Apparent Molecular Weight Distributions in Bituminous Binders
Abstract
:1. Introduction
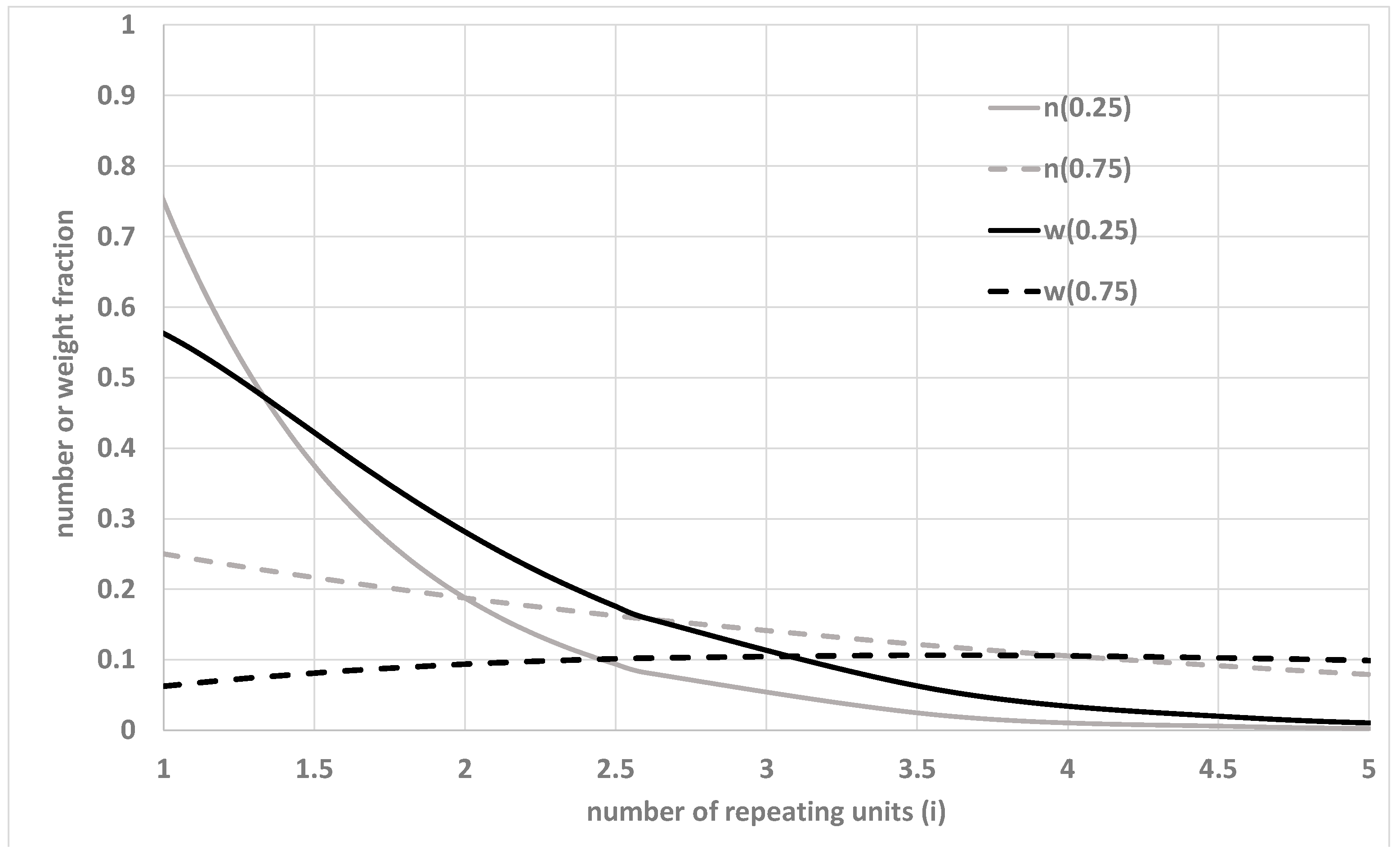
2. Molecular Weight Distributions
3. Main Differences between Solution and Bulk Distributions
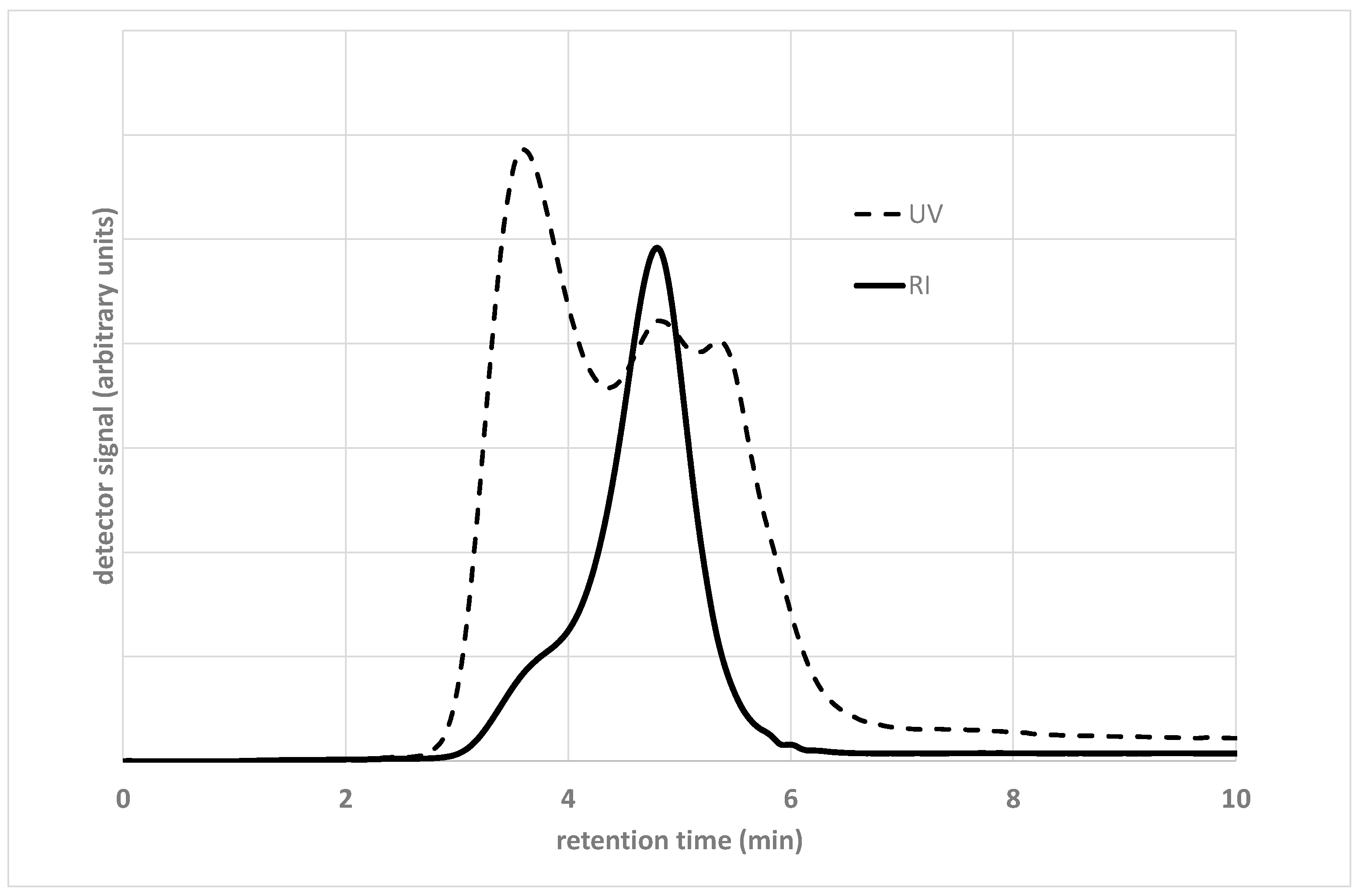
