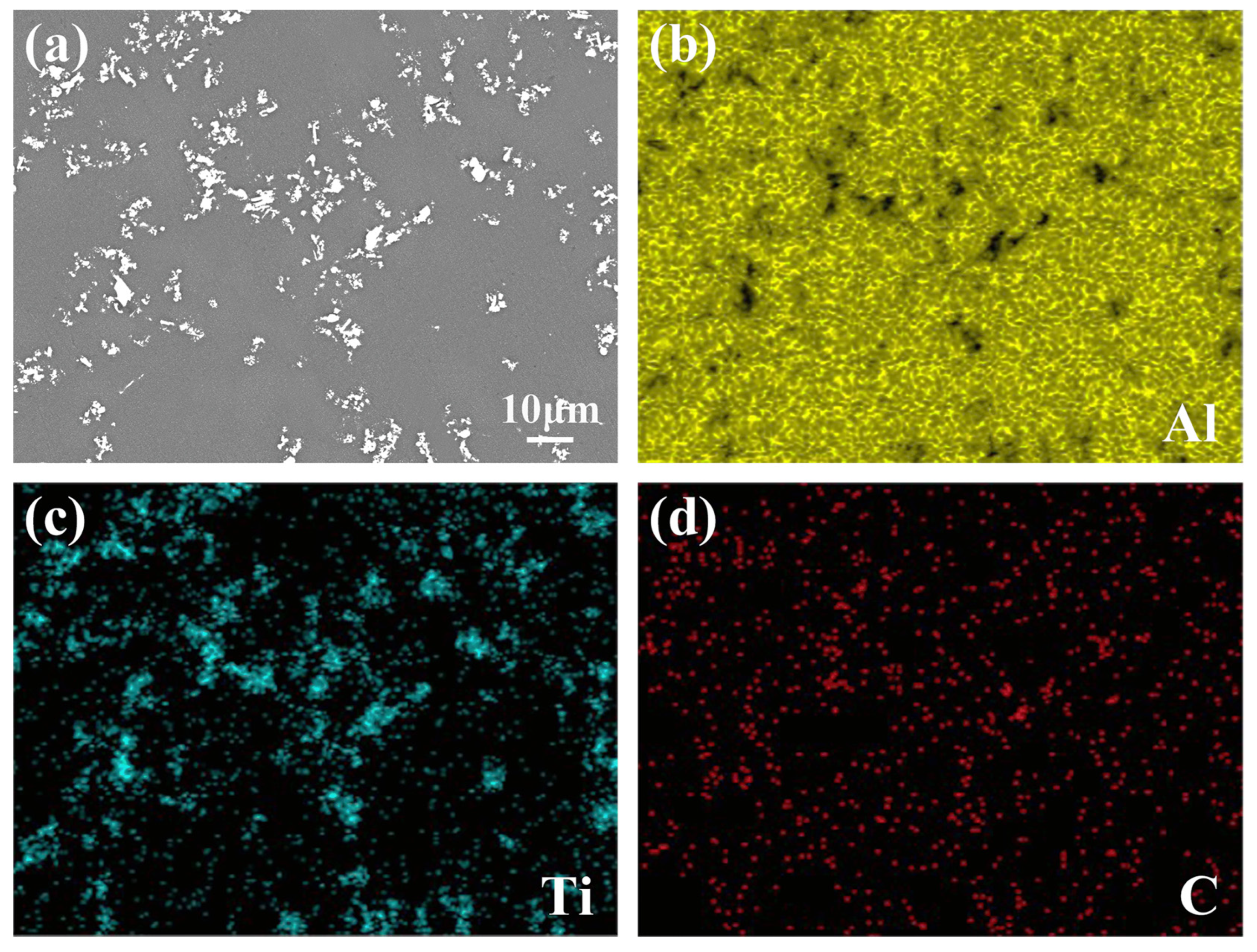
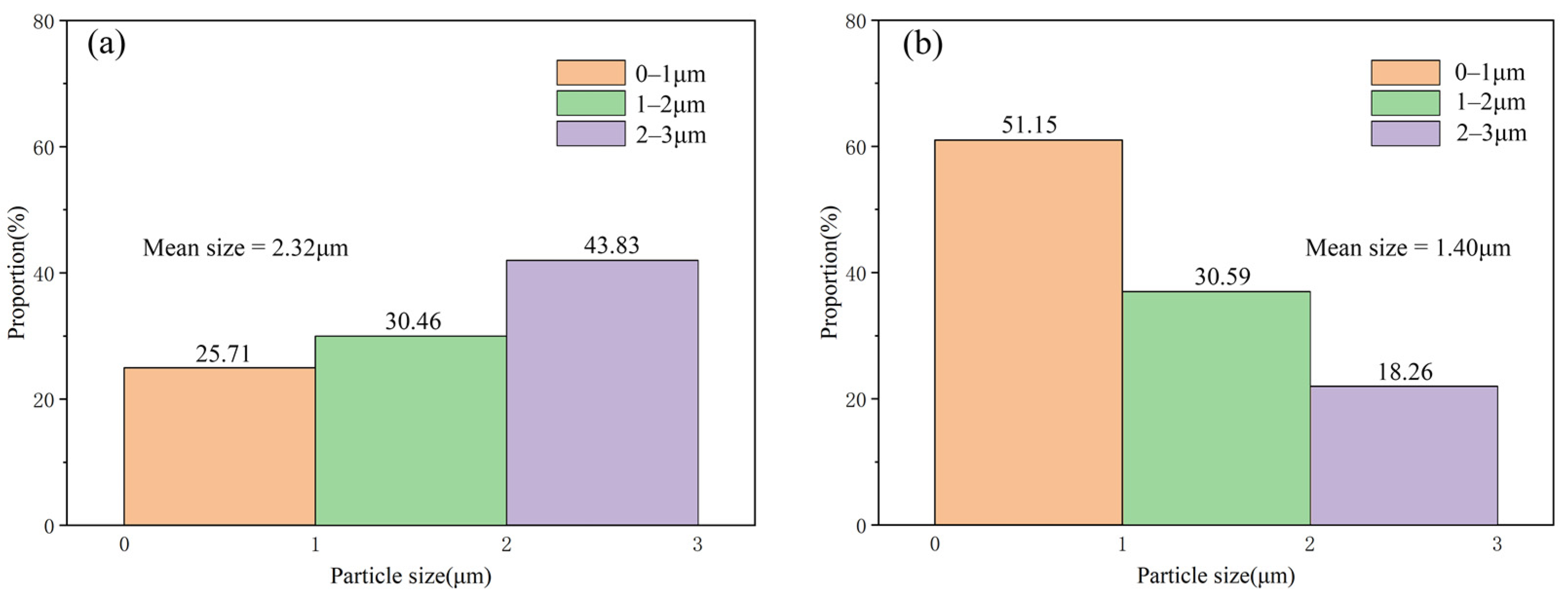
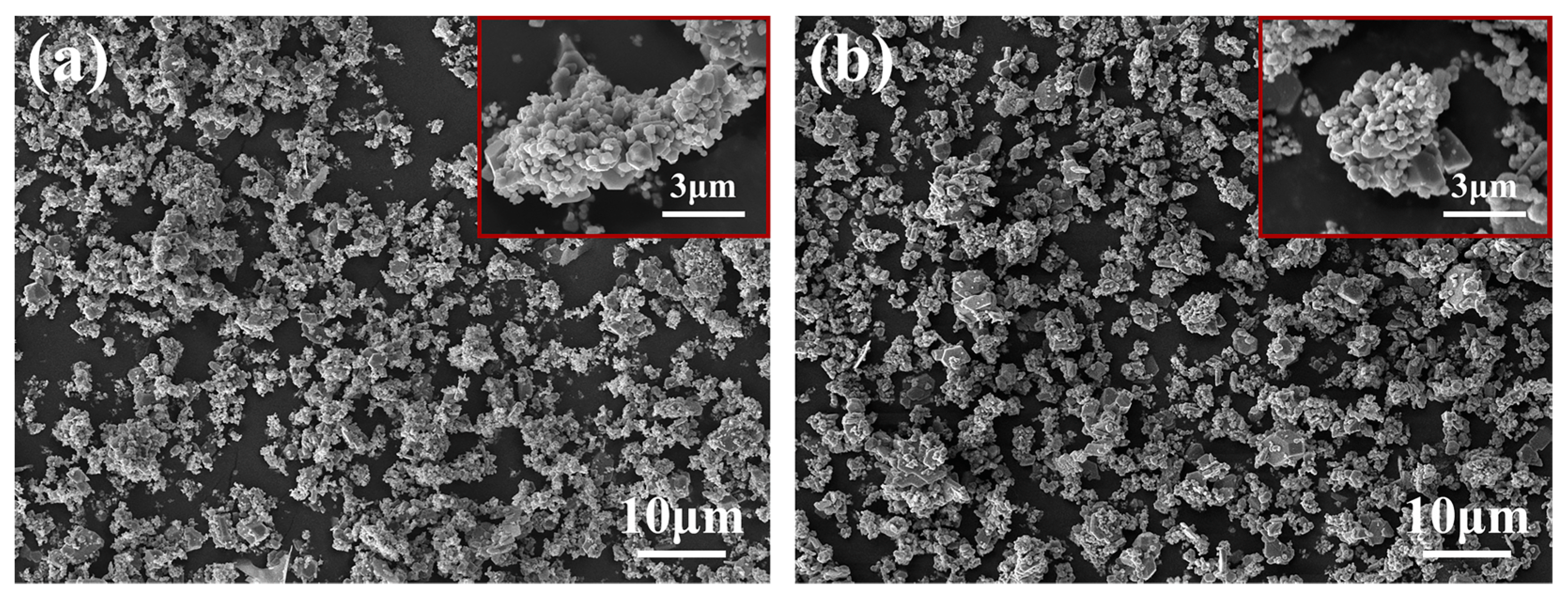
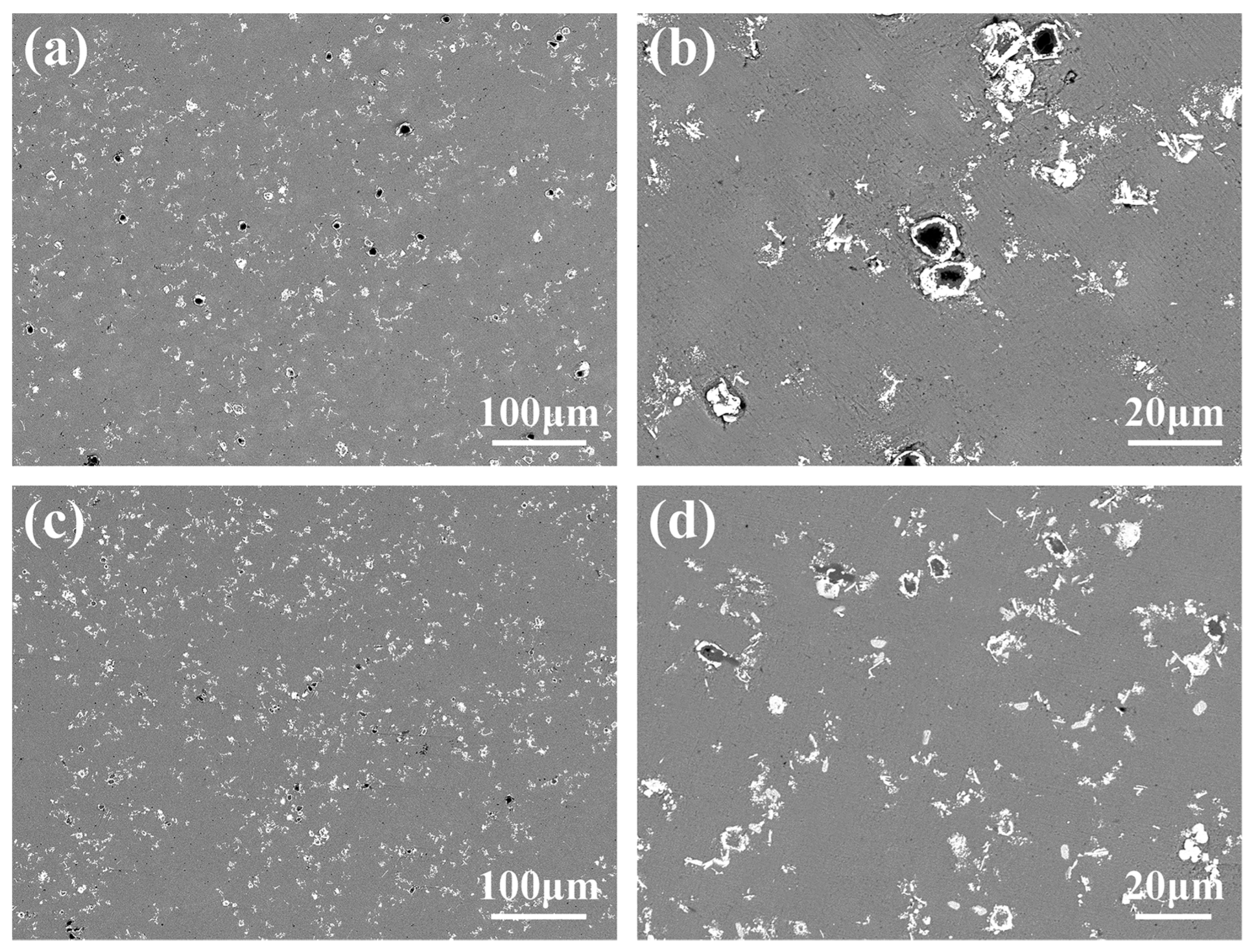
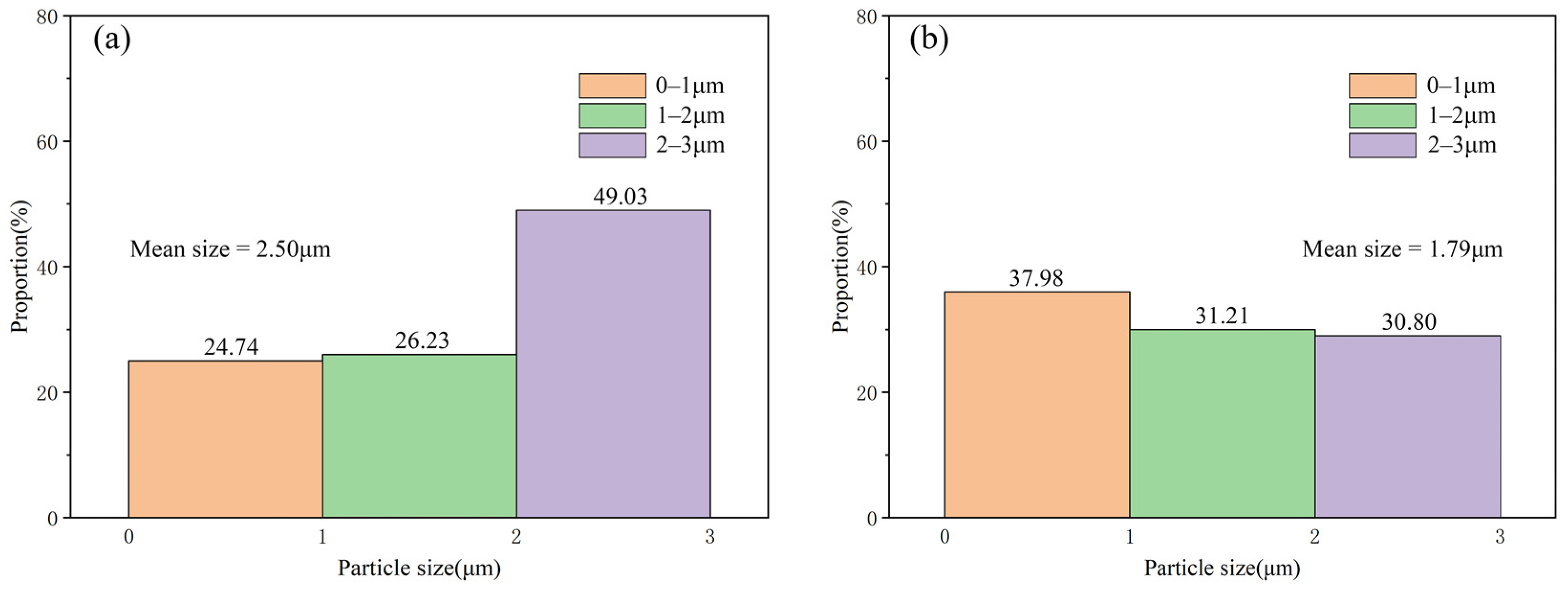
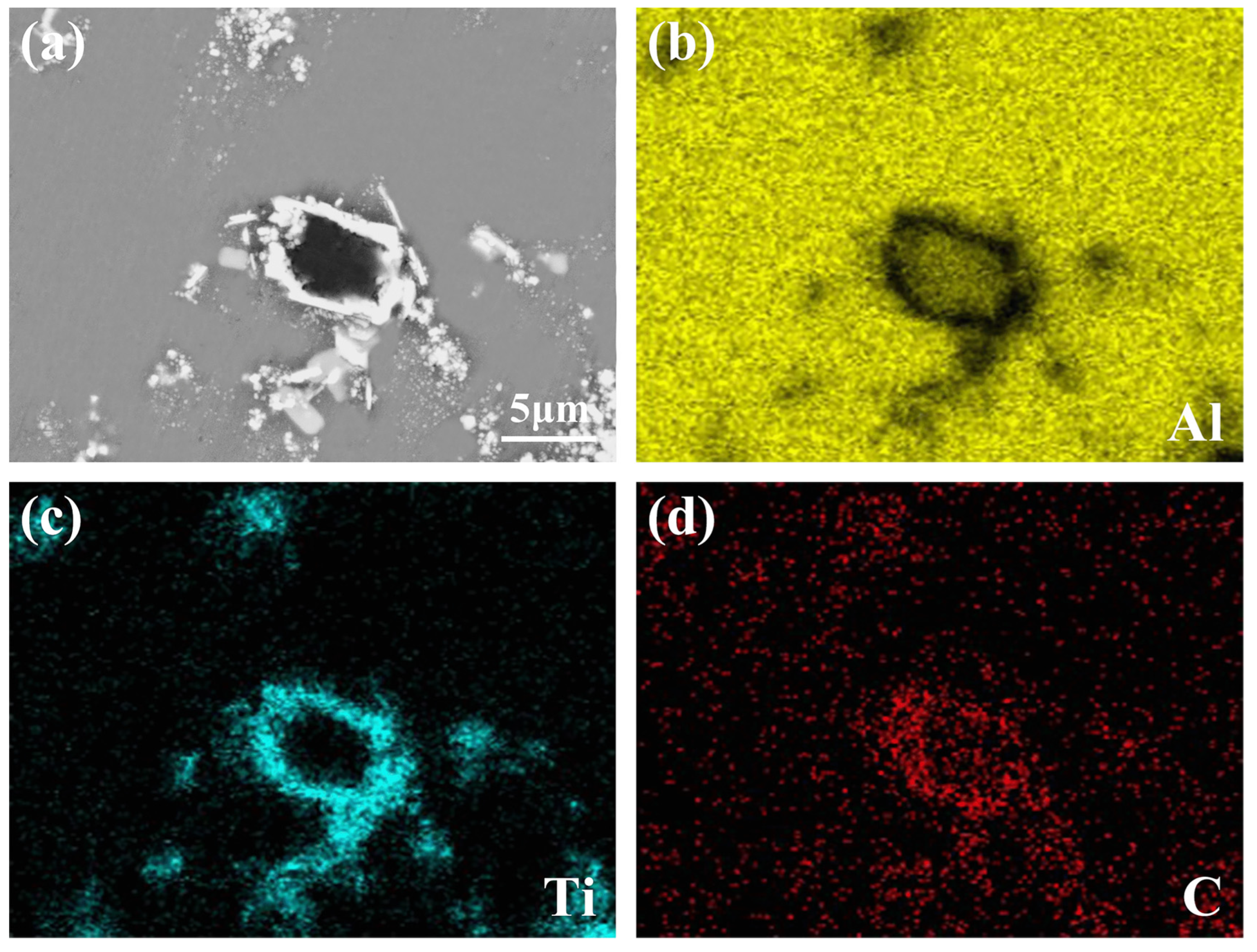
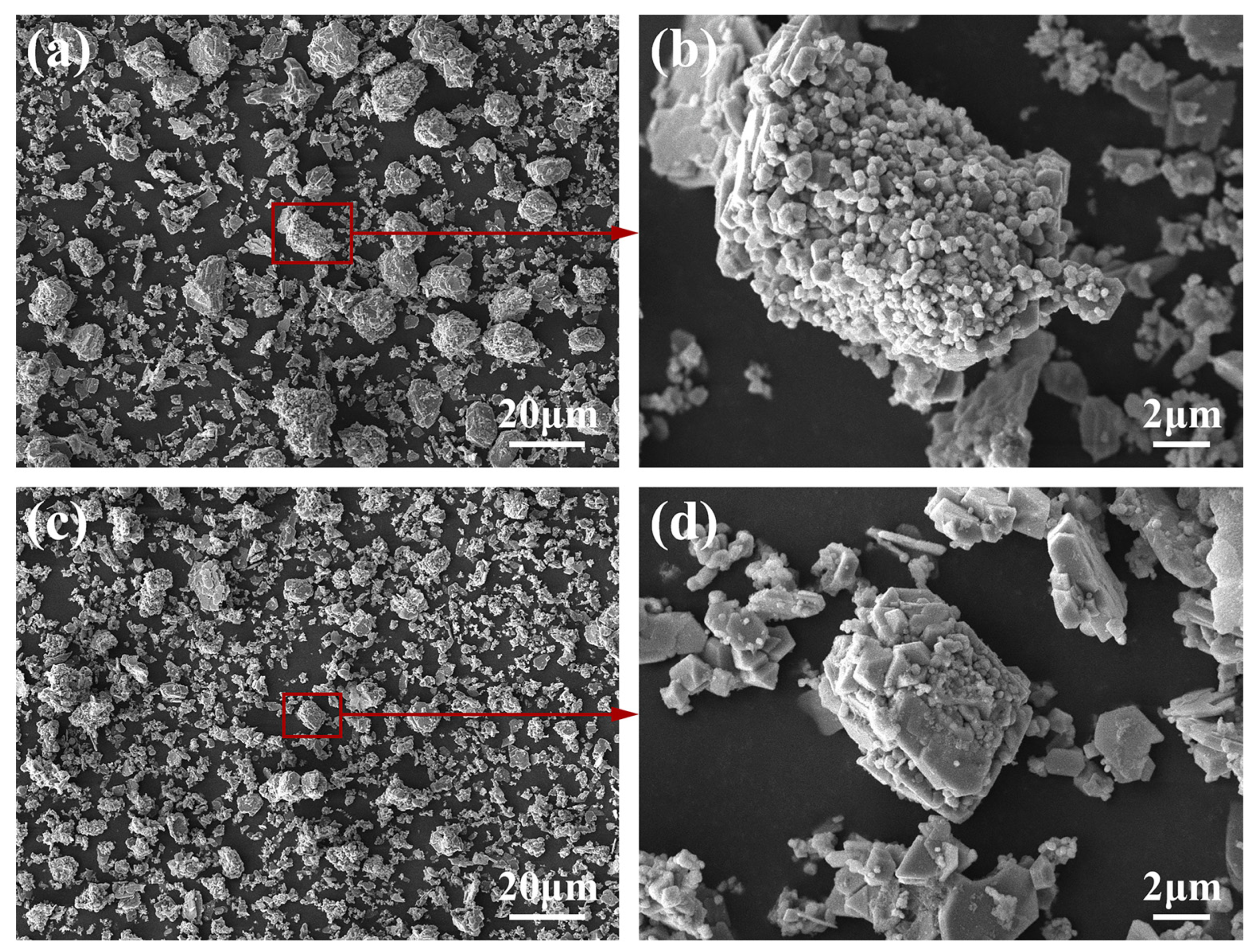
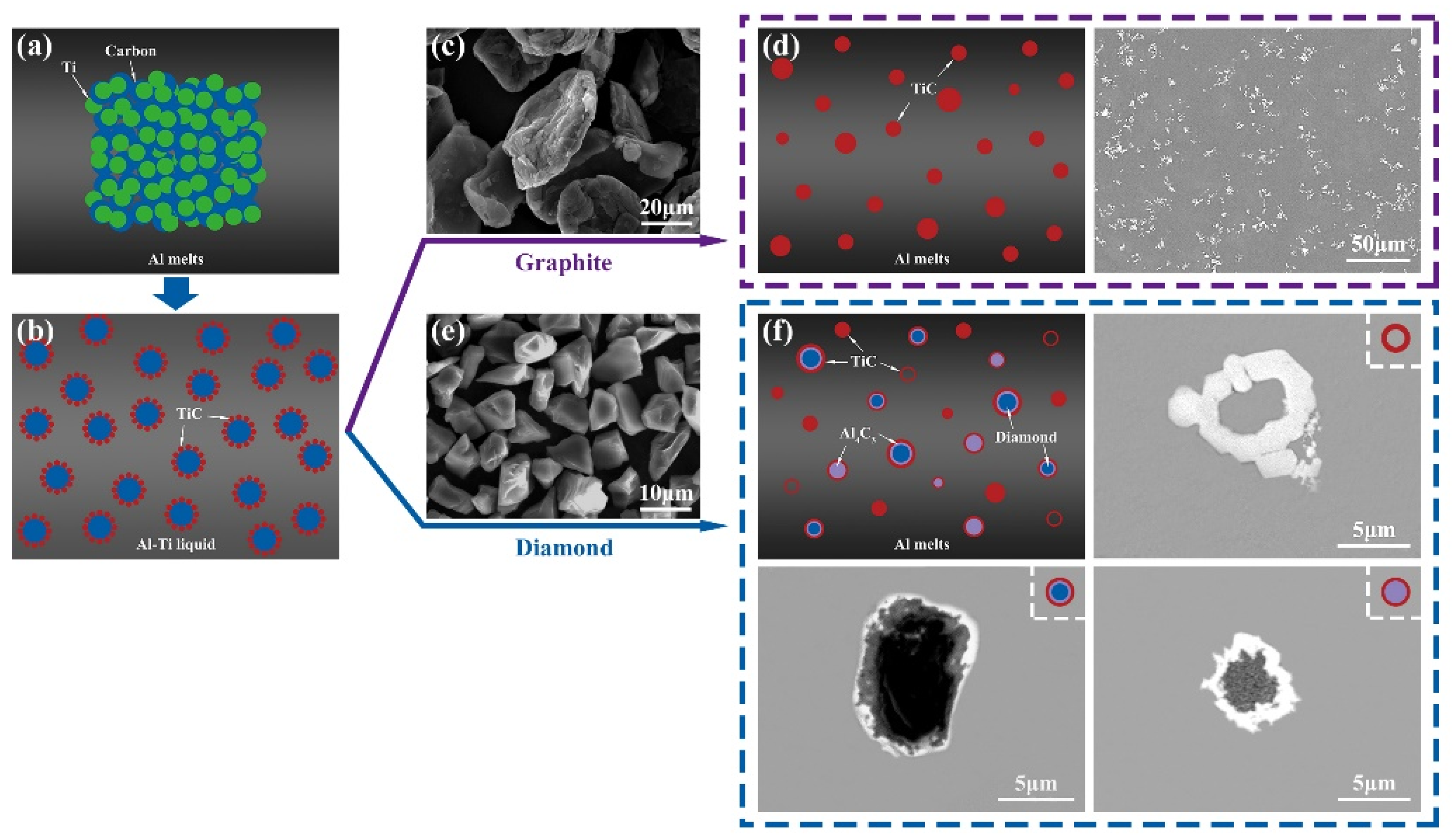
3.1. Microstructure and Distribution of TiC in Al Melt Synthesized by Graphite
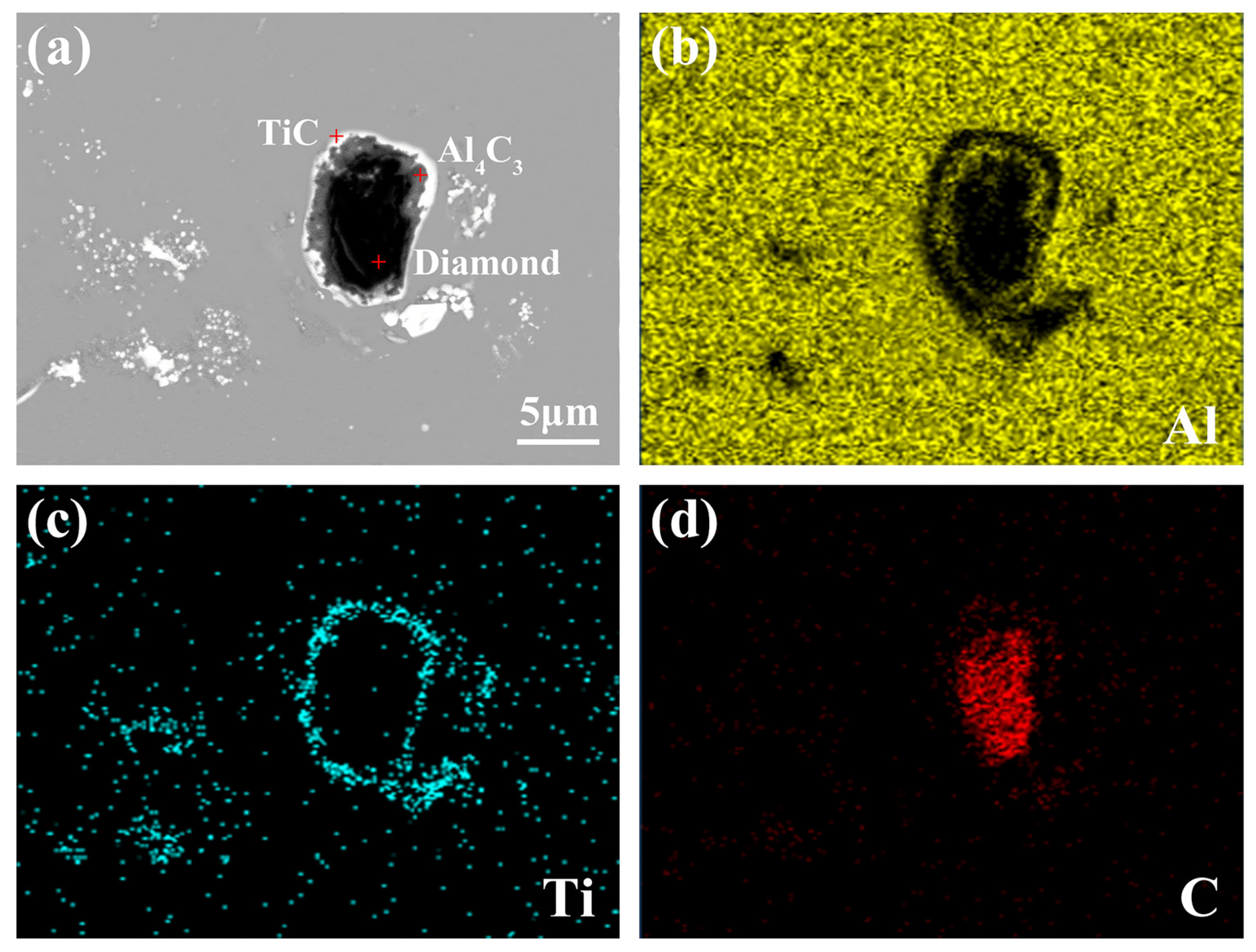
3.2. Microstructure and Distribution of TiC in Al Melt Prepared by Diamond
3.3. The Synthesis Processes of TiC in Al Melts Used Different Carbon Sources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; We, W.; He, X.; Lan, X.; Sha, A.; Hao, W. Effects of Strength and Distribution of SiC on the Mechanical Properties of SiCp/Al Composites. Materials 2022, 15, 1288. [Google Scholar] [CrossRef] [PubMed]
- Elshalakany, A.B.; Tirth, V.; El-Kashif, E.; Hussein, H.M.A.; Hoziefa, W. Characterization and mechanical properties of stir-rheo-squeeze cast AA5083/MWCNTs/GNs hybrid nanocomposites developed using a novel preform-billet method. J. Mater. Res. Technol. 2021, 10, 1195–1209. [Google Scholar] [CrossRef]
