Durability of Fibre Reinforced Polymers in Exposure to Dual Environment of Seawater Sea Sand Concrete and Seawater
Abstract
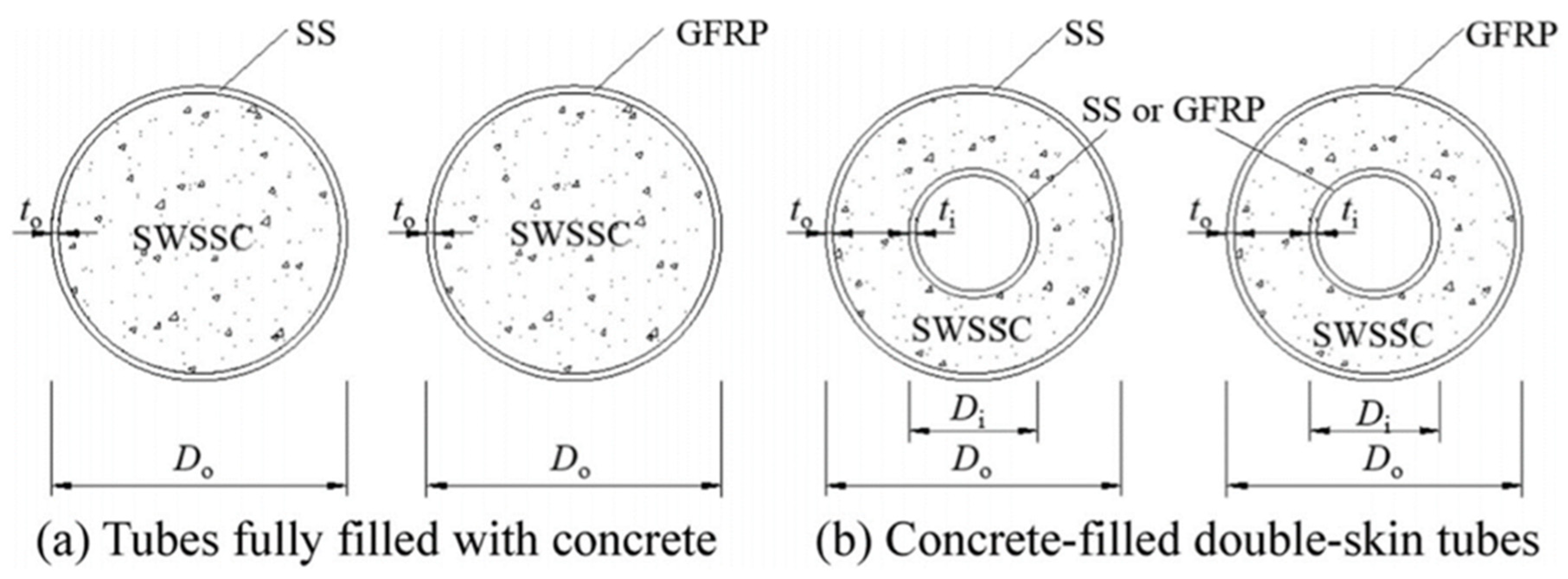
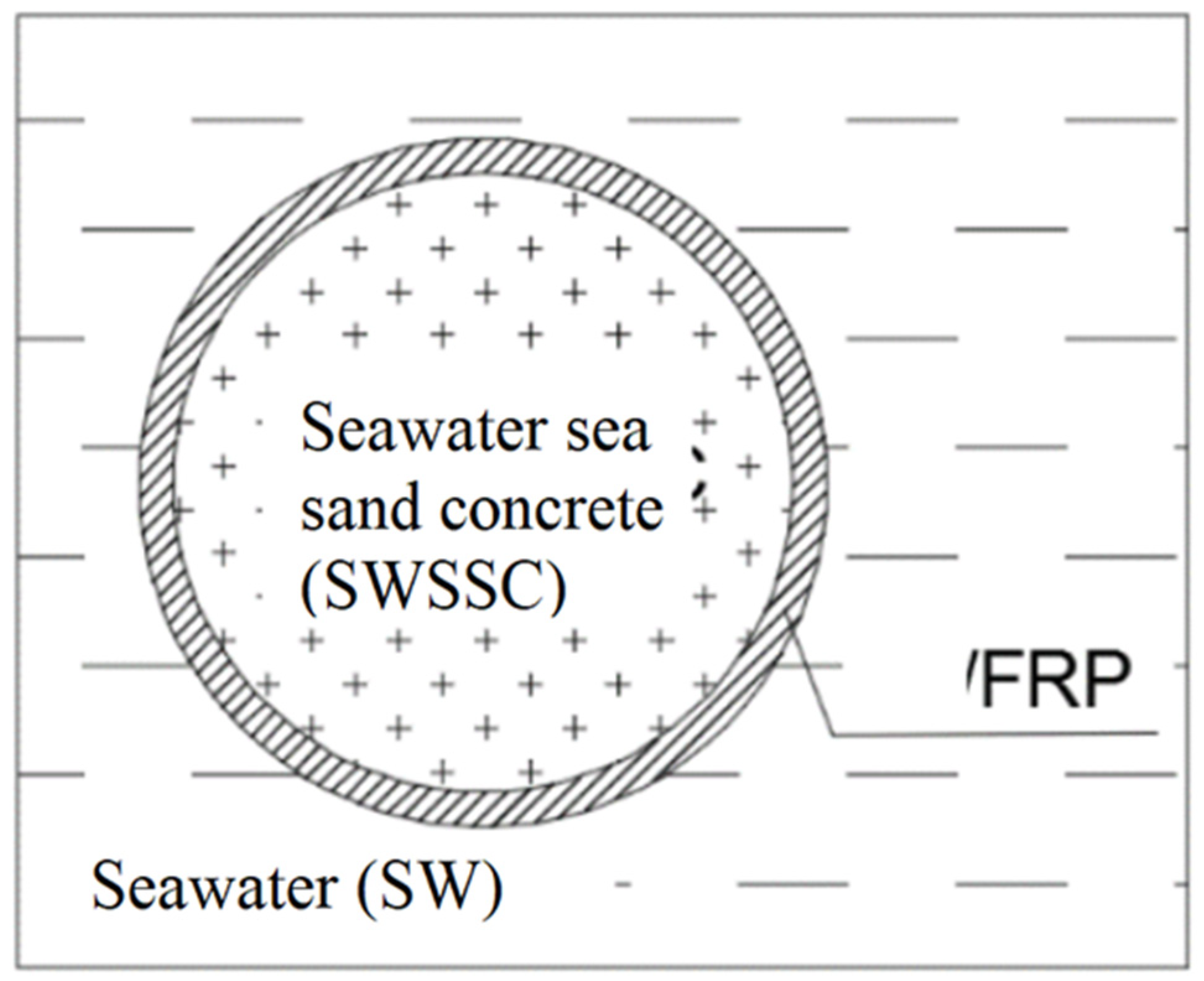
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimens
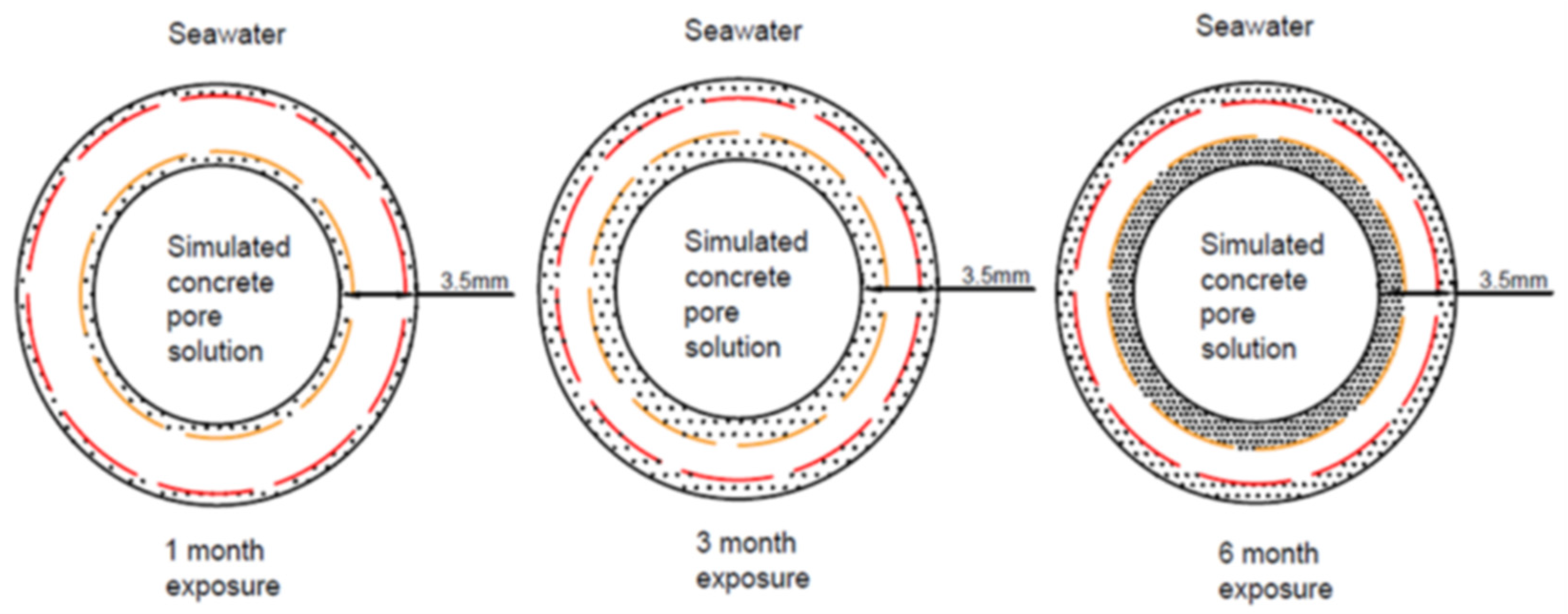
2.2. Dual Exposure Environment
2.3. Scanning Electron Microscopy (SEM)
2.4. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3. Results and Discussion
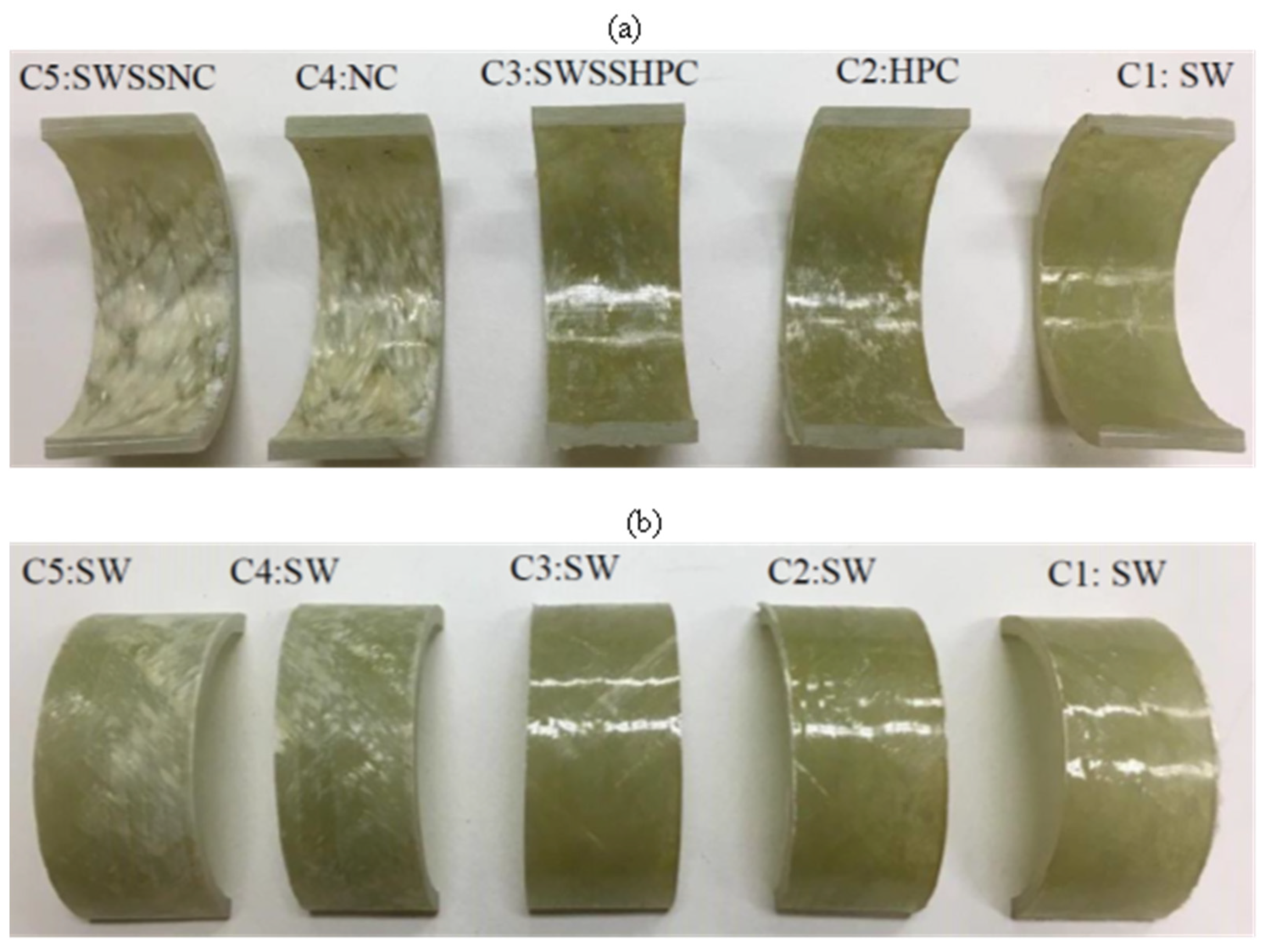
3.1. Visual Observation
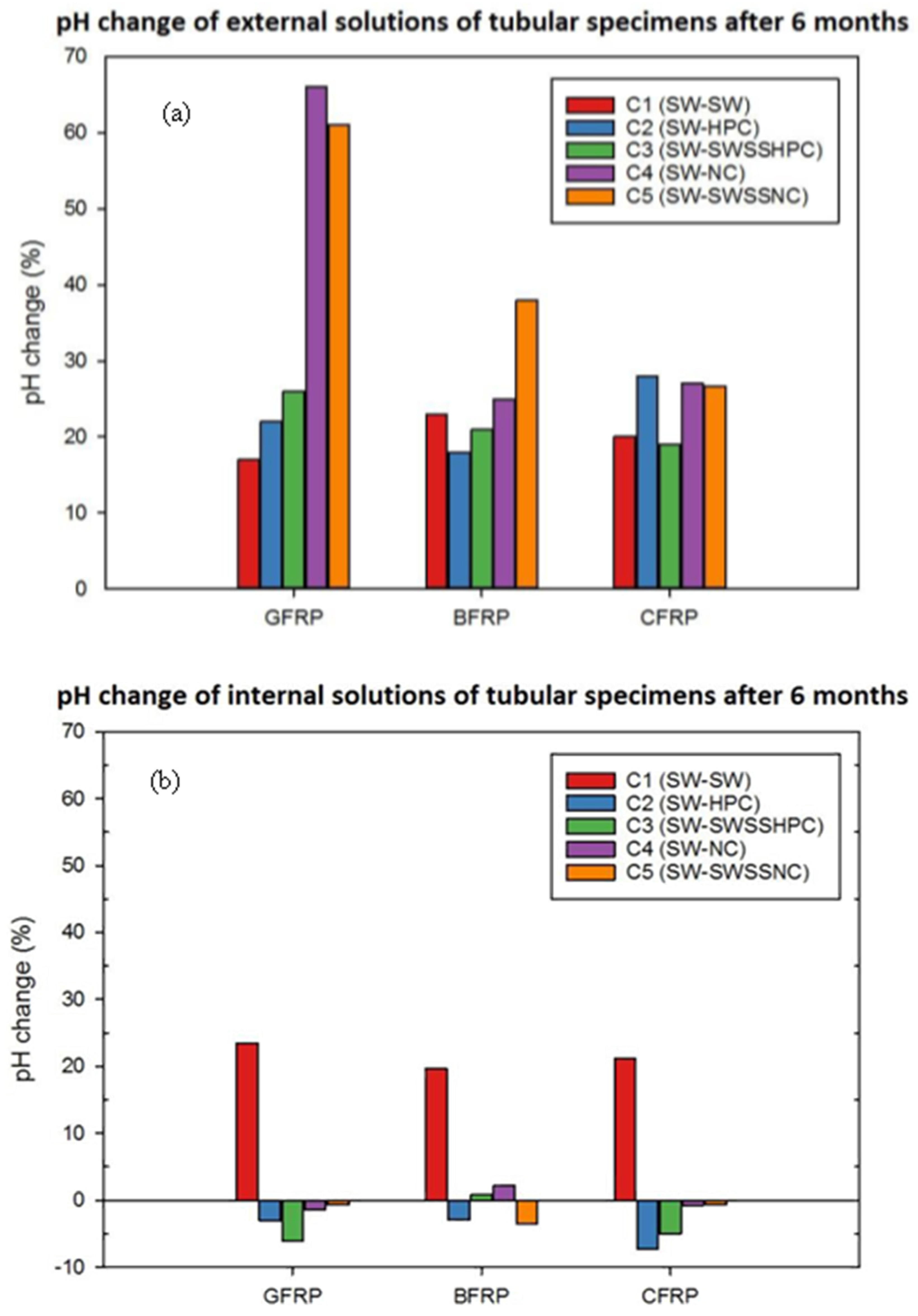
3.2. pH Changes
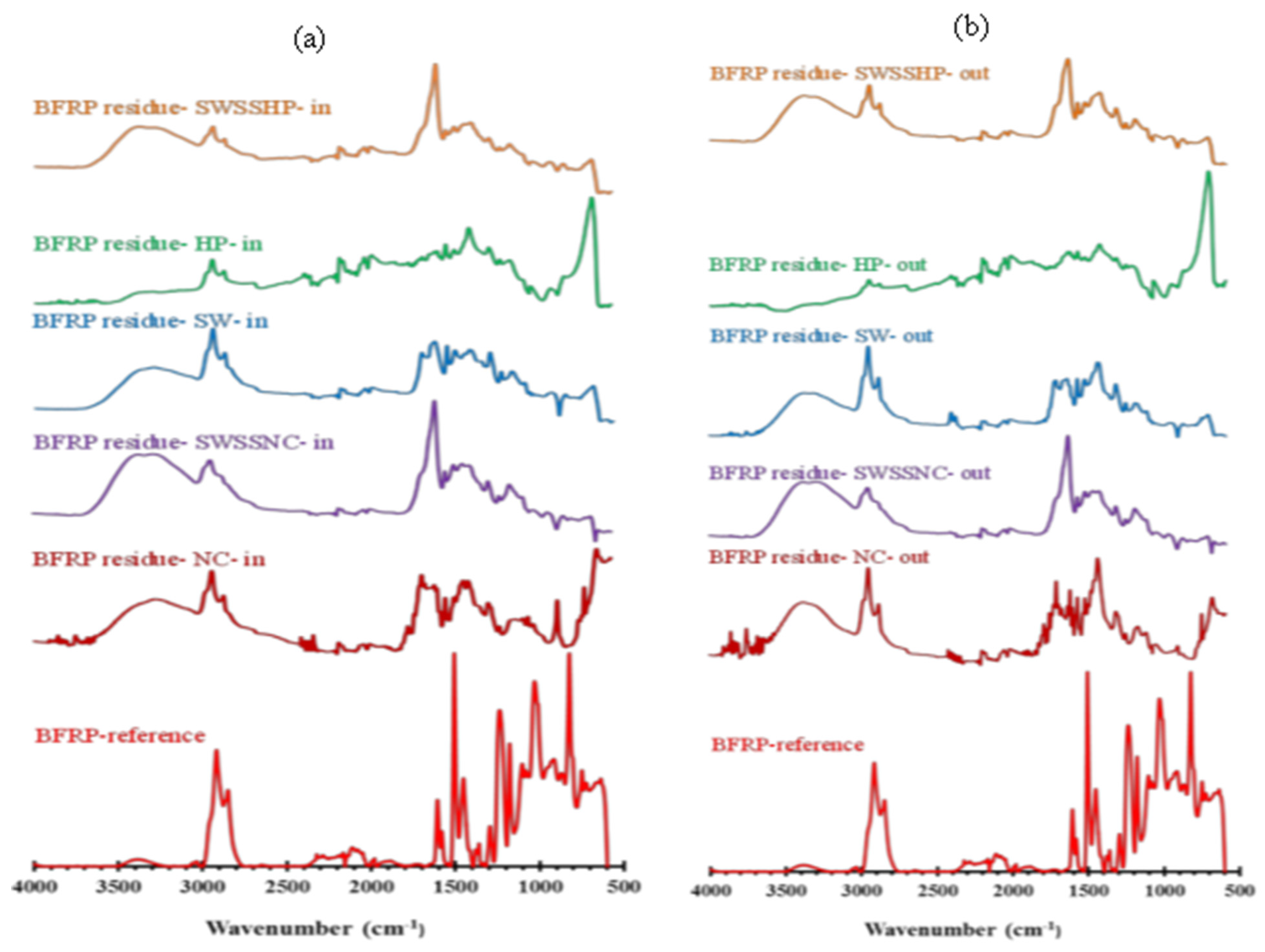
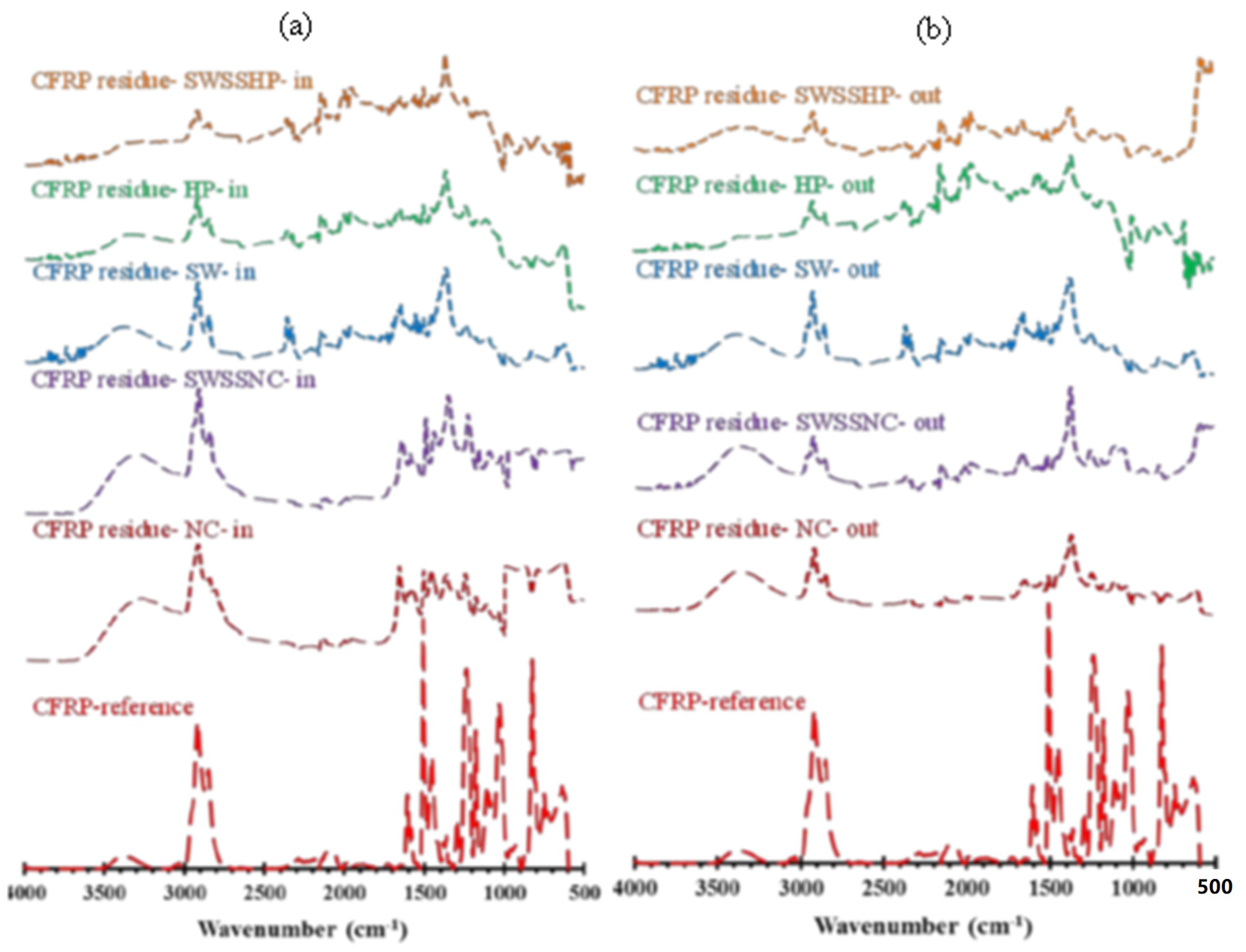
3.3. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
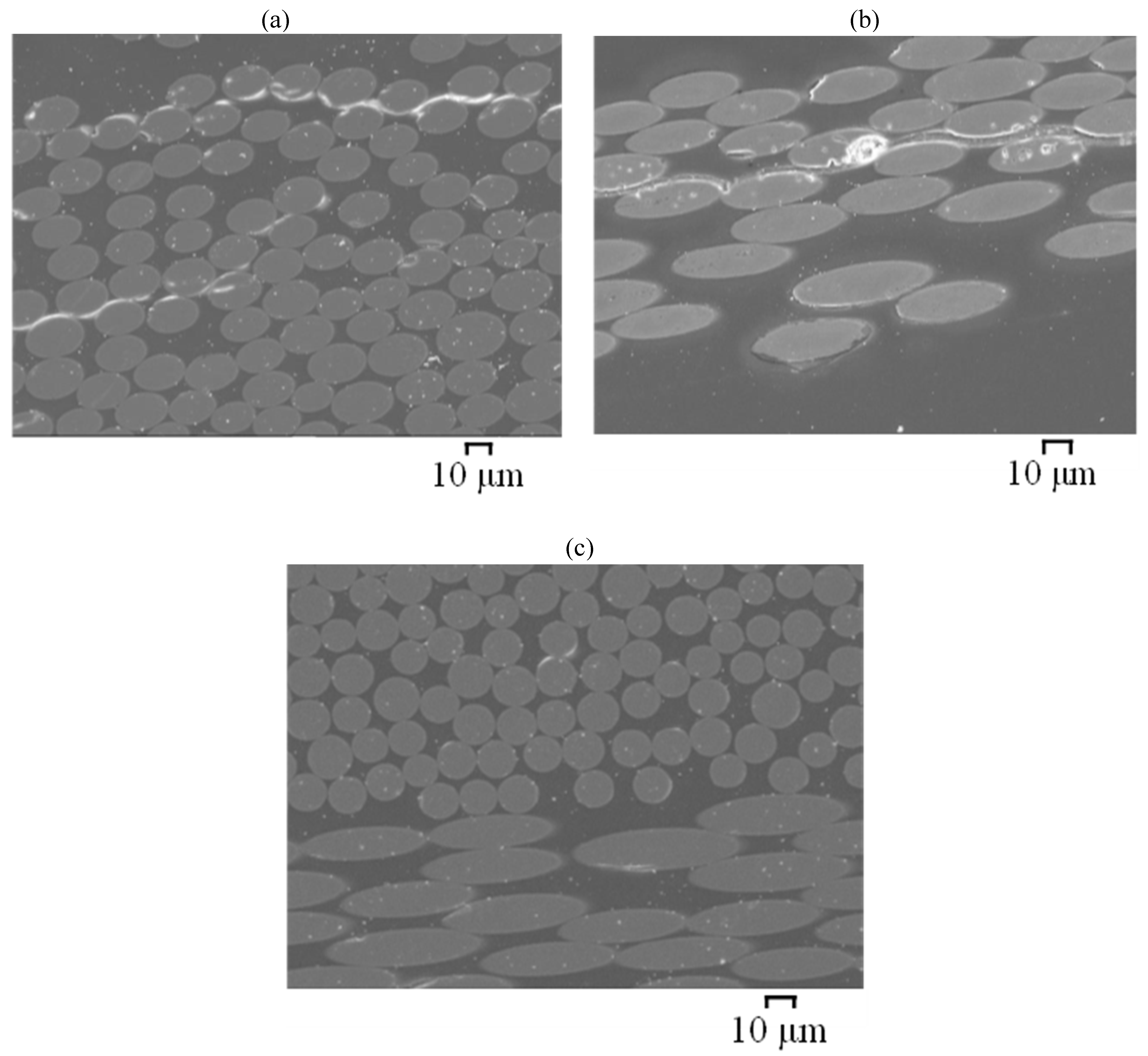
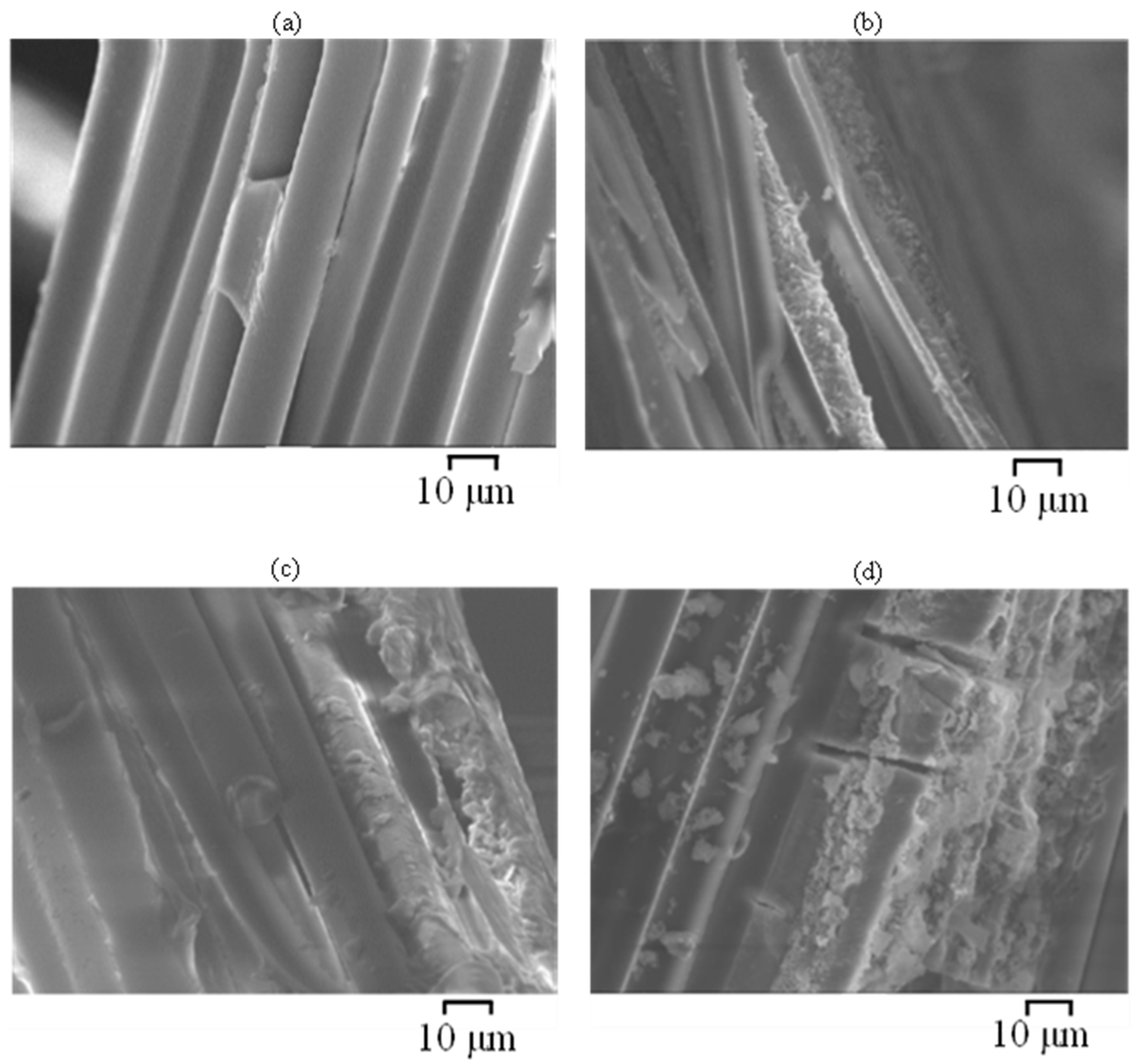
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
- Exposure of epoxy-based FRPs to seawater resulted in an ~20% pH increase in the exposure solution, which is attributed to the leaching of the polymer resins. A dual exposure environment of the simulated concrete pore solution internally, and the seawater externally, resulted in a greater pH increase in the external solution.
- For GFRP/BFRP exposed to seawater, both internally and externally, minor interface debondings were observed near the exposure surfaces, and basalt fibres largely remained clean and smooth, whereas glass fibres near the exposure surfaces were found to have slightly degraded.
- For BFRP and GFRP (E-glass) that were exposed to a dual environment of SWSSNC internally and SW externally, fibre–resin interface debondings joined to give the appearance of continuous cracks adjacent to both the internal and external surfaces as well as in the