Stabilizing Metallic Na Anodes via Sodiophilicity Regulation: A Review
Abstract
:1. Introduction
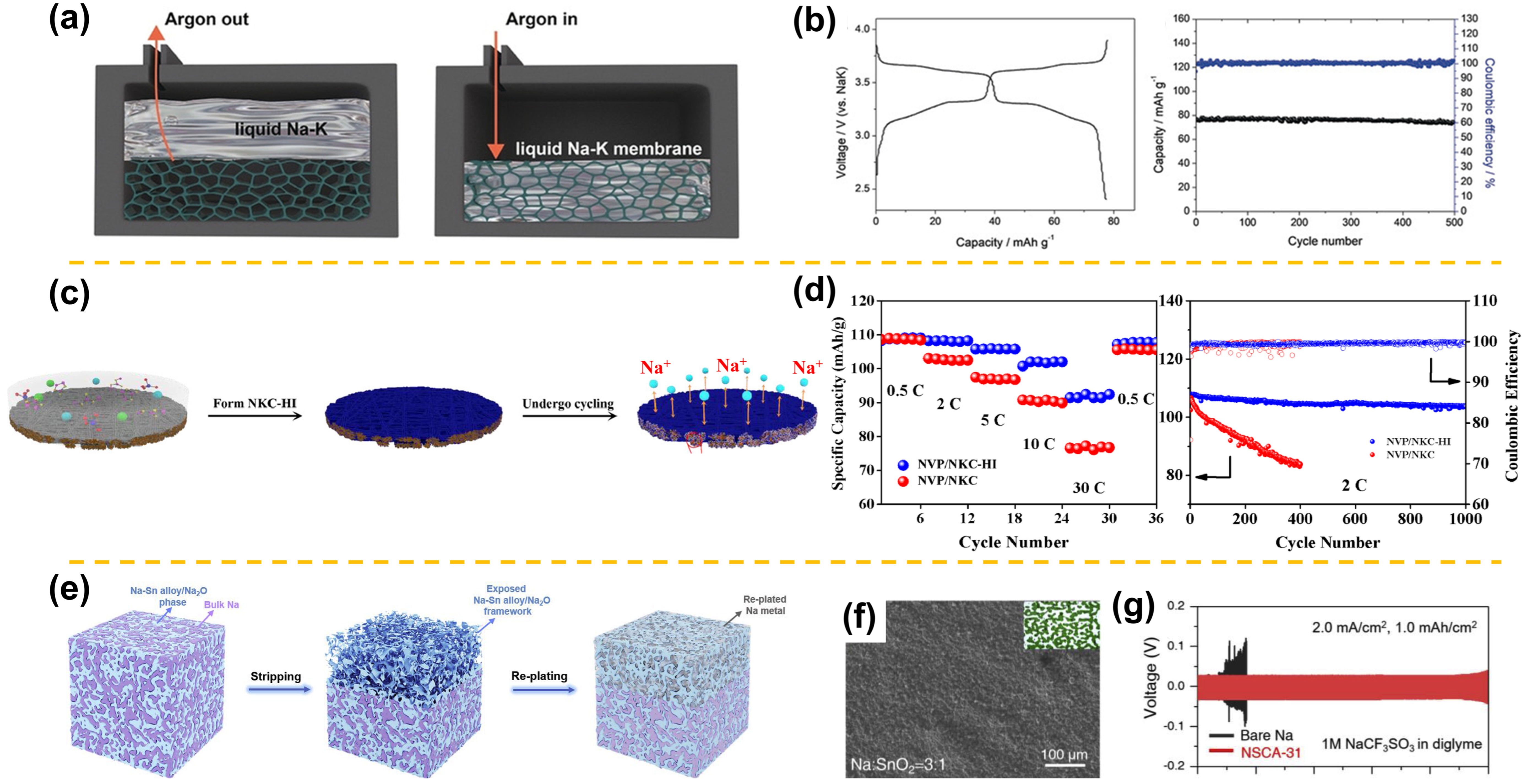
2. Molten Sodium-Metal Batteries
3. All-Solid-State Sodium-Metal Batteries
4. Conventional Sodium-Metal Batteries
4.1. Carbon-Based Hosts
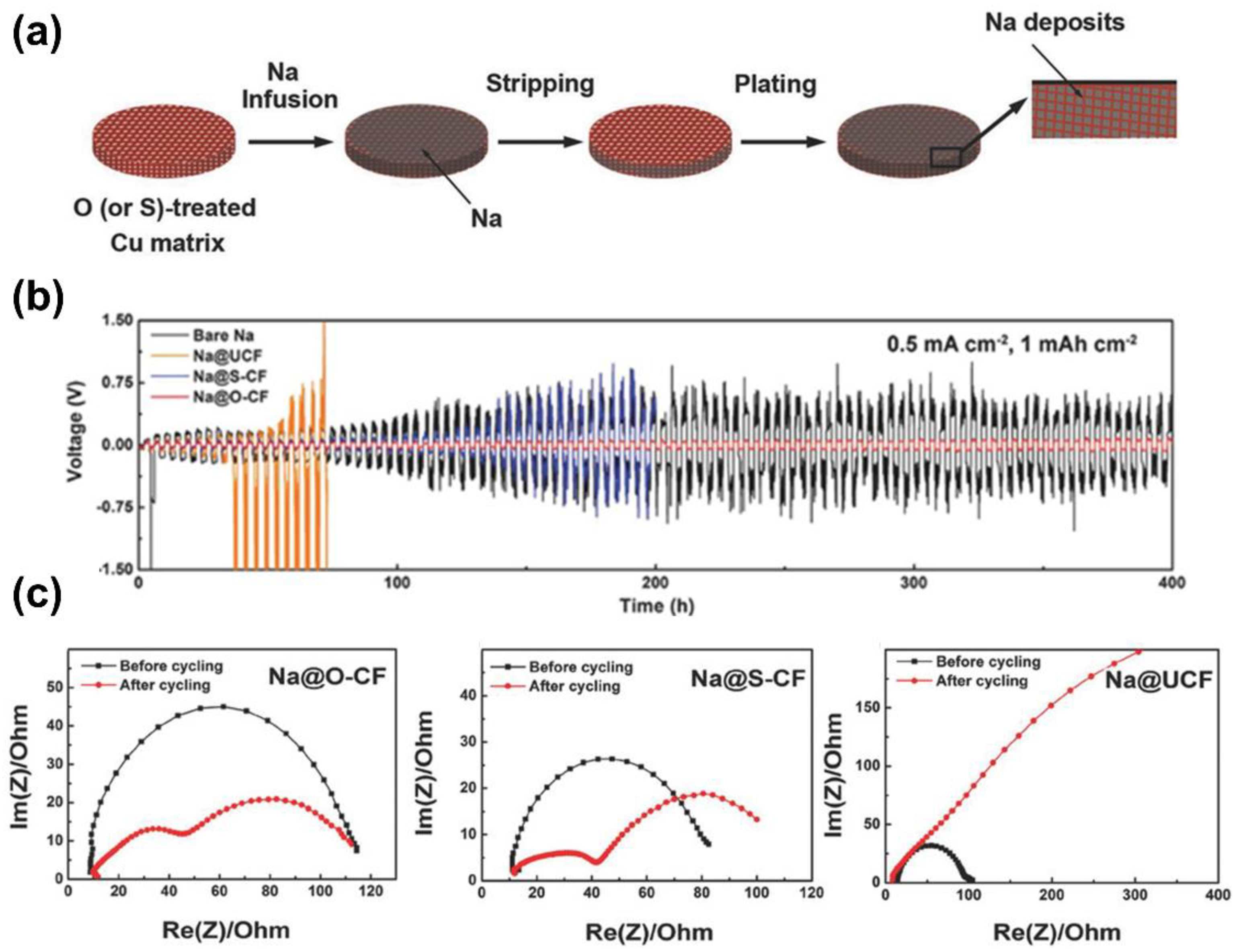
4.2. Alloy-Based Frameworks
4.3. Metal and MXene-Based Skeletons
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Shamim, N.; Thomsen, E.C.; Viswanathan, V.V.; Reed, D.M.; Sprenkle, V.L.; Li, G. Evaluating ZEBRA Battery Module under the Peak-Shaving Duty Cycles. Materials 2021, 14, 2280. [Google Scholar] [CrossRef]
- Zhan, X.; Li, M.M.; Weller, J.M.; Sprenkle, V.L.; Li, G. Recent Progress in Cathode Materials for Sodium-Metal Halide Batteries. Materials 2021, 14, 3260. [Google Scholar] [CrossRef]
- Reed, D.; Coffey, G.; Mast, E.; Canfield, N.; Mansurov, J.; Lu, X.; Sprenkle, V. Wetting of sodium on β″-Al2O3/YSZ composites for low temperature planar sodium-metal halide batteries. J. Power Sources 2013, 227, 94–100. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Mei, D.; Lemmon, J.P.; Sprenkle, V.L.; Liu, J. Liquid-metal electrode to enable ultra-low temperature sodium-beta alumina batteries for renewable energy storage. Nat. Commun. 2014, 5, 4578. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-J.; Lu, X.; Bonnett, J.F.; Canfield, N.L.; Han, K.; Engelhard, M.H.; Jung, K.; Sprenkle, V.L.; Li, G. Decorating β″-alumina solid-state electrolytes with micron Pb spherical particles for improving Na wettability at lower temperatures. J. Mater. Chem. A 2018, 6, 19703–19711. [Google Scholar] [CrossRef]
- Jin, D.; Choi, S.; Jang, W.; Soon, A.; Kim, J.; Moon, H.; Lee, W.; Lee, Y.; Son, S.; Park, Y.C.; et al. Bismuth Islands for Low-Temperature Sodium-Beta Alumina Batteries. ACS Appl. Mater. Interfaces 2019, 11, 2917–2924. [Google Scholar] [CrossRef]
- Yang, H.-L.; Zhang, B.-W.; Konstantinov, K.; Wang, Y.-X.; Liu, H.-K.; Dou, S.-X. Progress and Challenges for All-Solid-State Sodium Batteries. Adv. Energy Sustain. Res. 2021, 2, 2000057. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, L.; Chua, B.; Zeng, K.; Lu, L. Inorganic sodium solid-state electrolyte and interface with sodium metal for room-temperature metal solid-state batteries. Energy Storage Mater. 2021, 34, 28–44. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Zhu, K.; Zhang, Z.; Si, Y.; Wang, Y.; Jiao, L. Solid-State Electrolytes for Sodium Metal Batteries. Energy Fuels 2021, 35, 9063–9079. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, F.; Fu, C.; Chen, M.; Ma, Y.; Yin, G.; Wang, J. Interface Issues and Challenges in All-Solid-State Batteries: Lithium, Sodium, and Beyond. Adv. Mater. 2021, 33, 2000721. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, J.; Li, G.; Ferber, T.; Feng, J.; Li, Y.; Fu, H.; Jaegermann, W.; Monroe, C.W.; Huang, Y. TiO2 as Second Phase in Na3Zr2Si2PO12 to Suppress Dendrite Growth in Sodium Metal Solid-State Batteries. Adv. Energy Mater. 2022, 12, 2103607. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Guo, W.; Shao, Y.; Liu, L.; Lu, J.; Rong, X.; Han, X.; Li, H.; Chen, L.; et al. Hunting Sodium Dendrites in NASICON-Based Solid-State Electrolytes. Energy Mater. Adv. 2021, 2021, 9870879. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, J.; Yuan, H.; Fu, H.; Li, Y.; Li, Y.; Ferber, T.; Guhl, C.; Sun, H.; Jaegermann, W.; et al. Stabilizing Na3Zr2Si2PO12/Na Interfacial Performance by Introducing a Clean and Na-Deficient Surface. Chem. Mater. 2020, 32, 3970–3979. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium Metal Anodes: Emerging Solutions to Dendrite Growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10, 2000093. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Hu, A.; Mu, L.; Kautz, D.J.; Xu, Z.; Feng, Y.; Huang, H.; Lin, F. A Self-Sodiophilic Carbon Host Promotes the Cyclability of Sodium Anode. Adv. Funct. Mater. 2020, 31, 2007556. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Li, M.M.; Lu, X.; Zhan, X.; Engelhard, M.H.; Bonnett, J.F.; Polikarpov, E.; Jung, K.; Reed, D.M.; Sprenkle, V.L.; Li, G. High performance sodium-sulfur batteries at low temperature enabled by superior molten Na wettability. Chem. Commun. 2020, 57, 45–48. [Google Scholar] [CrossRef]
- Li, M.M.; Tripathi, S.; Polikarpov, E.; Canfield, N.L.; Han, K.S.; Weller, J.M.; Buck, E.C.; Engelhard, M.H.; Reed, D.M.; Sprenkle, V.L.; et al. Interfacial Engineering with a Nanoparticle-Decorated Porous Carbon Structure on beta″-Alumina Solid-State Electrolytes for Molten Sodium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 25534–25544. [Google Scholar] [CrossRef]
- Zhan, X.; Bonnett, J.F.; Engelhard, M.H.; Reed, D.M.; Sprenkle, V.L.; Li, G. A High-Performance Na–Al Battery Based on Reversible NaAlCl4 Catholyte. Adv. Energy Mater. 2020, 10, 2001378. [Google Scholar] [CrossRef]
- Zhan, X.; Bowden, M.E.; Lu, X.; Bonnett, J.F.; Lemmon, T.; Reed, D.M.; Sprenkle, V.L.; Li, G. A Low-Cost Durable Na-FeCl2 Battery with Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 1903472. [Google Scholar] [CrossRef]
- Viswanathan, L.; Virkar, A.V. Wetting characteristics of sodium on β″-alumina and on nasicon. J. Mater. Sci. 1982, 17, 753–759. [Google Scholar] [CrossRef]
- Ahlbrecht, K.; Bucharsky, C.; Holzapfel, M.; Tübke, J.; Hoffmann, M.J. Investigation of the wetting behavior of Na and Na alloys on uncoated and coated Na-β”-alumina at temperatures below 150 °C. Ionics 2017, 23, 1319–1327. [Google Scholar] [CrossRef]
- Wang, C.; Xie, H.; Zhang, L.; Gong, Y.; Pastel, G.; Dai, J.; Liu, B.; Wachsman, E.D.; Hu, L. Universal Soldering of Lithium and Sodium Alloys on Various Substrates for Batteries. Adv. Energy Mater. 2017, 8, 1701963. [Google Scholar] [CrossRef]
- Hu, Y.; Wen, Z.; Wu, X. Porous iron oxide coating on β″-alumina ceramics for Na-based batteries. Solid State Ionics 2014, 262, 133–137. [Google Scholar] [CrossRef]
- Jin, D.; Lee, H.G.; Choi, S.; Kim, S.; Lee, Y.; Son, S.; Park, Y.C.; Lee, J.S.; Jung, K.; Shim, W. Sparked Reduced Graphene Oxide for Low-Temperature Sodium-Beta Alumina Batteries. Nano Lett. 2019, 19, 8811–8820. [Google Scholar] [CrossRef]
- Landmann, D.; Graeber, G.; Heinz, M.V.F.; Haussener, S.; Battaglia, C. Sodium plating and stripping from Na-β"-alumina ceramics beyond 1000 mA/cm2. Mater. Today Energy 2020, 18, 100515. [Google Scholar] [CrossRef]
- Li, G.; Lu, X.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L. Improved cycling behavior of ZEBRA battery operated at intermediate temperature of 175 °C. J. Power Sources 2014, 249, 414–417. [Google Scholar] [CrossRef]
- Hu, Y.; Wen, Z.; Wu, X.; Lu, Y. Nickel nanowire network coating to alleviate interfacial polarization for Na-beta battery applications. J. Power Sources 2013, 240, 786–795. [Google Scholar] [CrossRef]
