Evaporation of As and Sn from Liquid Iron: Experiments and a Kinetic Model during Top-Blown Oxygen Steelmaking Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Apparatus and Procedure
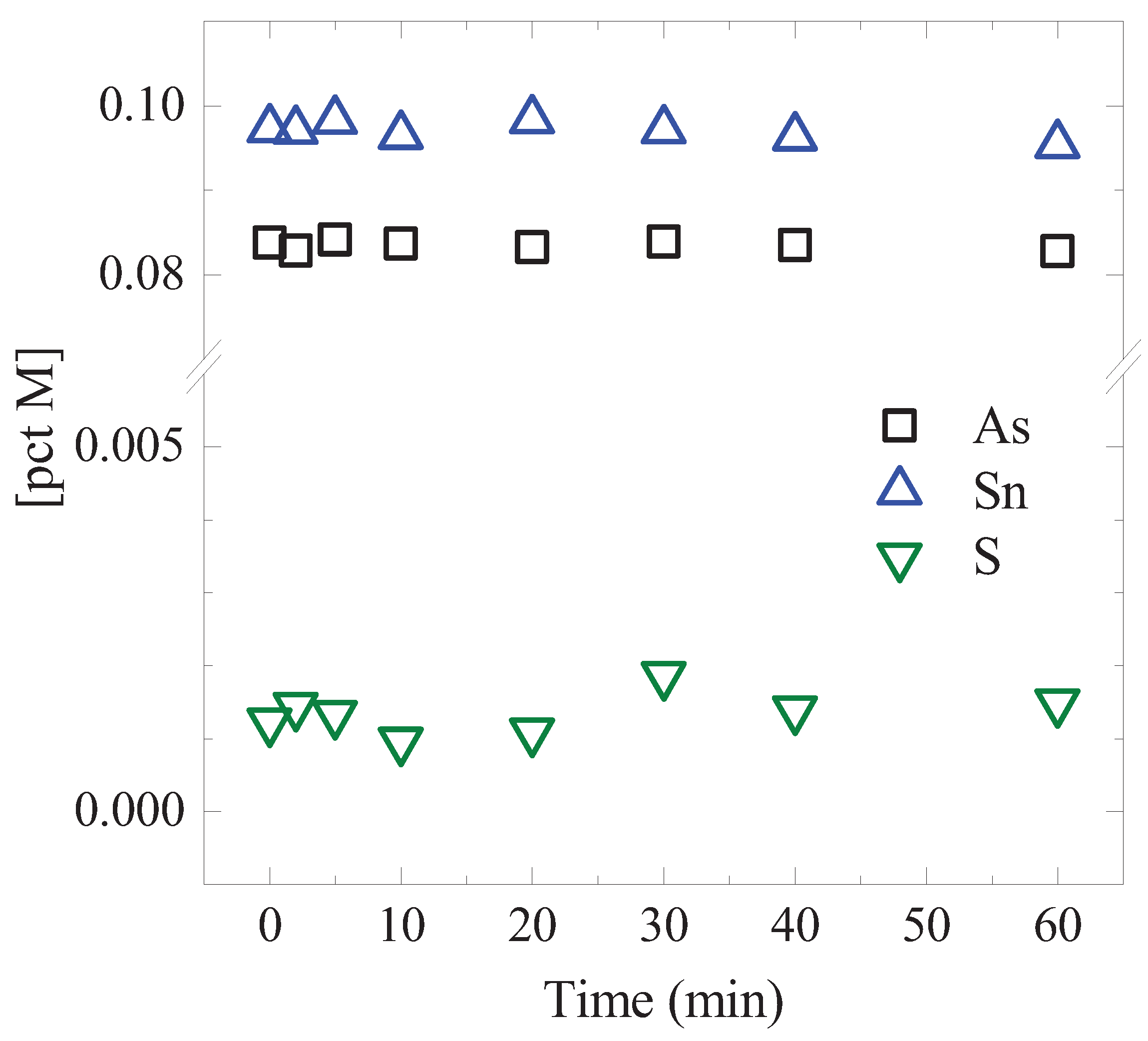
3. A Preliminary Test
4. Evaporation of As and Sn from a droplet