centre of the BFRP. Although a clear progressive degradation pattern cannot be identified from the ground-and-polished cross sections of the specimens, possibly due to the surface damages caused by the mechanical process, the fibre degradation was generally observed on fractured specimens. Basalt fibres were observed to have degraded adjacent to the internal exposure surface, whereas fibres at the rest parts remained clean and smooth; glass fibre degradation was found adjacent to both surfaces of the GFRP. The greater fibre degradation adjacent to the external surface of the GFRP (E-glass) than the BFRP might be attributed to a greater pH increase in the external exposure solution of the GFRP (E-glass) in this exposure environment of SWSSNC internally and SW externally.
- CFRP exhibited the best durability in a dual exposure environment of seawater sea sand normal concrete (SWSSNC) internally and seawater externally.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; Agricultural Development Economics Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Raman, R.K.S.; Guo, F.; Al-Saadi, S.; Zhao, X.-L.; Jones, R. Understanding Fibre-Matrix Degradation of FRP Composites for Advanced Civil Engineering Applications: An Overview. Corros. Mater. Degrad. 2020, 1, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Al-Saadi, S.; Singh Raman, R.K.; Zhao, X.L. Durability of fiber reinforced polymer (FRP) in simulated seawater sea sand concrete (SWSSC) environment. Corros. Sci. 2018, 141, 1–13. [Google Scholar] [CrossRef]
- Hanna, S.; Jones, R. Composite wraps for ageing infrastructure: Concrete columns. Compos. Struct. 1997, 38, 57–64. [Google Scholar] [CrossRef]
