Effect of Biochar Modification by Vitamin C, Hydrogen Peroxide or Silver Nanoparticles on Its Physicochemistry and Tetracycline Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Biochar and Chemically Upgraded Biochars
2.2. Physicochemical and Surface Properties of Biochars
2.3. Batch Adsorption/Desorption Experiment
2.4. Modeling of Adsorption Data
2.5. Statistical Analysis
3. Results and Discussion
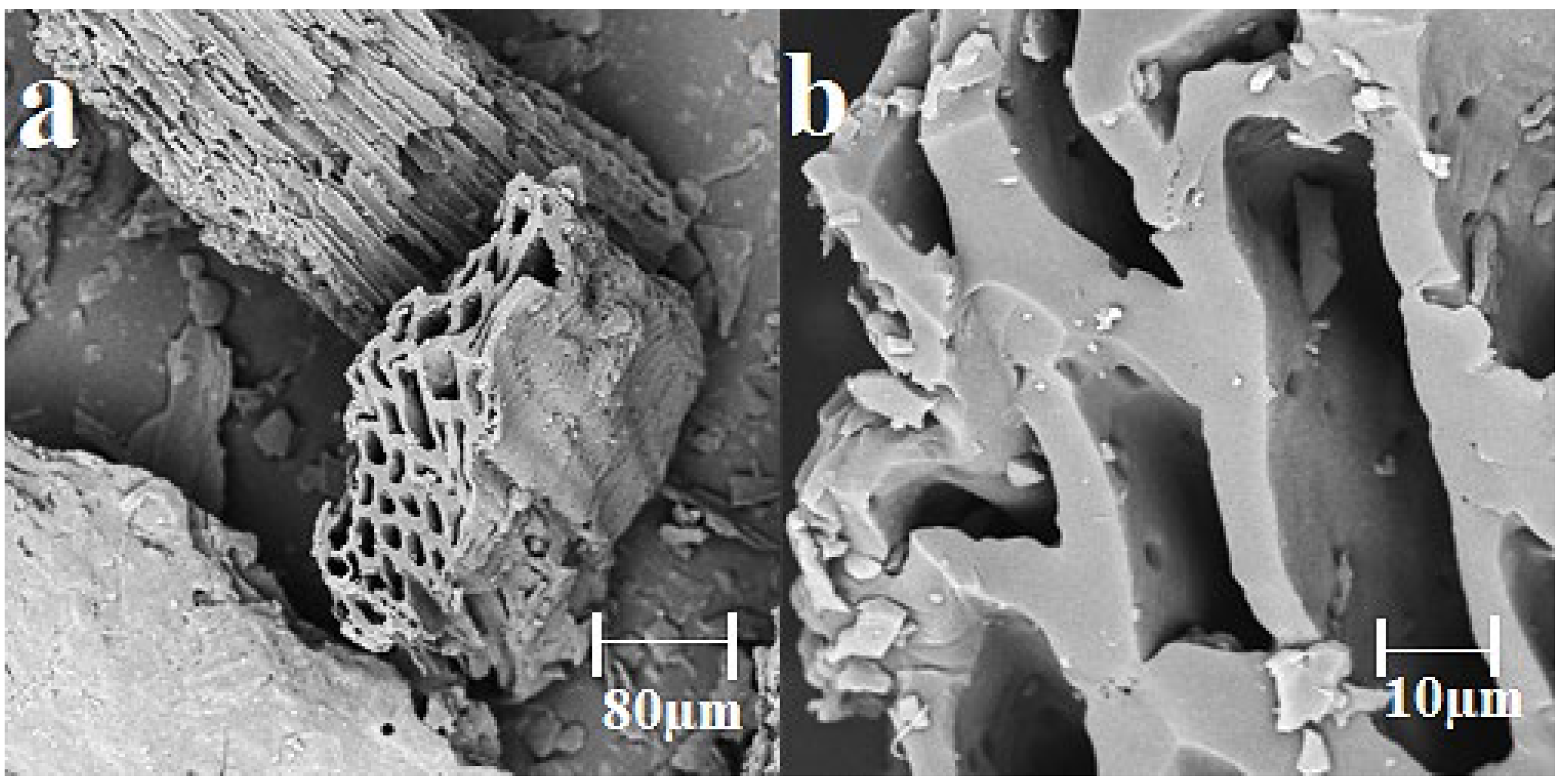
3.1. Characteristic of Biochar
3.2. Impact of Organic/Inorganic Modifiers on Biochar Properties
3.2.1. BET and Surface Chemistry Analysis
3.2.2. FTIR Analysis
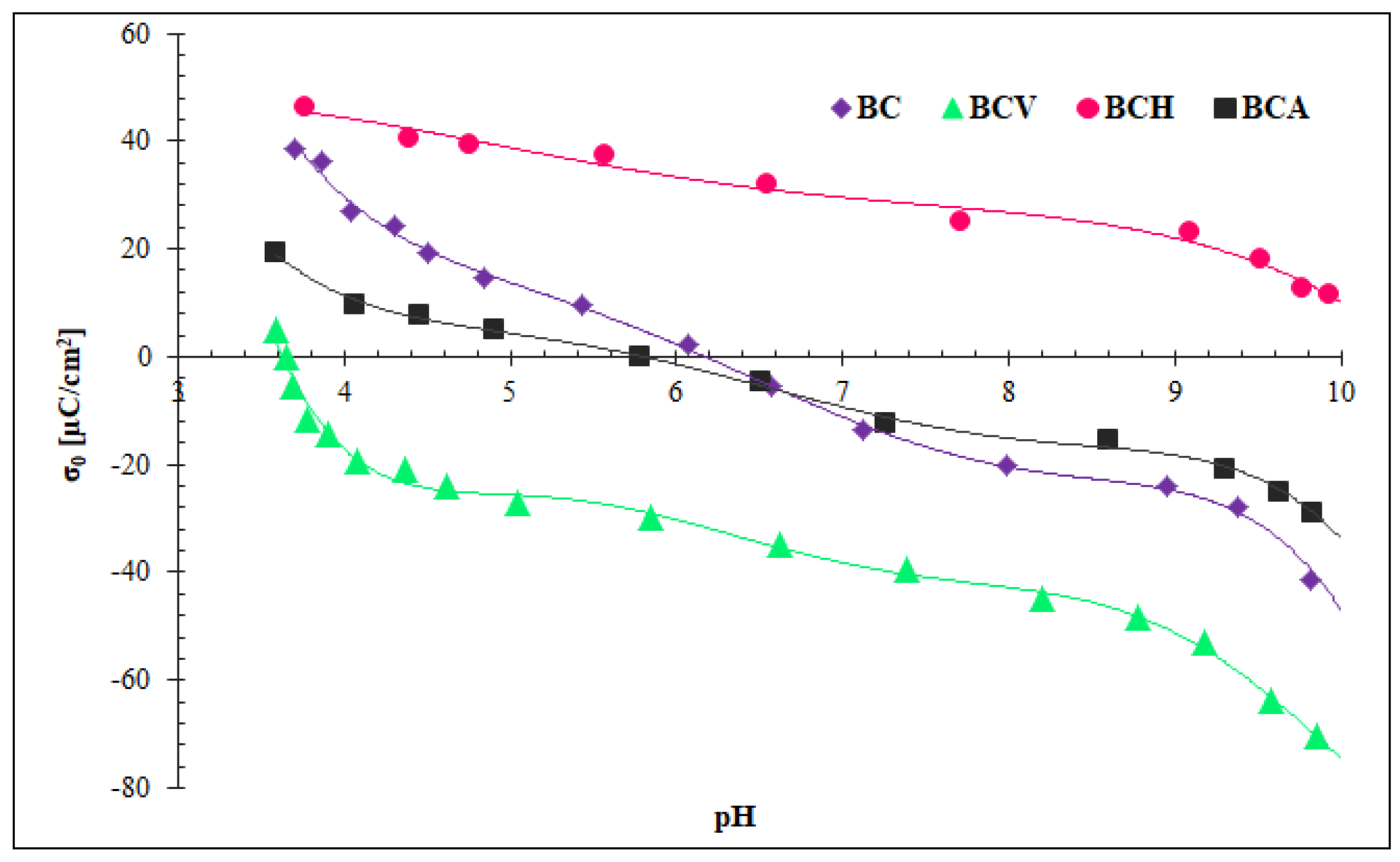
3.2.3. Surface Charge Density Analysis
3.3. Adsorption/Desorption Experimental Study
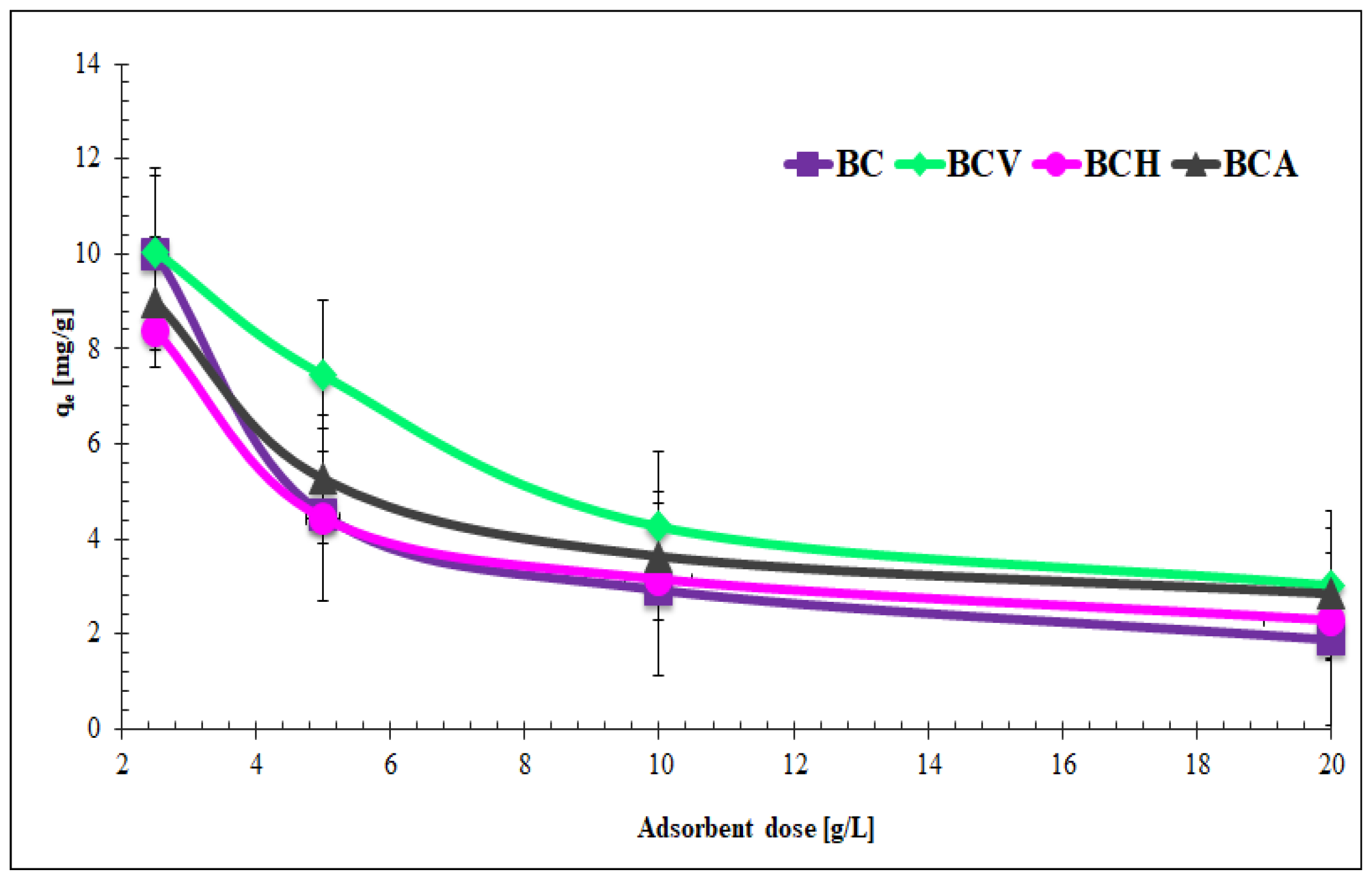
3.3.1. Dosage Effect
3.3.2. Contact Time Effect
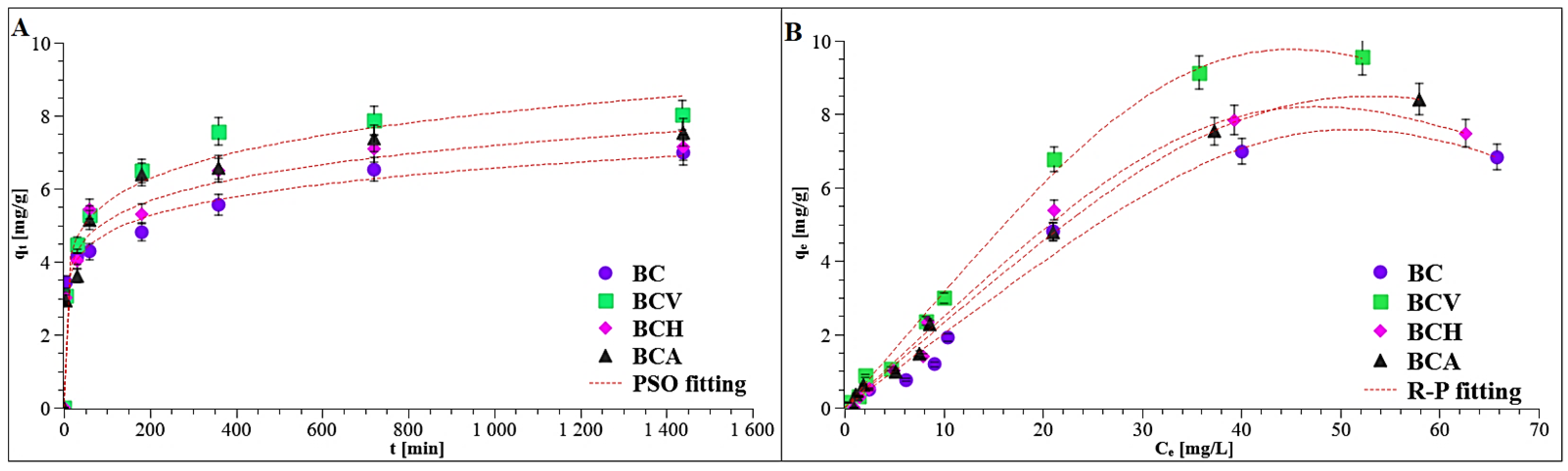
3.3.3. Kinetics and Equilibrium Adsorption
3.3.4. Mechanisms of Adsorption
3.3.5. Adsorption Efficiency of Biochars
3.3.6. Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Patyra, E.; Kwiatek, K. Substancje przeciwbakteryjne w nawozach organicznych–potencjalny problem skażenia środowiska. Życie Weter. 2018, 93, 796–799. (In Polish) [Google Scholar]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Wan, Y.; Yu, Q.; Xie, X. Effects of soil/solution ratios and cation types on adsorption and desorption of tetracycline in soils. Soil Sci. Soc. Am. J. 2010, 74, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Gopal, G.; Alex, S.A.; Chandrasekarana, N.; Mukherjee, A. A review on tetracycline removal from aqueous systems by advanced treatment techniques. RSC Adv. 2020, 10, 27081–27095. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Shakya, A.; Agarwal, T. Poultry Litter Biochar: An Approach towards Poultry Litter Management–A Review. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2657–2668. [Google Scholar] [CrossRef]