- Gross, M.M.; Small, L.J.; Peretti, A.S.; Percival, S.J.; Rodriguez, M.A.; Spoerke, E.D. Tin-based ionic chaperone phases to improve low temperature molten sodium–NaSICON interfaces. J. Mater. Chem. A 2020, 8, 17012–17018. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.-P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Materials Research Bulletin. Mat. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Gross, M.M.; Percival, S.J.; Lee, R.Y.; Peretti, A.S.; Spoerke, E.D.; Small, L.J. A high-voltage, low-temperature molten sodium battery enabled by metal halide catholyte chemistry. Cell Rep. Phys. Sci. 2021, 2, 100789. [Google Scholar] [CrossRef]
- Wu, T.; Wen, Z.; Sun, C.; Wu, X.; Zhang, S.; Yang, J. Disordered carbon tubes based on cotton cloth for modulating interface impedance in β″-Al2O3-based solid-state sodium metal batteries. J. Mater. Chem. A 2018, 6, 12623–12629. [Google Scholar] [CrossRef]
- Chi, X.; Hao, F.; Zhang, J.; Wu, X.; Zhang, Y.; Gheytani, S.; Wen, Z.; Yao, Y. A high-energy quinone-based all-solid-state sodium metal battery. Nano Energy 2019, 62, 718–724. [Google Scholar] [CrossRef]
- Lu, K.; Li, B.; Zhan, X.; Xia, F.; Dahunsi, O.J.; Gao, S.; Reed, D.M.; Sprenkle, V.L.; Li, G.; Cheng, Y. Elastic NaxMoS2-Carbon-BASE Triple Interface Direct Robust Solid–Solid Interface for All-Solid-State Na–S Batteries. Nano Lett. 2020, 20, 6837–6844. [Google Scholar] [CrossRef]
- Lai, H.; Wang, J.; Cai, M.; Song, Z.; Gao, X.; Wu, X.; Wen, Z. Double-functional 3D cross-linking carbon fiber with Sn particle coating layer for improving interfacial performance of Na-β″-Al2O3 batteries. Chem. Eng. J. 2022, 433, 133545. [Google Scholar] [CrossRef]
- Deng, T.; Ji, X.; Zou, L.; Chiekezi, O.; Cao, L.; Fan, X.; Adebisi, T.R.; Chang, H.J.; Wang, H.; Li, B.; et al. Interfacial-engineering-enabled practical low-temperature sodium metal battery. Nat. Nanotechnol. 2022, 17, 269–277. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Li, W.; Dolocan, A.; Manthiram, A. Interfacial Chemistry in Solid-State Batteries: Formation of Interphase and Its Consequences. J. Am. Chem. Soc. 2018, 140, 250–257. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Xin, S.; Goodenough, J.B. Rechargeable Sodium All-Solid-State Battery. ACS Cent. Sci. 2017, 3, 52–57. [Google Scholar] [CrossRef]
- Fu, H.; Yin, Q.; Huang, Y.; Sun, H.; Chen, Y.; Zhang, R.; Yu, Q.; Gu, L.; Duan, J.; Luo, W. Reducing Interfacial Resistance by Na-SiO2 Composite Anode for NASICON-Based Solid-State Sodium Battery. ACS Mater. Lett. 2019, 2, 127–132. [Google Scholar] [CrossRef]
- Oh, J.A.S.; Sun, J.; Goh, M.; Chua, B.; Zeng, K.; Lu, L. A Robust Solid–Solid Interface Using Sodium–Tin Alloy Modified Metallic Sodium Anode Paving Way for All-Solid-State Battery. Adv. Energy Mater. 2021, 11, 2101228. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Mao, Z.; Wang, D. In situ construction of a stable interface induced by the SnS2 ultra-thin layer for dendrite restriction in a solid-state sodium metal battery. J. Mater. Chem. A 2021, 9, 16039–16045. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Wu, J.; Gao, Z.; Hu, F.; Wei, Y.; Li, Y.; Liu, D.; Li, Z.; Huang, Y. Improving Na/Na3Zr2Si2PO12 Interface via SnOx /Sn Film for High-Performance Solid-State Sodium Metal Batteries. Small Methods 2021, 5, 2100339. [Google Scholar] [CrossRef]
- Yang, J.; Gao, Z.; Ferber, T.; Zhang, H.; Guhl, C.; Yang, L.; Li, Y.; Deng, Z.; Liu, P.; Cheng, C.; et al. Guided-formation of a favorable interface for stabilizing Na metal solid-state batteries. J. Mater. Chem. A 2020, 8, 7828–7835. [Google Scholar] [CrossRef]
- Miao, X.; Di, H.; Ge, X.; Zhao, D.; Wang, P.; Wang, R.; Wang, C.; Yin, L. AlF3-modified anode-electrolyte interface for effective Na dendrites restriction in NASICON-based solid-state electrolyte. Energy Storage Mater. 2020, 30, 170–178. [Google Scholar] [CrossRef]