- Karbhari, V.M. Introduction: The use of composites in civil structural applications. In Durability of Composites for Civil Structural Applications; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–10. [Google Scholar]
- Li, Y.L.; Zhao, X.L.; Singh, R.K.R.; Al-Saadi, S. Experimental study on seawater and sea sand concrete filled GFRP and stainless steel tubular stub columns. Thin-Walled Struct. 2016, 106, 390–406. [Google Scholar] [CrossRef]
- Robert, M.; Fam, A. Long-Term Performance of GFRP Tubes Filled with Concrete and Subjected to Salt Solution. J. Compos. Constr. 2012, 16, 217–224. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Lu, H.; Han, L.-H. Concrete-Filled Tubular Members and Connections, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Fam, A.; Greene, R.; Rizkalla, S. Field applications of concrete-filled FRP tubes for marine piles. Spec. Publ. 2003, 215, 161–180. [Google Scholar]
- Fam, A.Z.; Rizkalla, S.H. Flexural behavior of concrete-filled fiber-reinforced polymer circular tubes. J. Compos. Constr. 2002, 6, 123–132. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Singh, R.R.; Al-Saadi, S. Tests on seawater and sea sand concrete-filled CFRP, BFRP and stainless steel tubular stub columns. Thin-Walled Struct. 2016, 108, 163–184. [Google Scholar] [CrossRef]
- Eslami, S.; Honarbakhsh-Raouf, A.; Eslami, S. Effects of moisture absorption on degradation of E-glass fiber reinforced Vinyl Ester composite pipes and modelling of transient moisture diffusion using finite element analysis. Corros. Sci. 2015, 90, 168–175. [Google Scholar] [CrossRef]
- Ellyin, F.; Maser, R. Environmental effects on the mechanical properties of glass-fiber epoxy composite tubular specimens. Compos. Sci. Technol. 2004, 64, 1863–1874. [Google Scholar] [CrossRef]