- ISWA. ISWA Report; ISWA: Vienna, Austria, 2014. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M.; Czupryński, B.; Apiecionek, Ł. The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers 2019, 11, 1431. [Google Scholar] [CrossRef] [Green Version]
- Seiler, G.J.; Gulya, T.J. Sunflower: Overview. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar application to soil: Agronomic and environmental benefits and unintended consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar]
- Wiśniewska, M.; Nowicki, P.; Szewczuk-Karpisz, K.; Gęca, M.; Jędruchniewicz, K.; Oleszczuk, P. Simultaneous removal of toxic Pb(II) ions, poly(acrylic acid) and Triton X-100 from their mixed solution using engineered biochars obtained from horsetail herb precursor–Impact of post-activation treatment. Sep. Purif. Technol. 2021, 276, 119297. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Wernerc, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Nowicki, P.; Sokołowska, Z.; Pietrzak, R. Hay-based activated biochars obtained using two different heating methods as effective low-cost sorbents: Solid surface characteristics, adsorptive properties and aggregation in the mixed Cu(II)/PAM system. Chemosphere 2020, 250, 126312. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Z.; Yu, Q.; Cheng, Y.; Liu, Z.; Zhang, T.; Zhou, S. Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues. Environ. Res. 2020, 183, 109195. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, X.; Wang, L.; Gao, B.; Luo, J.; Fang, R.; Zou, W.; Meng, N. Sorption of tetracycline on H2O2-modified biochar derived from rape stalk. Env. Pollut. Bioavail. 2019, 31, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Gluba, Ł.; Rafalska-Przysucha, A.; Szewczak, K.; Łukowski, M.; Szlązak, R.; Vitková, J.; Kobyłecki, R.; Bis, Z.; Wichliński, M.; Zarzycki, R.; et al. Effect of Fine Size-Fractionated Sunflower Husk Biochar on Water Retention Properties of Arable Sandy Soil. Materials 2021, 14, 1335. [Google Scholar] [CrossRef]