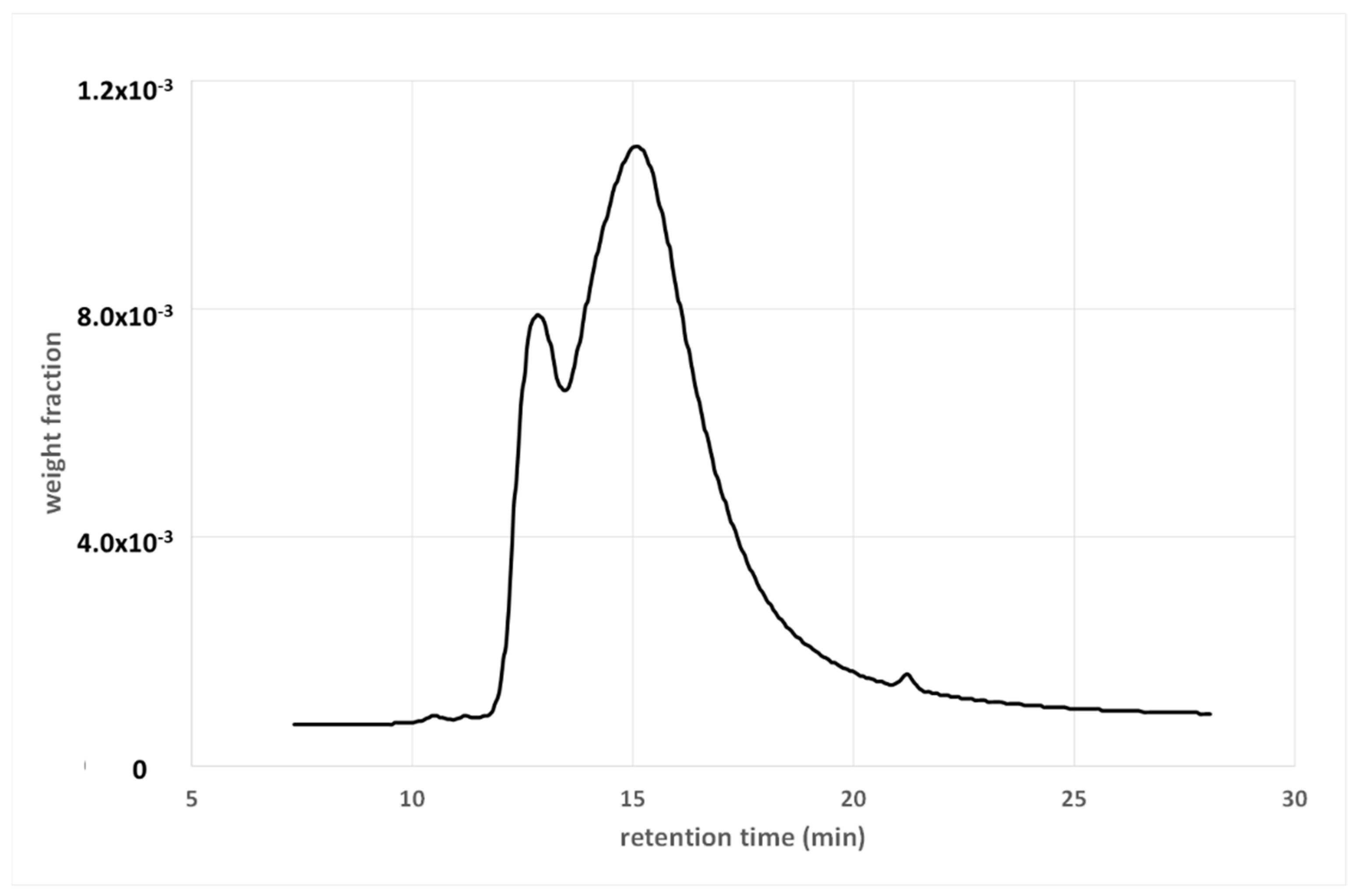
3.1. Gel Permeation Chromatography
- “size exclusion”, which means that the stationary phase works as a molecular sieve, without chemical interactions with the mobile phase;
- The detector records all the molecules;
- A calibration curve is available.