- Yao, J.; Ziegmann, G. Water absorption behavior and its influence on properties of GRP pipe. J. Compos. Mater. 2007, 41, 993–1008. [Google Scholar] [CrossRef]
- Mukherjee, A.; Arwikar, S. Performance of glass fiber-reinforced polymer reinforcing bars in tropical environments—Part II: Microstructural tests. ACI Struct. J. 2005, 102, 816. [Google Scholar]
- Wang, Z.; Zhao, X.-L.; Xian, G.; Wu, G.; Raman, R.S.; Al-Saadi, S.; Haque, A. Long-term durability of basalt-and glass-fibre reinforced polymer (BFRP/GFRP) bars in seawater and sea sand concrete environment. Constr. Build. Mater. 2017, 139, 467–489. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.-L.; Xian, G.; Wu, G.; Raman, R.S.; Al-Saadi, S. Durability study on interlaminar shear behaviour of basalt-, glass- and carbon-fibre reinforced polymer (B/G/CFRP) bars in seawater sea sand concrete environment. Constr. Build. Mater. 2017, 156, 985–1004. [Google Scholar] [CrossRef]
- Guo, F.; Al-Saadi, S.; Singh, R.; Zhao, X. Durability of Carbon Fiber Reinforced Polymer (Cfrp) With Combined Use Of Seawater Sea Sand Concrete (SWSSC). In Proceedings of the Sixth Asia-Pacific Conference on FRP in Structures (APFIS 2017), Singapore, 19–21 July 2017; pp. 19–21. [Google Scholar]
- Chen, Y.; Davalos, J.F.; Ray, I.; Kim, H.-Y. Accelerated aging tests for evaluations of durability performance of FRP reinforcing bars for concrete structures. Compos. Struct. 2007, 78, 101–111. [Google Scholar] [CrossRef]
- Chen, Y.; Davalos, J.F.; Ray, I. Durability prediction for GFRP reinforcing bars using short-term data of accelerated aging tests. J. Compos. Constr. 2006, 10, 279–286. [Google Scholar] [CrossRef]
