Improving the Mechanical Resistance of Hydroxyapatite/Chitosan Composite Materials Made of Nanofibers with Crystalline Preferential Orientation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Characterization of HAp Nanofibers
2.2.1. X-ray Diffraction
2.2.2. Scanning Electron Microscope
2.2.3. High-Resolution Electron Microscopy
2.3. Characterization of Composite Materials
2.3.1. X-ray Diffraction
2.3.2. Scanning Electron Microscopy
2.3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.4. Raman Spectroscopy
2.3.5. Mechanical Properties
3. Results
3.1. Characterization of HAp Nanofibers
3.1.1. X-ray Diffraction (XRD)
3.1.2. Scanning Electron Microscopy (SEM)
3.1.3. High-Resolution Transmission Electron Microscopy (HRTEM)
3.2. Characterization of Composite Material
3.2.1. X-ray Diffraction
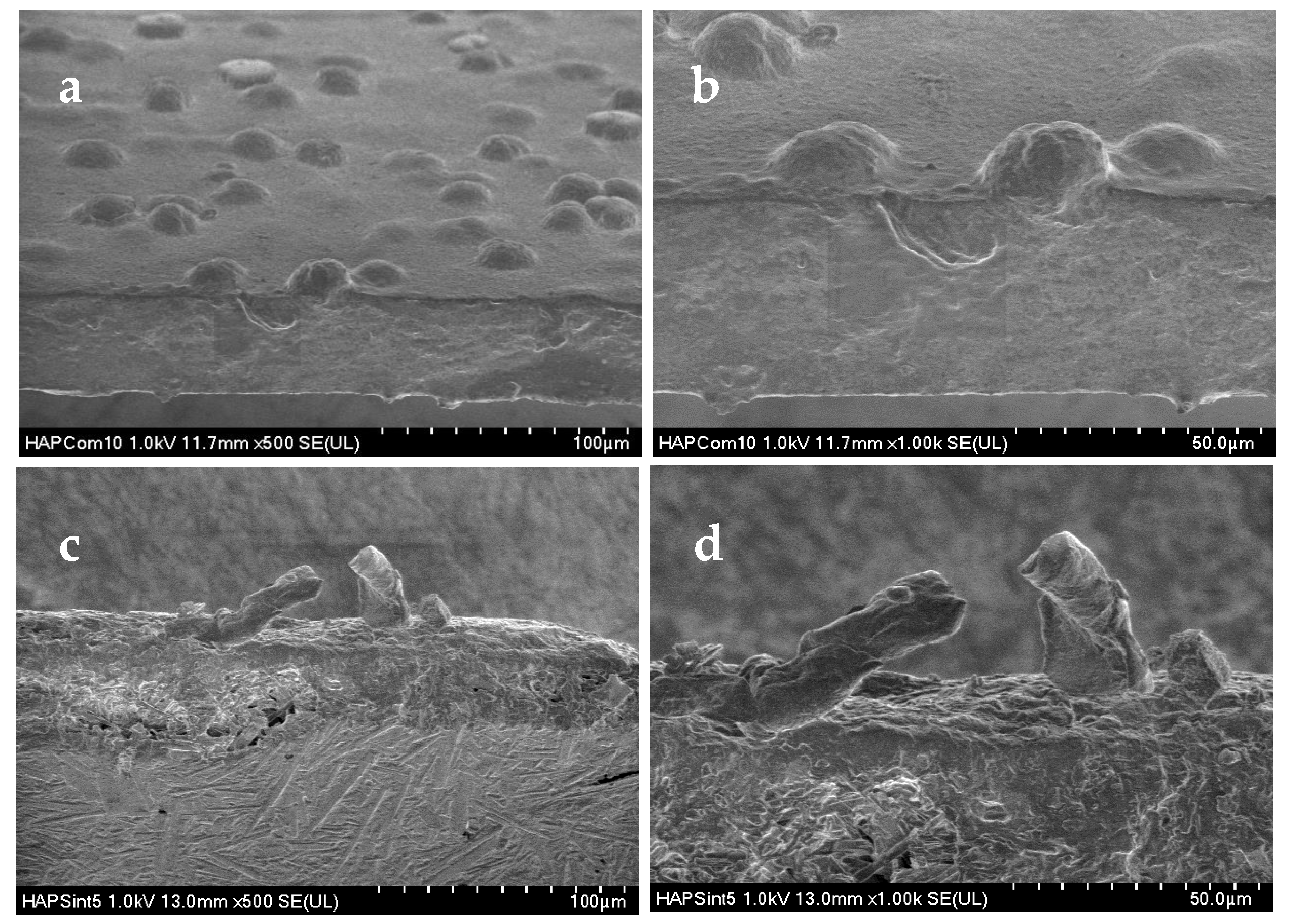
3.2.2. Scanning Electron Microscopy
3.2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2.4. Raman
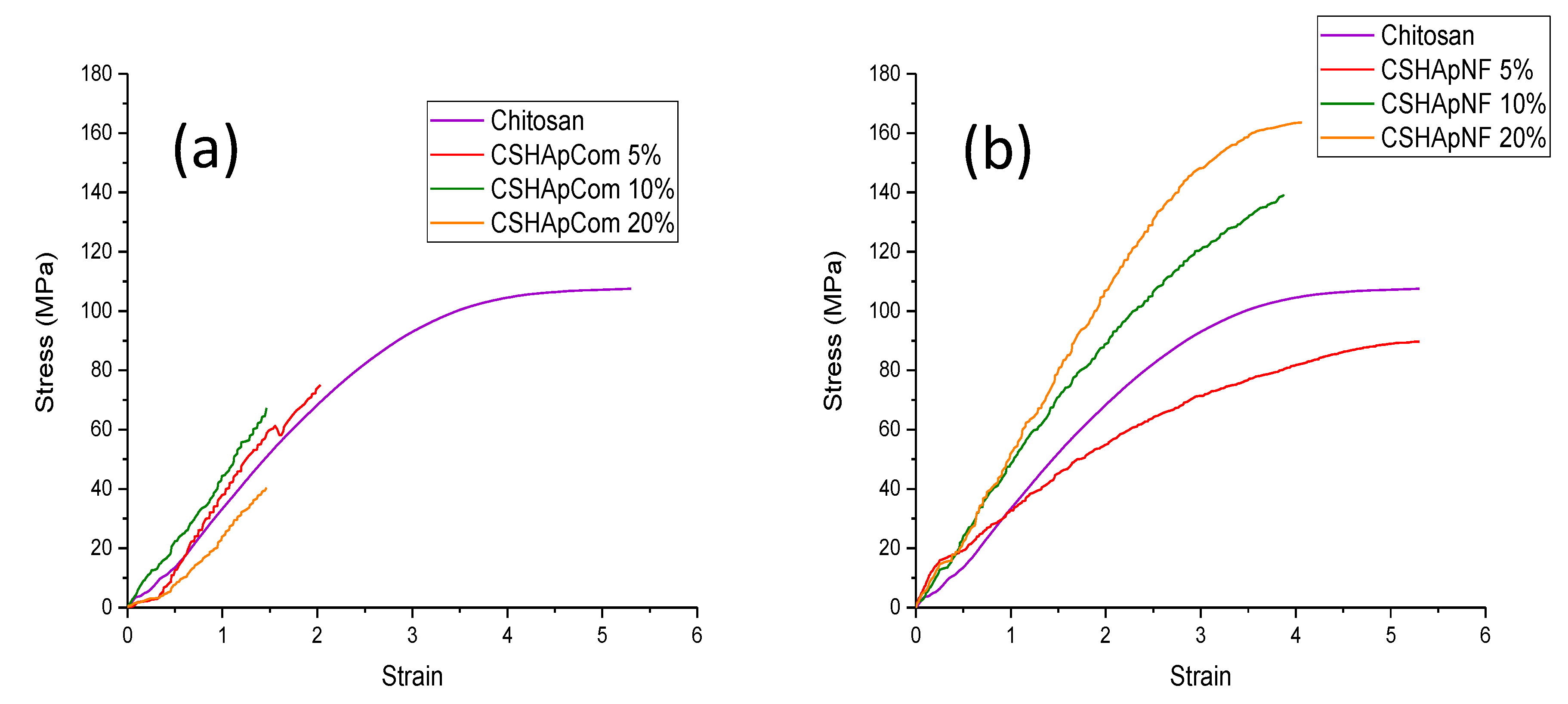
3.2.5. Mechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avashnee, C.; Ilse, W.; Marei, M.K.; Yasser, E.K.; Moussa Rania, M. Synthesis, properties and applications of hydroxyapatite. In Hydroxyapatite: Synthesis, Properties and Applications; Valeri, G., Demirchan Aleksandra, S., Eds.; Nova Sciences Publishers: New York, NY, USA, 2012; pp. 91–132. [Google Scholar]
- Campa, J.; Ulloa, S.; Bucio, L.; Belio, I.A.; Velazquez, R.; Rivera, E. Biomateriales: Fundamentos, Técnicas y Aplicaciones; Editoroal Universidad de Guadalajara: Guadalajara, Mexico, 2007. [Google Scholar]
- Bystrova, A.; Dekhtyar, Y.D.; Popov, A.; Coutinho, J.; Bystrov, V. Modified hydroxyapatite structure and properties: Modeling and synchrotron data analysis of modified hydroxyapatite structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.J. The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: An FT-IR microscopic investigation. Int. J. Artif. Organs 2005, 28, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tredwin, C.J.; Young, A.M.; Georgiou, G.; Shin, S.H.; Kim, H.W.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol–gel method. Optimisation, characterisation and rheology. Dent. Mater. 2013, 29, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tredwin, C.J.; Young, A.M.; Abou Neel, E.A.; Georgiou, G.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol–gel method: Dissolution behaviour and biological properties after crystallisation. J. Mater. Sci. Mater. Med. 2014, 25, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Tredwin, C.J.; Georgiou, G.; Kim, H.W.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol–gel method: Bonding to titanium and scanning electron microscopy. Dent. Mater. 2013, 29, 521–529. [Google Scholar] [CrossRef]