3.1.1. Size Exclusion
3.1.2. Detector
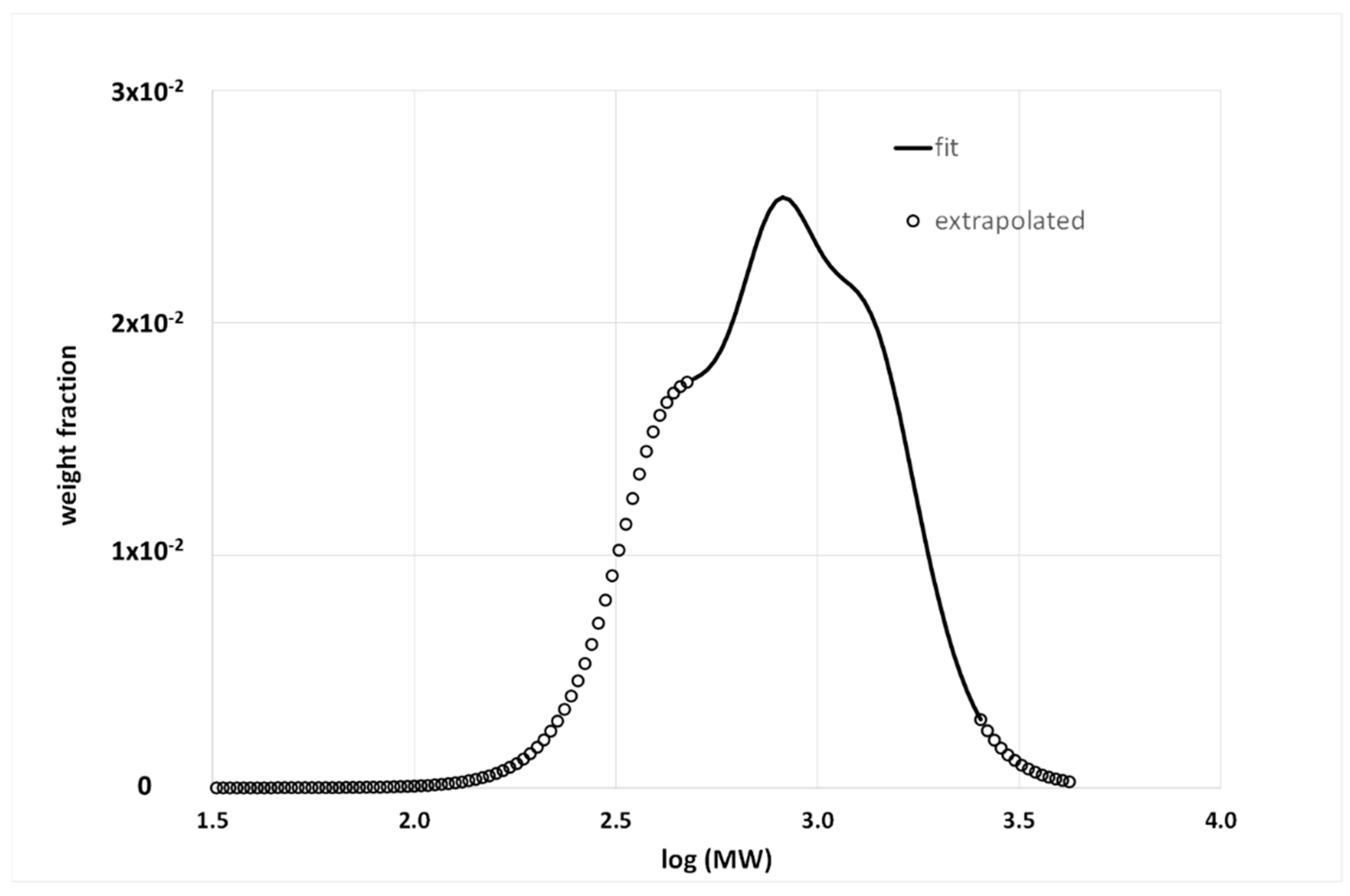
3.1.3. Calibration Curve
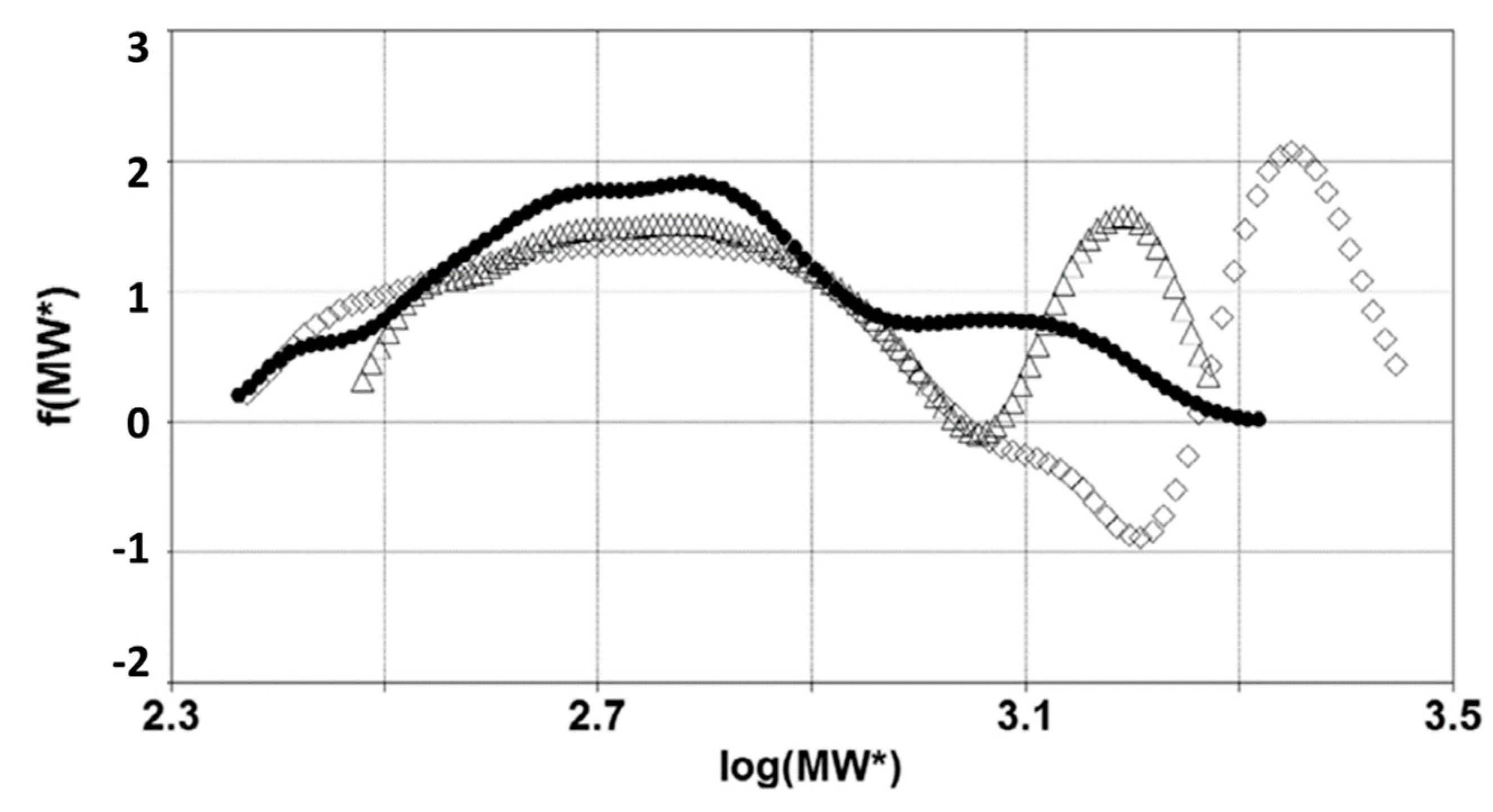
3.2. Inverse Mechanical Approach
4. Applications
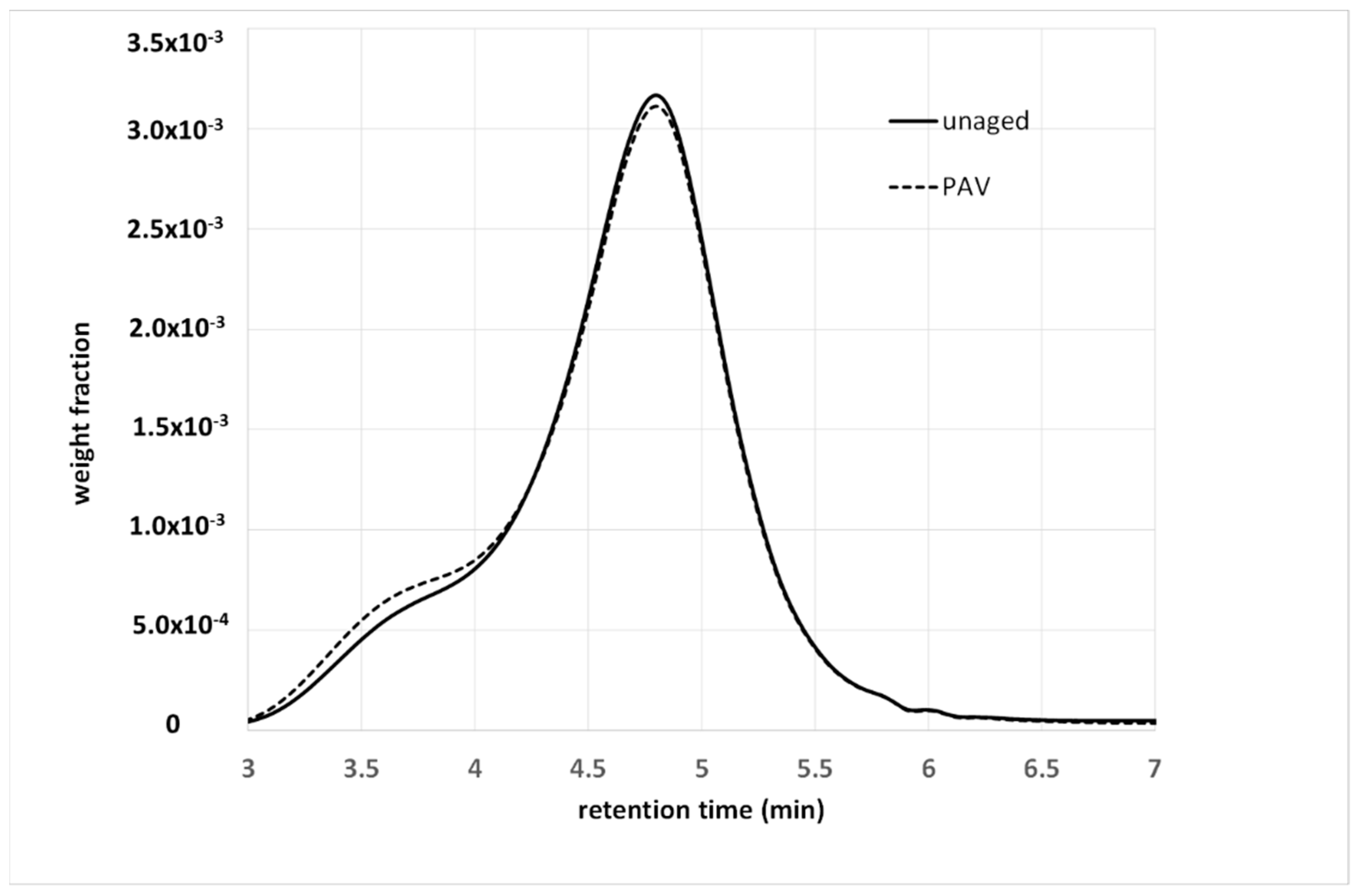
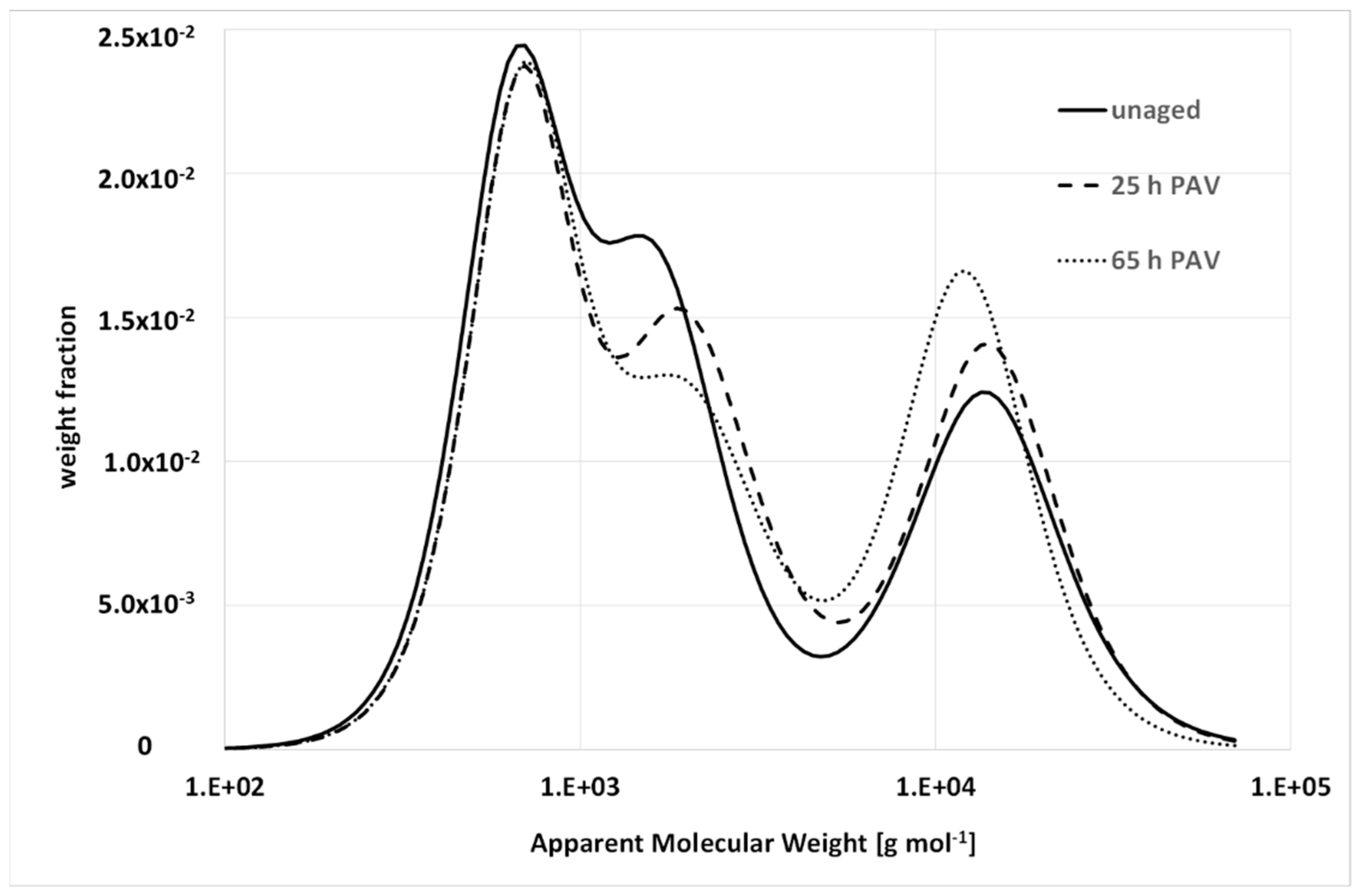
4.1. Aging
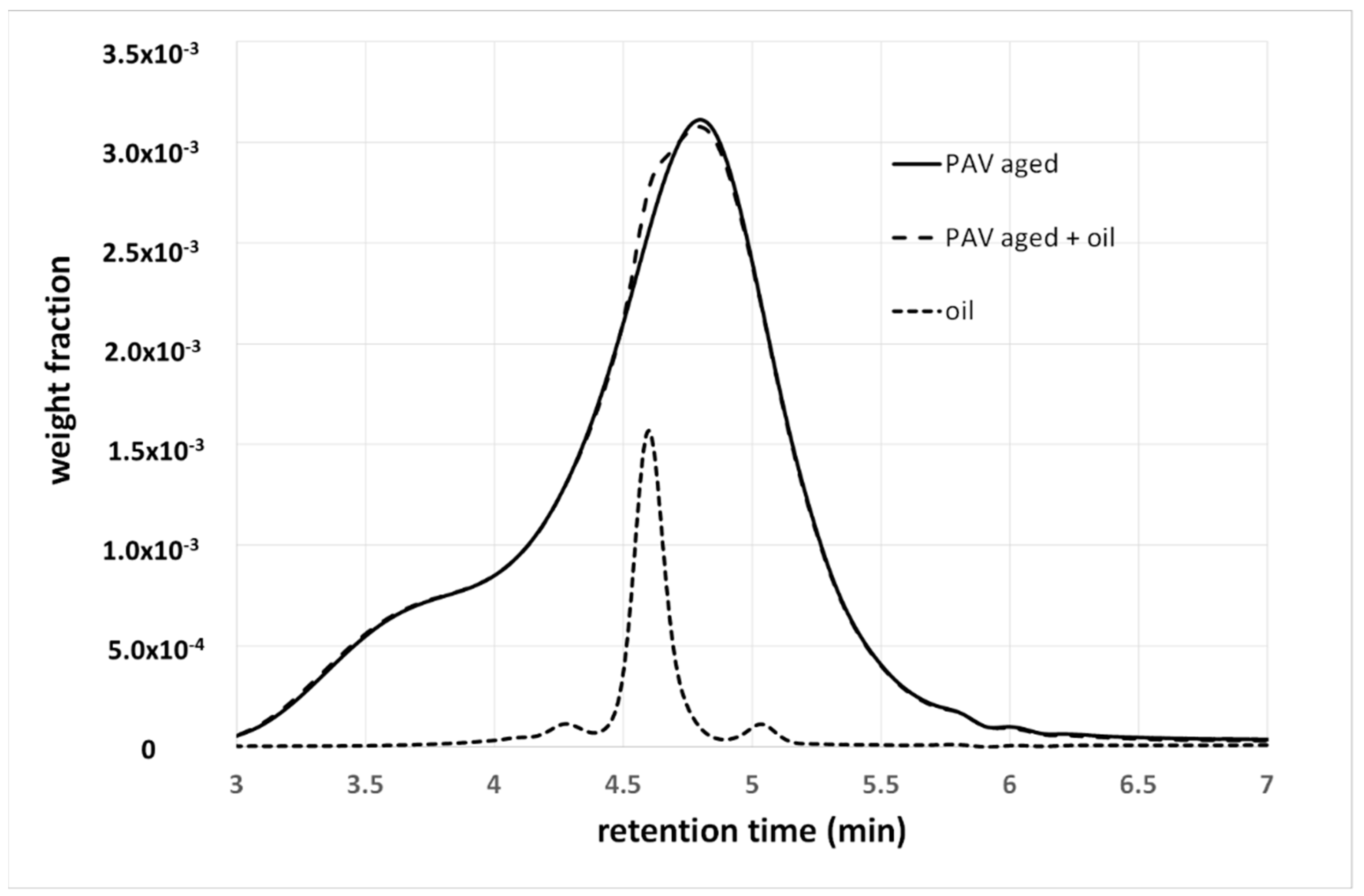
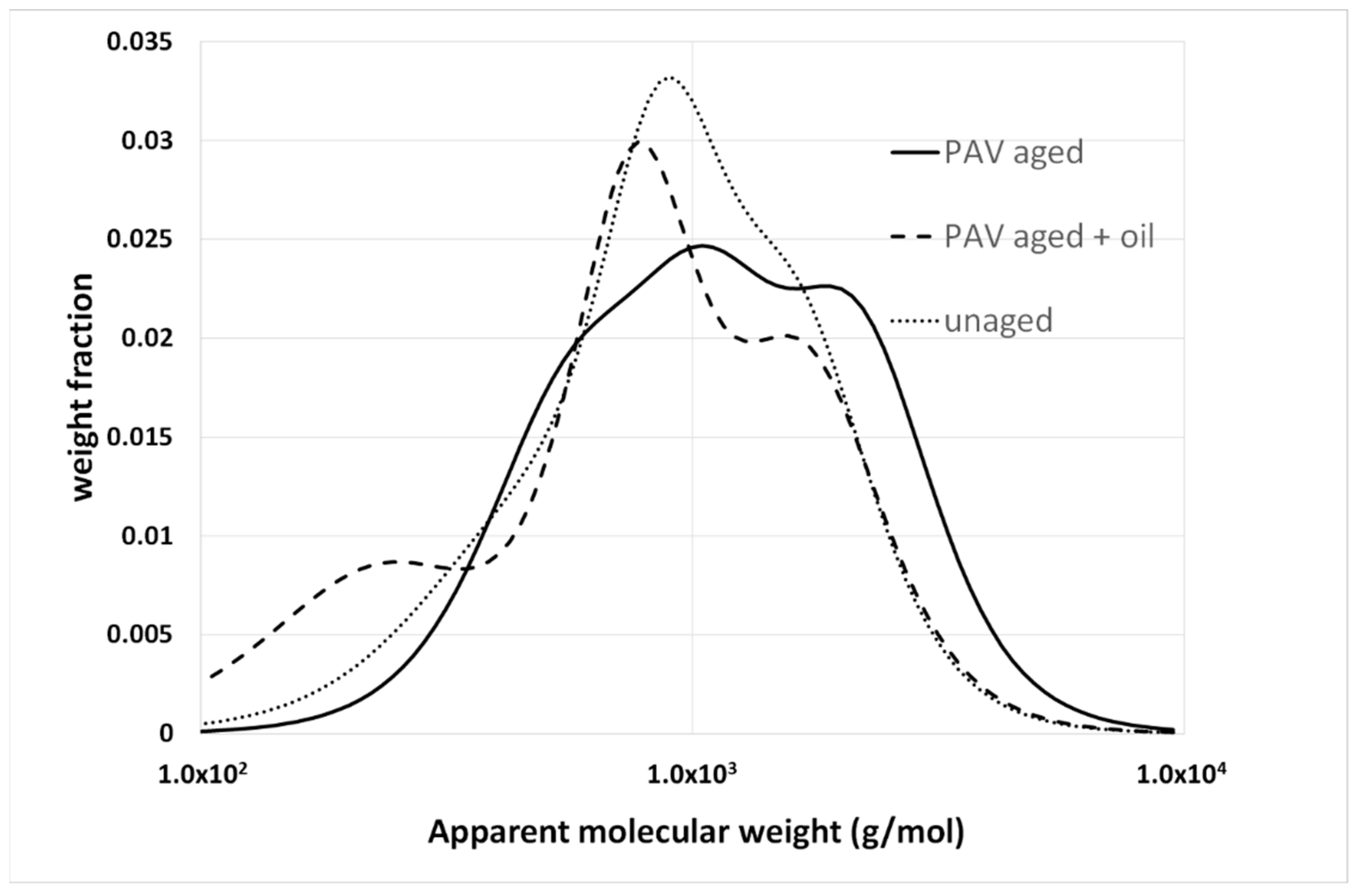
4.2. Additives or Modifiers
4.3. Polymer-Modified Bitumen (PMB)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Sun, G.; Sun, D.; Yu, F.; Hu, M.; Lu, T. Application of gel permeation chromatography technology in asphalt materials: A review. Constr. Build. Mater. 2021, 278, 122386. [Google Scholar] [CrossRef]
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0849339295. [Google Scholar]
- Domin, M.; Herod, A.; Kandiyoti, R.; Larsen, J.W.; Lazaro, M.-J.; Li, A.S.; Rahimi, P. A Comparative Study of Bitumen Molecular-Weight Distributions. Energy Fuels 1999, 13, 552–557. [Google Scholar] [CrossRef]
- Nciri, N.; Cho, N. A Thorough Study on the Molecular Weight Distribution in Natural Asphalts by Gel Permeation Chromatography (GPC): The Case of Trinidad Lake Asphalt and Asphalt Ridge Bitumen. Mater. Today Proc. 2018, 5, 23656–23663. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef]
- Thimm, W.; Friedrich, C.; Marth, M.; Honerkamp, J. An Analytical Relation between Relaxation Time Spectrum and Molecular Weight Distribution. J. Rheol. 1999, 43, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Tuminello, W.H. Molecular weight and molecular weight distribution from dynamic measurements of polymer melts. Polym. Eng. Sci. 1986, 26, 1339–1347. [Google Scholar] [CrossRef]