- Ngono, Y.; Maréchal, Y.; Mermilliod, N. Epoxy−Amine Reticulates Observed by Infrared Spectrometry. I: Hydration Process and Interaction Configurations of Embedded H2O Molecules. J. Phys. Chem. B 1999, 103, 4979–4985. [Google Scholar] [CrossRef]
- Lucovsky, G. Chemical effects on the frequencies of Si-H vibrations in amorphous solids. Solid State Commun. 1979, 29, 571–576. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Valadez-Gonzalez, A.; Cervantes-Uc, J.; Olayo, R.; Herrera-Franco, P. Chemical modification of henequen fibers with an organosilane coupling agent. Compos. Part B Eng. 1999, 30, 321–331. [Google Scholar] [CrossRef]
- Herrera-Franco, P.; Valadez-Gonzalez, A. A study of the mechanical properties of short natural-fiber reinforced composites. Compos. Part B Eng. 2005, 36, 597–608. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.-J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef] [PubMed]
- Rybin, V.A.; Utkin, A.V.; Baklanova, N.I. Alkali resistance, microstructural and mechanical performance of zirconia-coated basalt fibers. Cem. Concr. Res. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Rybin, V.; Utkin, A.; Baklanova, N. Corrosion of uncoated and oxide-coated basalt fibre in different alkaline media. Corros. Sci. 2016, 102, 503–509. [Google Scholar] [CrossRef]
- Scheffler, C.; Förster, T.; Mäder, E.; Heinrich, G.; Hempel, S.; Mechtcherine, V. Aging of alkali-resistant glass and basalt fibers in alkaline solutions: Evaluation of the failure stress by Weibull distribution function. J. Non-Cryst. Solids 2009, 355, 2588–2595. [Google Scholar] [CrossRef]
- Jantzen, C.M.; Brown, K.G.; Pickett, J.B. Durable glass for thousands of years. Int. J. Appl. Glass Sci. 2010, 1, 38–62. [Google Scholar] [CrossRef] [Green Version]