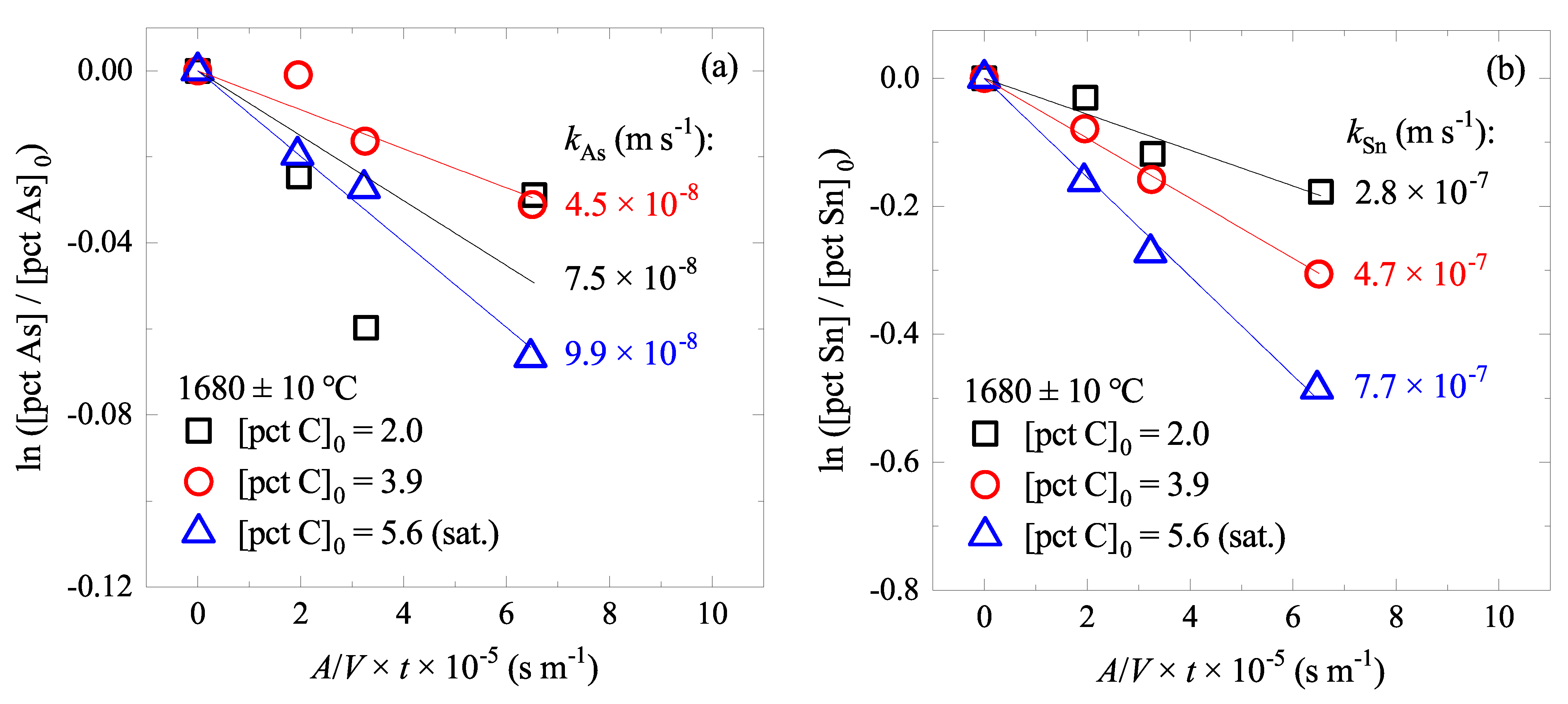
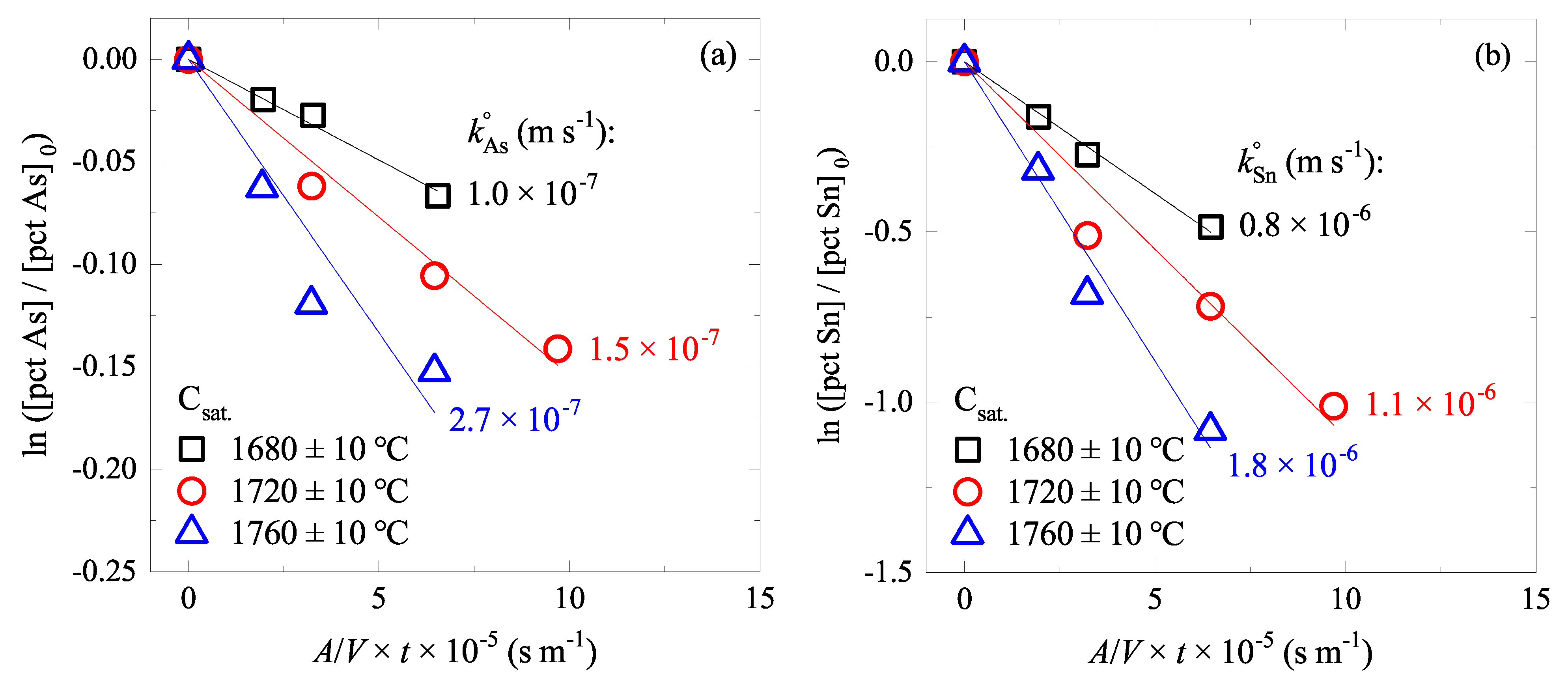
4.1. Effect of C Content on As and Sn Evaporations
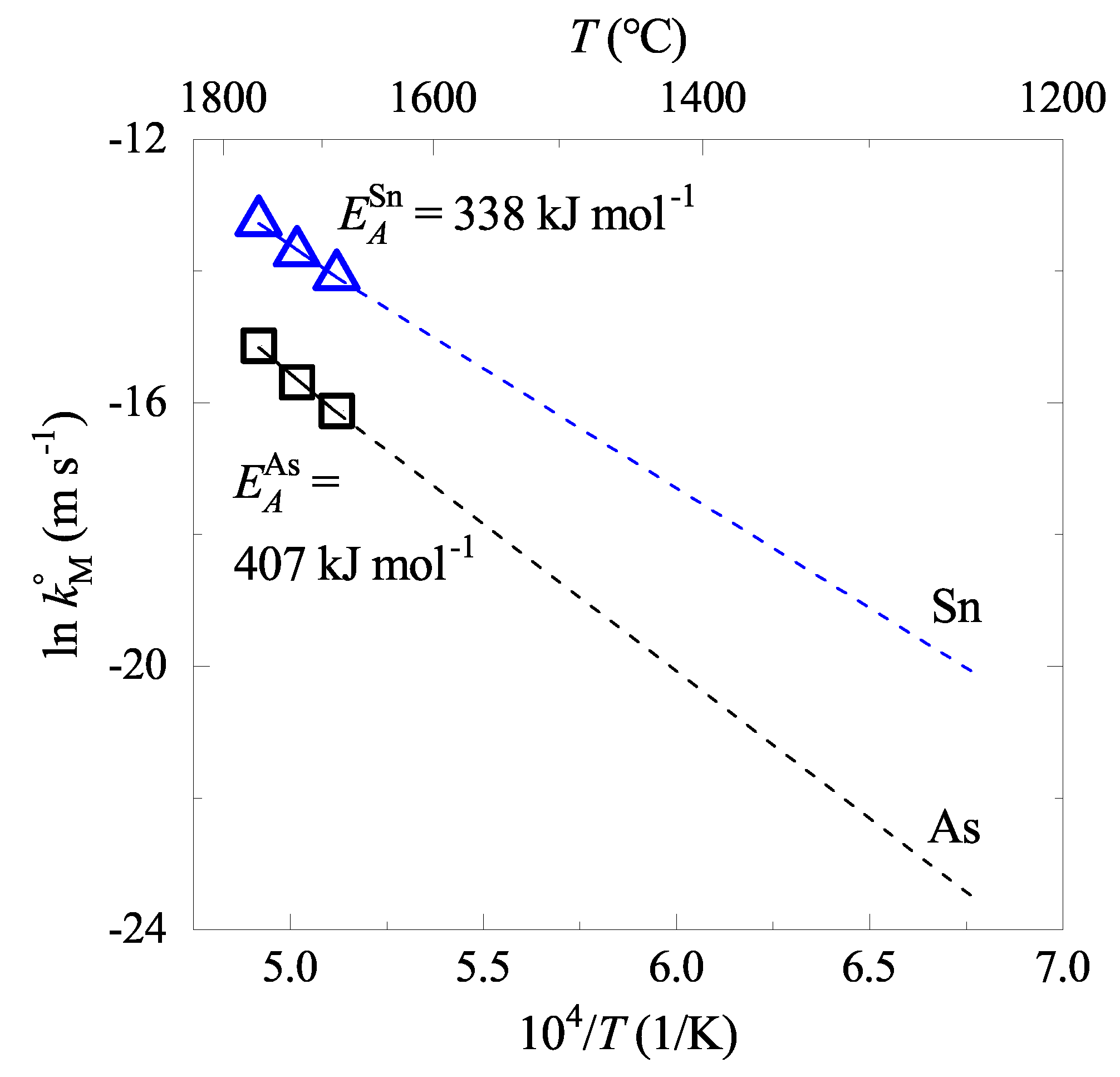
4.2. Effect of Temperature on As and Sn Evaporations
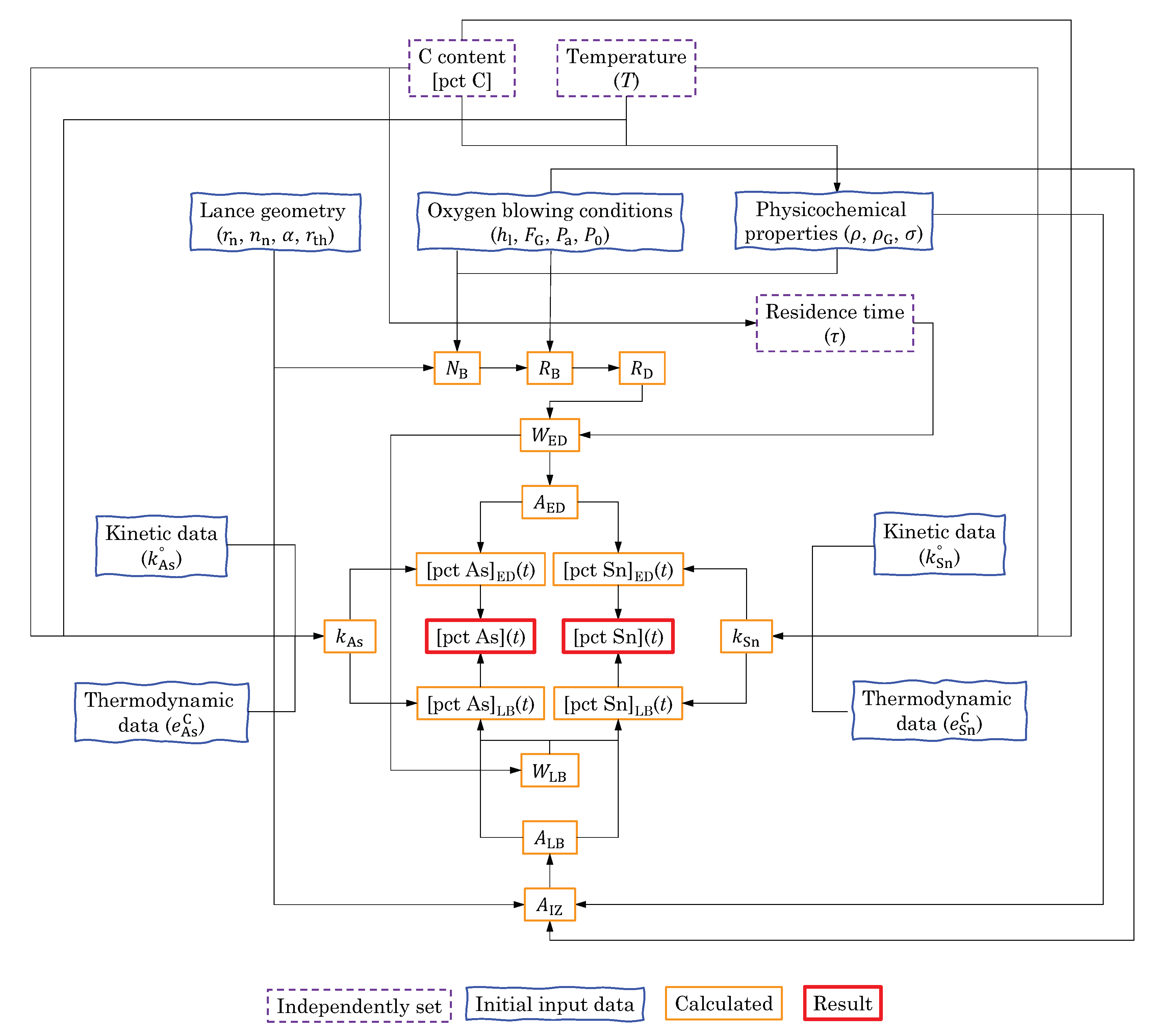
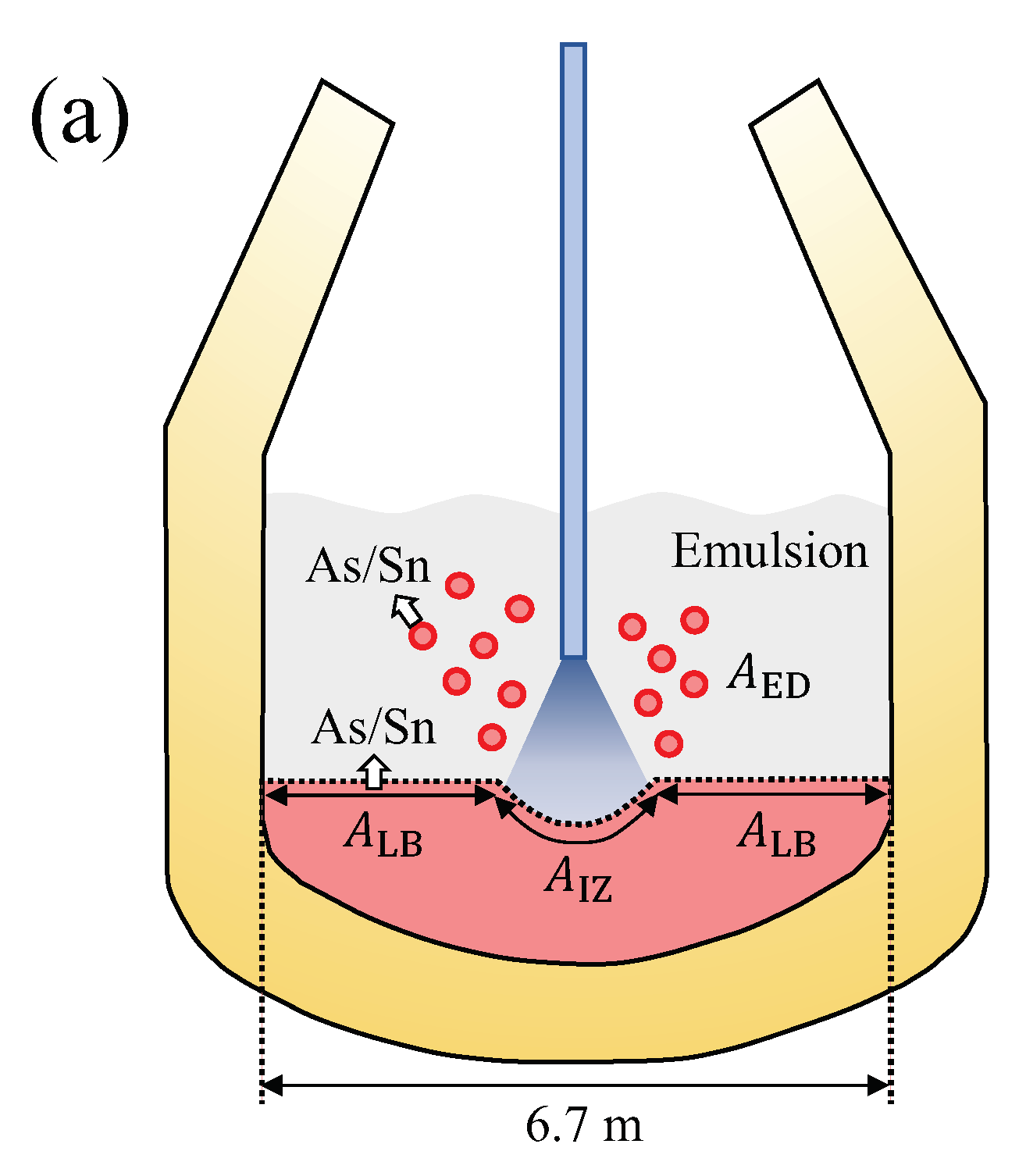
5. Evaporation of As and Sn from Liquid Iron during the Top-Blown Oxygen BOF Process
- (1)
- Ferrous scrap was charged into hot metal at the beginning of the BOF process with a top-blown oxygen lance. The scrap melts and the tramp elements in the scrap dissolve in the hot metal.