- Tuminello, W.H.; Cudré-Mauroux, N. Determining Molecular Weight Distributions from Viscosity versus Shear Rate Flow Curves. Polym. Eng. Sci. 1991, 31, 1496–1507. [Google Scholar] [CrossRef]
- Zanzotto, L.; Stastna, J.; Ho, S. Molecular weight distribution of regular asphalts from dynamic material functions. Mater. Struct. 1999, 32, 224–229. [Google Scholar] [CrossRef]
- Themeli, A.; Chailleux, E.; Farcas, F.; Chazallon, C.; Migault, B. Molecular weight distribution of asphaltic paving binders from phase-angle measurements. Road Mater. Pavement Des. 2015, 16, 228–244. [Google Scholar] [CrossRef]
- Yapp, M.T.; Durrani, A.Z.; Finn, F.N. HP-GPC and Asphalt Characterization, Literature Review; SHRP-A/UIR-91-503; National Research Council: Washington, DC, USA, 1991; Volume 143. [Google Scholar]
- Such, C.; Brule, B.; Baluja-Santos, C. Characterization of a Road Asphalt by Chromatographic Techniques (GPC and HPLC). J. Liq. Chromatogr. 1979, 2, 437–453. [Google Scholar] [CrossRef]
- Krolkral, K.; Haddadi, S.; Chailleux, E. Quantification of asphalt binder ageing from apparent molecular weight distributions using a new approximated analytical approach of the phase angle. Road Mater. Pavement Des. 2018, 21, 1045–1060. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Corbett, L.W. Composition of asphalt based on generic fractionation, using solvent deasphaltening, elution-adsorption chromatography, and densimetric characterization. Anal. Chem. 1969, 41, 576–579. [Google Scholar] [CrossRef]