- Rojas, J.I.; Siva, B.V.; Sahoo, K.L.; Crespo, D. Viscoelastic behavior of a novel aluminum metal matrix composite and comparison with pure aluminum, aluminum alloys, and a composite made of Al-Mg-Si alloy reinforced with SiC particles. J. Alloys Compd. 2018, 744, 445–452. [Google Scholar] [CrossRef]
- Singh, J.; Chauhan, A. Overview of wear performance of aluminium matrix composites reinforced with ceramic materials under the influence of controllable variables. Ceram Int. 2016, 42, 56–81. [Google Scholar] [CrossRef]
- Poovazhagan, L.; Kalaichelvan, K.; Rajadurai, A.; Senthilvelan, V. Characterization of hybrid silicon carbide and boron carbide nanoparticles-reinforced aluminum alloy composites. Procedia Eng. 2013, 64, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Song, M.S.; Huang, B.; Zhang, M.X.; Li, J.G. Study of formation behavior of TiC ceramic obtained by self-propagating high-temperature synthesis from Al-Ti-C elemental powders. Int. J. Refract. Met. Hard Mater. 2008, 9, 584–589. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Pozuelo, M.; Sokoluk, M.; Mathaudhu, S.; Roach, C.; Li, X.C.; Yang, J.M. Micro-mechanical properties of homogeneous- and inhomogeneous-structured pillars in Al-TiC nanocomposite: An in-situ study. Mater. Sci. Eng. A 2019, 762, 138084. [Google Scholar] [CrossRef]
- Anandajothi, M.; Ramanathan, S.; Ananthi, V.; Narayanasamy, P. Fabrication and characterization of Ti6A14V/TiB2-TiC composites by powder metallurgy method. Rare Met. 2017, 36, 806–811. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Simulation and experimental studies on microstructure evolution of resolidified dendritic TiCx in laser direct deposited Ti-TiC composite. Mater. Des. 2018, 159, 212–223. [Google Scholar] [CrossRef]
- Kumar, G.B.V.; Rao, C.S.P.; Selvaraj, N. Mechanical and tribological behavior of particulate reinforced aluminum metal matrix composites-a review. J. Miner. Mater. Charact. Eng. 2011, 10, 59–91. [Google Scholar] [CrossRef]
