Effects of Fly Ash Inclusion and Alkali Activation on Physical, Mechanical, and Chemical Properties of Clay
Abstract
:1. Introduction
- (1)
- Dissolution of amorphous aluminosilicate materials from the source material through the action of alkali hydroxide;
- (2)
- Transportation or condensation of precursor ions into monomers;
- (3)
- Poly(sialate) Si:Al = 1(-Si-O-Al-O-);
- Poly(sialate-siloxo) Si:Al = 2(-Si-O-Al-O-Si-O-);
- Poly(sialate-disiloxo) Si:Al = 3(-Si-O-Al-O-Si-O-Si-O-) [15].
- Mn [−(SiO2)z − AlO2]n.wH2O
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
- The required amount of Na2O was determined based on 8%, 12%, 16%, or 20% of M+ (M+ = Na2O/fly ash);
- The required amount of SiO2 was evaluated for SM values of 1, 1.25, 1.5, or 1.75 (SM = SiO2/Na2O);
- The required amount of SS solution was determined based on the required SiO2 (SiO2 required/SiO2 % in solution);
- The chemical composition of SS includes 14% of Na2O. Based on this, the required amount of Na2O was determined according to the ‘required SS solution’;
- The shortfall of Na2O amount in SS solution was completed using SH pellets. Therefore, SH pellets were used to achieve 8%, 12%, 16%, or 20% of M+, considering that the Na2O content in SH was 77.5%;
- The required amount of SH pellets was dissolved in distilled water to obtain a solution based on the additional water. The additional water content was determined as:additional water = water required (OMC)–water in SS solution;