- Javadinejad, H.R.; Ebrahimi-Kahrizsangi, R. Thermal and kinetic study of hydroxyapatite formation by solid-state reaction. Int. J. Chem. Kinet. 2021, 53, 583–595. [Google Scholar] [CrossRef]
- Bazin, T.; Magnaudeix, A.; Mayet, R.; Carles, P.; Julien, I.; Demourgues, A.; Gaudon, M.; Champion, E. Sintering and biocompatibility of copper-doped hydroxyapatite bioceramics. Ceram. Int. 2021, 47, 13644–13654. [Google Scholar] [CrossRef]
- Molino, G.; Palmieri, M.C.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application: A short review. Biomed. Mater. 2020, 15, 022001. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Zhao, W.; Sahai, N. A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J. Mater. Chem. B 2015, 3, 9157–9167. [Google Scholar] [CrossRef]
- Mohandes, F.; Salavati-Niasari, M. Particle size and shape modification of hydroxyapatite nanostructures synthesized via a complexing agent-assisted route. Mater. Sci. Eng. C 2014, 40, 288–298. [Google Scholar] [CrossRef]
- Amer, W.; Abdelouahdi, K.; Ramananarivo, H.R.; Zahouily, M.; Fihri, A.; Coppel, Y.; Varma, R.S.; Solhy, A. Synthesis of mesoporous nano-hydroxyapatite by using zwitterions surfactant. Mater. Lett. 2013, 107, 189–193. [Google Scholar] [CrossRef]
- Moreno-Perez, B.; Matamoros-Veloza, Z.; Rendon-Angeles, J.C.; Yanagisawa, K.; Onda, A.; Pérez-Terrazas, J.E.; Rodríguez-Reyes, M. Synthesis of silicon-substituted hydroxyapatite using hydrothermal process. Boletín Soc. Española Cerámica Vidr. 2020, 59, 50–64. [Google Scholar] [CrossRef]
- Qi, Y.; Shen, J.; Jiang, Q.; Jin, B.; Chen, J.; Zhang, X. The morphology control of hydroxyapatite microsphere at high pH values by hydrothermal method. Adv. Powder Technol. 2015, 26, 1041–1046. [Google Scholar] [CrossRef]
- Nosrati, H.; Mamoory, R.S.; Le, D.Q.S.; Bünger, C.E.; Emameh, R.Z.; Dabir, F. Gas injection approach for synthesis of hydroxyapatite nanorods via hydrothermal method. Mater. Charact. 2020, 159, 110071. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.; Yang, M.; Wang, W.; Bi, Y. Synthesis of mesoporous hydroxyapatite via a vitamin C templating hydrothermal route. Mater. Lett. 2018, 218, 52–55. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Deng, H.; Yao, S.; Wang, Y. Regulatory synthesis and characterization of hydroxyapatite nanocrystals by a microwave-assisted hydrothermal method. Ceram. Int. 2020, 46, 2185–2193. [Google Scholar] [CrossRef]