- Lu, Y.; Alonso, J.A.; Yi, Q.; Lu, L.; Wang, Z.L.; Sun, C. A High-Performance Monolithic Solid-State Sodium Battery with Ca2+ Doped Na3Zr2Si2PO12 Electrolyte. Adv. Energy Mater. 2019, 9, 1901205. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Gao, X.; Zhao, Y. Stabilizing the Na/Na3Zr2Si2PO12 interface through intrinsic feature regulation of Na3Zr2Si2PO12. Cell Rep. Phys. Sci. 2021, 2, 100478. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Z.; Zhao, Y.; Wang, B.; Shao, C.; Sun, C.; Zhao, Y.; Li, J.; Jin, H.; Qu, L. Grain Boundary Design of Solid Electrolyte Actualizing Stable All-Solid-State Sodium Batteries. Small 2021, 17, 2103819. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, C.; Lv, X.; Lu, Y.; Lin, K.; Zhang, S.; Kang, F.; Hu, Y.-S.; Li, B. Stabilizing a sodium-metal battery with the synergy effects of a sodiophilic matrix and fluorine-rich interface. J. Mater. Chem. A 2019, 7, 24857–24867. [Google Scholar] [CrossRef]
- Go, W.; Kim, M.H.; Park, J.; Lim, C.H.; Joo, S.H.; Kim, Y.; Lee, H.W. Nanocrevasse-Rich Carbon Fibers for Stable Lithium and Sodium Metal Anodes. Nano Lett. 2019, 19, 1504–1511. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Wang, Y.; Xu, T.; Kong, D.; Jiang, Y.; Wu, D.; Tang, Y.; Li, X.; Lee, C.S. Fabricating Na/In/C Composite Anode with Natrophilic Na-In Alloy Enables Superior Na Ion Deposition in the EC/PC Electrolyte. Nanomicro Lett. 2021, 14, 23. [Google Scholar] [CrossRef]
- Ye, S.; Liu, F.; Xu, R.; Yao, Y.; Zhou, X.; Feng, Y.; Cheng, X.; Yu, Y. RuO2 Particles Anchored on Brush-Like 3D Carbon Cloth Guide Homogenous Li/Na Nucleation Framework for Stable Li/Na Anode. Small 2019, 15, 1903725. [Google Scholar] [CrossRef]
- Xiong, W.S.; Jiang, Y.; Xia, Y.; Qi, Y.; Sun, W.; He, D.; Liu, Y.; Zhao, X.Z. A robust 3D host for sodium metal anodes with excellent machinability and cycling stability. Chem. Commun. 2018, 54, 9406–9409. [Google Scholar] [CrossRef]
- Xiong, W.S.; Xia, Y.; Jiang, Y.; Qi, Y.; Sun, W.; He, D.; Liu, Y.; Zhao, X.Z. Highly Conductive and Robust Three-Dimensional Host with Excellent Alkali Metal Infiltration Boosts Ultrastable Lithium and Sodium Metal Anodes. ACS Appl. Mater. Interfaces 2018, 10, 21254–21261. [Google Scholar] [CrossRef]
- Lu, C.; Gao, Z.; Liu, B.; Shi, Z.; Yi, Y.; Zhao, W.; Guo, W.; Liu, Z.; Sun, J. Synchronous Promotion in Sodiophilicity and Conductivity of Flexible Host via Vertical Graphene Cultivator for Longevous Sodium Metal Batteries. Adv. Funct. Mater. 2021, 31, 2101233. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Kuo, L.Y.; Kaghazchi, P.; Sun, Q.; Liang, J.; Wang, B.; Lushington, A.; Li, R.; Zhang, H.; et al. High Capacity, Dendrite-Free Growth, and Minimum Volume Change Na Metal Anode. Small 2018, 14, 1703717. [Google Scholar] [CrossRef]
- Wu, W.; Hou, S.; Zhang, C.; Zhang, L. A Dendrite-free Na-Na2S-Carbon Hybrid toward a Highly Stable and Superior Sodium Metal Anode. ACS Appl. Mater. Interfaces 2020, 12, 27300–27306. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Pastel, G.; Kuang, Y.; Xie, H.; Li, Y.; Liu, B.; Luo, W.; Chen, C.; Hu, L. 3D Wettable Framework for Dendrite-Free Alkali Metal Anodes. Adv. Energy Mater. 2018, 8, 1800635. [Google Scholar] [CrossRef]
- Li, S.; Liu, Q.; Zhou, J.; Pan, T.; Gao, L.; Zhang, W.; Fan, L.; Lu, Y. Hierarchical Co3O4 Nanofiber–Carbon Sheet Skeleton with Superior Na/Li-Philic Property Enabling Highly Stable Alkali Metal Batteries. Adv. Funct. Mater. 2019, 29, 1808847. [Google Scholar] [CrossRef]