- Mallegni, N.; Cappello, M.; Filippi, S.; Leandri, P.; Polacco, G.; Losa, M. Use of Recycled Oils as Rejuvenators for Bituminous Binders. Chem. Eng. Trans. 2021, 86, 1249–1254. [Google Scholar] [CrossRef]
- Cuciniello, G.; Mallegni, N.; Cappello, M.; Filippi, S.; Leandri, P.; Polacco, G.; Losa, M. Classification and selection of exhausted oils for rejuvenating bituminous blends. Constr. Build. Mater. 2021, 278, 122387. [Google Scholar] [CrossRef]
- Branthaver, J.F.; Petersen, J.C.; Robertson, R.E.; Duvall, J.J.; Kim, S.S.; Harnsberger, P.M.; Mill, T.; Ensley, E.K.; Barbour, F.A.; Schabron, J.F. Binder Characterization and Evaluation. Volume 2: Chemistry; National Research Council: Washington, DC, USA, 1993. [Google Scholar]
- Le Guern, M.; Chailleux, E.; Farcas, F.; Dreessen, S.; Mabille, I. Physico-chemical analysis of five hard bitumens: Identification of chemical species and molecular organization before and after artificial aging. Fuel 2010, 89, 3330–3339. [Google Scholar] [CrossRef]
- Chailleux, E.; Queffélec, C.; Borghol, I.; Farcas, F.; Marceau, S.; Bujoli, B. Bitumen fractionation: Contribution of the individual fractions to the mechanical behavior of road binders. Constr. Build. Mater. 2020, 271, 121528. [Google Scholar] [CrossRef]
- Ninomiya, K.; Fujita, H. Stress-relaxation behavior of polyvinyl acetate films. J. Colloid Sci. 1957, 12, 204–229. [Google Scholar] [CrossRef]
- Fujita, H.; Ninomiya, K. Note on the Calculation of the Molecular Weight Distribution of a Linear Amorphous Polymer from its Relaxation Distribution in the Rubbery Region. J. Phys. Chem. 1957, 61, 814–817. [Google Scholar] [CrossRef]