- Qi, C.; Tang, Q.L.; Zhu, Y.J.; Zhao, X.Y.; Chen, F. Microwave-Assisted Hydrothermal Rapid Synthesis of Hydroxyapatite Nanowires Using Adenosine 5’-Triphosphate Disodium Salt as Phosphorus Source. Mater. Lett. 2012, 85, 71–73. [Google Scholar] [CrossRef]
- Kumar, G.S.; Karunakaran, G.; Girija, E.K.; Kolesnikov, E.; Van Minh, N.; Gorshenkov, M.V.; Kuznetsov, D. Size and morphology-controlled synthesis of mesoporous hydroxyapatite nanocrystals by microwave-assisted hydrothermal method. Ceram. Int. 2018, 44, 11257–11264. [Google Scholar] [CrossRef]
- Yu, H.P.; Zhu, Y.J.; Lu, B.Q. Highly efficient and environmentally friendly microwave-assisted hydrothermal rapid synthesis of ultralong hydroxyapatite nanowires. Ceram. Int. 2018, 44, 12352–12356. [Google Scholar] [CrossRef]
- Montoya-Cisneros, K.L.; Rendón-Ángeles, J.C.; Matamoros-Veloza, Z.; Yanagisawa, K. Rapid synthesis and characterization of Zn substituted hydroxyapatite nanoparticles via a microwave-assisted hydrothermal method. Mater. Lett. 2017, 195, 5–9. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.J.; Cheng, G.F.; Ruan, Y.J.; Sun, T.W.; Chen, F.; Wu, J.; Zhao, X.; Ding, G.J. Microwave-assisted hydrothermal rapid synthesis of amorphous calcium phosphate nanoparticles and hydroxyapatite microspheres using cytidine 5′-triphosphate disodium salt as a phosphate source. Mater. Lett. 2014, 124, 208–211. [Google Scholar] [CrossRef]
- Venkatesan, J.; Pallela, R.; Bhatnagar, I.; Kim, S.K. Chitosan–amylopectin/hydroxyapatite and chitosan–chondroitin sulphate/hydroxyapatite composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Bahrololoom, M.E.; Vashaee, D.; Tayebi, L. In situ preparation of iron oxide nanoparticles in natural hydroxyapatite/chitosan matrix for bone tissue engineering application. Ceram. Int. 2015, 41, 3094–3100. [Google Scholar] [CrossRef]
- Chudhuri, B.; Bhadra, D.; Dash, S.; Sardar, G.; Pramanik, K.; Chaudhuri, B.K. Hydroxyapatite and hydroxyapatite-chitosan composite from crab shell. J. Biomater. Tissue Eng. 2013, 3, 653–657. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Z.; Yang, Y.; Jian, Y.; Li, R.; Dai, X.; Wu, W.; Zhong, J.; Chen, C. Synthesis, characterization and biological performance study of Sr-doped hydroxyapatite/chitosan composite coatings. Mater. Chem. Phys. 2021, 270, 124752. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Wu, Q.; Zuo, J.; Qin, Y.; Wang, J. Novel Mesoporous Hydroxyapatite/Chitosan Composite for Bone Repair. J. Bionic Eng. 2012, 9, 243–251. [Google Scholar] [CrossRef]
- Sutha, S.; Kavitha, K.; Karunakaran, G.; Rajendran, V. In-Vitro Bioactivity, Biocorrosion and Antibacterial Activity of Silicon Integrated Hydroxyapatite/Chitosan Composite Coating on 316L Stainless Steel Implants. Mater. Sci. Eng. 2013, 33, 4046–4054. [Google Scholar] [CrossRef]