- Tomczyk, A.; Szewczuk-Karpisz, K.; Sokołowska, Z.; Kercheva, M.; Dimitrov, E. Purification of Aqueous Media by Biochars: Feedstock Type Effect on Silver Nanoparticles Removal. Molecules 2020, 25, 2930. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Sholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. C 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Janusz, W. Electrical double layer at the metal oxide/electrolyte interface in interfacial forces and fields: Theory and applications. In Surfactant Science Series; Decker, M., Ed.; ScienceOpen Inc.: New York, NY, USA, 1994; Volume 85. [Google Scholar]
- Szewczuk-Karpisz, K.; Wiśniewska, M.; Myśliwiec, D. Albumin adsorption influence on the stability of the mesoporous zirconia suspension. J. Ind. Eng. Chem. 2015, 32, 113–119. [Google Scholar] [CrossRef]
- Sahoo, D.; Remya, N. Influence of operating parameters on the microwave pyrolysis of rice husk: Biochar yield, energy yield, and property of biochar. Biomass Conv. Bioref. 2020, 12, 3447–3456. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. Kungl. Svenska Vetenskapsakad. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption process. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advances in water pollution research: Removal of biologically resistant pollutant from waste water by adsorption. In Proceedings of 1st International Conference on Water Pollution Symposium 2; Pergamon Press: Oxford, UK, 1962; pp. 231–266. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Celińska, M.; Sokołowska, Z.; Kuśmierz, M. Carboxin and Diuron Adsorption Mechanism on Sunflower Husks Biochar and Goethite in the Single/Mixed Pesticide Solutions. Materials 2021, 14, 2584. [Google Scholar] [CrossRef]