- Lipatov, Y.V.; Gutnikov, S.; Manylov, M.; Zhukovskaya, E.; Lazoryak, B. High alkali-resistant basalt fiber for reinforcing concrete. Mater. Des. 2015, 73, 60–66. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.; Cano, M.; Marcos, M.; Sánchez-Amaya, J.; González-Rovira, L. Behaviour of the alloy AA2017 in aqueous solutions of NaCl. Part I: Corrosion mechanisms. Corros. Sci. 2009, 51, 518–524. [Google Scholar] [CrossRef]
- Zhu, D.; van Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl) propyl] tetrasulfide in neutral sodium chloride solution. Part 1: Corrosion of AA 2024-T3. Corros. Sci. 2003, 45, 2163–2175. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Corrosion and corrosion inhibition of aluminum in Arabian Gulf seawater and sodium chloride solutions by 3-amino-5-mercapto-1, 2, 4-triazole. Int. J. Electrochem. Sci. 2011, 6, 1479–1492. [Google Scholar]
- Stumm, W.; Lee, G.F. Oxygenation of ferrous iron. Ind. Eng. Chem. 1961, 53, 143–146. [Google Scholar] [CrossRef]
- Lemire, R.; Berner, U.; Musikas, C.; Palmer, D.; Taylor, P.; Tochiyama, O. Chemical Thermodynamics of Iron. Part 1. V. 13a of Chemical Thermodynamics; OECD Publ.: Paris, France, 2013. [Google Scholar]
- Strahm, U.; Patel, R.C.; Matijevic, E. Thermodynamics and kinetics of aqueous iron (III) chloride complexes formation. J. Phys. Chem. 1979, 83, 1689–1695. [Google Scholar] [CrossRef]





| Types | Chemical Composition (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | MnO | P2O5 | ZnO | CuO | BaO | |
| E-glass | 46.7 | 10 | 0.31 | 17.1 | 2.09 | 0.30 | 0.27 | 0.23 | 0.01 | 0.04 | 0.01 | <0.01 | 0.02 |
| Basalt | 46.7 | 14.6 | 8.51 | 6.69 | 2.83 | 2.07 | 1.31 | 0.97 | 0.14 | 0.16 | 0.04 | 0.01 | 0.03 |
| Exposure Environment | ||