- Bazargan-Lari, R.; Zafarani, H.R.; Bahrololoom, M.E.; Nemati, A. Removal of Cu(II) Ions from Aqueous Solutions by Low-Cost Natural Hydroxyapatite/Chitosan Composite: Equilibrium, Kinetic and Thermodynamic Studies. J. Taiwan Inst. Chem. Eng. 2014, 45, 1642–1648. [Google Scholar] [CrossRef]
- Bambaeero, A.; Bazargan-Lari, R. Simultaneous removal of copper and zinc ions by low cost natural snail shell/hydroxyapatite/chitosan composite. Chin. J. Chem. Eng. 2021, 33, 221–230. [Google Scholar] [CrossRef]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of Nano-Hydroxyapatite/Chitosan Composite for Cadmium Ions Removal in Wastewater Treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Maddila, S.; Gangu, K.K.; Maddila, S.N.; Jonnalagadda, S.B. A Facile, Efficient, and Sustainable Chitosan/CaHAp Catalyst and One-Pot Synthesis of Novel 2,6-Diamino-Pyran-3,5-Dicarbonitriles. Mol. Divers. 2017, 21, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Samant, A.; Misra, P.K.; Saxena, M. Nanocrystalline Hydroxyapatite: A Potent Material for Adsorption, Biological and Catalytic Studies. Mater. Today Proc. 2019, 9, 689–698. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Wang, J.; Qu, Y.; Liu, G. In Vivodrug Release and Antibacterial Properties of Vancomycin Loaded Hydroxyapatite/Chitosan Composite. Drug Deliv. 2019, 19, 264–269. [Google Scholar] [CrossRef]
- Uskoković, V.; Tejal, A.D. In Vitro Analysis of Nanoparticulate Hydroxyapatite/Chitosan Composites as Potential Drug Delivery Platforms for the Sustained Release of Antibiotics in the Treatment of Osteomyelitis. J. Pharm. Sci. 2014, 103, 567–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Jin, H.; Cai, J. Preparation and characterization of nano-hydroxyapatite/chitosan composite with enhanced compressive strength by urease-catalyzed method. Mater. Lett. 2014, 116, 293–295. [Google Scholar] [CrossRef]
- Mohamed, K.R.; Beherei, H.H.; El-Rashidy, Z.M. In vitro study of nano-hydroxyapatite/chitosan–gelatin composites for bio-applications. J. Adv. Res. 2014, 5, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Said, H.A.; Noukrati, H.; Ben Youcef, H.; Bayoussef, A.; Oudadesse, H.; Barroug, A. Mechanical behavior of hydroxyapatite-chitosan composite: Effect of processing parameters. Minerals 2021, 11, 213. [Google Scholar] [CrossRef]
- Tavakol, S.; Kikpour, M.R.; Armani, A.; Soltani, M.; Rabiee, S.M.; Rezayat, S.M.; Jahanshahi, M. Bone Regeneration Based on Nano-Hydroxyapatite and Hydroxyapatite/Chitosan Nanocomposites: An in Vitro and in Vivo Comparative Study. J. Nanoparticle Res. 2013, 15, 1373. [Google Scholar] [CrossRef]