- Kumara, N.T.R.N.; Hamdan, N.; Petra, M.I.; Tennakoon, K.U.; Ekanayake, P. Equilibrium Isotherm Studies of Adsorption of Pigments Extracted from Kuduk-kuduk (Melastoma malabathricum L.) Pulp onto TiO2 Nanoparticles. J. Chem. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Malucelli, L.C.; Silvestre, G.F.; Carneiro, J.; Vasconcelos, E.C.; Guiotoku, M.; Maia, C.M.; Carvalho Filho, M.A. Biochar higher heating value estimative using thermogravimetric analysis. J. Therm. Anal. Calorim. 2020, 139, 2215–2220. [Google Scholar] [CrossRef]
- Saleh, M.E.; El-Refaey, A.A.; Mahmoud, A.H. Effectiveness of sunflower seed husk biochar for removing copper ions from wastewater: A comparative study. Soil Water Res. 2016, 11, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Lin, Q.; Irfan, M.; Chen, Q.; Zhao, X.; Li, G. Slow pyrolysis as a promising approach for producing biochar from sunflower straw. BioResources 2018, 13, 7455–7469. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Global Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Mitan, N.M.M.; Dimin, M.F. Characterization of Biochar Derived from Rubber Wood Sawdust through Slow Pyrolysis on Surface Porosities and Functional Groups. Procedia Eng. 2013, 68, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Zaitun, Z.; Halim, A.; Sa’dah, Y.; Cayhadi, R. Surface morphology properties of biochar feedstock for soil amendment. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 012034. [Google Scholar] [CrossRef]
- Suárez-Hernández, L.; Ardila-A, A.N.; Barrera-Zapata, R. Morphological and physicochemical characterization of biochar produced by gasification of selected forestry species. Rev. Fac. Ing. 2017, 26, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Jinsha, Y.; Xin, L.; Ruisen, L. Adsorption Performance of Two Kinds of Biochar for Cd2+ in Water. Int. J. Environ. Protect. Pol. 2022, 10, 67–72. [Google Scholar]