- Menefee, E.; Peticolas, W.L. Molecular Weight Distribution from the Normal Coordinate Theory of Viscoelasticity. J. Chem. Phys. 1961, 35, 946–950. [Google Scholar] [CrossRef]
- Des Cloizeaux, J. Double Reptation vs Simple Reptation in Polymer Melts. Europhysics Lett. 1988, 5, 437–442. [Google Scholar] [CrossRef]
- Stastna, J.; Zanzotto, L.; Ho, K. Fractional complex modulus manifested in asphalts. Rheol. Acta 1994, 33, 344–354. [Google Scholar] [CrossRef]
- Cuciniello, G.; Filippi, S.; Cappello, M.; Leandri, P.; Polacco, G. A Revised Relationship between Molecular Weight and Re-duced Angular Frequency in δ-Method Applied to Unmodified Petroleum Bitumens. Road Mater. Pavement Des. 2022, 23, 491–508. [Google Scholar] [CrossRef]
- Oeser, M.; Pellinien, T. Computational framework for common visco-elastic models in engineering based on the theory of rheology. Comput. Geotech. 2012, 42, 145–156. [Google Scholar] [CrossRef]
- Yusoff, N.I.M.; Monieur, D.N.I.; Airey, G.D. The 2S2P1D: An Excellent Linear Viscoelastic Model. J. Civ. Eng. Sci. Technol. 2010, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Md. Yusoff, N.I.; Mounier, D.; Marc-Stéphane, G.; Rosli Hainin, M.; Airey, G.D.; Di Benedetto, H. Modelling the rheological properties of bituminous binders using the 2S2P1D Model. Constr. Build. Mater. 2013, 38, 395–406. [Google Scholar] [CrossRef]
- Jasso, M.; Stastna, J.; Polacco, G.; Cuciniello, G. Development of internal structure of polymer-modified asphalts via transformations of the reduced frequency. J. Appl. Polym. Sci. 2020, 138, 50037. [Google Scholar] [CrossRef]