- Liang, B.Y.; Han, X.; Zhou, Q.; Zhao, Y.; Wang, M. TiC/Ti3SiC2 composite prepared by mechanical alloying. Int. J. Refract. Met. Hard Mater. 2008, 10, 664–666. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, F.; Zhao, Q.; Wang, H.; Jiang, Q. Simultaneously increasing the elevated-temperature tensile strength and plasticity of in situ nano-sized TiCx/Al-Cu-Mg composites. Mater. Charact. 2017, 125, 7–12. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.P.; Wang, X.; Koizumi, Y.; Kenta, Y.; Chiba, A. In-situ fabrication and characterization of ultrafine structured Cu-TiC composites with high strength and high conductivity by mechanical milling. J. Alloys Compd. 2016, 657, 122–132. [Google Scholar] [CrossRef]
- Chak, V.; Chattopadhyay, H.; Dora, T.L. A review on fabrication methods, reinforcements and mechanical properties of aluminum matrix composites. J. Manuf. Processes 2020, 56, 1059–1074. [Google Scholar] [CrossRef]
- Hu, Z.-J.; Shen, P.; Jiang, Q.-C. Developing high-performance laminated Cu/TiC composites through melt infiltration of Ni-doped freeze-cast preforms. Ceram. Int. 2019, 45, 11686–11693. [Google Scholar] [CrossRef]