- Chi, S.-S.; Qi, X.-G.; Hu, Y.-S.; Fan, L.-Z. 3D Flexible Carbon Felt Host for Highly Stable Sodium Metal Anodes. Adv. Energy Mater. 2018, 8, 1702764. [Google Scholar] [CrossRef]
- Wang, A.; Hu, X.; Tang, H.; Zhang, C.; Liu, S.; Yang, Y.W.; Yang, Q.H.; Luo, J. Processable and Moldable Sodium-Metal Anodes. Angew. Chem. Int. Ed. Engl. 2017, 56, 11921–11926. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, J.; Luo, R.; Huang, Y.; Mei, Y.; Xie, M.; Chen, R. Reduced graphene oxide aerogel as stable host for dendrite-free sodium metal anode. Energy Storage Mater. 2019, 22, 376–383. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Y.; Xu, S.; Dai, J.; Hitz, E.; Li, Y.; Yang, C.; Chen, C.; Liu, B.; Hu, L. Encapsulation of Metallic Na in an Electrically Conductive Host with Porous Channels as a Highly Stable Na Metal Anode. Nano Lett. 2017, 17, 3792–3797. [Google Scholar] [CrossRef]
- Li, T.; Sun, J.; Gao, S.; Xiao, B.; Cheng, J.; Zhou, Y.; Sun, X.; Jiang, F.; Yan, Z.; Xiong, S. Superior Sodium Metal Anodes Enabled by Sodiophilic Carbonized Coconut Framework with 3D Tubular Structure. Adv. Energy Mater. 2020, 11, 2003699. [Google Scholar] [CrossRef]
- Liu, H.; Osenberg, M.; Ni, L.; Hilger, A.; Chen, L.; Zhou, D.; Dong, K.; Arlt, T.; Yao, X.; Wang, X.; et al. Sodiophilic and conductive carbon cloth guides sodium dendrite-free Na metal electrodeposition. J. Energy Chem. 2021, 61, 61–70. [Google Scholar] [CrossRef]
- Liang, J.; Wu, W.; Xu, L.; Wu, X. Highly stable Na metal anode enabled by a multifunctional hard carbon skeleton. Carbon 2021, 176, 219–227. [Google Scholar] [CrossRef]
- Mubarak, N.; Rehman, F.; Wu, J.; Ihsan-Ul-Haq, M.; Li, Y.; Zhao, Y.; Shen, X.; Luo, Z.; Huang, B.; Kim, J.-K. Morphology, chemistry, performance trident: Insights from hollow, mesoporous carbon nanofibers for dendrite-free sodium metal batteries. Nano Energy 2021, 86, 106132. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Li, S.M.; Li, B.; Gong, Y.J.; Du, Z.G.; Yang, S.B. Homogeneous guiding deposition of sodium through main group II metals toward dendrite-free sodium anodes. Sci. Adv. 2019, 5, eaau6264. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Gao, H.; Li, Y.; Goodenough, J.B. Cathode Dependence of Liquid-Alloy Na-K Anodes. J. Am. Chem. Soc. 2018, 140, 3292–3298. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhou, W.; Xin, S.; Gao, H.; Li, Y.; Zhou, A.; Goodenough, J.B. Room-Temperature Liquid Na-K Anode Membranes. Angew. Chem. Int. Ed. Engl. 2018, 57, 14184–14187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, S.; Ding, Y.; Guo, X.; Qian, Y.; Celio, H.; He, G.; Yu, G. A graphite intercalation compound associated with liquid Na–K towards ultra-stable and high-capacity alkali metal anodes. Energy Environ. Sci. 2019, 12, 1989–1998. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, J.; Zhang, Z. A stable carbon host engineering surface defects for room-temperature liquid Na K anode. J. Electroanal. Chem. 2020, 856, 113676. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, R.; Zuo, P.; Gao, Y.; Yin, G.; Du, C.; Wang, J.; Huo, H.; Ma, Y. An Interphase-enhanced Liquid Na-K Anode for Dendrite-free Alkali Metal Batteries Enabled by SiCl4 Electrolyte Additive. Energy Storage Mater. 2021, 37, 199–206. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Deng, W.; Chen, J.; Tian, Y.; Gao, X.; Deng, X.; Yi, S.; Li, S.; Chen, L.; et al. Liquid Alloying Na-K for Sodium Metal Anodes. J. Phys. Chem. Lett. 2021, 12, 9321–9327. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, W.; Wang, Z.; Huang, L.; Geng, S.; Wen, J.; Luo, W.; Huang, Y. Embedding a percolated dual-conductive skeleton with high sodiophilicity toward stable sodium metal anodes. Nano Energy 2020, 69, 104387. [Google Scholar] [CrossRef]