- (2)
- Once the oxygen blowing starts, some portion of hot metal splashes by forming small sizes of droplets. Each droplet stays out of the hot metal bath for a certain residence time (), which depends on the size and C content in the droplet. The temperature and C content in the droplets are different from those of the remaining hot metal bath. Those reported in the literature were used in the present study [21,22,23,27,28].
- (3)
- As and Sn evaporate during the steelmaking process. These elements were considered to evaporate both from the liquid bath and the ejected droplets, where the latter were thought to contribute considerably to the evaporation kinetics of these elements, due to the large surface-to-volume ratio and high temperature.
- (4)
- Two routes for the evaporation (liquid bath and droplets) for As and Sn take place independently, but the contents of these elements in the bath and the droplets are coupled with each other.
- (5)
- The droplet surface is open to the gas phase, thereby allowing free evaporation of As and Sn without physical blocking by BOF slag. This is not unlikely as the bloated droplets are covered by decarburized gas [21,29]. On the other hand, the other surface blocking (chemically) by surface adsorption of O [30] was neglected, as its effect would appear at the last stage of the reaction.
- (6)
5.1. Ejected Droplets (ED) during the Oxygen Blowing
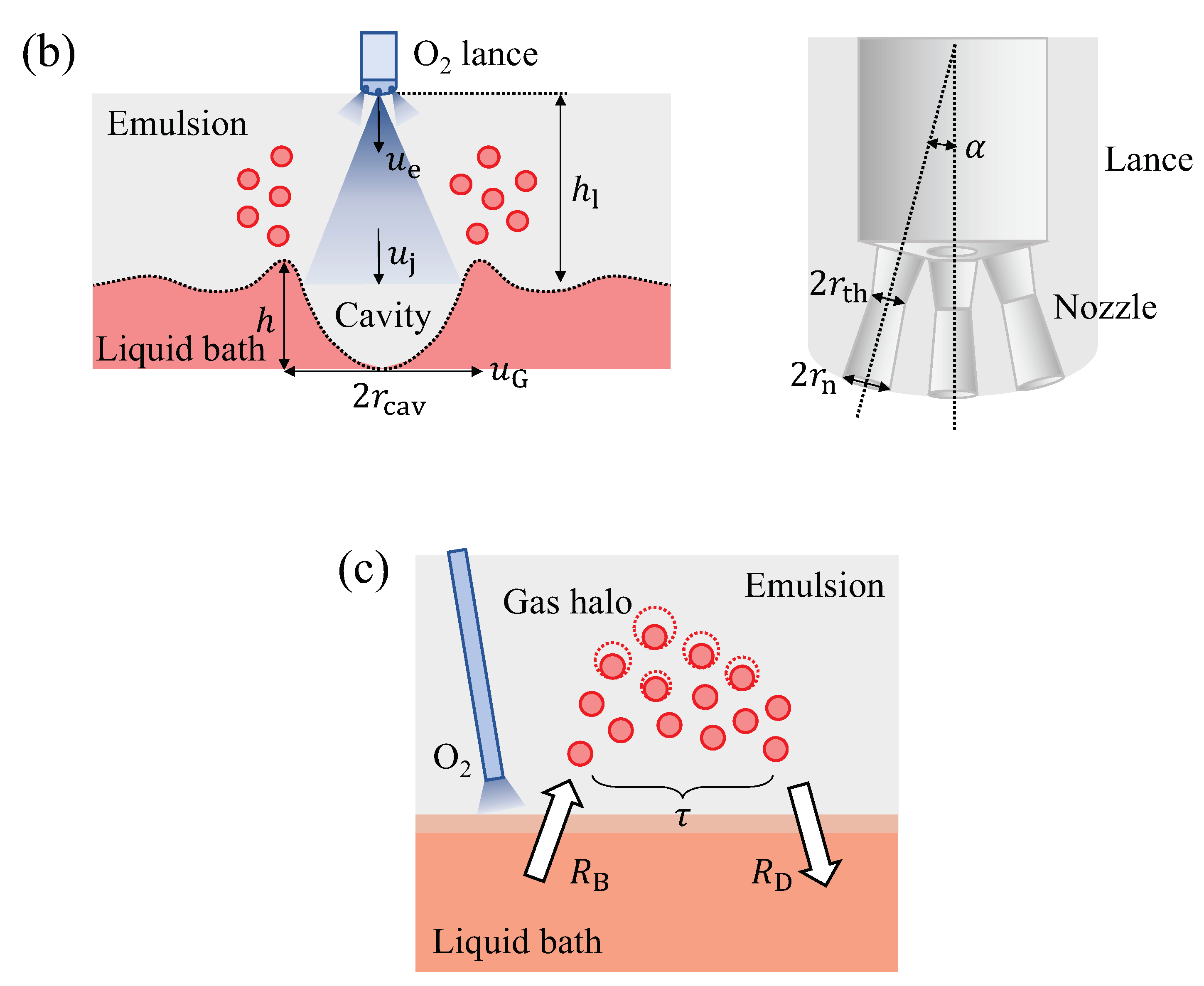
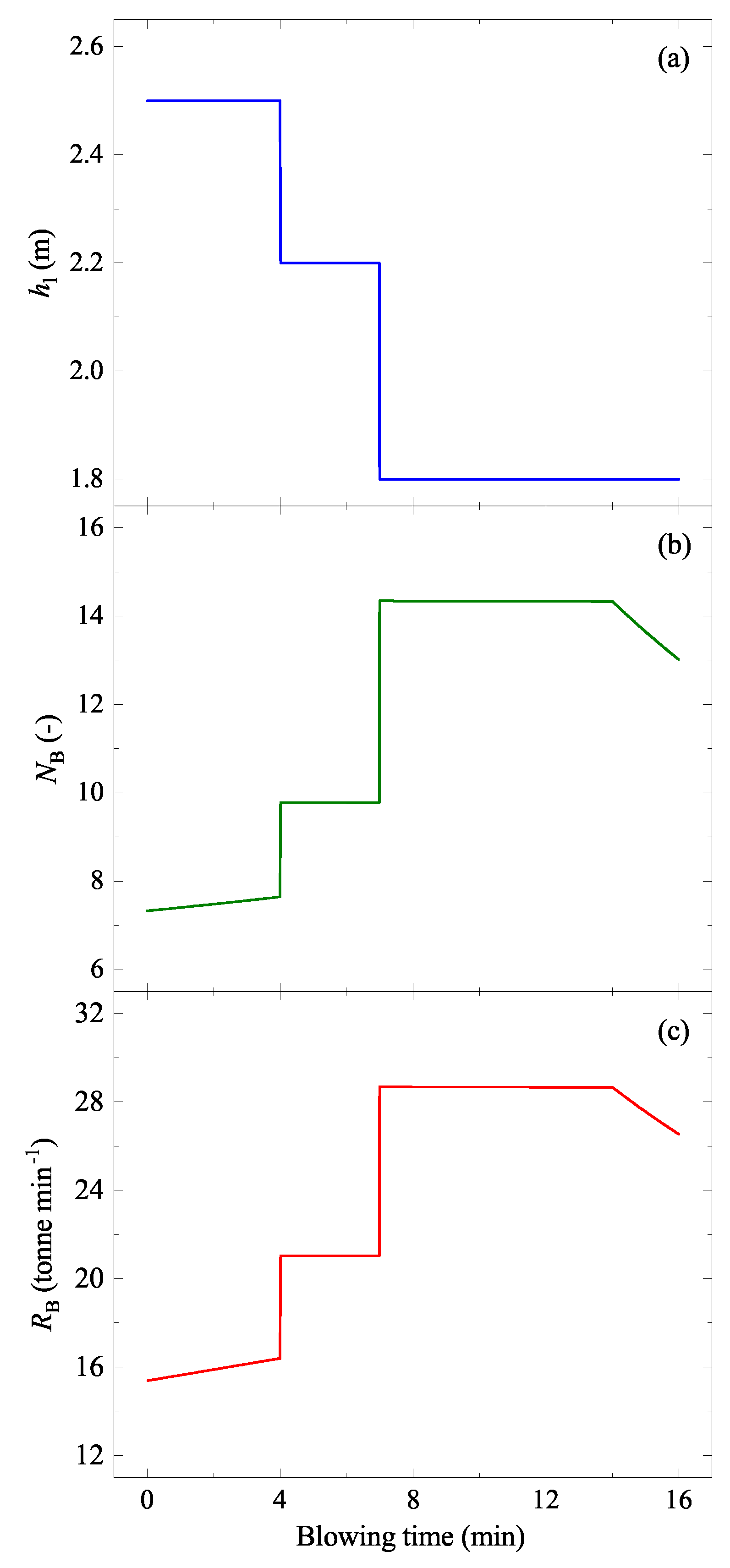
5.1.1. Droplet Generation Rate
5.1.2. Residence Time of the Ejected Droplets ()

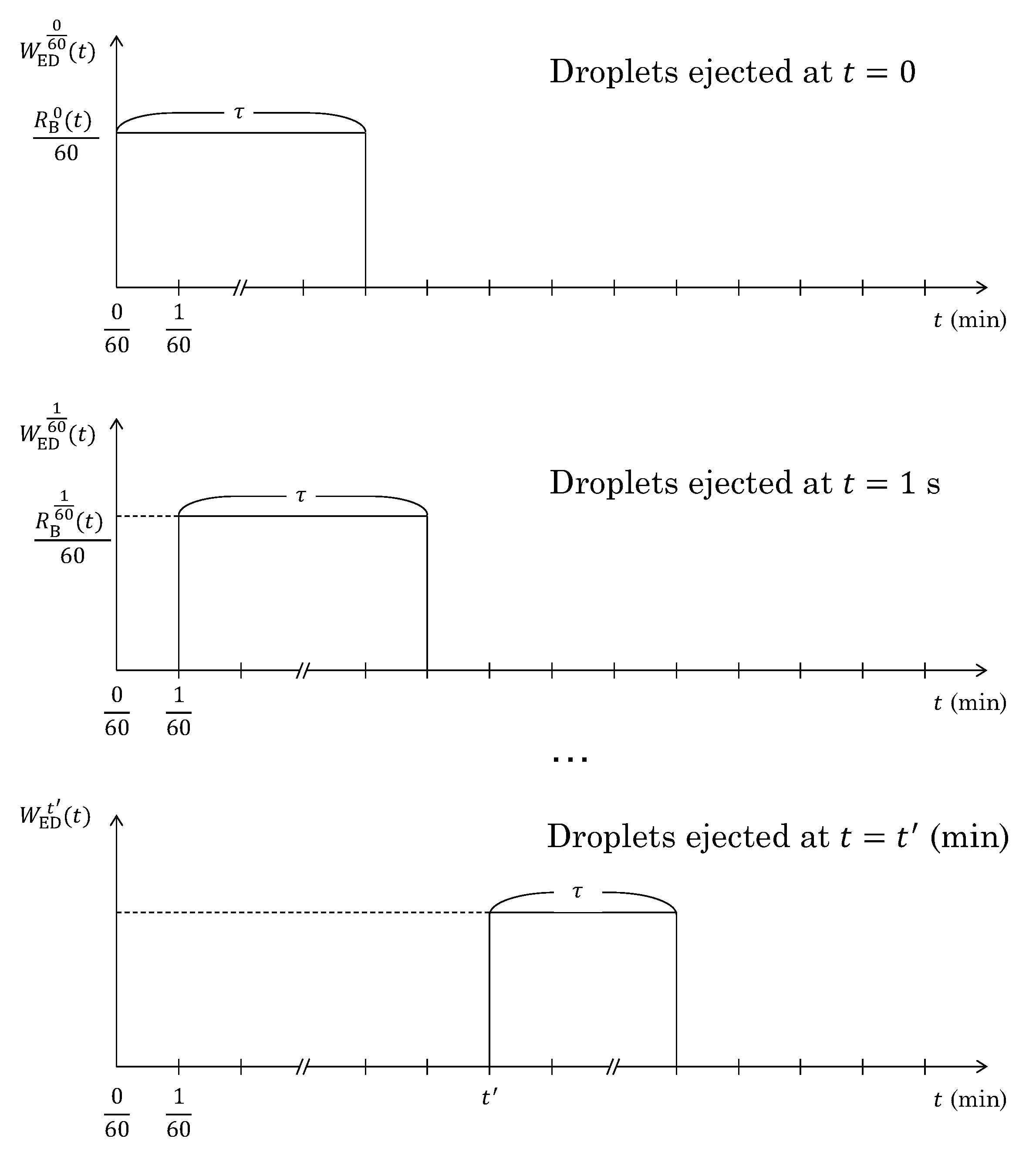


5.1.3. Mass of the Ejected Droplets ()
5.1.4. Area of the Ejected Droplets ()
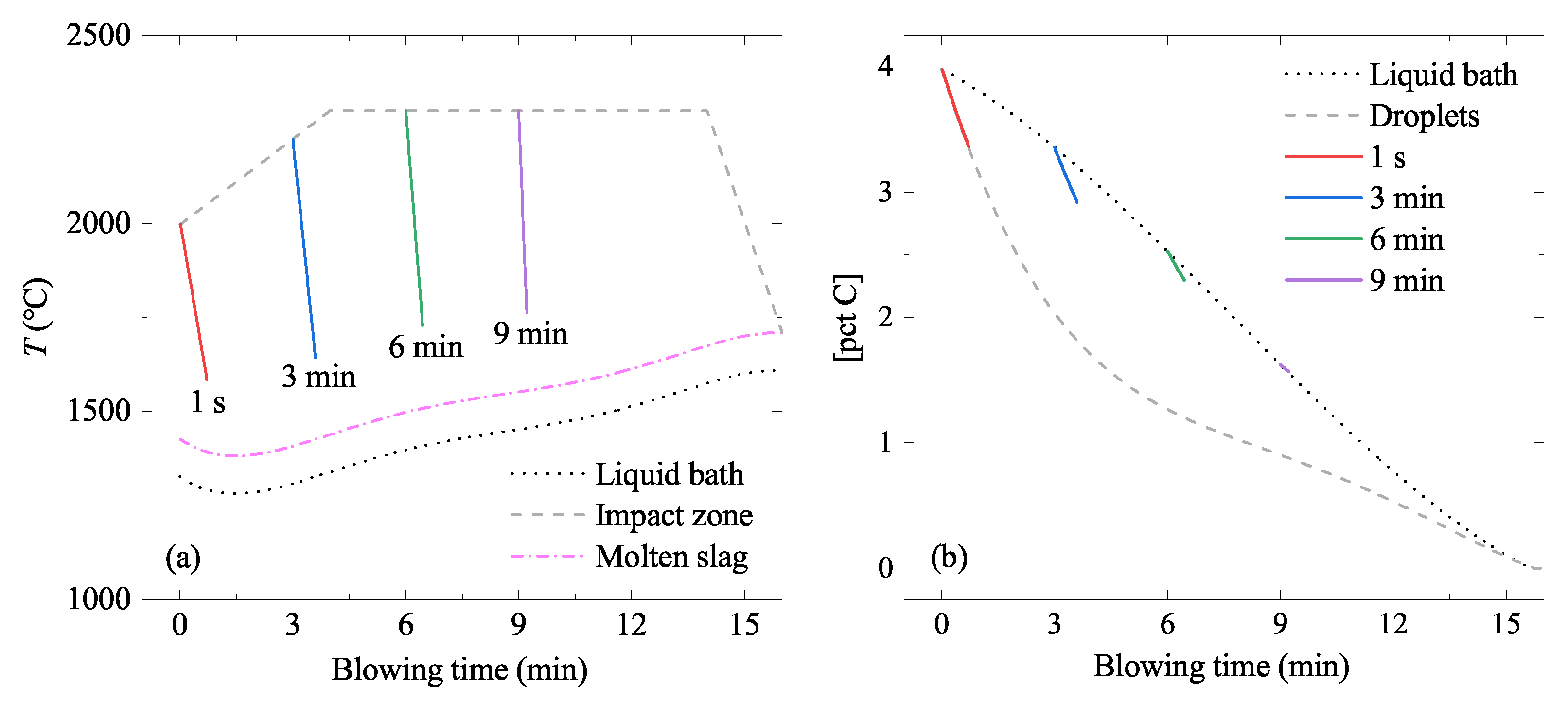
5.1.5. Temperature Change of the Ejected Droplets
5.1.6. [pct C] of the Ejected Droplets
5.2. Liquid Bath in the Converter
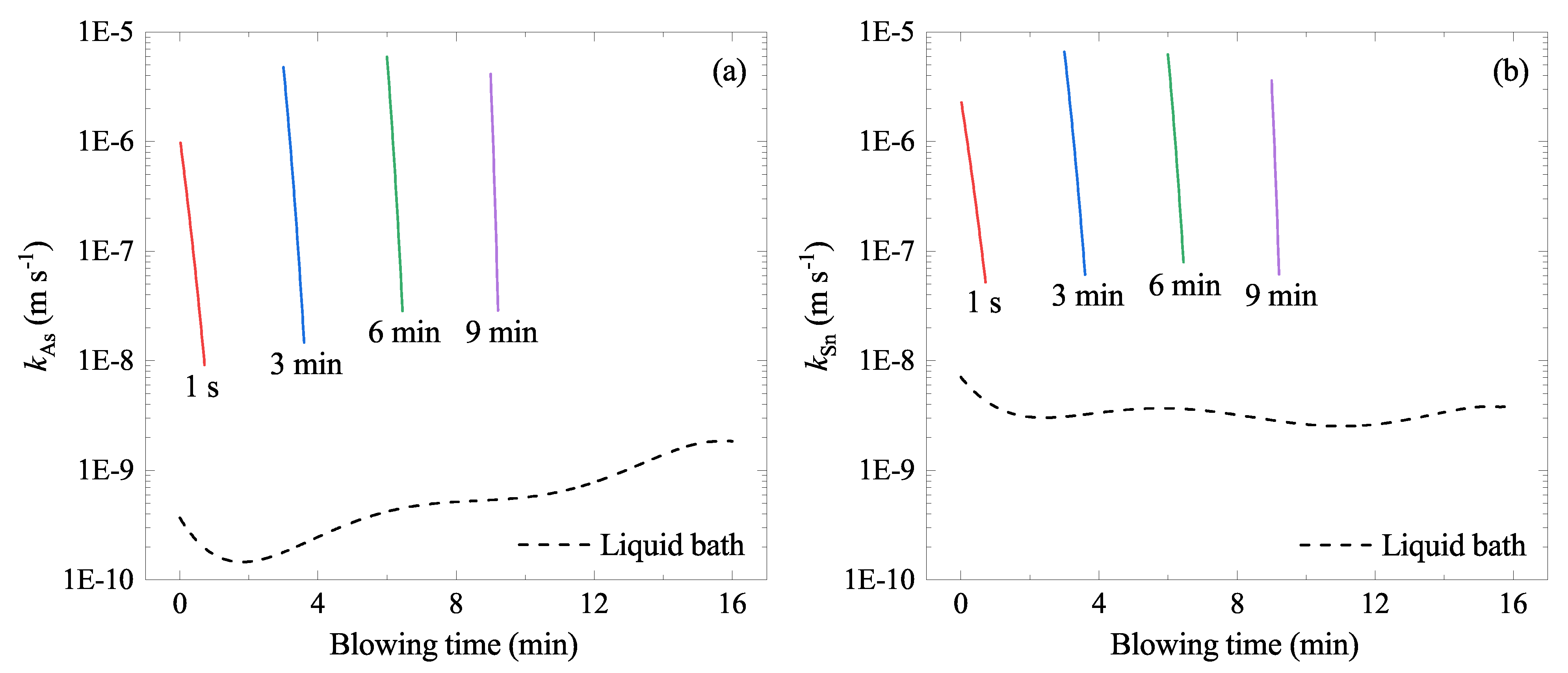
5.3. Rate Constant
5.4. Evaporation Rate of As and Sn
- Wider reaction area than (Figure 11).