- Polacco, G.; Vacin, O.J.; Biondi, D.; Stastna, J.; Zanzotto, L. Dynamic Master Curves of Polymer Modified Asphalt from Three Different Geometries. Appl. Rheol. 2003, 13, 118–124. [Google Scholar] [CrossRef]
- Cappello, M.; Filippi, S.; Hung, Y.; Losa, M.; Polacco, G. Apparent Molecular Weight Distributions for Investigating Aging in Polymer-Modified Bitumen. Adv. Polym. Technol. 2021, 2021, 3660646. [Google Scholar] [CrossRef]
- Cappello, M.; Polacco, G.; Crépier, J.; Hung, Y.; Filippi, S. Use of Linear Viscoelastic Functions to Estimate the Aging Resistance and Internal Structure of Bituminous Binders. Appl. Sci. 2021, 11, 7483. [Google Scholar] [CrossRef]
- Petersen, J.C.; Glaser, R. Asphalt Oxidation Mechanisms and the Role of Oxidation Products on Age Hardening Revisited. Road Mater. Pavement Des. 2011, 12, 795–819. [Google Scholar] [CrossRef]
- Petersen, J.C. Quantitative method using differential infrared spectrometry for the determination of compound types absorbing in the carbonyl region in asphalts. Anal. Chem. 1975, 47, 112–117. [Google Scholar] [CrossRef]
- Dorrence, S.M.; Barbour, F.A.; Petersen, J.C. Direct evidence of ketones in oxidized asphalts. Anal. Chem. 1974, 46, 2242–2244. [Google Scholar] [CrossRef]
- Lin, P.; Huang, W.; Tang, N.; Xiao, F.; Li, Y. Understanding the low temperature properties of Terminal Blend hybrid asphalt through chemical and thermal analysis methods. Constr. Build. Mater. 2018, 169, 543–552. [Google Scholar] [CrossRef]