- Huff, M.D.; Lee, J.W. Biochar-surface oxygenation with hydrogen peroxide. J. Environ. Manage. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Han, Q.; Yang, Y.; Wang, R.; Zhang, K.; Liu, N.; Hong, M. Biochar Derived from Agricultural Wastes as a Means of Facilitating the Degradation of Azo Dyes by Sulfides. Catalysts 2021, 11, 434. [Google Scholar] [CrossRef]
- Nikraftar, N.; Ghorbani, F. Adsorption of As(V) Using Modified Magnetic Nanoparticles with Ascorbic Acid: Optimization by Response Surface Methodology. Water Air Soil Pollut. 2016, 227, 168. [Google Scholar] [CrossRef]
- Shen, C.; Wen, Y.; Kang, X.; Liu, W. H2O2-induced surface modification: A facile, effective and environmentally friendly pretreatment of chitosan for dyes removal. Chem. Eng. J. 2011, 166, 474–482. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Xian, Q.; Shen, F.; Wu, J.; Zhang, Y. Removal of Congo Red and Methylene Blue from Aqueous Solutions by Vermicompost-Derived Biochars. PLoS ONE 2016, 11, e0154562. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Wiśniewska, M.; Medykowska, M.; Galaburda, M.V.; Bogatyrov, V.M.; Oranska, I.O.; Błachnio, M.; Oleszczuk, P. Simultaneous adsorption of Cu(II) ions and poly(acrylic acid) on the hybrid carbon-mineral nanocomposites with metallic elements. J. Hazard. Mater. 2020, 412, 125138. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Grygorczuk-Płaneta, K.; Naveed, S. Rhizobium leguminosarum bv. trifolii exopolysaccharide and sunflower husk biochar as factors affecting immobilization of both tetracycline and Cd ions on soil solid phase. J. Soil. Sediment. 2022. [Google Scholar] [CrossRef]