- Guo, Y.P.; Guan, J.J.; Yang, J.; Wang, Y.; Zhang, C.Q.; Ke, Q.F. Hybrid nanostructured hydroxyapatite–chitosan composite scaffold: Bioinspired fabrication, mechanical properties and biological properties. J. Mater. Chem. B 2015, 3, 4679–4689. [Google Scholar] [CrossRef]
- Liaw, B.S.; Chang, T.T.; Chang, H.K.; Liu, W.K.; Chen, P.Y. Fish scale-extracted hydroxyapatite/chitosan composite scaffolds fabricated by freeze casting—An innovative strategy for water treatment. J. Hazard. Mater. 2020, 382, 121082. [Google Scholar] [CrossRef]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.K. In Vivo Study of Chitosan-Natural Nano Hydroxyapatite Scaffolds for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, J.; Ke, Q.; Gao, Y.; Zhang, C.; Gou, Y. Bioinspired Fabrication of Carbonated Hydroxyapatite/Chitosan Nanohybrid Scaffolds Loaded with TWS119 for Bone Regeneration. Chem. Eng. J. 2018, 341, 112–125. [Google Scholar] [CrossRef]
- Alanís, J.R.; Rivera, E.M.; Cervantes, J.S.; Almanza, R.H.; Nava, M.R.; Cortes, R.C.; Velázquez, C.R. Synthesis of Micro and Nano-Sized Hydroxyapatite Fibers Through the Microwave Assisted Hydrothermal Method. J. Nanosci. Nanotechnol. 2016, 16, 7557–7566. [Google Scholar] [CrossRef]
- Méndez, L.N.; Velázquez, C.R.; Rivera, M.E.; Bucio, G.L.; Mondragón, G.G.; Manzano, R.A.; Apátiga, L.M. Crystal growth and structural analysis of hydroxyapatite nanofibers synthesized by the hydrothermal microwave-assisted method. Ceram. Int. 2017, 43, 451–457. [Google Scholar] [CrossRef]
- Alanís-Gómez, J.R.; Rivera-Muñoz, E.M.; Peza-Ledesma, C.; Manzano-Ramírez, A.; Velázquez-Castillo, R. A comparison of mechanical properties of different hydroxyapatite (HAp) based nanocomposites: The influence of morphology and preferential orientation. J. Nanosci. Nanotechnol. 2020, 20, 1968–1976. [Google Scholar] [CrossRef]
- Kusrini, E.; Sontang, M. Characterization of X-ray diffraction and electron spin resonance: Effects of sintering time and temperature on bovine hydroxyapatite. Radiat. Phys. Chem. 2012, 81, 118–125. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- An, L.; Li, W.; Xu, Y.; Zeng, D.; Cheng, Y.; Wang, G. Controlled additive-free hydrothermal synthesis and characterization of uniform hydroxyapatite nanobelts. Ceram. Int. 2016, 42, 3104–3112. [Google Scholar] [CrossRef]
- Jaber, H.L.; Hammood, A.S.; Parvin, N. Synthesis and characterization of hydroxyapatite powder from natural camelus bone. J. Aust. Ceram. Soc. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Mohandes, F.; Salavati-Niasari, M.; Fathi, M.; Fereshteh, Z. Hydroxyapatite nanocrystals: Simple preparation, characterization and formation mechanism. Mater. Sci. Eng. C 2014, 45, 29–36. [Google Scholar] [CrossRef]