- Finally, the quantities of SH, SS, and water determined in the previous steps were mixed, and the resulting activating solution was added to the dry soil–fly ash mixture.
2.3. Testing Methods
3. Results and Discussion
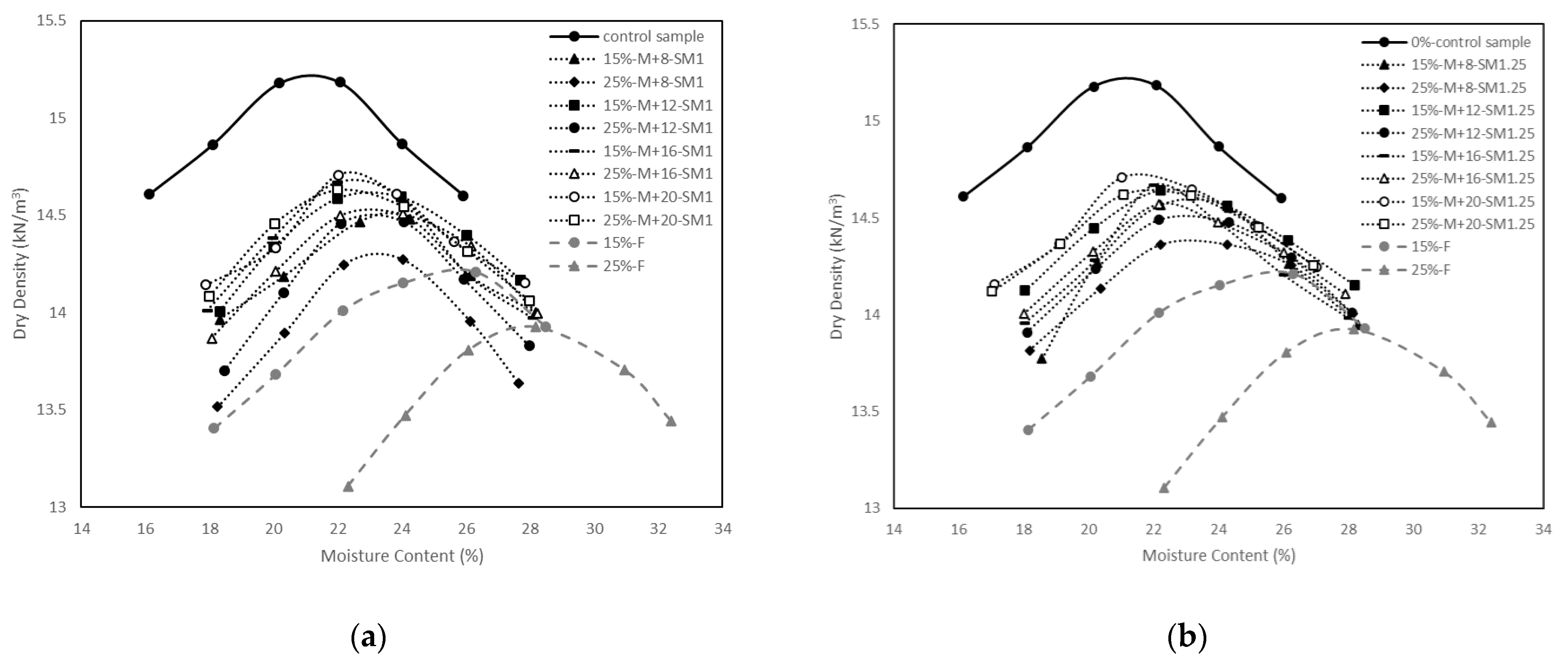
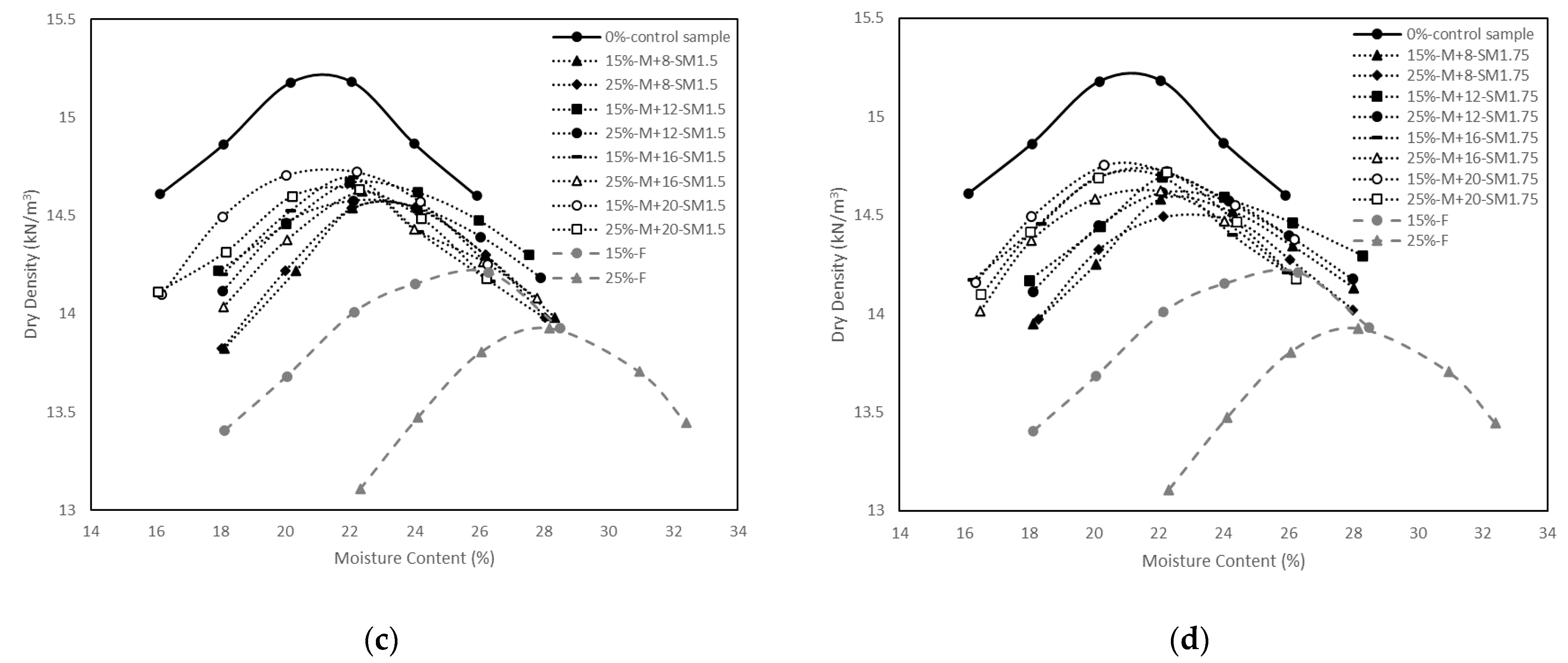
3.1. Compaction Tests
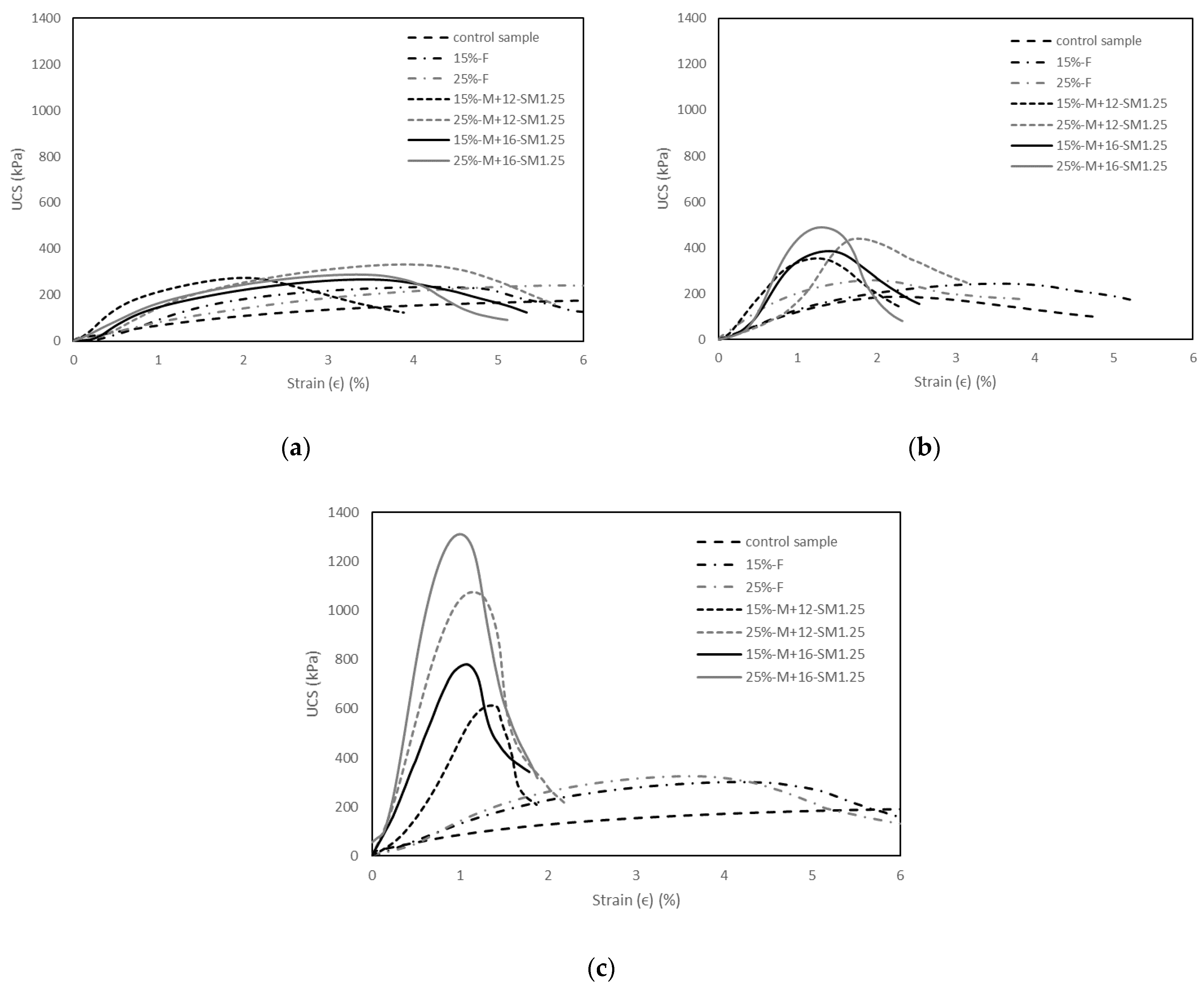
3.2. Unconfined Compressive Strength (UCS) Tests
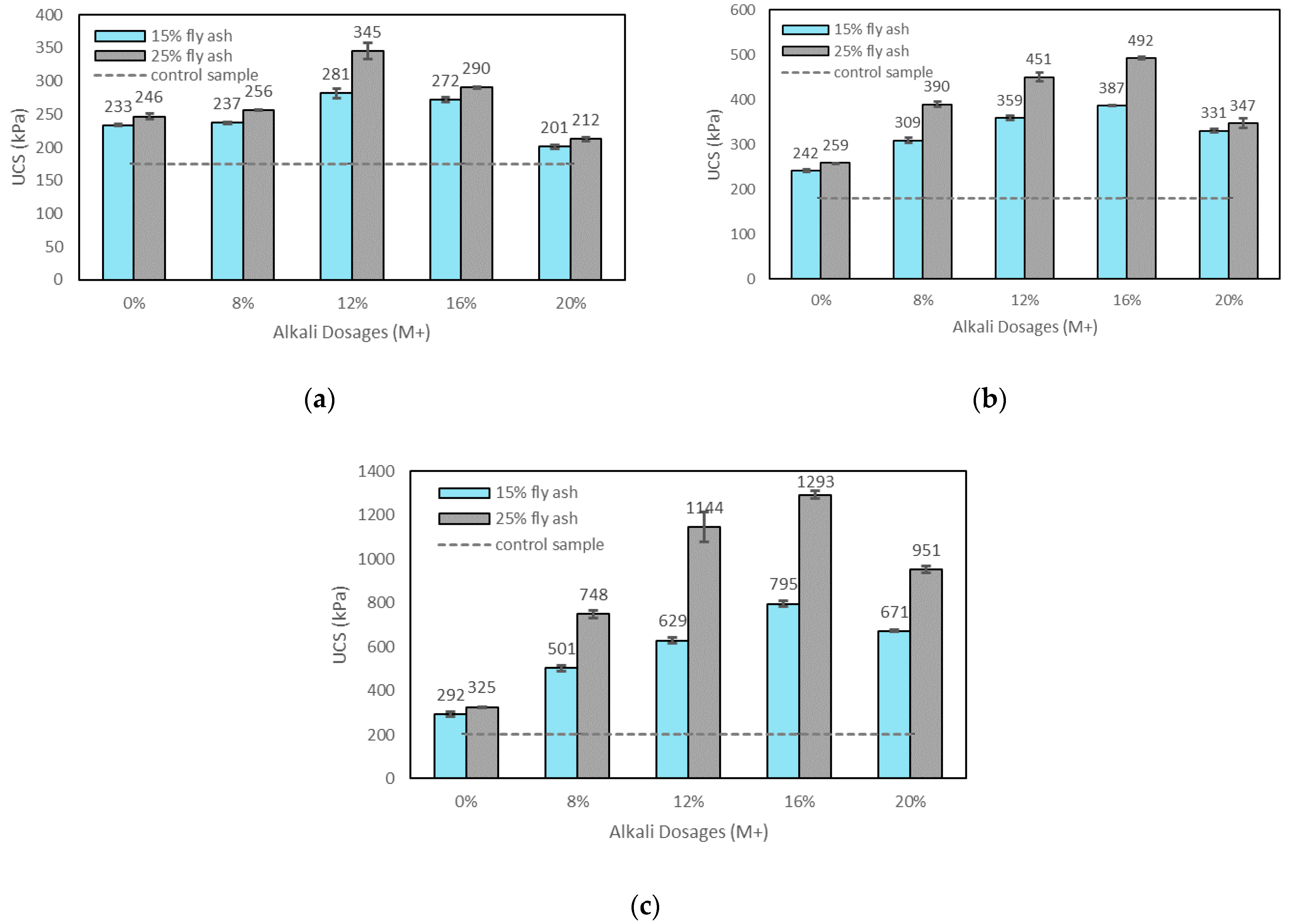
3.2.1. Effects of M+ on UCS
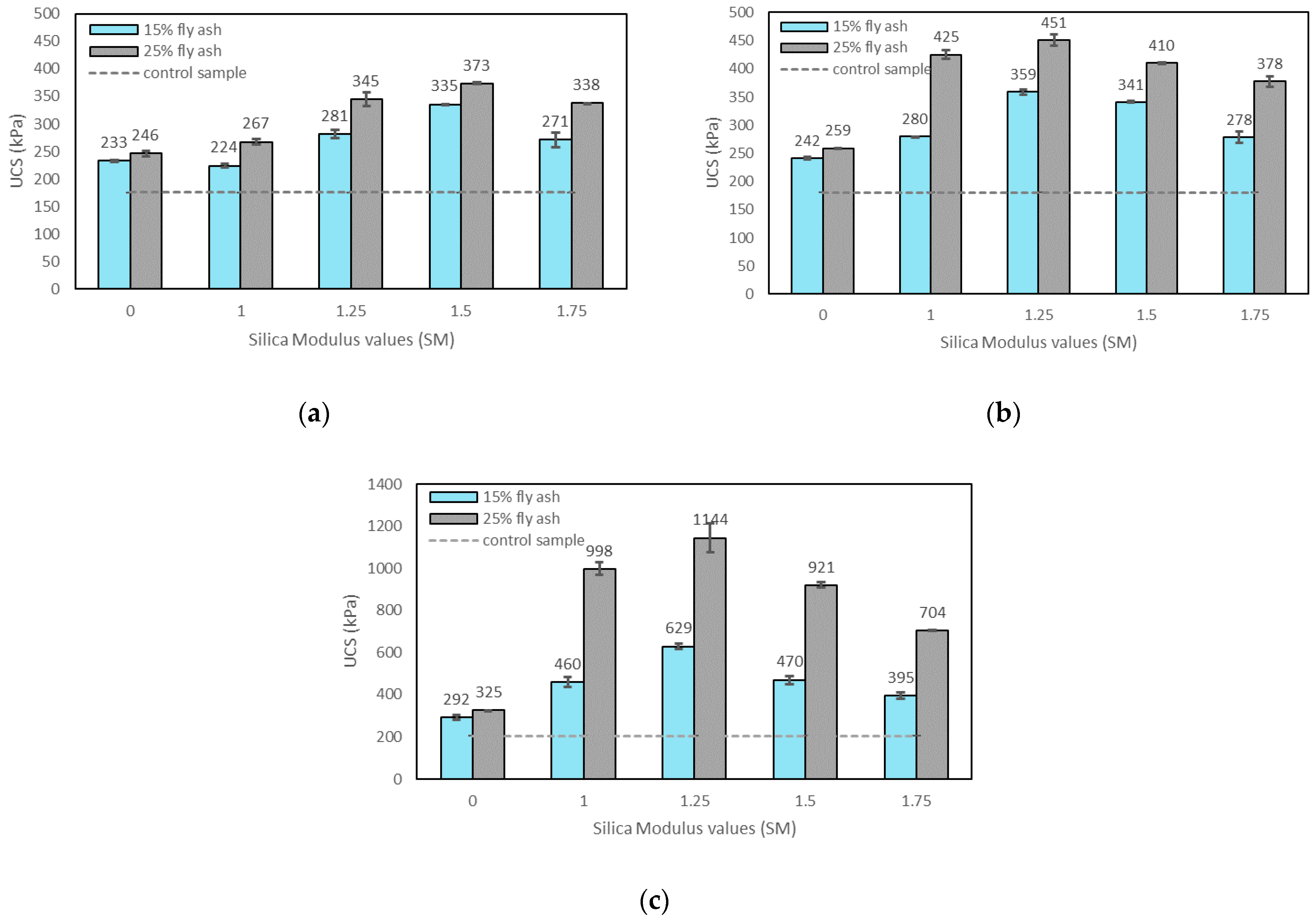
3.2.2. Effects of SM on UCS
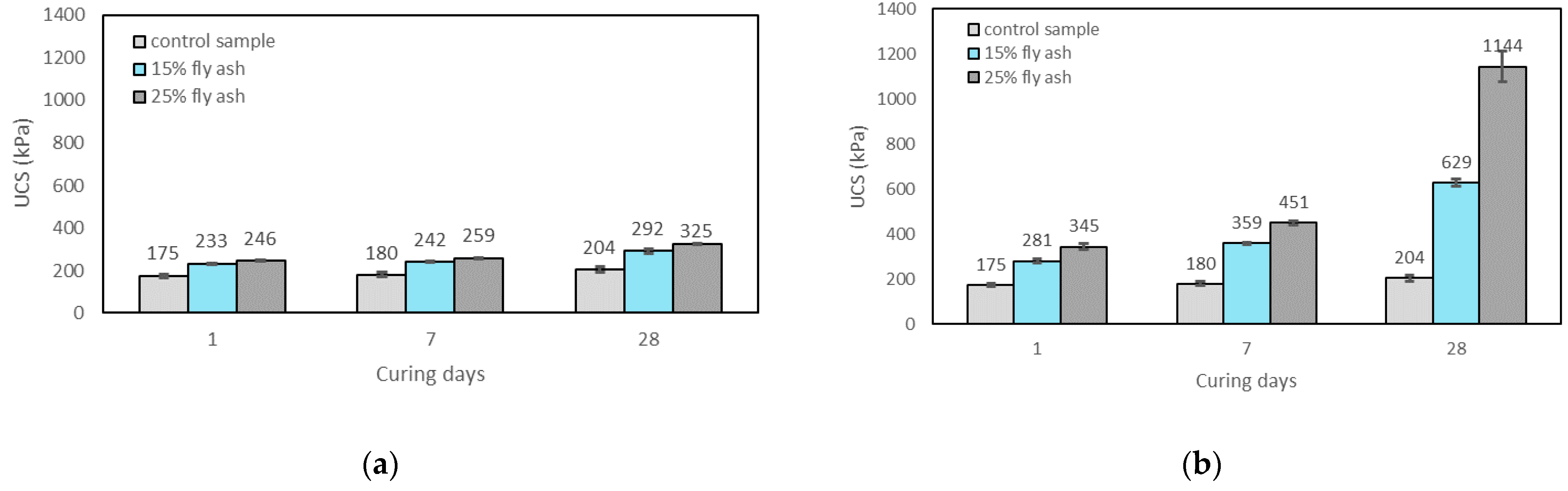
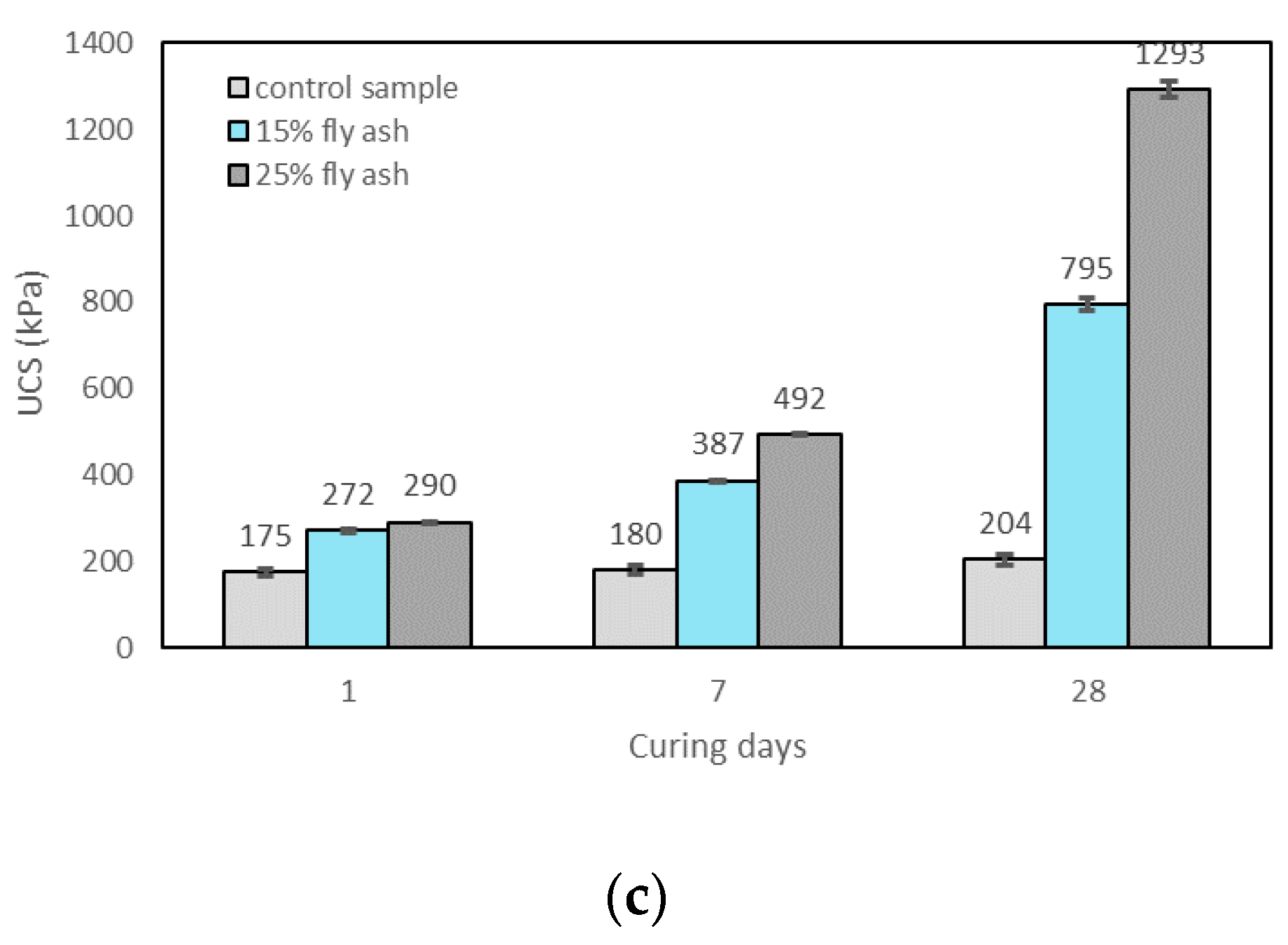
3.2.3. Effects of Curing Time on UCS
3.2.4. Effects of Fly Ash on UCS
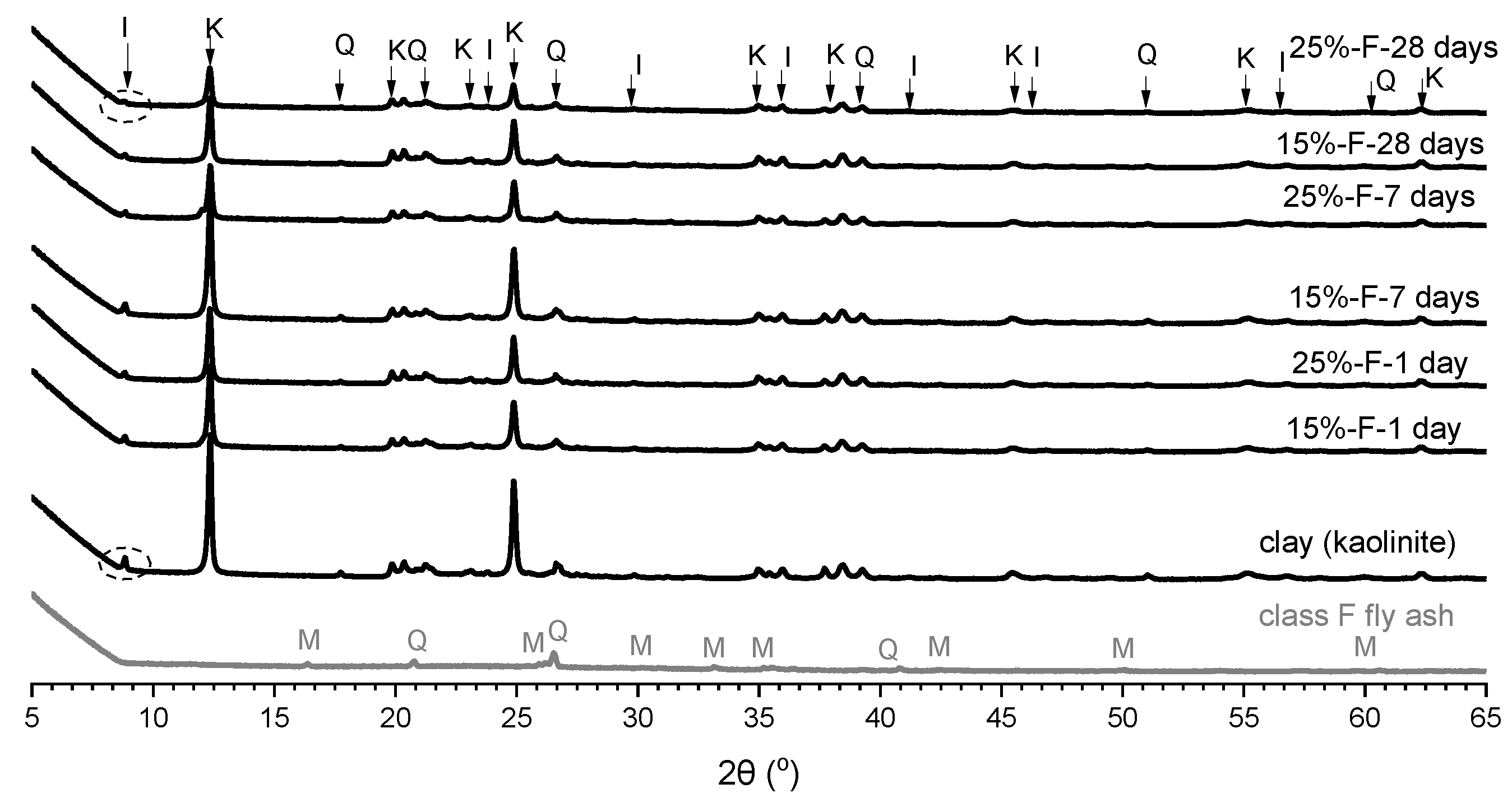
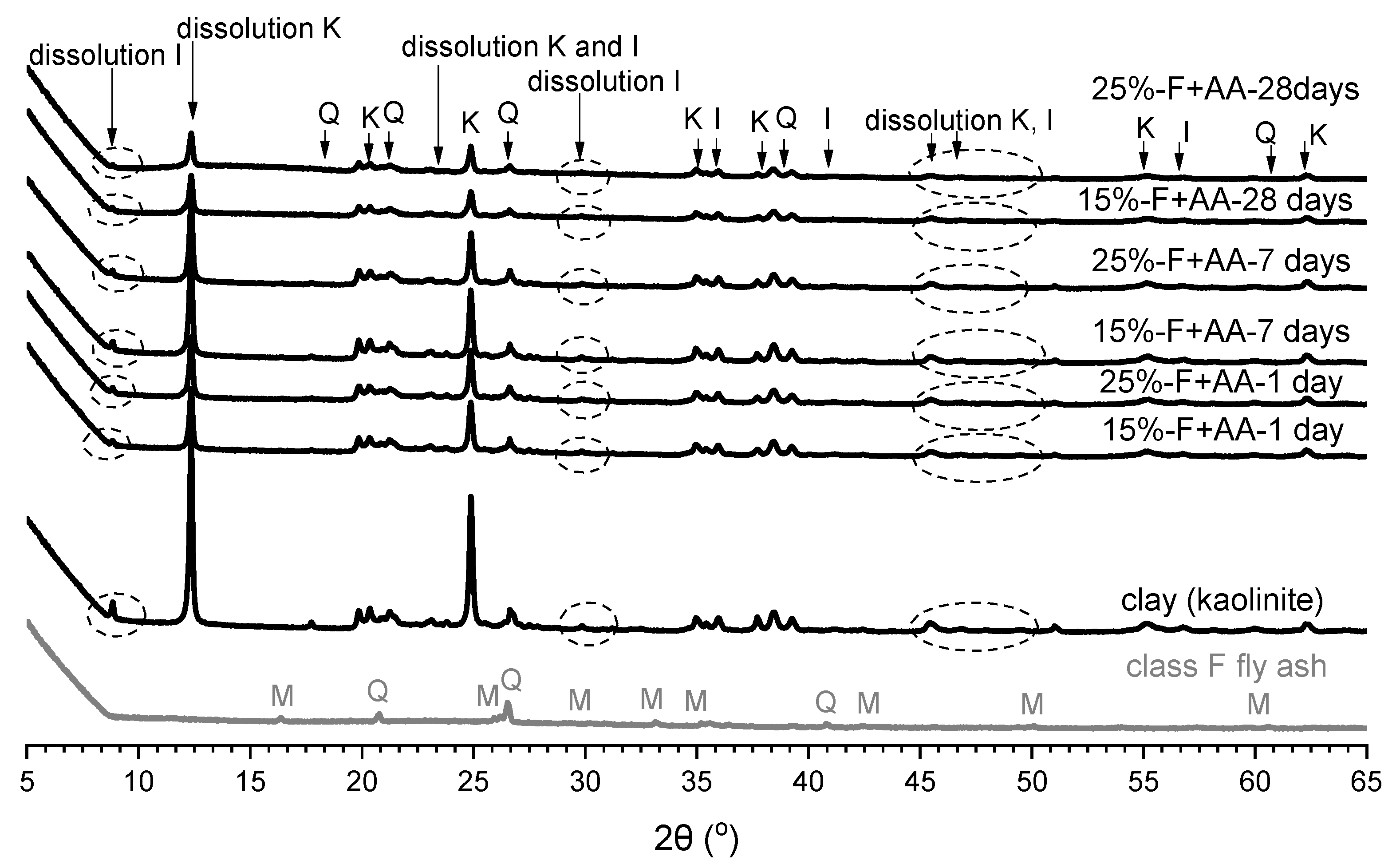
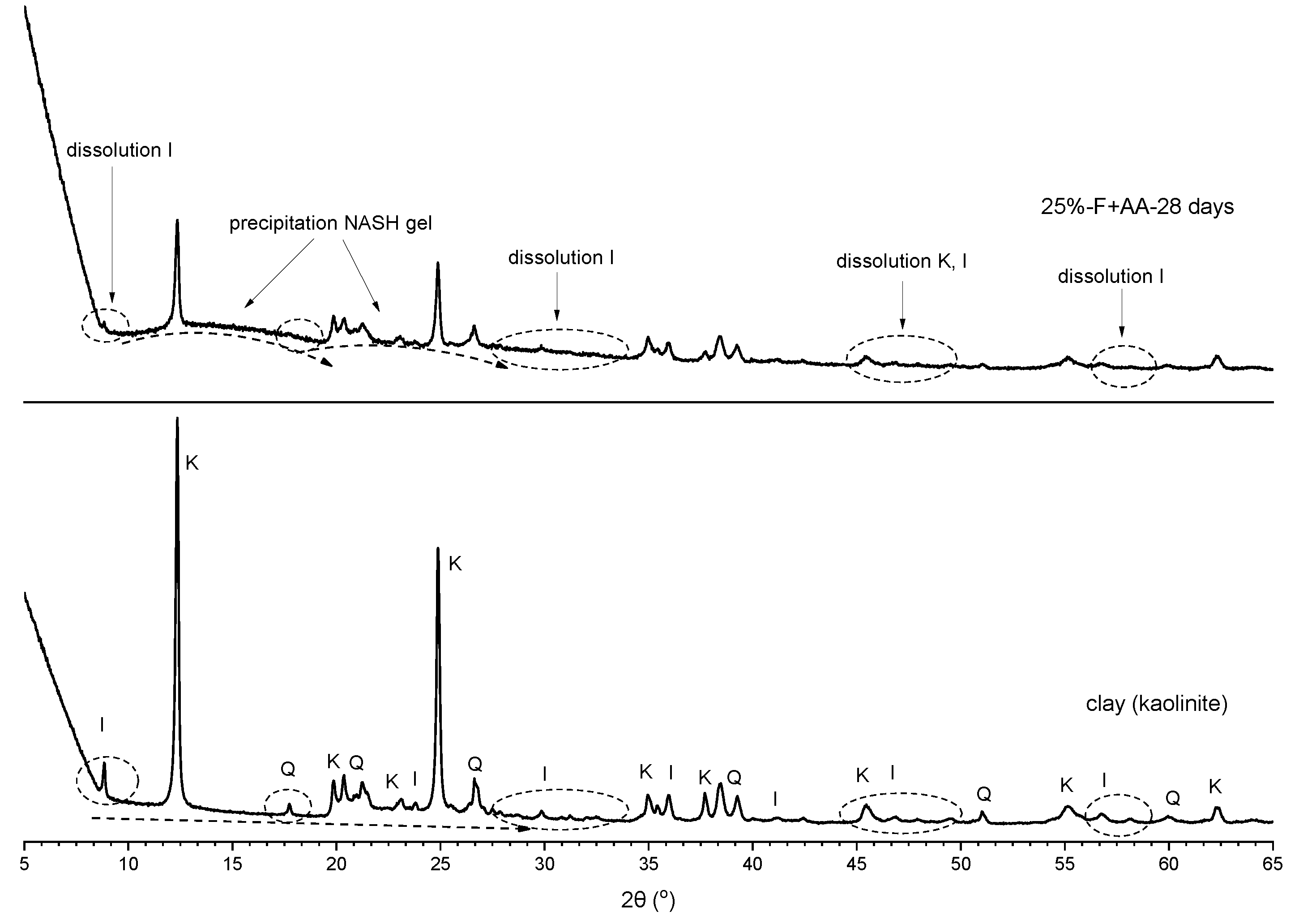
3.3. X-ray Diffraction Analysis
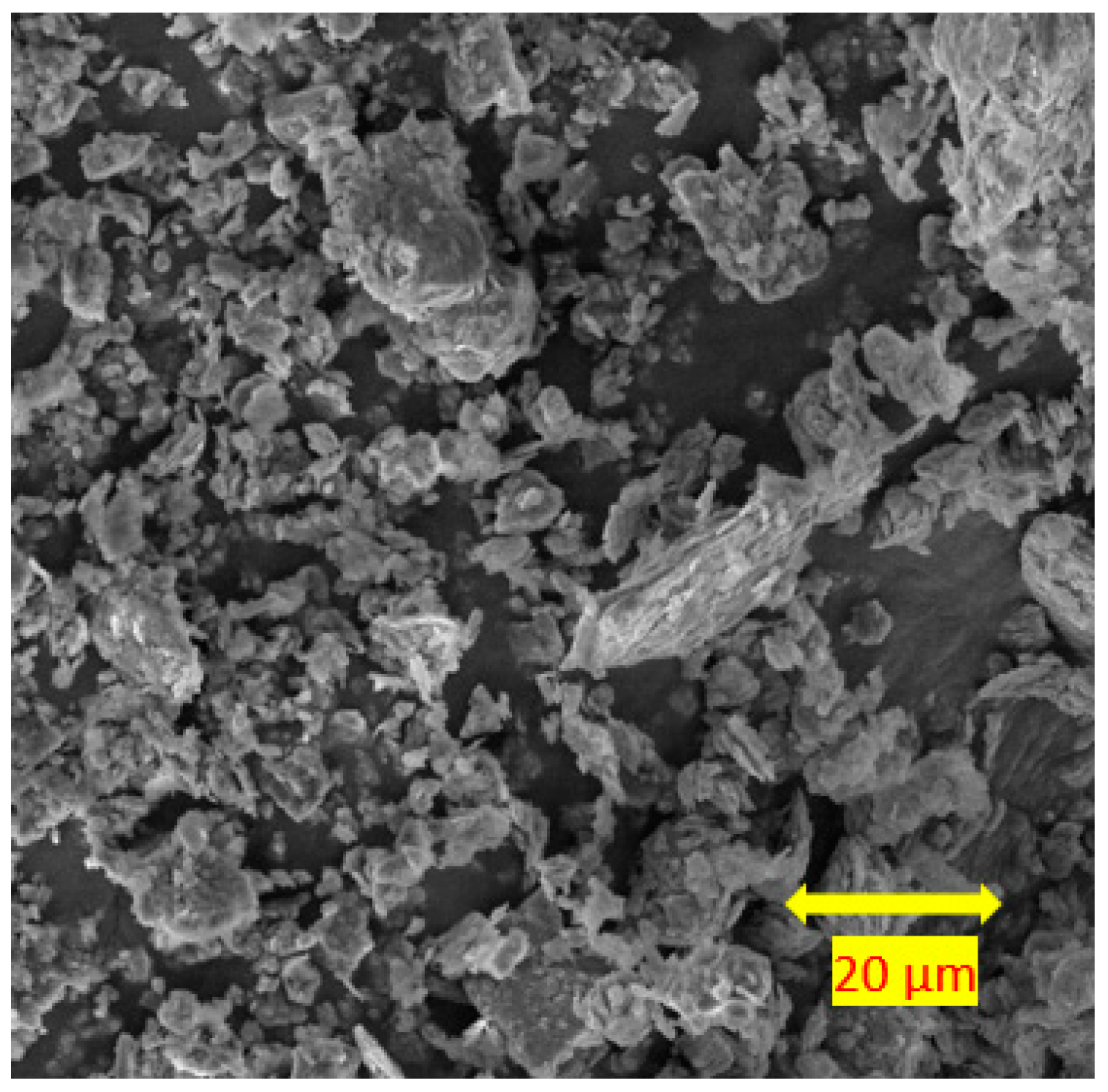
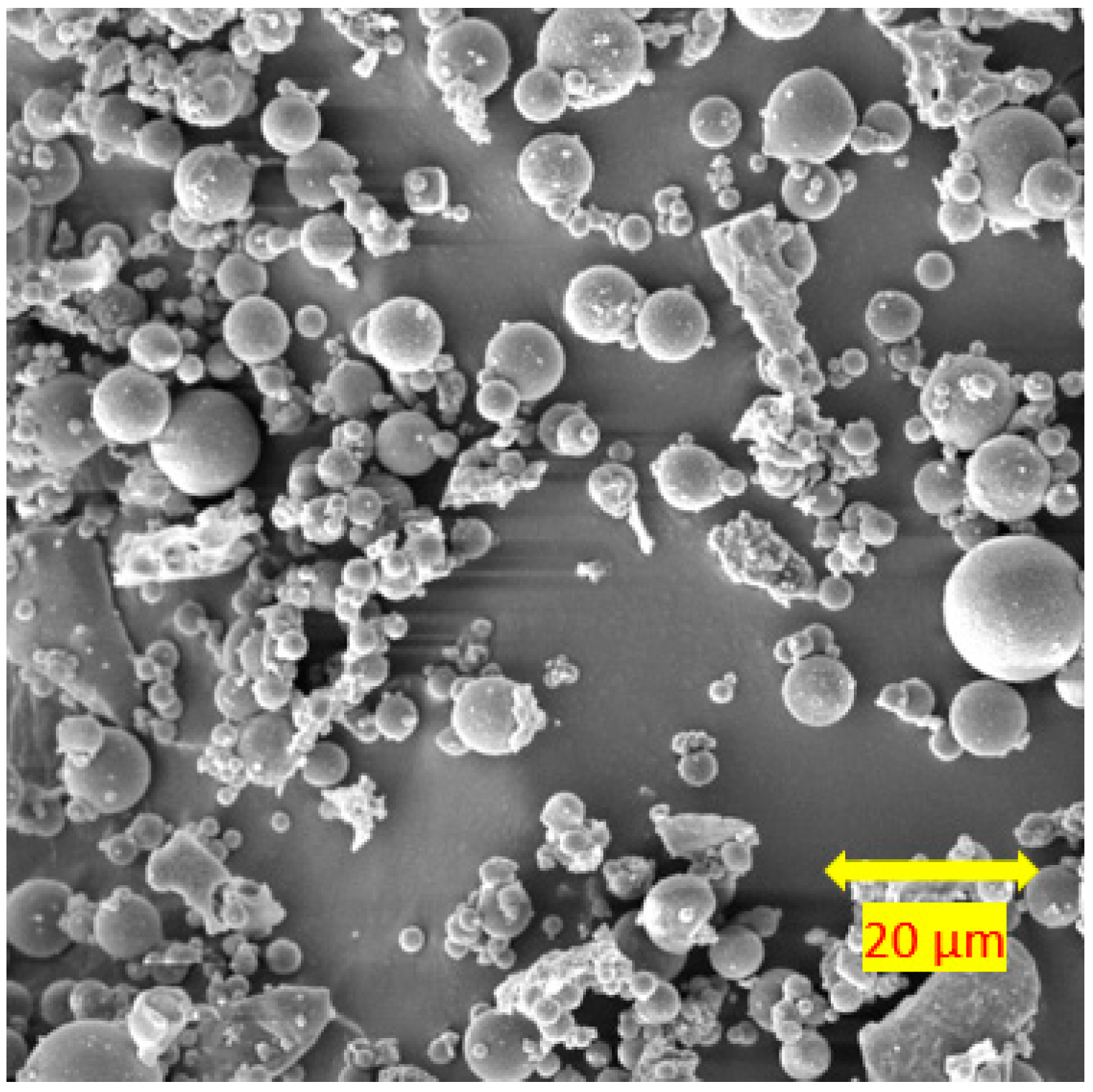
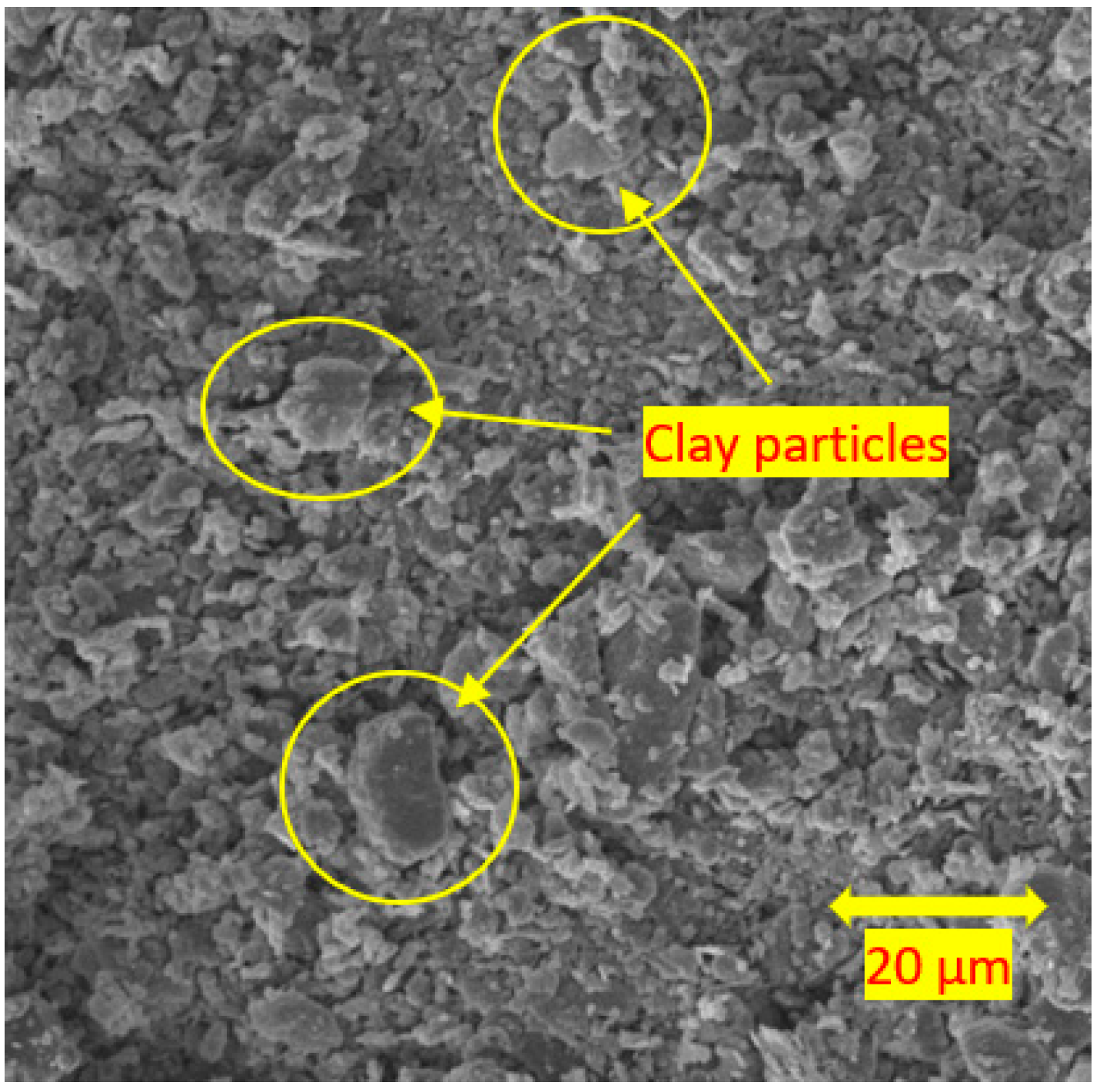
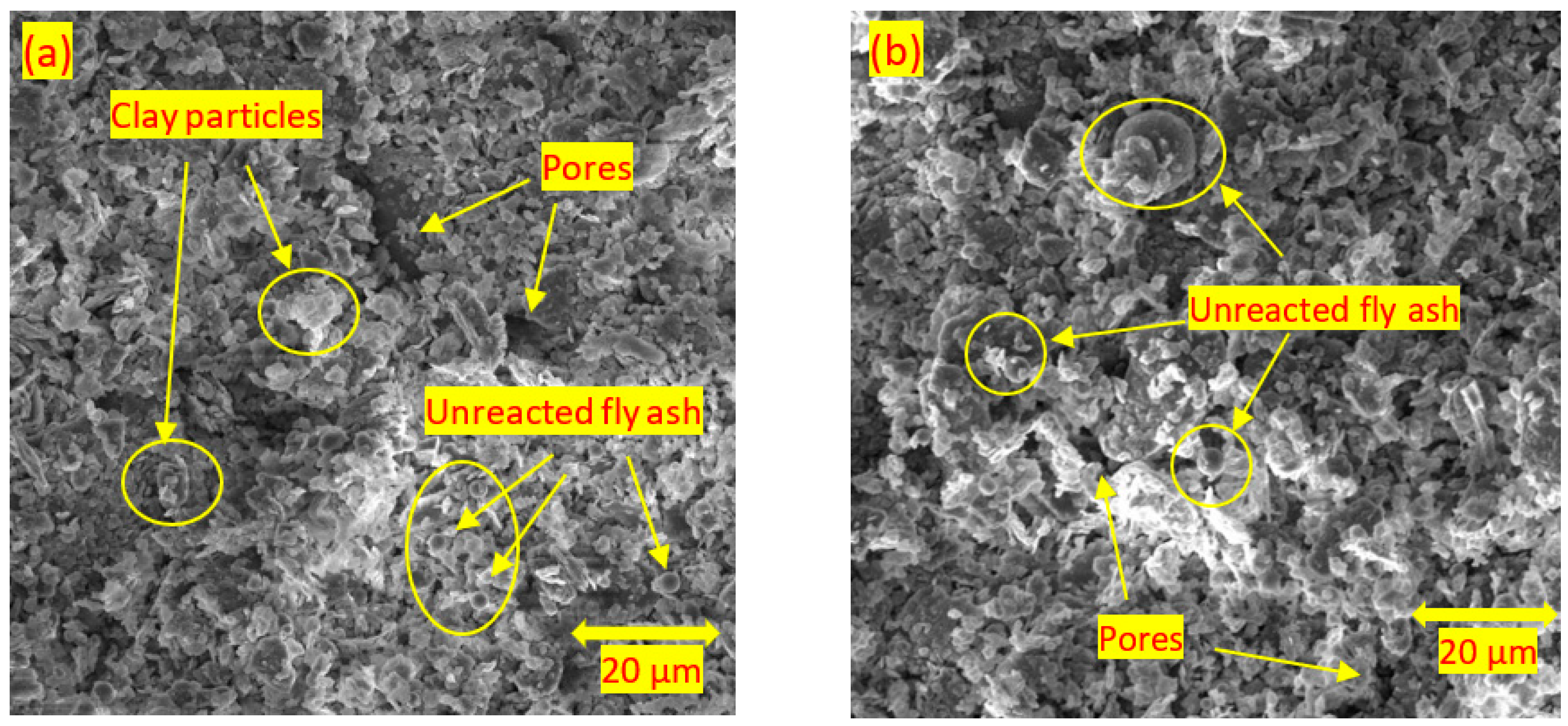
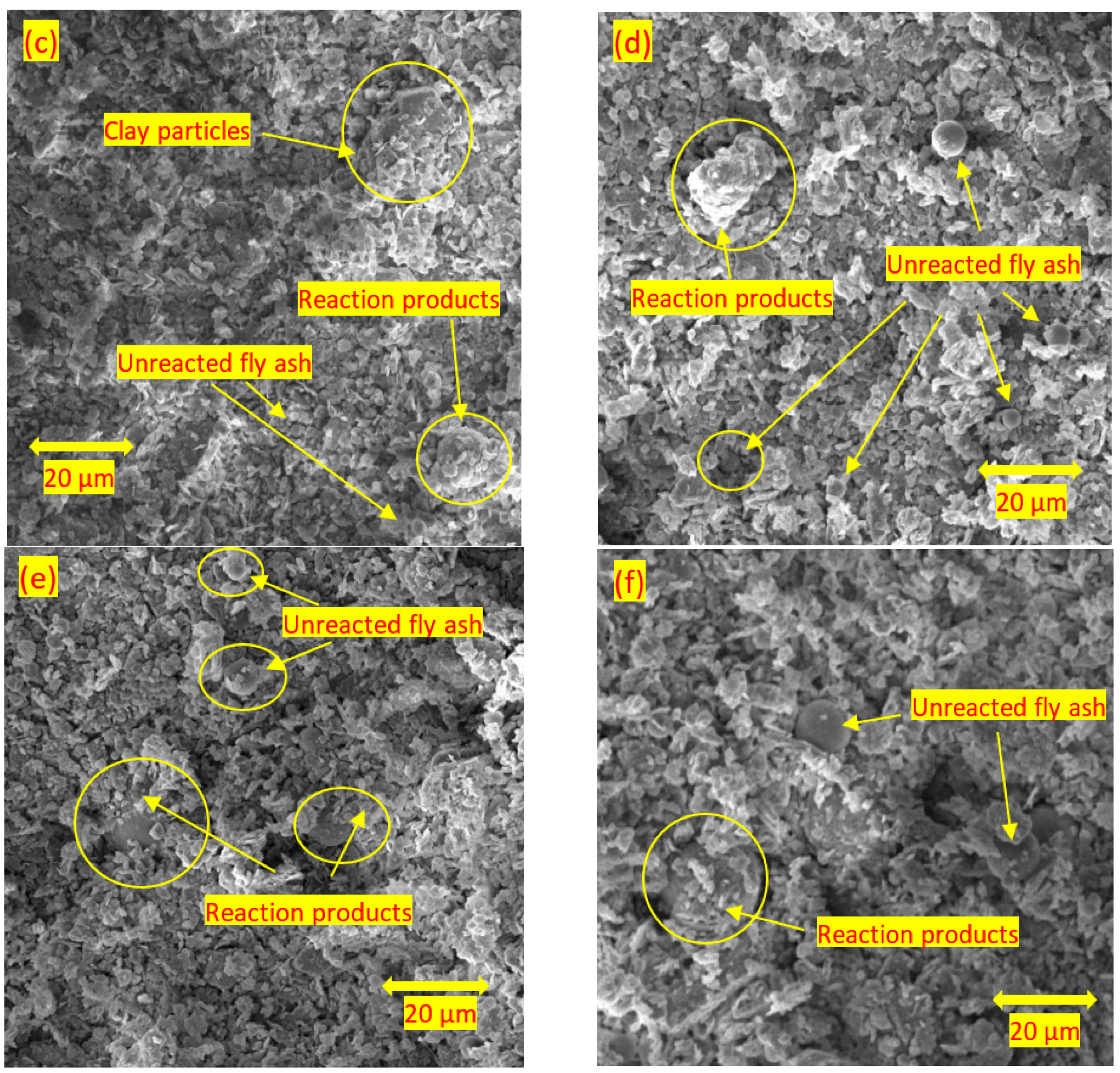
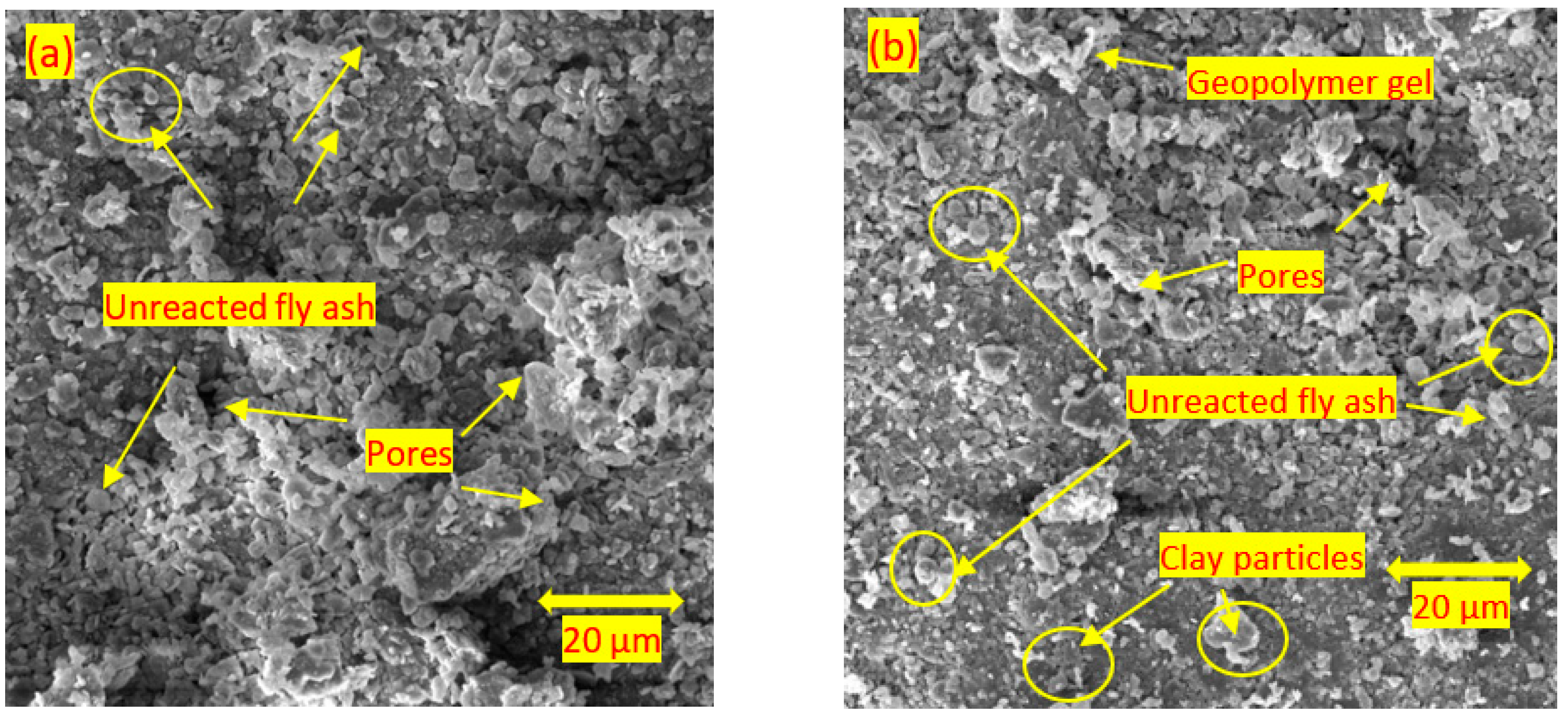
3.4. Scanning Electron Microscopy
4. Conclusions
- The control sample had higher MDD and lower OMC than the soil stabilised with fly ash and the soil stabilised with alkali-activated fly ash. The modest increase in MDD ranging from 14.3 to 14.7 kN/m3 and the modest decrease in OMC ranging from 23.8 to 21.6% were observed with the increase in M+ and SM;
- The UCS of the soil increased with the addition of alkali activators, fly ash content, and the curing time. The highest UCS result was found with 25% fly ash content, M+ of 16% and SM of 1.25 at 28 days of curing which can improve the UCS by about 6.3 times over the control sample;