- Saremi, F.; Miroliaei, M.R.; Nejad, M.S.; Sheibani, H. Adsorption of tetracycline antibiotic from aqueous solutions onto vitamin B6-upgraded biochar derived from date palm leaves. J. Mol. Liq. 2020, 318, 114126. [Google Scholar] [CrossRef]
- Han, X.; Li, R.; Miao, P.; Gao, J.; Hu, G.; Zhao, Y.; Chen, T. Design, Synthesis and Adsorption Evaluation of Bio-Based Lignin/Chitosan Beads for Congo Red Removal. Materials 2022, 15, 2310. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Gao, J.; Hu, G.; Zhao, Y.; Tang, X.; Han, X. Efficient Removal of Methylene Blue by Bio-Based Sodium Alginate/Lignin Composite Hydrogel Beads. Polymers 2022, 14, 2917. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Sang, Y.-n.; Tang, M.; Hu, G.-w.; Han, X.-b.; Gao, J.; Ma, R. Facile synthesis of magnetic CS-g-SPSS microspheres via electron beam radiation for efficient removal of methylene blue. J. Saudi Chem. Soc. 2021, 25, 101299. [Google Scholar] [CrossRef]


| Equation | Formula | References |
|---|---|---|
| Pseudo-first order (PFO) | (7) | [26] |
| Pseudo-second order (PSO) | (8) | [27] |
| Particle internal diffusion model (IPD) | (9) | [28] |
| Langmuir isotherm | (10) | [29] |
| Langmuir-Freundlich isotherm | (11) | [30] |
| Redlich-Peterson isotherm (R-P) | (12) | [31] |
| Sample | M [%] | Ash [%] | H/C | O/C | (O+N)/C | HHV [MJ/kg] | ED [%] |
|---|---|---|---|---|---|---|---|
| Sunflower husks | 9.38 | 2.43 | 0.12 | 0.84 | 0.85 | 16.31 | 2.01 |
| Biochar | 0.49 | 35.4 | 0.04 | 0.02 | 0.04 | 32.75 |
| Sample | SBET [m2/g] | Vm [cm3/g] | Vt [m2/g] | D [nm] | TOC [%] | Acidic Groups [mmol/g] | Basic Groups [mmol/g] |
|---|---|---|---|---|---|---|---|
| BC | 7.02 | 0.004 | 7.98 | 3.54 | 82.15 | 2.90 | 3.20 |
| BCV | 1.30 | 0.001 | 3.40 | 36.37 | 82.92 | 3.10 | 3.40 |
| BCH | 2.19 | 0.002 | 4.07 | 5.11 | 80.72 | 5.20 | 6.90 |