- Luo, S.; Tian, J.; Liu, Z.; Lu, Q.; Zhong, K.; Yang, X. Rapid determination of styrene-butadiene-styrene (SBS) content in modified asphalt based on Fourier transform infrared (FTIR) spectrometer and linear regression analysis. Measurement 2019, 151, 107204. [Google Scholar] [CrossRef]
- Cuciniello, G.; Leandri, P.; Filippi, S.; Presti, D.L.; Polacco, G.; Losa, M.; Airey, G. Microstructure and rheological response of laboratory-aged SBS-modified bitumens. Road Mater. Pavement Des. 2019, 22, 372–396. [Google Scholar] [CrossRef]
- Syroezhko, A.M.; Begak, O.Y.; Fedorov, V.V.; Gusarova, E.N. Modification of Paving Asphalts with Sulfur. Russ. J. Appl. Chem. 2003, 76, 491–496. [Google Scholar] [CrossRef]
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polacco, G.; Cappello, M.; Cuciniello, G.; Filippi, S. Apparent Molecular Weight Distributions in Bituminous Binders. Materials 2022, 15, 4700. https://doi.org/10.3390/ma15134700
Polacco G, Cappello M, Cuciniello G, Filippi S. Apparent Molecular Weight Distributions in Bituminous Binders. Materials. 2022; 15(13):4700. https://doi.org/10.3390/ma15134700
Chicago/Turabian StylePolacco, Giovanni, Miriam Cappello, Giacomo Cuciniello, and Sara Filippi. 2022. "Apparent Molecular Weight Distributions in Bituminous Binders" Materials 15, no. 13: 4700. https://doi.org/10.3390/ma15134700
APA StylePolacco, G., Cappello, M., Cuciniello, G., & Filippi, S. (2022). Apparent Molecular Weight Distributions in Bituminous Binders. Materials, 15(13), 4700. https://doi.org/10.3390/ma15134700