6. Discussions
7. Conclusions
- Evaporation rates of As and Sn from liquid iron were slow: a small crucible test showed that As and Sn hardly evaporated at 1400 C.
- The electromagnetic levitation test showed that the evaporation rates increased by increasing temperature and C content in the liquid iron. The evaporation rate constants (, ) were formulated as functions of temperature and C content using available thermodynamic data.
- Mass, volume, and surface area of liquid iron in a 200-tonne BOF during the top-blowing were modeled using available information in the literature. The surface area of the liquid increased enormously (up to ∼163 times). This increased the site for evaporation, thereby increasing the evaporation rates.
- Temperature of liquid steel at the impact zone was significantly high [23]. This resulted in high temperature in the ejected droplets, thereby increasing the evaporation rates.
- Considering the enlarged surface area and the high temperature of many numbers of ejected droplets could enhance the evaporation. However, due to the too-low vapor pressure of As and Sn, resultant evaporation rates were not acceptable in practical operation. This suggests additional actions, including increasing vapor pressure of the tramp elements, e.g., adding S and decreasing ambient pressure, etc.
Author Contributions
Funding
Conflicts of Interest
References
- Roche, C.; Mudd, G. An overview of mining and the environment in western Australia. In Resource Curse or Cure ? Brueckner, M., Durey, A., Mayes, R., Pforr, C., Eds.; Sustainability, Ethics & Governance; CSR: Boca Raton, FL, USA; Springer: Berlin/Heidelberg, Germany, 2014; pp. 179–194. [Google Scholar] [CrossRef]
- Isawa, T.; Wakssugi, T.; Noguchi, K.; Sano, N. Thermodynamics of antimony, arsenic, and tin in CaO–CaF2 melts. Steel Res. 1987, 58, 296–302. [Google Scholar] [CrossRef]
- Street, S.J.; Coley, K.S.; Irons, G.A. Tin solubility in CaO–bearing slags. Scand. J. Metall. 2001, 30, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Cho, Y.-M.; Park, S.-C.; Kim, S.-Y.; Kang, Y.-B. Dissolution behavior of Sn in CaO–CaF2 molten flux and its distribution ratio between CaO–CaF2 molten flux and liquid Iron. JOM 2021, 73, 1080–1089. [Google Scholar] [CrossRef]
- Kim, D.-H.; Cho, Y.-M.; Kang, Y.-B. POSTECH, Pohang-si, Korea. 2019; Unpublished research. [Google Scholar]
- Jung, S.-H.; Kang, Y.-B.; Seo, J.-D.; Park, J.-K.; Choi, J. Evaporation mechanism of Sn and SnS from liquid Fe: Part I: Experiment and adsorption of S on reaction site. Metall. Trans. B 2015, 46, 250–258. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, Y.-B.; Seo, J.-D.; Park, J.-K.; Choi, J. Evaporation mechanism of Sn and SnS from liquid Fe: Part II: Residual site and evaporation kinetics via Sn (g) and SnS (g). Metall. Trans. B 2015, 46, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-H.; Kang, Y.-B.; Seo, J.-D.; Park, J.-K.; Choi, J. Evaporation mechanism of Sn and SnS from liquid Fe: Part III: Effect of C on Sn removal. Metall. Trans. B 2015, 46, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-H.; Kang, Y.-B. Evaporation mechanism of Cu from liquid Fe containing C and S. Metall. Trans. B 2016, 47, 2164–2176. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, Y.-B. Simultaneous evaporation of Cu and Sn from liquid steel. Metall. Trans. B 2016, 47, 2564–2570. [Google Scholar] [CrossRef] [Green Version]
- Tafwidli, F.; Choi, M.-E.; Yi, S.-H.; Kang, Y.-B. Evaporation of Cu, Sn, and S from Fe–C–Cu–Sn–S liquid alloys in the temperature range from 1513K to 1873K (1240 °C to 1600 °C). Metall. Trans. B 2018, 49, 1089–1100. [Google Scholar] [CrossRef]
- Morales, D.R.; Sano, N. Alloying effect on vaporization rate of copper and tin from molten iron alloys. Ironmak. Steelmak. 1982, 9, 64–76. [Google Scholar]
- Liu, X.; Jeffes, J.H.E. Vaporisation of tin sulphide from molten iron. I. mass transport processes. Ironmak. Steelmak. 1988, 15, 21–26. [Google Scholar]
- Liu, X.; Jeffes, J.H.E. Vaporisation of tin sulphide from molten iron. II. chemical reaction process. Ironmak. Steelmak. 1988, 15, 27–32. [Google Scholar]
- Savov, L.; Tu, S.; Janke, D. Methods of increasing the rate of tin evaporation from iron-based melts. ISIJ Int. 2000, 40, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Maya, K.; Nishi, T.; Shinme, K.; Ueno, A.; Anezaki, S. Removal of copper and tin in molten iron with decarburization under reduced pressure. ISIJ Int. 1996, 36, S62–S65. [Google Scholar] [CrossRef] [Green Version]
- El-Kadah, N.; Szekely, J. The electromagnetic force field, fluid flow field, and temperature profiles in levitated metal droplets. Metall. Trans. B 1983, 14B, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Huo, Y.; Li, B.Q. Three-dimensional Marangoni convection in electrostatically positioned droplets under microgravity. Int. J. Heat Mass Transfer 2004, 47, 3533–3547. [Google Scholar] [CrossRef]
- Lin, Z.; Guthrie, R.I.L. Modeling of metallurgical emulsions. Metall. Trans. B 1994, 25, 855–864. [Google Scholar] [CrossRef]
- Subagyo; Brooks, G.A.; Coley, K.S.; Irons, G.A. Generation of droplets in slag-metal emulsions through top gas blowing. ISIJ Int. 2003, 43, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Dogan, N.; Brooks, G.A.; Rhamdhani, M.A. Comprehensive model of oxygen steelmaking part 2: Application of bloated droplet theory for decarburization in emulsion zone. ISIJ Int. 2011, 51, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Dering, D.; Swartz, C.; Dogan, N. Dynamic modeling and simulation of basic oxygen furnace (BOF) operation. Processes 2020, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Chiba, K.; Ono, A.; Saeki, M.; Yamauchi, M.; Kanamoto, M. Development of direct analysis method for molten iron in converter: Hotspot radiation spectrometry. Ironmak. Steelmak. 1993, 20, 215–220. [Google Scholar]