- Yang, H.; Gao, T.; Wu, Y.; Zhang, H.; Nie, J.; Liu, X. Microstructure and mechanical properties at both room and high temperature of in-situ TiC reinforced Al-4.5Cu matrix nanocomposite. J. Alloys Compd. 2018, 767, 606–616. [Google Scholar] [CrossRef]
- Gao, M.; Gao, P.; Wang, Y.; Lei, T.; Ouyang, C. Study on Metallurgically Prepared Copper-Coated Carbon Fibers Reinforced Aluminum Matrix Composites. Met. Mater. Int. 2021, 27, 5425–5435. [Google Scholar] [CrossRef]
- Taha, M.A. Practicalization of cast metal matrix composites (MMCCs). Mater. Des. 2000, 22, 431–441. [Google Scholar] [CrossRef]
- Lapin, J.; Štamborská, M.; Pelachová, T.; Bajana, O. Fracture behaviour of cast in-situ TiAl matrix composite reinforced with carbide particles. Mater. Sci. Eng. A 2018, 721, 1–7. [Google Scholar] [CrossRef]
- Mortimer, D.A.; Nicholas, M. The wetting of carbon by copper and copper alloys. J. Mater. Sci. 1970, 5, 149–155. [Google Scholar] [CrossRef]
- So, K.P.; Lee, I.H.; Duong, D.L.; Kim, T.H.; Lim, S.C.; An, K.H.; Lee, Y.H. Improving the wettability of aluminum on carbon nanotubes. Acta Mater. 2011, 59, 3313–3320. [Google Scholar] [CrossRef]