| BCA | 0.10 | 0.001 | 3.84 | 17.68 | 82.64 | 4.00 | 5.20 |
| FTIR Adsorption Peak (cm−1) | Vibrations | References |
|---|---|---|
| 3432 | O-H stretching–carboxylic acid, hydroxyl groups and phenol | [21] |
| 2922 | C-H stretching–methyl and methylene groups | [40] |
| 1611 | C=O stretching–carboxylic and lactone groups and C=C stretching in aromatic rings | [30] |
| 1384 | N-O stretching–pyridinic-N oxide | [41] |
| 1164 | C-O stretching–saturated ester | [42] |
| 1107 | C-O-C stretching–fatty ether | [42] |
| 1050 | C-N and C=C stretching–secondary amines and aromatic structure | [41] |
| 880 | aromatic C–H stretching | [40] |
| 811 | aromatic C–H stretching | [40] |
| 668 | aromatic C–H stretching | [40] |
| 580 | chloride and CO3 = groups | [14] |
| Equation | Parameter | BC | BCV | BCH | BCA |
|---|---|---|---|---|---|
| PFO | qe [mg/g] | 5.10 ±0.49 | 7.28 ±0.34 | 6.67 ±1.16 | 6.96 ±0.29 |
| k1 [1/min] | 0.11 × 10−2 ±0.02 × 10−2 | 0.28 × 10−2 ±0.17 × 10−2 | 0.12 × 10−2 ±0.09 × 10−2 | 0.21 × 10−2 ±0.16 × 10−2 | |
| R2 | 0.69 | 0.61 | 0.86 | 0.85 | |
| PSO | qe [mg/g] | 6.99 ±0.88 | 7.85 ±0.61 | 7.14 ±0.35 | 7.55 ±0.54 |
| k2 [g/mg·min] | 0.29 × 10−2 ±0.13 × 10−2 | 0.54 × 10−2 ±0.05 × 10−2 | 0.41 × 10−2 ±0.15 × 10−2 | 0.47 × 10−2 ±0.02 × 10−2 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | |
| IPD | b [mg/g] | 3.55 ±0.72 | 4.29 ±0.99 | 3.98 ±0.88 | 4.11 ±0.92 |
| kD [g/mg·min1/2] | 0.19 × 10−2 ±0.13 × 10−2 | 0.33 × 10−2 ±0.17 × 10−2 | 0.29 × 10−2 ±0.16 × 10−2 | 0.32 × 10−2 ±0.16 × 10−2 | |
| R2 | 0.47 | 0.38 | 0.36 | 0.41 | |
| Langmuir | KL [L/mg] | 1.02 × 10−2 ±0.98 × 10−2 | 2.09 × 10−2 ±0.69 × 10−2 | 1.24·10−2 ±0.87 × 10−2 | 1.64 × 10−2 ±0.47 × 10−2 |
| Qm [mg/g] | 7.26 ±1.61 | 10.56 ±2.82 | 7.93 ±1.16 | 8.09 ±2.19 | |
| R2 | 0.94 | 0.98 | 0.95 | 0.98 | |