- Grigoriu, C.; Wang, W.-N.; Martin, D.; Biswas, P. Capture of particles from an iron and steel smelter with a pulse-energized electrostatic precipitator. Aerosol Air Qual. Res. 2012, 12, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Wada, H.; Saito, T. Interaction parameters of alloying elements in molten iron. Trans. Jpn. Inst. Met. 1961, 2, 15–20. [Google Scholar] [CrossRef]
- Jimbo, I.; Cramb, A. The density of liquid iron–carbon alloys. Metall. Trans. B 1993, 24, 5–10. [Google Scholar] [CrossRef]
- Cicutti, C.; Valdez, M.; Perez, T.; Donayo, R.; Petroni, J. Analysis of slag foaming during the operation of an industrial converter. Lat. Am. Appl. Res. 2002, 32, 237–240. [Google Scholar]
- Madhavan, N.; Brooks, G.A.; Rhamdhani, M.A.; Rout, B.K.; Overbosch, A.; Gu, K.; Kadrolkar, A.; Dogan, N. Droplet heat transfer in oxygen steelmaking. Metall. Mater. Trans. B 2021, 52B, 4141–4156. [Google Scholar] [CrossRef]
- Min, D.-J.; Fruehan, R.J. Rate of reduction of FeO in slag by Fe–C drops. Metall. Trans. B 1992, 23, 29–37. [Google Scholar] [CrossRef]
- Ogino, K.; Nogi, K.; Koshida, Y. Effect of oxygen on the wettability of solid oxide with molten iron. Tetsu-to-Hagane 1973, 59, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Price, D.J. LD steelmaking: Significance of the emulsion in carbon removal. In Proceedings of the Process Engineering of Pyrometallurgy Symposium, London, UK, 8–15 January 1974; pp. 8–15. [Google Scholar]
- Cicutti, C.; Valez, M.; Pérez, T.; Petroni, J.; Gomez, A.; Donayo, R.; Ferro, L. Study of slag-metal reactions in an LD-LBE converter. In Proceedings of the 6th International Conference on Molten Slags, Fluxes and Salts, Helsinki, Finland, 12–17 June 2000; p. 367. [Google Scholar]
- Dutta, S.K.; Chokshi, Y.B. Basic Concepts of Iron and Steel Making; Springer Nature Singapore Pte Ltd.: Singapore, 2020; Chapter 15; pp. 344–346. ISBN 978-981-15-2436-3. [Google Scholar] [CrossRef]
- Deo, B.; Boom, R. Fundamentals of Steelmaking Metallurgy; Prentice Hall International: Upper Saddle River, NJ, USA, 1993; Chapter 5; pp. 168–187. ISBN 978-0133453805. [Google Scholar]
- Keene, B.J. A survey of extant data for the surface tension of iron and its binary alloys. NPL Rep. DMA (A) 1983, 67. [Google Scholar]
- Standish, N.; He, Q.L. Drop generation due to an impinging jet and the effect of bottom blowing in the steelmaking vessel. ISIJ Int. 1989, 29, 455–461. [Google Scholar] [CrossRef] [Green Version]
- He, Q.L.; Standish, N. A model study of residence time of metal droplets in the slag in bof steelmaking. ISIJ Int. 1990, 30, 356–361. [Google Scholar] [CrossRef] [Green Version]
- He, Q.L.; Standish, N. A model study of droplet generation in the BOF steelmaking. ISIJ Int. 1990, 30, 305–309. [Google Scholar] [CrossRef]
- Li, R.; Harris, R.L. Interaction of gas jets with model process liquids. In Proceedings of the Pyrometallurgy’ 95, London, UK, 10–12 July 1995; pp. 107–124. [Google Scholar]
- Cheslak, F.R.; Nicholls, J.A.; Sichel, M. Cavities formed on liquid surfaces by impinging gaseous jets. J. Fluid Mech. 1969, 36, 55–63. [Google Scholar] [CrossRef]
- Rout, B.K.; Brooks, G.; Rhamdhani, M.A.; Li, Z.; Schrama, F.N.; Sun, J. Dynamic model of basic oxygen steelmaking process based on multi-zone reaction kinetics: Model derivation and validation. Metall. Trans. B 2018, 49, 537–557. [Google Scholar] [CrossRef] [Green Version]
- Koria, S.C.; Lange, K.W. Penetrability of impinging gas jets in molten steel bath. Steel Res. 1987, 58, 421–426. [Google Scholar] [CrossRef]
- Lupis, C.H.P. Chemical Thermodynamics of Materials; Prentice Hall, Inc.: Englewood Cliffs, NJ, USA, 1993; p. 528. ISBN 981-3026-75-8. [Google Scholar]
| Case | Temperature (C) | [pct C] | [pct As] | [pct Sn] | Crucible |
|---|---|---|---|---|---|
| 1-1 | 1680 (±10) | 2.0 | 0.18 | 0.18 | Magnesia |
| 1-2 | 3.9 | ||||
| 1-3 | 5.6 (sat.) | 0.16 | 0.19 | Graphite | |
| 2-1 | |||||
| 2-2 | 1720 (±10) | 5.8 (sat.) | |||
| 2-3 | 1760 (±10) | 5.9 (sat.) |
| Input Parameters | Unit | Value |
|---|---|---|
| Diameter of converter | m | 6.7 [33] |
| Lance height, | m | 1.8–2.5 (Figure 7) [27,32] |
| Nozzle angle, | deg() | 17.5 [21] |
| Throat radius of nozzle, | m | 0.017 [21] |
| Exit radius of nozzle, | m | 0.023 [21] |
| Number of nozzles, | - | 6 [27,32] |
| Supply pressure, | Pa | 10 [21] |
| Ambient pressure, | Pa | 10 [34] |
| Oxygen flow rate, | Nm min | 620 [27,32] |
| Mass of iron | tonne | 200 [27,32,33] |
| Density of iron, | kg m | 7100 − 73.2 [pct C] − (0.828 − 0.0874 [pct C]) |
| × (T − 1550) [26] | ||
| Surface tension of iron, | N m | [(2367 ± 500) − 0.34 T ] / 1000 [35] |
| Density of oxygen, | kg min | 1.429 at STP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Park, W.-B.; Park, S.-C.; Kang, Y.-B. Evaporation of As and Sn from Liquid Iron: Experiments and a Kinetic Model during Top-Blown Oxygen Steelmaking Process. Materials 2022, 15, 4771. https://doi.org/10.3390/ma15144771
Kim D-H, Park W-B, Park S-C, Kang Y-B. Evaporation of As and Sn from Liquid Iron: Experiments and a Kinetic Model during Top-Blown Oxygen Steelmaking Process. Materials. 2022; 15(14):4771. https://doi.org/10.3390/ma15144771
Chicago/Turabian StyleKim, Dong-Hyun, Won-Bum Park, Sang-Chae Park, and Youn-Bae Kang. 2022. "Evaporation of As and Sn from Liquid Iron: Experiments and a Kinetic Model during Top-Blown Oxygen Steelmaking Process" Materials 15, no. 14: 4771. https://doi.org/10.3390/ma15144771