- Bao, S.; Tang, K.; Kvithyld, A.; Engh, T.; Tangstad, M. Wetting of pure aluminium on graphite, SiC and Al2O3 in aluminium filtration. Trans. Nonferrous Met. Soc. China 2012, 22, 1930–1938. [Google Scholar] [CrossRef]
- Yang, L.; Shen, P.; Lin, Q.; Qiu, F.; Jiang, Q. Effect of Cr on the wetting in Cu/graphite system. Appl. Surf. Sci. 2011, 257, 6276–6281. [Google Scholar] [CrossRef]
- Landry, K.; Kalogeropoulou, S.; Eustathopoulos, N. Wettability of carbon by aluminum and aluminum alloys. Mater. Sci. Eng. A 1998, 254, 99–111. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Brampton, B. The reactive wetting and incorporation of B4C particles into molten aluminium. Scr. Mater. 2001, 44, 1077–1082. [Google Scholar] [CrossRef]
- Liu, Q.; Miao, W.; Ding, H.; Glandut, N.; Jia, H.; Li, C. The introduction of SiC into Cu melts based on Ti–SiC system and its transformation. J. Mater. Res. Technol. 2020, 9, 2881–2891. [Google Scholar] [CrossRef]
- Ding, H.; Jia, H.; Chu, W.; Liu, Q.; Chu, K.; Wang, J. The in-situ synthesized of TiC in Al melts based on Ti–Si-diamond system and its morphologies. J. Mater. Res. Technol. 2020, 9, 3865–3874. [Google Scholar] [CrossRef]