- Szatkowski, T.; Kołodziejczak-Radzimska, A.; Zdarta, J.; Szwarc-Rzepka, K.; Paukszta, D.; Wysokowski, M.; Ehrlich, H.; Jesionowski, T. Synthesis and characterization of hydroxyapatite/chitosan composites. Physicochem. Probl. Miner. Processing 2015, 51, 575–585. [Google Scholar]
- Nikpour, M.R.; Rabiee, S.M.; Jahanshahi, M.J.C.P.B.E. Synthesis and characterization of hydroxyapatite/chitosan nanocomposite materials for medical engineering applications. Compos. Part B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Bita, B.; Stancu, E.; Stroe, D.; Dumitrache, M.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Groza, A. The effects of electron beam irradiation on the morphological and physicochemical properties of magnesium-doped hydroxyapatite/chitosan composite coatings. Polymers 2022, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.; Ravichandran, Y.D.; Nambi Raj, N.A.; Senthilkumar, N. Development of a biodegradable composite (hydroxyapatite-chitosan-coir pith) as a packing material. Polym.-Plast. Technol. Eng. 2014, 53, 1105–1110. [Google Scholar] [CrossRef]
- Mohonta, S.K.; Maria, K.H.; Rahman, S.; Das, H.; Hoque, S.M. Synthesis of hydroxyapatite nanoparticle and role of its size in hydroxyapatite/chitosan–gelatin bio-composite for bone grafting. Int. Nano Lett. 2021, 11, 381–393. [Google Scholar] [CrossRef]
- Jin, H.H.; Lee, C.H.; Lee, W.K.; Lee, J.K.; Park, H.C.; Yoon, S.Y. In situ formation of the hydroxyapatite/chitosan-alginate composite scaffolds. Mater. Lett. 2008, 62, 1630–1633. [Google Scholar] [CrossRef]
- Ruksudjarit, A.; Pengpat, K.; Rujijanagul, G.; Tunkasiri, T. Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr. Appl. Phys. 2008, 8, 270–272. [Google Scholar] [CrossRef]
- Osman, Z.; Arof, A.K. FTIR studies of chitosan acetate-based polymer electrolytes. Electrochim. Acta 2003, 48, 993–999. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.C.; Lin, C.J. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nanocomposites for use in biomedical materials. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- De Aza, P.N.; Guitian, F.; Santos, C.; De Aza, S.; Cusco, R.; Artus, L. Vibrational properties of calcium phosphate compounds. 2. Comparison between hydroxyapatite and β-tricalcium phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier transform Raman spectroscopy of synthetic and biological calcium phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Djosic, M.; Jankovic, A.; Kojic, V.; Vukasinovic-Sekulic, M.; Stojanovic, J.; Odovic, J.; Crevar-Sakac, M.; Kyong-Yop, R.; Miskovic-Stankovic, V. Antibacterial graphene-based hydroxyapatite/chitosan coating with gentamicin for pottencial applications in bone tissue engineering. J. Biomed. Mater. Res. Part A 2020, 108, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2004. [Google Scholar]