- M+ = 12% was found to be optimal at 1 day of curing, whereas M+ = 16% gave the highest strengths at 7 and 28 days of curing. However, the increase rate of UCS from M+ of 12% to M+ of 16% was generally found to be marginal; thus M+ = 12% was chosen for further tests;
- SM = 1.25 resulted in the highest strength improvement at 7 and 28 days of curing, and was therefore chosen for further tests;
- The XRD analysis showed that, in the soil stabilised with fly ash, the peak intensities of kaolinite and illite slightly decreased at 28 days of curing, whereas in the soil stabilised with alkali-activated fly ash, the peak intensities of kaolinite and illite significantly decreased at all curing times;
- For the soils stabilised with alkali-activated fly ash, the microstructure of the soil was altered with the addition of alkali-activated fly ash, resulting in pores and hollow cavities at an early age. During the curing, aggregated-coarser particles were observed, leading to a denser fabric. On the other hand, the soils stabilised with fly ash showed insignificant microstructural behaviour.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turan, C.; Javadi, A.; Vinai, R.; Cuisinier, O.; Russo, G.; Consoli, N.C. Mechanical Properties of Calcareous Fly Ash Stabilized Soil. In Proceedings of the EUROCOALASH Conference, Dundee, UK, 10–12 June 2019. [Google Scholar]