- Liang, X.; Jia, C.; Chu, K.; Chen, H. Predicted interfacial thermal conductance and thermal conductivity of diamond/Al composites with various interfacial coatings. Rare Met. 2011, 30, 544–549. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Qi, F.; Liu, Q.; Fan, X.; Shi, Y. Mechanism of in situ synthesis of TiC in Cu melts and its microstructures. J. Alloys Compd. 2017, 695, 3410–3418. [Google Scholar] [CrossRef]
- Ding, H.; Chu, W.; Liu, Q.; Wang, H.; Hao, C.; Jia, H.; Wang, J.; Ci, T. Microstructure evolution of Cu-TiC composites with the change of Ti/C ratio. Results Phys. 2019, 14, 102369. [Google Scholar] [CrossRef]
- Dong, C.-G.; Cui, R.; Wang, R.-C.; Peng, C.-Q.; Cai, Z.-Y. Microstructures and mechanical properties of Al 2519 matrix composites reinforced with Ti-coated SiC particles. Trans. Nonferrous Met. Soc. China 2020, 30, 863–871. [Google Scholar] [CrossRef]
- Li, S.-B.; Xiang, W.-H.; Zhai, H.-X.; Zhou, Y. Formation of TiC hexagonal platelets and their growth mechanism. Powder Technol. 2007, 185, 49–53. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Jiang, L.; Yang, W. Reaction procedure of a graphite fiber reinforced Ti-Al composite produced by squeeze casting-in situ reaction. Rare Met. 2010, 29, 98–101. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Wu, J.; Jia, H.; Liu, F.; Wang, J. The Influence of Carbon Sources on the Microstructures of In Situ-Synthesized TiC in Al Melts. Materials 2022, 15, 4610. https://doi.org/10.3390/ma15134610
Ding H, Wu J, Jia H, Liu F, Wang J. The Influence of Carbon Sources on the Microstructures of In Situ-Synthesized TiC in Al Melts. Materials. 2022; 15(13):4610. https://doi.org/10.3390/ma15134610
Chicago/Turabian StyleDing, Haimin, Jiangmin Wu, Haoran Jia, Fang Liu, and Jinfeng Wang. 2022. "The Influence of Carbon Sources on the Microstructures of In Situ-Synthesized TiC in Al Melts" Materials 15, no. 13: 4610. https://doi.org/10.3390/ma15134610
APA StyleDing, H., Wu, J., Jia, H., Liu, F., & Wang, J. (2022). The Influence of Carbon Sources on the Microstructures of In Situ-Synthesized TiC in Al Melts. Materials, 15(13), 4610. https://doi.org/10.3390/ma15134610