- Cao, K.; Ma, Q.; Tietz, F.; Xu, B.B.; Yan, M.; Jiang, Y. A robust, highly reversible, mixed conducting sodium metal anode. Sci. Bull. 2021, 66, 179–186. [Google Scholar] [CrossRef]
- Ye, S.; Wang, L.; Liu, F.; Shi, P.; Yu, Y. Integration of homogeneous and heterogeneous nucleation growth via 3D alloy framework for stable Na/K metal anode. eScience 2021, 1, 75–82. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Matios, E.; Hu, X.; Li, W. A Chemically Engineered Porous Copper Matrix with Cylindrical Core–Shell Skeleton as a Stable Host for Metallic Sodium Anodes. Adv. Funct. Mater. 2018, 28, 1802282. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, F.; Liang, J.; Fang, K.; Wu, W.; Wu, X. In-situ constructing a supersodiophilic fluffy surface layer on a Cu foam host for stable Na metal anodes. J. Alloy Compd. 2021, 853, 157371. [Google Scholar] [CrossRef]
- Fang, Y.; Lian, R.; Li, H.; Zhang, Y.; Gong, Z.; Zhu, K.; Ye, K.; Yan, J.; Wang, G.; Gao, Y.; et al. Induction of Planar Sodium Growth on MXene (Ti3C2Tx)-Modified Carbon Cloth Hosts for Flexible Sodium Metal Anodes. ACS Nano 2020, 14, 8744–8753. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Y.; Zhu, K.; Lian, R.; Gao, Y.; Yin, J.; Ye, K.; Cheng, K.; Yan, J.; Wang, G.; et al. Lithiophilic Three-Dimensional Porous Ti3C2Tx-rGO Membrane as a Stable Scaffold for Safe Alkali Metal (Li or Na) Anodes. ACS Nano 2019, 13, 14319–14328. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Lu, K.; Cai, W.; Jie, Y.; Huang, F.; Li, X.; Cao, R.; Jiao, S. Engineering rGO/MXene Hybrid Film as an Anode Host for Stable Sodium-Metal Batteries. Energy Fuels 2021, 35, 4587–4595. [Google Scholar] [CrossRef]
- Reis, G.S.D.; Oliveira, H.P.; Larsson, S.H.; Thyrel, M.; Claudio Lima, E. A Short Review on the Electrochemical Performance of Hierarchical and Nitrogen-Doped Activated Biocarbon-Based Electrodes for Supercapacitors. Nanomaterials 2021, 11, 424. [Google Scholar] [CrossRef]
- Li, J.; Qian, Y.; Wang, L.; He, X. Nitrogen-Doped Carbon for Red Phosphorous Based Anode Materials for Lithium Ion Batteries. Materials 2018, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Dou, H.; Zhao, W.; Zhao, X.; Wan, B.; Wang, J.; Yan, Y.; Wang, X.; Ma, Z.-F.; Yang, X. Three dimensional frameworks of super ionic conductor for thermodynamically and dynamically favorable sodium metal anode. Nano Energy 2020, 70, 104479. [Google Scholar] [CrossRef]
- Kim, B.-R.; Jeong, G.; Kim, A.; Kim, Y.; Kim, M.G.; Kim, H.; Kim, Y.-J. High Performance Na-CuCl2Rechargeable Battery toward Room Temperature ZEBRA-Type Battery. Adv. Energy Mater. 2016, 6, 1600862. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, X.; Reed, D.M.; Sprenkle, V.L.; Li, G. Emerging soluble organic redox materials for next-generation grid energy-storage applications. MRS Commun. 2020, 10, 215–229. [Google Scholar] [CrossRef]
- Sarkar, S.; Thangadurai, V. Critical Current Densities for High-Performance All-Solid-State Li-Metal Batteries: Fundamentals, Mechanisms, Interfaces, Materials, and Applications. ACS Energy Lett. 2022, 7, 1492–1527. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Nie, L.; Hu, X.; Huang, Y.; Yu, Y.; Liu, W. All-Solid-State Batteries with a Limited Lithium Metal Anode at Room Temperature using a Garnet-Based Electrolyte. Adv. Mater. 2021, 33, 2002325. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Yuan, C.; Cui, X.; Zhang, K.; Yu, Y.; Ren, X.; Zhan, X.; Gao, S. A dendrite-suppressed and utilization-improved metallic Li anode enabled by lithiophilic nano-Pb decoration on carbon cloth. J. Mater. Chem. A 2022, 10, 8424–8431. [Google Scholar] [CrossRef]