- Turan, C.; Javadi, A.; Vinai, R.; Shariatmadari, N.; Farmani, R. Use of class C fly ash for stabilization of fine-grained soils. In Proceedings of the EUNSAT Conference, Lisbon, Portugal, 19–21 October 2020. [Google Scholar]
- Abdullah, H.H.; Shahin, M.A.; Sarker, P. Use of Fly-Ash Geopolymer Incorporating Ground Granulated Slag for Stabilisation of Kaolin Clay Cured at Ambient Temperature. Geotech. Geol. Eng. 2018, 37, 721–740. [Google Scholar] [CrossRef]
- Corrêa-Silva, M.; Araujo, N.; Cristelo, N.; Miranda, T.; Gomes, A.T.; Coelho, J. Improvement of a clayey soil with alkali activated low-calcium fly ash for transport infrastructures applications. Road Mater. Pavement Des. 2018, 20, 1912–1926. [Google Scholar] [CrossRef] [Green Version]
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Ridtirud, C.; Leekongbub, S.; Chindaprasirt, P. Compressive Strength of Soil Cement Base Mixed with Fly Ash–Based Geo-polymer. Int. J. GEOMATE 2018, 14, 82–88. [Google Scholar] [CrossRef]
- Firdous, R.; Stephan, D. Effect of silica modulus on the geopolymerization activity of natural pozzolans. Constr. Build. Mater. 2019, 219, 31–43. [Google Scholar] [CrossRef]
- Wong, B.Y.F.; Wong, K.S.; Phang, I.R.K. A review on geopolymerisation in soil stabilization. IOP Conf. Series Mater. Sci. Eng. 2019, 495, 012070. [Google Scholar] [CrossRef]
- Murmu, A.L.; Dhole, N.; Patel, A. Stabilisation of black cotton soil for subgrade application using fly ash geopolymer. Road Mater. Pavement Des. 2018, 21, 867–885. [Google Scholar] [CrossRef]