|---|---|---|
| Specimen Identification | Internal Solution | External Solution |
| C1 | SW | SW |
| C2 | HPC | SW |
| C3 | SWSSHPC | SW |
| C4 | NC | SW |
| C5 | SWSSNC | SW |
| Test Solutions | Simulated Environment | Test Solution Composition (g/L) | pH | |||
|---|---|---|---|---|---|---|
| NaOH | KOH | Ca(OH)2 | NaCl | |||
| 1 a | SWSSNC | 2.4 | 19.6 | 2.0 | 35 | ~13.4 |
| 2 b | NC | 2.4 | 19.6 | 2.0 | ~13.4 | |
| 3 a | SWSSHPC | 0.6 | 1.4 | 0.037 | 35 | ~12.7 |
| 4 b | HPC | 0.6 | 1.4 | 0.037 | ~12.7 | |
| 5 | DW | ~7.5 | ||||
| Solutions Original pH | 3 Months pH | 6 Months pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E | I | E | E-C | I-C | E | E-C | I | I-C | |||
| GFRP | Seawater (C1) | 7.5 | 9.33 | +24% | 9.65 | +29% | 8.80 | +17% | 9.26 | +23% | |
| HPC (C2) | 12.7 | 9.46 | +26% | 11.98 | −6% | 9.16 | +22% | 12.31 | −3% | ||
| SWSSHPC (C3) | 7.5 | 12.7 | 10.25 | +37% | 12.36 | −3% | 9.46 | +26% | 11.92 | −6% | |
| NC (C4) | 13.4 | 11.04 | +47% | 13.37 | 0% | 12.43 | +66% | 13.22 | −1% | ||
| SWSSNC (C5) | 13.4 | 11.81 | +57% | 13.20 | −1% | 12.08 | +61% | 13.31 | −1% | ||
| BFRP | Seawater (C1) | 7.5 | 9.17 | +22% | 9.40 | +25% | 9.26 | +23% | 8.98 | +20% | |
| HPC (C2) | 12.7 | 9.27 | +24% | 12.64 | −1% | 8.83 | +18% | 12.32 | −3% | ||
| SWSSHPC (C3) | 7.5 | 12.7 | 9.23 | +23% | 12.27 | −3% | 9.11 | +21% | 12.8 | 1% | |
| NC (C4) | 13.4 | 9.44 | +26% | 13.20 | −1% | 9.35 | +25% | 13.70 | 2% | ||
| SWSSNC (C5) | 13.4 | 10.60 | +41% | 13.13 | −2% | 10.32 | +38% | 12.94 | −3% | ||
| CFRP | Seawater (C1) | 7.5 | 9.11 | +21% | 9.39 | +25% | 9.03 | +20% | 9.09 | +21% | |
| HPC (C2) | 12.7 | 9.40 | +25% | 12.46 | −2% | 9.61 | +28% | 11.78 | −7% | ||