- Li, C.; Liu, S.; Li, G.; Bai, J.; Wang, W.; Du, Q. Hydrothermal synthesis of large-sized hydroxyapatite whiskers regulated by glutamic acid in solutions with low supersaturation of precipitation. Adv. Powder Technol. 2011, 22, 537–543. [Google Scholar] [CrossRef]
- Shulin, W. Human enamel structure studied by high resolution electron microscopy. Electron Microsc. Rev. 1989, 2, 1–16. [Google Scholar] [CrossRef]
- Brès, E.F.; Voegel, J.C.; Frank, R.M. High-resolution electron microscopy of human enamel crystals. J. Microsc. 1990, 160, 183–201. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Bres, E.F. Crystallographic structure of human tooth enamel by electron microscopy and x-ray diffraction: Hexagonal or monoclinic? J. Microsc. 2012, 248, 102–109. [Google Scholar] [CrossRef]
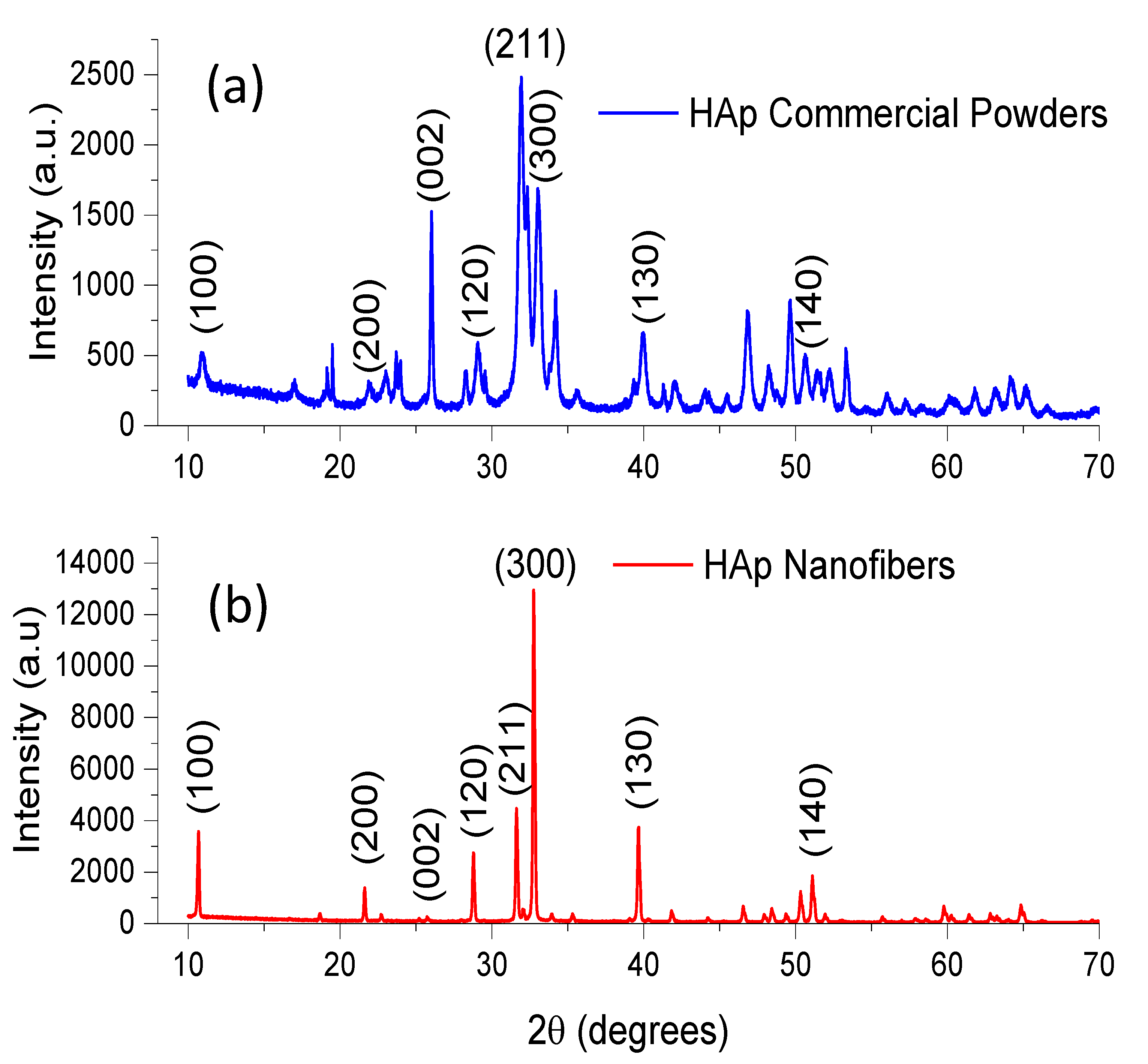



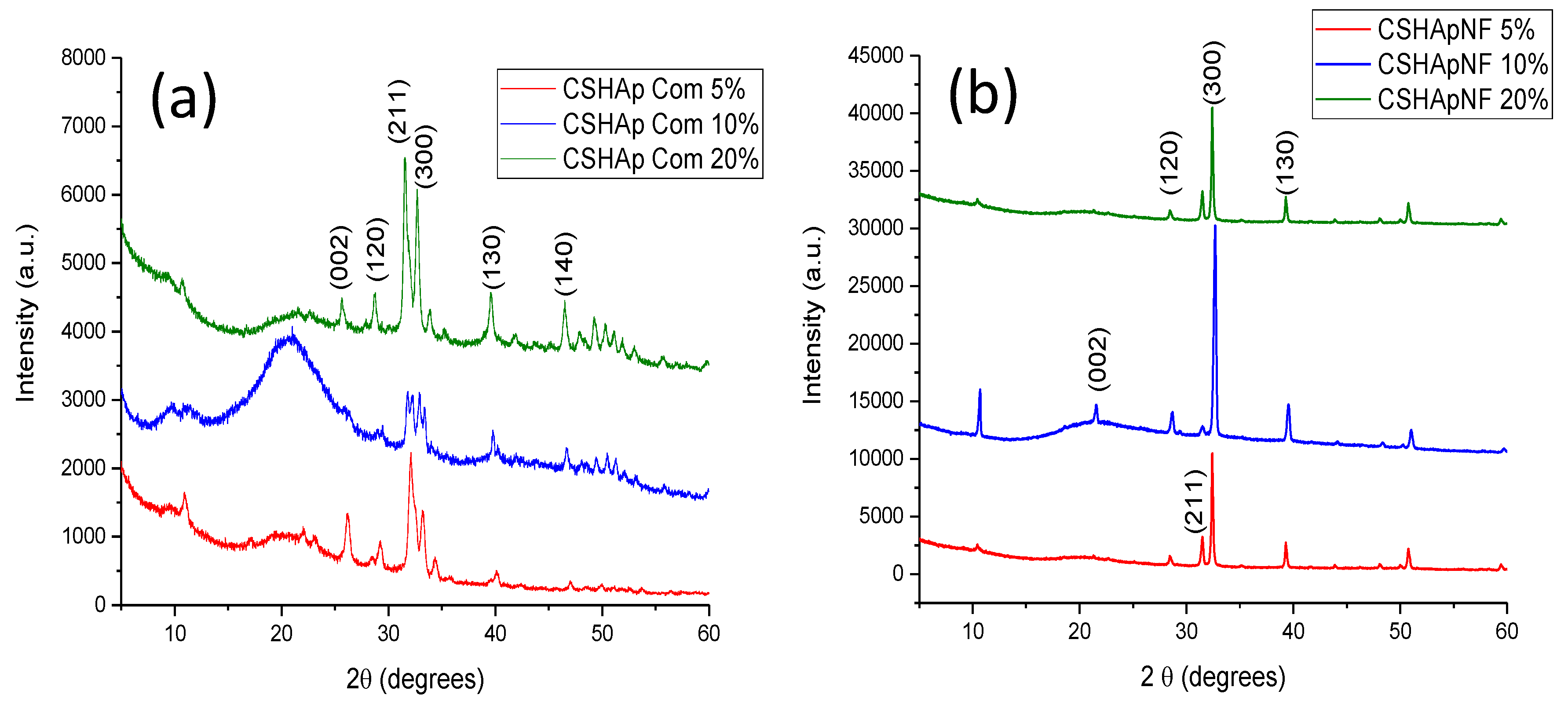
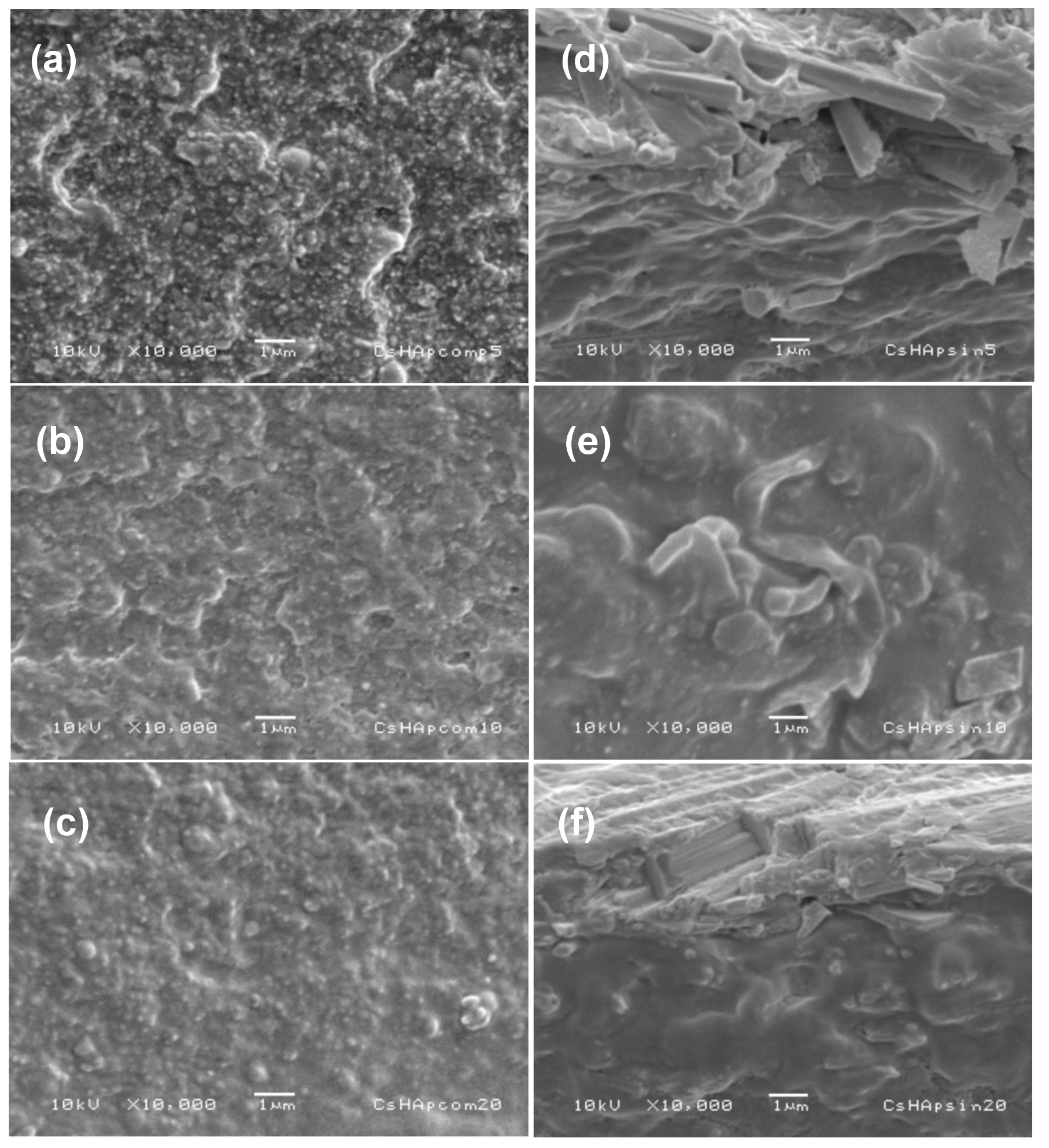
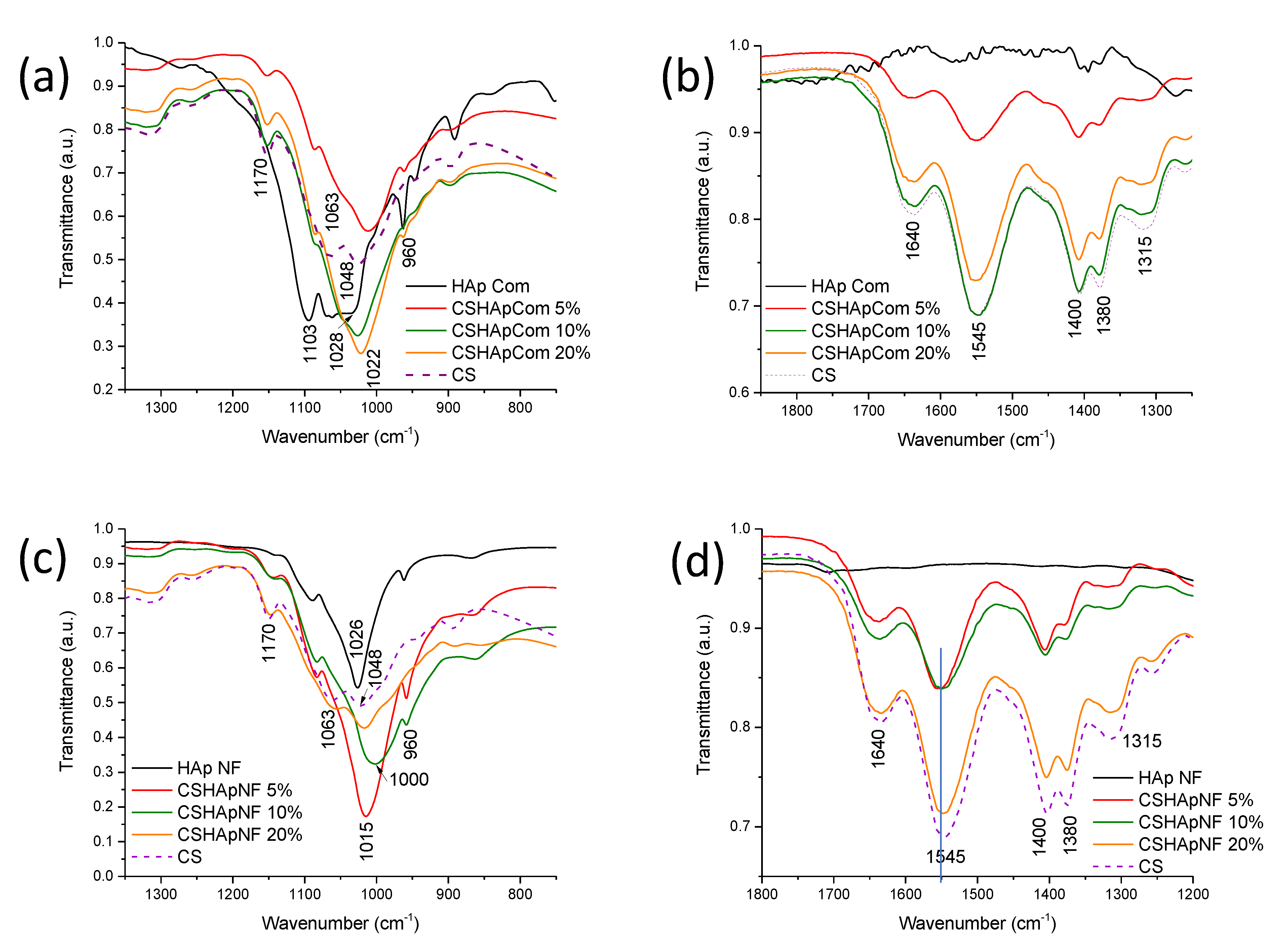

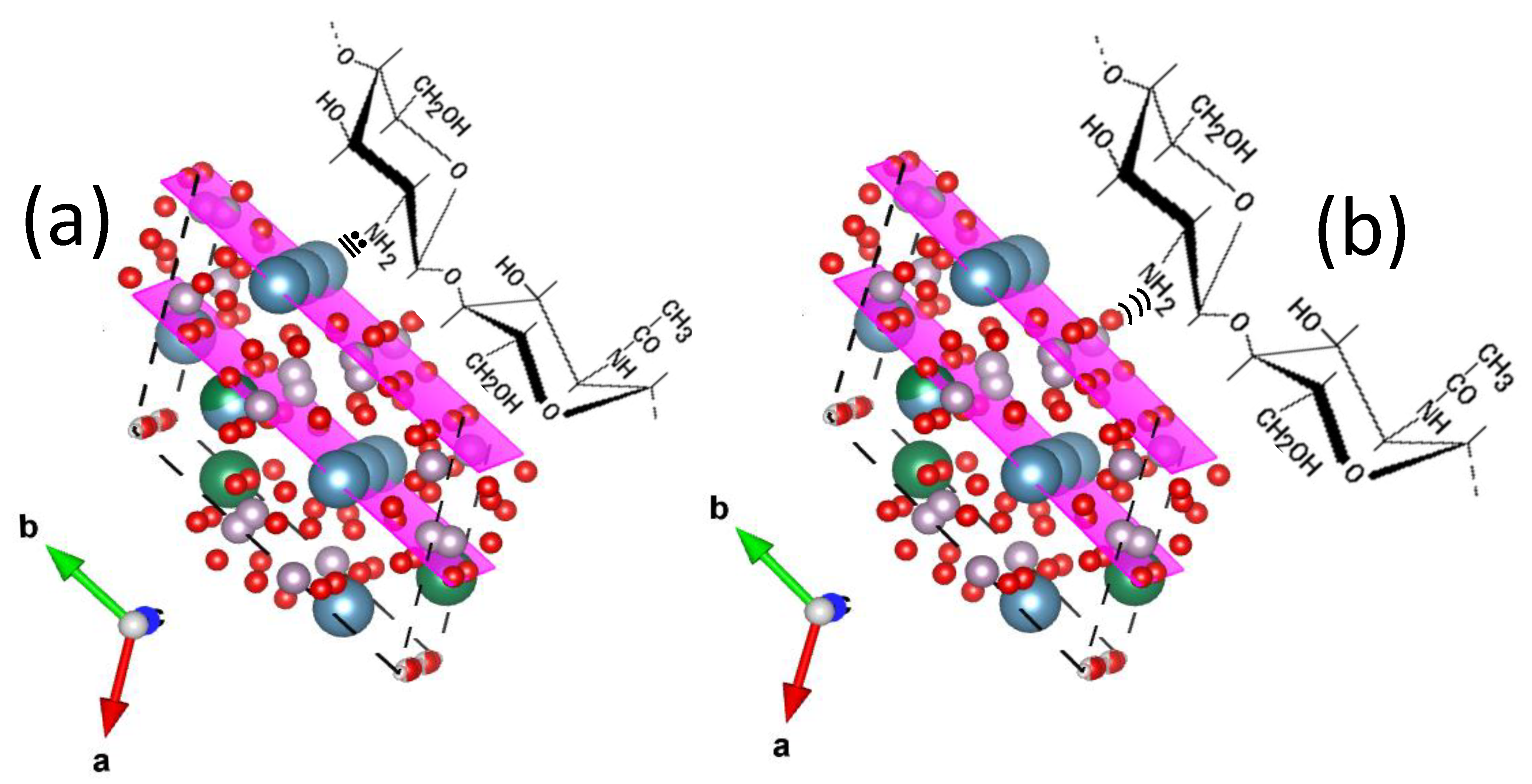
| Signal at (cm−1) in Pure HAp or Pure Chitosan | Group | Type of Vibration | Signal at (cm−1) in Composite Material and Type of Variation in the Signal | References |
|---|---|---|---|---|
| 960 | Phosphate | Symmetrical deformation | 960; transmittance variation | [58,59] |
| 1103 | Phosphate | Asymmetrical deformation | 1130; transmittance variations | |
| 1028 Commercial HAp | Phosphate | Position shift to 1022 RM 1 1024 RM 2 and 3 | ||
| 1026 HAp Nanofibers (narrower than comm. HAP) | Phosphate | Asymmetrical deformation | Consistent widening and position shift to: 1015 in CNF with 5% NF; 1000 in CNF with 10% NF; 1015 in CNF with 20% NF | |
| 1048 | -C-O | Vibration of glucose ring | Transmittance variations in RM Overlap with signal at 1026 in CNF | [55,56,58,59] |
| 1063 | ||||
| 1315 | C=O | Symmetrical vibration | 1315; transmittance variation in RM; widening and transmittance changes in CNF | [60] |
| 1380 | C-O | Vibration of (-CH2-OH) group | 1380; unexpected transmittance variations in RM; shift in CNF | [61] |
| 1400 | C-O | Vibration of (-CH-OH) group | 1400; unexpected transmittance variations in RM; shift in CNF | |
| 1545 | -N-H | Vibration of amines | 1545; unexpected transmittance variations in RM; widening in CNF | |
| 1640 | C=O | Strong vibration of amides | 1640; transmittance variation in RM; widening and expected transmittance variation in CNF | [60] |