- Behnood, A. Soil and clay stabilization with calcium- and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Ibrahim, W.M.W. Mechanism and Chemical Reaction of Fly Ash Geopolymer Cement—A Review. Int. J. Pure Appl. Sci. Technol. 2011, 6, 35–44. [Google Scholar]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Al Bakri, A.M.; Faheem, T.M.; Sandhu, A.V.; Alida, A.; Salleh, M.A.A.M.; Ruzaidi, C. Microstructure Studies on Different Types of Geopolymer Materials. Appl. Mech. Mater. 2013, 421, 384–389. [Google Scholar] [CrossRef]
- Phummiphan, I.; Horpibulsuk, S.; Sukmak, P.; Chinkulkijniwat, A.; Arulrajah, A.; Shen, S.-L. Stabilisation of marginal lateritic soil using high calcium fly ash-based geopolymer. Road Mater. Pavement Des. 2016, 17, 877–891. [Google Scholar] [CrossRef]
- Damrongwiriyanupap, N.; Srikhamma, T.; Plongkrathok, C.; Phoo-Ngernkham, T.; Sae-Long, W.; Hanjitsuwan, S.; Sukontasukkul, P.; Li, L.-Y.; Chindaprasirt, P. Assessment of equivalent substrate stiffness and mechanical properties of sustainable alkali-activated concrete containing recycled concrete aggregate. Case Stud. Constr. Mater. 2022, 16, e00982. [Google Scholar] [CrossRef]
- de Azevedo, A.; Cruz, A.; Marvila, M.; de Oliveira, L.; Monteiro, S.; Vieira, C.; Fediuk, R.; Timokhin, R.; Vatin, N.; Daironas, M. Natural Fibers as an Alternative to Synthetic Fibers in Reinforcement of Geopolymer Matrices: A Comparative Review. Polymers 2021, 13, 2493. [Google Scholar] [CrossRef]