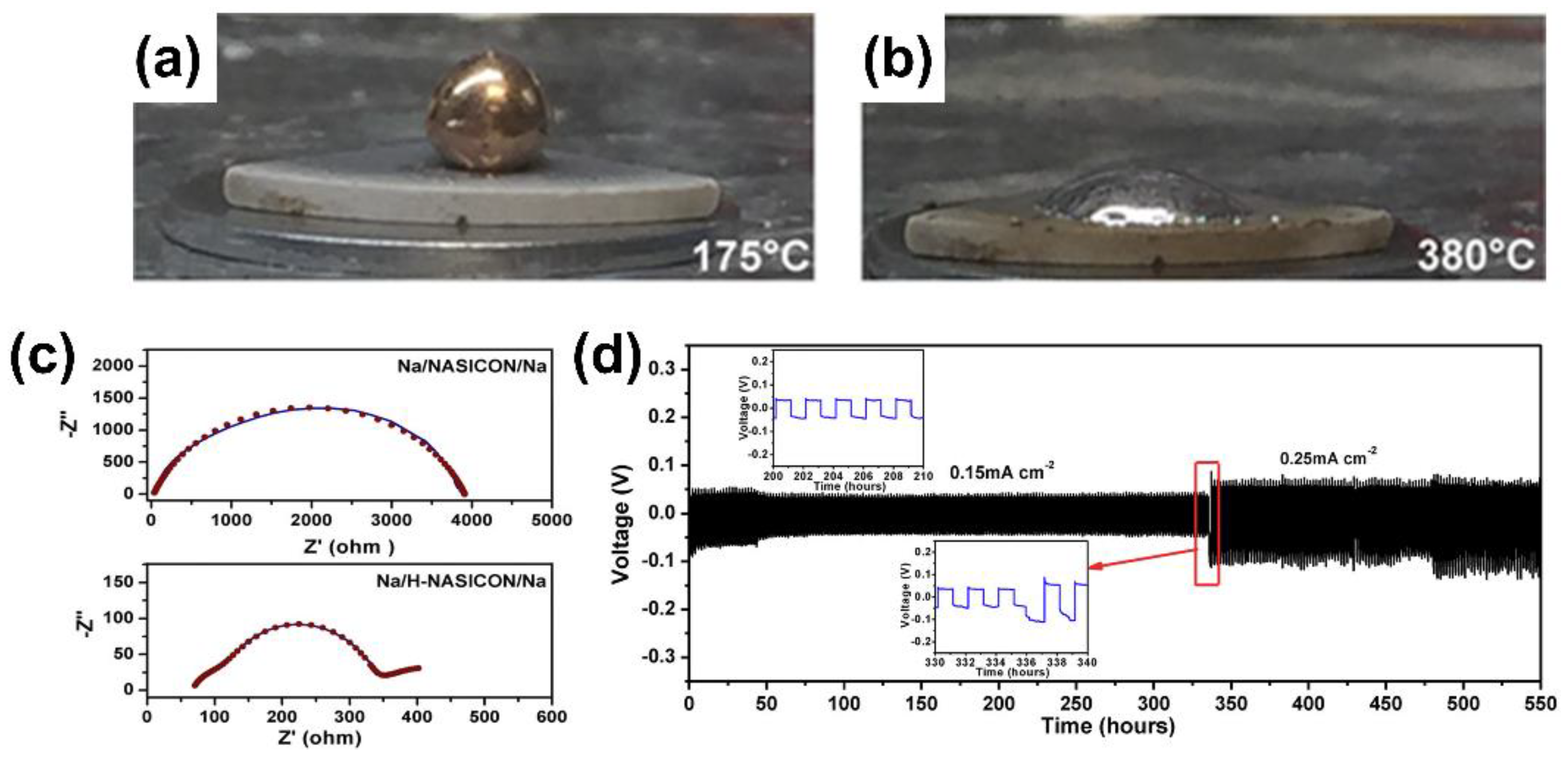
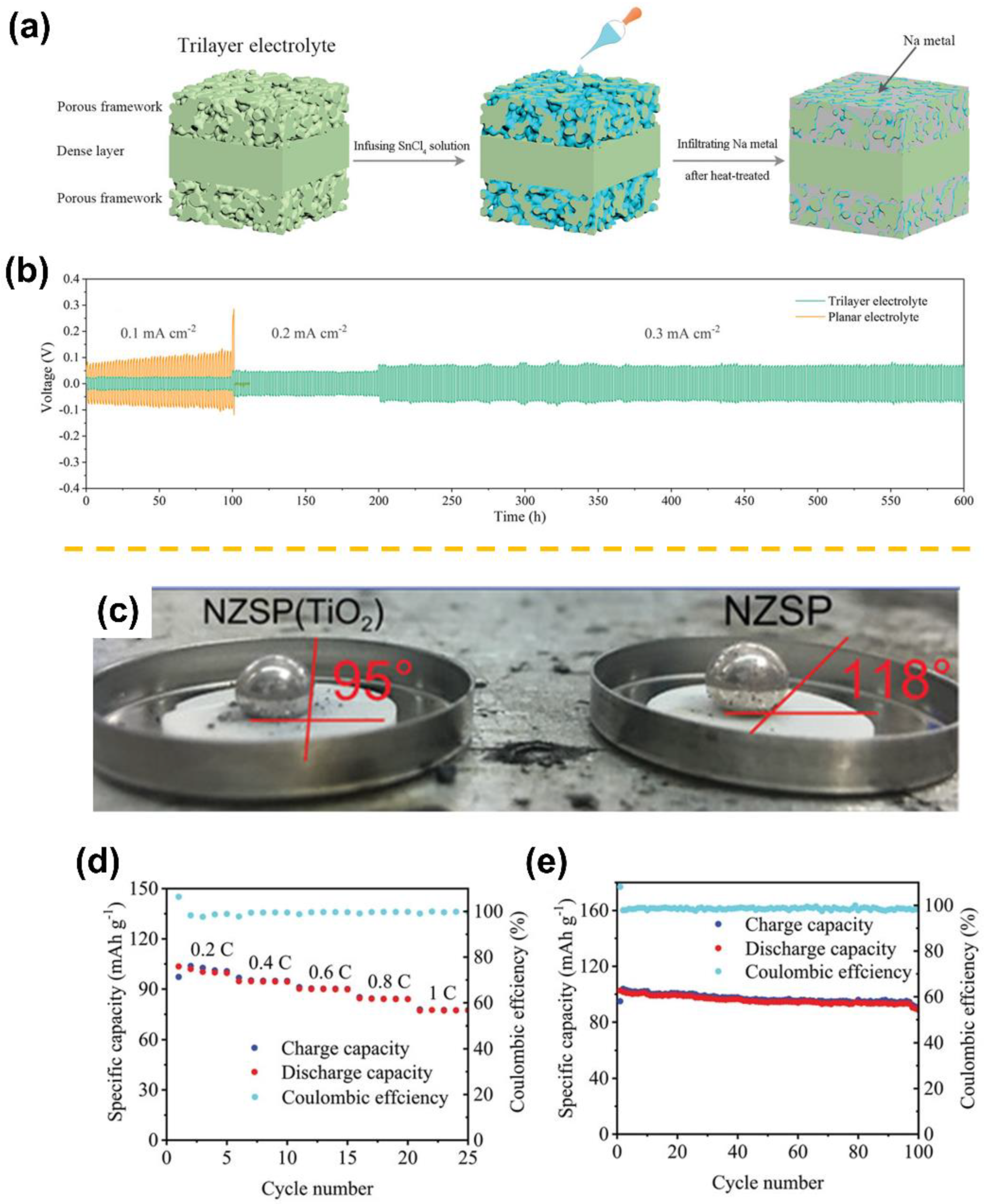

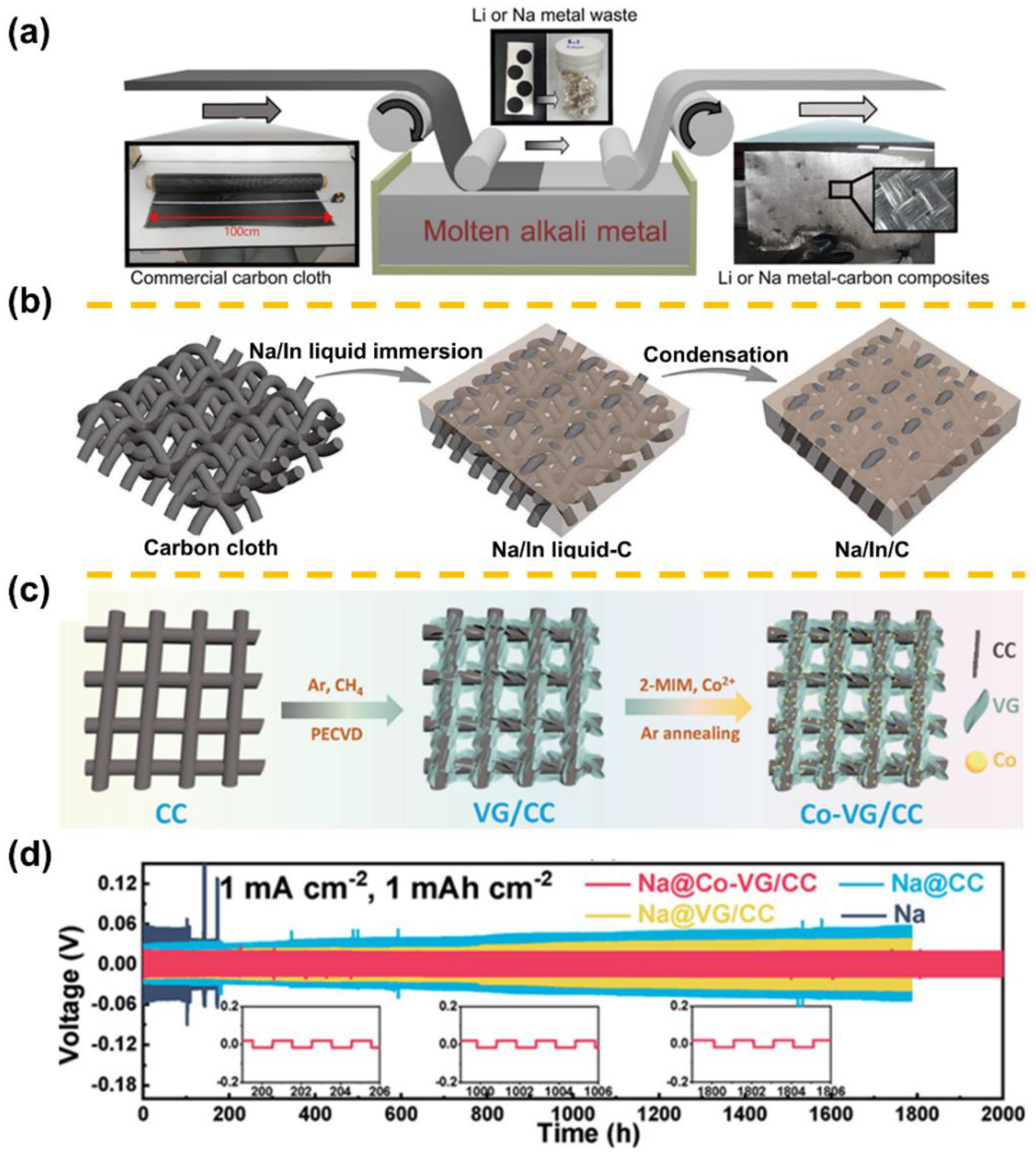
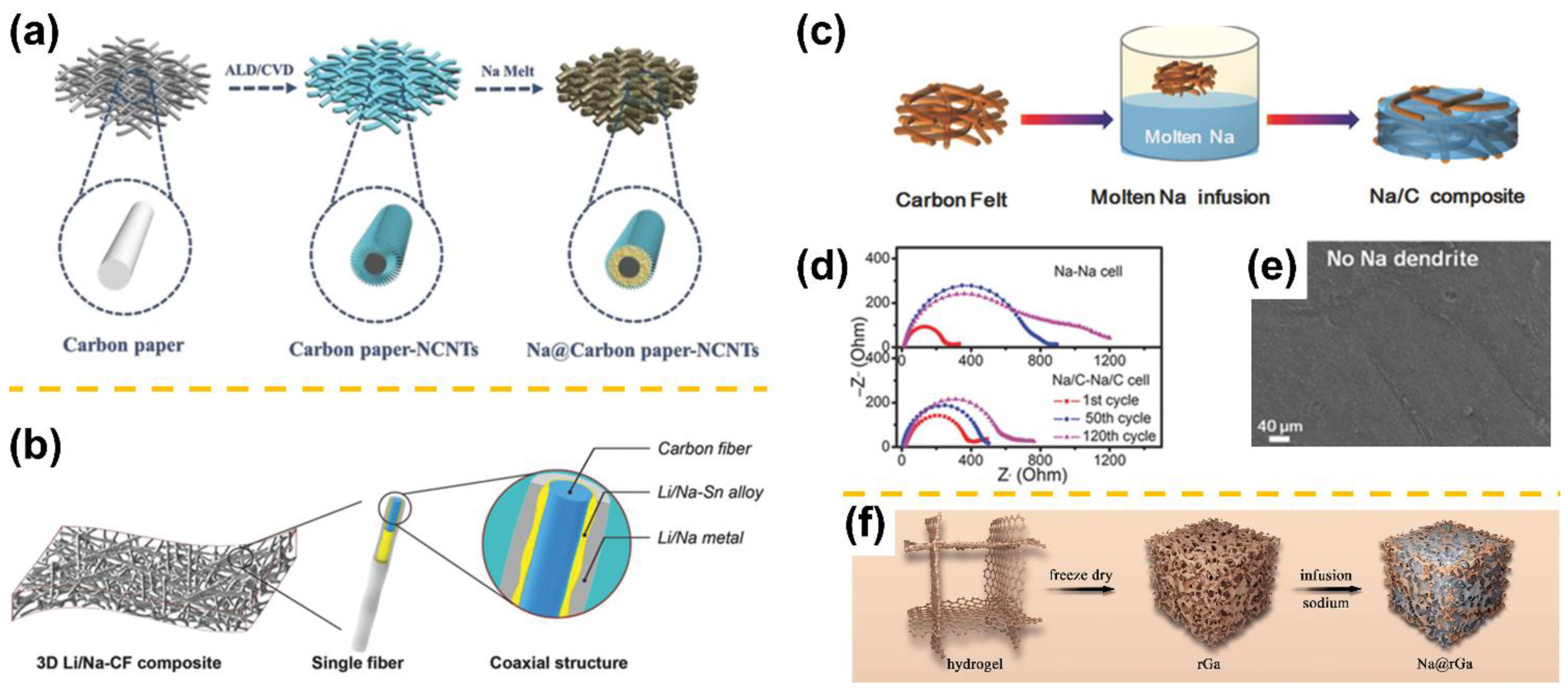


| Sodiophilicity Regulation Strategy | Testing Temperature | Interfacial Resistance (Ω cm2) | CCD (mA cm−2) | Lifespan in Symmetric Cells | Ref. |
|---|---|---|---|---|---|
| 450 °C heat-treated NZSP | 25 °C | 636 | / | 1500 h at 0.1 mA cm−2; 250 h at 0.3 mA cm−2 | [15] |
| Na-NZSP reaction at 380 °C | 65 °C | Total resistance: 400 Ω cm−2 | / | 213 h at 0.25 mA cm−2 | [41] |
| Na-SiO2 composite | 25 °C | 101 | 0.5 | 85 h at 0.2 mA cm−2 | [42] |
| NZSP-TiO2 composite | 25 °C | 149 | 1 | 750 h at 0.1 mA cm−2 | [13] |
| Fluorinated amorphous carbon-regulated NZSP | 75 °C | 100 | / | 100 h at 0.5 mA cm−2 | [14] |
| Na5Sn composite | 25 °C | 8.5 | 2.5 | 500 h at 0.3/0.5 mA cm−2 | [43] |
| SnS2@NZSP | 25 °C | Total resistance: 280 | 0.9 | 800 h at 0.1 mA cm−2 | [44] |
| SnOx/Sn@NZSP | 25 °C | 3 | 1 | 1500 h at 0.1 mA cm−2; 500 h at 0.3 mA cm−2 | [45] |
| TiO2@NZSP | 23 °C | 101 | / | 860 h at 0.1 mA cm−2 | [46] |
| AlF3@NZSP | 60 °C | / | 1.2 | 150 h at 0.25 mA cm−2 | [47] |
| Porous|dense|porous trilayer NZSP | 25 °C | Total resistance: 175 | / | 400 h at 0.3 mA cm−2 | [48] |
| Na3.4Zr1.6Sc0.4Si2PO12 | 25 °C | 63 | / | 700 h at 0.1/0.2 mA cm−2 | [49] |
| Na3.4Zr1.8Mg0.2PO12 | 25 °C | 93 | 0.95 | ~5000 h at 0.3 mA cm−2 | [50] |
| Disordered carbon tubes @BASE | 58 °C | 150 Ω cm−2 | / | 1000 h at 0.1 mA cm−2 | [35] |
| Sn@BASE | 60 °C | 9.6 Ω cm−2 | / | 1000 h at 0.5 mA cm−2 | [36] |
| NaxMoS2-carbon-BASE triple junction | 80 °C | / | / | 200 h at 0.3 mA cm−2 | [37] |
| carbon fiber with Sn particles@BASE | 25 °C | 6.6 | 1.3 | 3000 h at 0.2 mA cm−2; 400 h at 0.5 mA cm−2 | [38] |
| yttria-stabilized zirconia (YSZ)-BASE composite | 80 °C | 3.6 | ~7 | / | [39] |
| Sodiophilicity Regulation Strategy | Electrolyte | Current Density (mA cm−2) | Areal Capacity (mAh cm−2) | Overpotential (mV) | Cycle Life (h) | Cathode | Full-Cell Electrochemical Performance | Ref. |
|---|---|---|---|---|---|---|---|---|
| Na-carbon composite | 1 M NaPF6 in EC/DMC; 1 M NaPF6 in FEC/DMC | 1 | 0.5 | 80; 50 | 140; 600 | Na3V2(PO4)3 |
| [51] |
| Na-C composite | 1 M NaCF3SO3 in DME | 1; 3 | / | 18; 25 | 200; 80 | Seawater |
| [52] |
| Na-In-C composite | 1 M NaClO4 in EC/PC with 5 wt% FEC | 1; 2; 5; 1 | 1; 1; 1; 5 | 51; 100; 250; 50 | 870; 710; 560; 600 | Na3V2O2(PO4)2F |
| [53] |
| Na-Ru-cabon cloth | 1 M NaClO4 in carbonate electrolyte with 5% FEC | 1 | 1 | 12.5 | 250 | / | / | [54] |
| Na-Fe2O3-carbon textile | 1 M NaClO4 in EC/DMC | 1; 3; 5 | 1; 1; 1 | 20; 70; 120 | 333; 222; 139 | / | / | [55] |
| Na-3D SnO2 carbon textiles | 1 M NaClO4 in EC and DMC | 1 | 1 | 50 | 222 | / | / | [56] |
| Na-Co nanoparticle-N-doped carbon | 1 M NaClO4 in EC/DMC/EMC with 2 wt% FEC | 1; 3; 5 | 1; 6; 5 | 270; 26; 18 | 280; 1000; 2000 | Na3V2(PO4)3 |
| [57] |
| Na-carbon paper-N-doped carbon nanotubes | 1 M NaPF6 in EC/PC | 3; 5; 5 | 1; 1; 3 | 200; 120; 200 | 180; 140; 90 | / | / | [58] |
| Na-Na2S-carbon composite | 1 M NaClO4 in EC/DEC with 10% FEC | 1; 4 | 0.5; 2 | 50; 89 | 300; 150 | Na3V2(PO4)3 |
| [59] |
| Na-carbon fiber composite | 1 M NaClO4 in EC/DMC/EMC with 5% FEC | 0.5 | 1 | 50 | 300 | / | / | [60] |