| Signal at (cm−1) in Pure HAp or Pure Chitosan | Group | Type of Vibration | Signal at (cm−1) in Composite Material and Type of Variation of the Signal | References |
|---|---|---|---|---|
| 895 | C-O-C | Symmetric stretch | 895; irregular intensity reduction in RM 895; expected intensity reduction in CNF | [27,57,65] |
| 1030 | C-O | Deformation | 1030; irregular intensity reduction in RM 1030; expected intensity reduction in CNF | |
| 1080 | 1080; irregular intensity reduction in RM 1080; expected intensity reduction in CNF | |||
| 1115 | C-O-C | Asymmetric stretch | 1030; irregular intensity reduction in RM 1030; expected intensity reduction in CNF | |
| 960 | Phosphate | Symmetrical deformation | 960; intensity and width variation in RM 960; inconsistent intensity reduction |
| Sample | Young’s Modulus (MPa) | Ultimate Strength (MPa) |
|---|---|---|
| Reference 1 | 21.1927 ± 1.102 | 40.316 ± 2.177 |
| Reference 2 | 42.5532 ± 2.16 | 71.5969 ± 3.56 |
| Reference 3 | 43.2241 ± 2.171 | 74.6025 ± 3.954 |
| CSHApNF 5% | 23.7201 ± 1.138 | 89.664 ± 3.855 |
| CSHApNF 10% | 43.611 ± 1.831 | 138.881 ± 5.833 |
| CSHApNF 20% | 54.345 ± 2.44 | 163.603 ± 6.567 |
| Chitosan | 35.6892 ± 1.788 | 107.5055 ± 5.396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanis-Gómez, R.P.; Rivera-Muñoz, E.M.; Luna-Barcenas, G.; Alanis-Gómez, J.R.; Velázquez-Castillo, R. Improving the Mechanical Resistance of Hydroxyapatite/Chitosan Composite Materials Made of Nanofibers with Crystalline Preferential Orientation. Materials 2022, 15, 4718. https://doi.org/10.3390/ma15134718
Alanis-Gómez RP, Rivera-Muñoz EM, Luna-Barcenas G, Alanis-Gómez JR, Velázquez-Castillo R. Improving the Mechanical Resistance of Hydroxyapatite/Chitosan Composite Materials Made of Nanofibers with Crystalline Preferential Orientation. Materials. 2022; 15(13):4718. https://doi.org/10.3390/ma15134718
Chicago/Turabian StyleAlanis-Gómez, Ricardo Pascual, Eric Mauricio Rivera-Muñoz, Gabriel Luna-Barcenas, José Rafael Alanis-Gómez, and Rodrigo Velázquez-Castillo. 2022. "Improving the Mechanical Resistance of Hydroxyapatite/Chitosan Composite Materials Made of Nanofibers with Crystalline Preferential Orientation" Materials 15, no. 13: 4718. https://doi.org/10.3390/ma15134718
APA StyleAlanis-Gómez, R. P., Rivera-Muñoz, E. M., Luna-Barcenas, G., Alanis-Gómez, J. R., & Velázquez-Castillo, R. (2022). Improving the Mechanical Resistance of Hydroxyapatite/Chitosan Composite Materials Made of Nanofibers with Crystalline Preferential Orientation. Materials, 15(13), 4718. https://doi.org/10.3390/ma15134718