- Azimi, E.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials 2020, 13, 1015. [Google Scholar] [CrossRef] [Green Version]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P.; Faheem, M.T.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Rios, S.; Cristelo, N.; da Fonseca, A.V.; Ferreira, C. Structural Performance of Alkali-Activated Soil Ash versus Soil Cement. J. Mater. Civ. Eng. 2016, 28, 04015125. [Google Scholar] [CrossRef] [Green Version]
- Van Deventer, S.J.S.J.; Lukey, G.C.; Xu, H. Effect of Curing Temperature and Silicate Concentration on Fly-Ash-Based Ge-opolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Łach, M.; Mikuła, J. Alkali Activation of Waste Clay Bricks: Influence of The Silica Modulus, SiO2/Na2O, H2O/Na2O Molar Ratio, and Liquid/Solid Ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidovits, J. Properties of Geopolymer Cements. In Proceedings of the First International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 11–14 October 1994; Volume 1, pp. 131–149. [Google Scholar]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Noguchi, T. Alkali-silica reaction (ASR) in the alkali-activated cement (AAC) system: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119105. [Google Scholar] [CrossRef]
- Trinh, S.H.; Bui, Q.A.T. Influencing of Clay and Binder Content on Compression Strength of Soft Soil Stabilized by Geo-polymer Based Fly Ash. Int. J. Appl. Eng. Res. 2018, 13, 7954–7958. [Google Scholar]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. Strength Development of Soil–Fly Ash Geopolymer: Assessment of Soil, Fly Ash, Alkali Activators, and Water. J. Mater. Civ. Eng. 2018, 30, 04018171. [Google Scholar] [CrossRef] [Green Version]