| SWSSHPC (C3) | 7.5 | 12.7 | 9.47 | +26% | 12.30 | −3% | 8.89 | +19% | 12.07 | −5% | |
| NC (C4) | 13.4 | 9.46 | +26% | 13.58 | +1% | 9.55 | +27% | 13.30 | −1% | ||
| SWSSNC (C5) | 13.4 | 9.51 | +27% | 13.48 | +1% | 9.50 | +27% | 13.31 | −1% | ||
| Adsorption Bands (cm−1) | Assignment |
|---|---|
| 3400 | O-H stretching band |
| ~2930 and ~2900 | Stretching vibration of C-H group |
| ~1610 | C=C stretching band (alkane) |
| ~1509 | C=C (aromatic nucleus) |
| ~1415 | O-H stretching (alcohol) |
| ~1300 | C-N stretching band |
| ~1245 | Asymmetric stretching vibration C-O-Φ |
| ~1220 | C-O-H (of guaiacyl ring) |
| ~1040 | symmetric stretching vibration C-O-Φ |
| ~827 | Out of plane bending of C-H (benzene) |
| ~800 | CH2 rocking |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Al-Saadi, S.; Singh Raman, R.K.; Zhao, X. Durability of Fibre Reinforced Polymers in Exposure to Dual Environment of Seawater Sea Sand Concrete and Seawater. Materials 2022, 15, 4967. https://doi.org/10.3390/ma15144967
Guo F, Al-Saadi S, Singh Raman RK, Zhao X. Durability of Fibre Reinforced Polymers in Exposure to Dual Environment of Seawater Sea Sand Concrete and Seawater. Materials. 2022; 15(14):4967. https://doi.org/10.3390/ma15144967
Chicago/Turabian StyleGuo, Fan, Saad Al-Saadi, R. K. Singh Raman, and Xiaoling Zhao. 2022. "Durability of Fibre Reinforced Polymers in Exposure to Dual Environment of Seawater Sea Sand Concrete and Seawater" Materials 15, no. 14: 4967. https://doi.org/10.3390/ma15144967
APA StyleGuo, F., Al-Saadi, S., Singh Raman, R. K., & Zhao, X. (2022). Durability of Fibre Reinforced Polymers in Exposure to Dual Environment of Seawater Sea Sand Concrete and Seawater. Materials, 15(14), 4967. https://doi.org/10.3390/ma15144967