| Langmuir-Freundlich | KLF [L/mg] | 3.36 × 10−2 ±0.21 × 10−2 | 3.88 × 10−2 ±0.14 × 10−2 | 3.64 × 10−2 ±0.19 × 10−2 | 3.42 × 10−2 ±0.96 × 10−2 |
| Am [mg/g] | 6.17 ±0.46 | 9.13 ±0.15 | 8.09 ±0.21 | 8.39 ±0.22 | |
| m | 1.17 ±0.17 | 1.19 ±0.11 | 1.17 ±0.14 | 1.21 ±0.05 | |
| R2 | 0.94 | 0.98 | 0.95 | 0.98 | |
| Redlich-Peterson | KR [L/mg] | 2.81 ±0.29 | 8.65 ±0.08 | 4.17 ±0.21 | 6.33 ±0.22 |
| aR [(L/mg)β] | 2.32 ±0.55 | 5.66 ±0.95 | 4.81 ±0.24 | 3.74 ±0.51 | |
| β | 0.24 ±0.06 | 0.39 ±0.13 | 0.29 ±0.09 | 0.31 ±0.05 | |
| R2 | 0.98 | 0.99 | 0.98 | 0.99 |
| Sample | Deionized Water | HCl | NaOH | ||
|---|---|---|---|---|---|
| 5 | 7 | 9 | |||
| BC | 18.20% | 19.24% | 20.42% | 66.28% | 3.69% |
| BCV | 11.71% | 11.56% | 14.76% | 73.82% | 8.23% |
| BCH | 12.15% | 12.75% | 15.45% | 71.33% | 4.83% |
| BCA | 13.82% | 16.67% | 17.22% | 62.88% | 2.95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, A.; Szewczuk-Karpisz, K. Effect of Biochar Modification by Vitamin C, Hydrogen Peroxide or Silver Nanoparticles on Its Physicochemistry and Tetracycline Removal. Materials 2022, 15, 5379. https://doi.org/10.3390/ma15155379
Tomczyk A, Szewczuk-Karpisz K. Effect of Biochar Modification by Vitamin C, Hydrogen Peroxide or Silver Nanoparticles on Its Physicochemistry and Tetracycline Removal. Materials. 2022; 15(15):5379. https://doi.org/10.3390/ma15155379
Chicago/Turabian StyleTomczyk, Agnieszka, and Katarzyna Szewczuk-Karpisz. 2022. "Effect of Biochar Modification by Vitamin C, Hydrogen Peroxide or Silver Nanoparticles on Its Physicochemistry and Tetracycline Removal" Materials 15, no. 15: 5379. https://doi.org/10.3390/ma15155379
APA StyleTomczyk, A., & Szewczuk-Karpisz, K. (2022). Effect of Biochar Modification by Vitamin C, Hydrogen Peroxide or Silver Nanoparticles on Its Physicochemistry and Tetracycline Removal. Materials, 15(15), 5379. https://doi.org/10.3390/ma15155379