- Dungca, J.R.; Codilla, E.E.T., II. Fly-ash-based geopolymer as stabilizer for silty sand embankment materials. Int. J. Geomate 2018, 14, 143–149. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S.-L.; Chindaprasirt, P.; Suksiripattanapong, C. Factors influencing strength development in clay–fly ash geopolymer. Constr. Build. Mater. 2013, 47, 1125–1136. [Google Scholar] [CrossRef]
- Chi, M. Effects of modulus ratio and dosage of alkali-activated solution on the properties and micro-structural characteristics of alkali-activated fly ash mortars. Constr. Build. Mater. 2015, 99, 128–136. [Google Scholar] [CrossRef]
- Karakoç, M.B.; Türkmen, I.; Maraş, M.M.; Kantarci, F.; Demirboğa, R.; Toprak, M.U. Mechanical properties and setting time of ferrochrome slag based geopolymer paste and mortar. Constr. Build. Mater. 2014, 72, 283–292. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Impacts of silica modulus on the early strength of alkaline activated ground slag/ultrafine palm oil fuel ash based concrete. Mater. Struct. 2014, 48, 733–741. [Google Scholar] [CrossRef]
- Zahmak, A.; Abdallah, M.; Jarah, B.; Arab, M.G. Environmental performance of alkali-activated binders for ground improvement. Transp. Geotech. 2021, 31, 100631. [Google Scholar] [CrossRef]
- BS 1377-2; Methods of Test for Soils for Civil Engineering Purposes. Classification Tests, BSI Standard Publications: London, UK, 1990.
- BS EN ISO 14688-2; Geotechnical Investigation and Testing–Identification and Classification of Soil, Part 2: Principles for a Classification. British Standard Institution: London, UK, 2018.