| Na-Co3O4 nanofiber-carbon sheet | 1 M NaClO4 in EC/DMC/EMC with 5% FEC | 1; 2; 1 | 1; 1; 3 | 80; 110; 70 | 250; 140; 240 | Na3V2(PO4)2F3 |
| [61] |
| Na-carbon felt composite | 1 M NaClO4 in EC/PC | 1; 3; 5 | 2; 2; 2 | 20; 50; 100 | 120; 120; 120 | Na0.67Ni0.33Mn0.67O2 |
| [62] |
| Na-reduced graphene oxide | 1 M NaPF6 in EC/PC | 0.25; 0.5 | 0.25; 0.25 | 90; 110 | 300; 60 | Na3V2(PO4)3 |
| [63] |
| Na-reduced graphene oxide aerogel | 1 M NaClO4 in EC/DEC | 0.5; 3; 5 | 0.5; 2; 1 | 35; 120; 50 | 350; 120; 400 | Na0.67Ni0.25Mn0.75O2 |
| [64] |
| Na-carbonized wood | 1 M NaClO4 in EC/DEC | 0.5; 1; 1 | 0.25; 0.5; 1 | 30; 62.5; 70 | 250; 250; 500 | / | / | [65] |
| Na-oxygen-containing carbonized coconut framework | 1 M NaPF6 in diglyme | 10; 30; 50 | 1; 1; 1 | 5.3; 12; 22 | 700; 675; 400 | Na3V2(PO4)3 |
| [66] |
| Na-carbon cloth | 1 M NaClO4 in EC/DEC with 5% FEC | 0.3 | / | 4.8 | 1600 | Na3V2(PO4)3 |
| [67] |
| Na-N-functionalized hard carbon | 1 M NaCF3SO3 in diglyme | 1; 2 | 1; 2 | 32; 76 | 1700; 800 | Carbon-coated NaTi2(PO4)3 |
| [68] |
| Na-hollow and mesoporous carbon nanofiber | 1 M NaCF3SO3 in DEG/DME | 3; 5 | 3; 5 | 40; 50 | 2400; 1000 | Na3V2(PO4)2F3 |
| [69] |
| Na-3D hierarchical structure | 1 M NaClO4 in EC/DEC with 5% FEC | 0.5; 1 | 1; 1 | 27; 60 | 1350; 380 | Na3V2(PO4)3 |
| [70] |
| Liquid Na-K | 1 M NaClO4 in PC with 10 wt% FEC | / | / | / | / | Na3V2(PO4)3 |
| [71] |
| Na-K-Al | 1 M NaClO4 in PC with 10 wt% FEC | / | / | / | / | Na2/3Ni1/3Mn2/3O2 |
| [72] |
| Na-K-graphite intercalation compound | 1 M NaClO4 in 1:1 EC/DEC | 0.4 | 0.4 | 380 | 400 | Na2MnFe(CN)6 |
| [73] |
| NaK-Carbon cloth | 1 M NaClO4 in PC with 5 wt% FEC | 2 | 2 | 150 | 1800 | Na3V2(PO4)3 |
| [74] |
| Na-K treated by 0.1 M SiCl4-contained electrolyte | 0.8 M NaFSI in EC/DMC | 1 | 1 | 200 | 2000 | Na3V2(PO4)3 |
| [75] |
| NaK-Na | 1 M NaClO4 in EC/DMC/EMC with 5 wt% FEC | 20; 40 | 1; 3 | 25; 25 | 1200; 2800 | Na3V2(PO4)3 |
| [76] |
| Na-SnO2 weight ratio of 3:1 | 1 M NaClO4 in EC/PC | 0.5; 1 | 1; 1 | 13; 50 | 160; 300 | Na3V2(PO4)3 |
| [77] |
| Bulk hybrid Na-metal | 1 M NaPF6 in EC/DEC/PC with 5 wt% FEC | 1; 1; 3 | 1; 5; 3 | 35; 36; 25 | 750; 700; 150 | Na3V2(PO4)3/C |
| [78] |
| 3D-Na3Bi alloy host | 1 M NaClO4 in EC/DEC with 5 wt% FEC | 1 | 1 | 80 | 700 | Na3V2(PO4)3 |
| [79] |
| Na-oxygen treated Cu foam | 1 M NaClO4 in EC/DEC | 0.5; 1; 2 | 1; 1; 3 | 50; 100; 120 | 400; 200; 300 | Na3V2(PO4)3 |
| [80] |
| Na-CuO/Cu2O surface layer on the Cu foam | 1 M NaCF3SO3 in diglyme | 0.5; 0.5; 1 | 0.5; 2.5; 1 | 12; 12; 19 | 1000; 1000; 400 | NaTi2(PO4)3 |
| [81] |
| Na-Ti3C2Tx-carbon cloth | 1 M NaClO4 in EC/DMC/EMC with 5.0% FEC | 5; 3 | 1; 1 | 20; 62 | 300; 300 | Na3V2(PO4)3 |
| [82] |
| Na-Ti3C2Tx-reduced graphene oxide | 1 M NaClO4 in EC/DMC/EMC with 5.0% FEC | 1; 3 | 1; 1 | 20; 33 | 1200; 500 | / | / | [83] |
| Na-reduced graphene oxide/MXene | 1 M NaPF6 in diglyme | 1; 3 | 1; 1 | 34; 85 | 1700; 1600 | Na3V2(PO4)3 |
| [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Li, R.; Zhan, X.; Sprenkle, V.L.; Li, G. Stabilizing Metallic Na Anodes via Sodiophilicity Regulation: A Review. Materials 2022, 15, 4636. https://doi.org/10.3390/ma15134636
Yuan C, Li R, Zhan X, Sprenkle VL, Li G. Stabilizing Metallic Na Anodes via Sodiophilicity Regulation: A Review. Materials. 2022; 15(13):4636. https://doi.org/10.3390/ma15134636
Chicago/Turabian StyleYuan, Chenbo, Rui Li, Xiaowen Zhan, Vincent L. Sprenkle, and Guosheng Li. 2022. "Stabilizing Metallic Na Anodes via Sodiophilicity Regulation: A Review" Materials 15, no. 13: 4636. https://doi.org/10.3390/ma15134636