- BS 1377-4; Methods of Test for Soils for Civil Engineering Purposes. Compaction-Related Tests, BSI Standard Publications: London, UK, 1990.
- ASTM International. ASTM standard specification for coal fly ash and raw or calcined natural pozzolan for use in concrete (C618-05). In Annual Book of ASTM Standards, Concrete and Aggregates; American Society for Testing Materials: West Conshohocken, PA, USA, 2005; Volume 4. [Google Scholar]
- Soutsos, M.; Boyle, A.P.; Vinai, R.; Hadjierakleous, A.; Barnett, S. Factors influencing the compressive strength of fly ash based geopolymers. Constr. Build. Mater. 2016, 110, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Guidelines for mix proportioning of fly ash/GGBS based alkali activated concretes. Constr. Build. Mater. 2017, 147, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, H.H.; Shahin, M.A.; Sarker, P. Stabilisation of Clay with Fly-Ash Geopolymer Incorporating GGBFS. In Proceedings of the 2nd World Congress on Civil, Structural, and Environmental Engineering, Barcelona, Spain, 2–4 April 2017. [Google Scholar] [CrossRef]
- Mahvash, S.; López-Querol, S.; Bahadori-Jahromi, A. Effect of class F fly ash on fine sand compaction through soil stabilization. Heliyon 2017, 3, e00274. [Google Scholar] [CrossRef] [Green Version]
- BS 1377-7; Methods of Test for Soils for Civil Engineering Purposes. Shear Strength Tests (Total Stress), BSI Standard Pub-lications: London, UK, 1990.
- Prabakar, J.; Dendorkar, N.; Morchhale, R. Influence of fly ash on strength behavior of typical soils. Constr. Build. Mater. 2004, 18, 263–267. [Google Scholar] [CrossRef]
- Mir, B.A.; Sridharan, A. Physical and Compaction Behaviour of Clay Soil–Fly Ash Mixtures. Geotech. Geol. Eng. 2013, 31, 1059–1072. [Google Scholar] [CrossRef]
- Seyrek, E. Engineering behavior of clay soils stabilized with class C and class F fly ashes. Sci. Eng. Compos. Mater. 2018, 25, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Savaş, H.; Türköz, M.; Seyrek, E.; Ünver, E. Comparison of the effect of using class C and F fly ash on the stabilization of dispersive soils. Arab. J. Geosci. 2018, 11, 612. [Google Scholar] [CrossRef]
- Abdullah, H.; Shahin, M.A.; Walske, M.L.; Karrech, A. Systematic approach to assessing the applicability of fly-ash-based geopolymer for clay stabilization. Can. Geotech. J. 2020, 57, 1356–1368. [Google Scholar] [CrossRef]
- Vitale, E.; Russo, G.; Deneele, D. Use of Alkali-Activated Fly Ashes for Soil Treatment. Springer Nat. 2019, 40, 723–733. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Arulrajah, A.; Suksiripattanapong, C.; Udomchai, A. Strength development in soft marine clay stabilized by fly ash and calcium carbide residue based geopolymer. Appl. Clay Sci. 2016, 127–128, 134–142. [Google Scholar] [CrossRef]
- Runci, A.; Serdar, M. Effect of curing time on the chloride diffusion of alkali-activated slag. Case Stud. Constr. Mater. 2022, 16, e00927. [Google Scholar] [CrossRef]
- Syed, M.; GuhaRay, A.; Kar, A. Stabilization of Expansive Clayey Soil with Alkali Activated Binders. Geotech. Geol. Eng. 2020, 38, 6657–6677. [Google Scholar] [CrossRef]
- Parhi, P.S.; Garanayak, L.; Mahamaya, M.; Das, S.K. Stabilization of an Expansive Soil Using Alkali Activated Fly Ash Based Geopolymer. In International Congress and Exhibition” Sustainable Civil Infrastructures: Innovative Infrastructure Geotechnology”; Springer: Cham, Switzerland, 2017; pp. 36–50. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L. Geo-mechanical behavior of clay soils stabilized at ambient temperature with fly-ash geopolymer-incorporated granulated slag. Soils Found. 2019, 59, 1906–1920. [Google Scholar] [CrossRef]
- Turan, C.; Javadi, A.A.; Vinai, R. Effects of Class C and Class F Fly Ash on Mechanical and Microstructural Behavior of Clay Soil—A Comparative Study. Materials 2022, 15, 1845. [Google Scholar] [CrossRef]
- Kamruzzaman, A.H.M.; Chew, S.H.; Lee, F.H. Structuration and Destructuration Behaviour of Cement-Treated Singapore Marine Clay. J. Geotech. Geoenviron. Eng. 2009, 135, 573–589. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Latifi, N.; Meehan, C.L.; Majid, M.A.; Horpibulsuk, S. Strengthening montmorillonitic and kaolinitic clays using a calcium-based non-traditional additive: A micro-level study. Appl. Clay Sci. 2016, 132–133, 182–193. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Fly ash-slag interaction during alkaline activation: Influence of activators on phase assemblage and microstructure formation. Constr. Build. Mater. 2016, 122, 594–606. [Google Scholar] [CrossRef] [Green Version]
- Marsh, A.; Heath, A.; Patureau, P.; Evernden, M.; Walker, P. Alkali activation behaviour of un-calcined montmorillonite and illite clay minerals. Appl. Clay Sci. 2018, 166, 250–261. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Gado, R.; Khattab, R.M. Optimization of Slag Content and Properties Improvement of Metakaolin-slag Geopolymer Mixes. Open Mater. Sci. J. 2018, 12, 40–57. [Google Scholar] [CrossRef]
- Khan, H.A.; Castel, A.; Khan, M. Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 2020, 168, 108586. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L.; Karrech, A. Cyclic behaviour of clay stabilised with fly-ash based geopolymer incorporating ground granulated slag. Transp. Geotech. 2020, 26, 100430. [Google Scholar] [CrossRef]
- Raj, S.S.; Sharma, A.K.; Anand, K.B. Performance appraisal of coal ash stabilized rammed earth. J. Build. Eng. 2018, 18, 51–57. [Google Scholar] [CrossRef]
- Jaditager, M.; Sivakugan, N. Influence of Fly Ash–Based Geopolymer Binder on the Sedimentation Behavior of Dredged Mud. J. Waterw. Port Coast. Ocean Eng. 2017, 143, 04017012. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, D.; Ji, W.; Zhao, X. Experiment on the Properties of Soda Residue-Activated Ground Granulated Blast Furnace Slag Mortars with Different Activators. Materials 2022, 15, 3578. [Google Scholar] [CrossRef]




| Chemical Composition | Clay | Fly Ash |
|---|---|---|
| CaO (%) | 0.1 | 2.2 |
| SiO2 (%) | 54.8 | 48.6 |
| Al2O3 (%) | 41.1 | 22.5 |
| Fe2O3 (%) | 1.0 | 9.2 |
| K2O (%) | 2.1 | 4.1 |
| MgO (%) | 0.4 | 1.3 |
| Na2O (%) | 0.1 | 0.9 |
| P2O5 (%) | 0.1 | 0.2 |
| SO3 (%) | - | 0.9 |
| TiO2 (%) | 0.1 | 1.1 |
| 1 Day Curing | 7 Days Curing | 28 Days Curing | |||||
|---|---|---|---|---|---|---|---|
| Fly Ash Content | Fly Ash Content | ||||||
| M+ | SM | 15% | 25% | 15% | 25% | 15% | 25% |
| 0% | 0 | 233 | 246 | 242 | 259 | 292 | 325 |
| 8% | 1 | 210 | 253 | 255 | 373 | 422 | 687 |
| 1.25 | 237 | 256 | 309 | 390 | 501 | 748 | |
| 1.5 | 244 | 276 | 262 | 385 | 413 | 621 | |
| 1.75 | 230 | 263 | 236 | 311 | 384 | 504 | |
| 12% | 1 | 224 | 267 | 280 | 425 | 460 | 998 |
| 1.25 | 281 | 345 | 359 | 451 | 629 | 1144 | |
| 1.5 | 335 | 373 | 341 | 410 | 470 | 921 | |
| 1.75 | 271 | 338 | 278 | 378 | 395 | 704 | |
| 16% | 1 | 224 | 265 | 306 | 472 | 566 | 1106 |
| 1.25 | 272 | 290 | 387 | 492 | 795 | 1293 | |
| 1.5 | 305 | 335 | 342 | 483 | 539 | 1097 | |
| 1.75 | 281 | 297 | 313 | 436 | 485 | 838 | |
| 20% | 1 | 172 | 201 | 269 | 296 | 441 | 834 |
| 1.25 | 201 | 212 | 331 | 347 | 671 | 951 | |
| 1.5 | 208 | 219 | 277 | 298 | 404 | 782 | |
| 1.75 | 172 | 197 | 247 | 284 | 352 | 677 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turan, C.; Javadi, A.A.; Vinai, R.; Russo, G. Effects of Fly Ash Inclusion and Alkali Activation on Physical, Mechanical, and Chemical Properties of Clay. Materials 2022, 15, 4628. https://doi.org/10.3390/ma15134628
Turan C, Javadi AA, Vinai R, Russo G. Effects of Fly Ash Inclusion and Alkali Activation on Physical, Mechanical, and Chemical Properties of Clay. Materials. 2022; 15(13):4628. https://doi.org/10.3390/ma15134628
Chicago/Turabian StyleTuran, Canan, Akbar A. Javadi, Raffaele Vinai, and Giacomo Russo. 2022. "Effects of Fly Ash Inclusion and Alkali Activation on Physical, Mechanical, and Chemical Properties of Clay" Materials 15, no. 13: 4628. https://doi.org/10.3390/ma15134628
APA StyleTuran, C., Javadi, A. A., Vinai, R., & Russo, G. (2022). Effects of Fly Ash Inclusion and Alkali Activation on Physical, Mechanical, and Chemical Properties of Clay. Materials, 15(13), 4628. https://doi.org/10.3390/ma15134628







