Use of Organic Materials to Limit the Potential Negative Effect of Nitrogen on Maize in Different Soils
Abstract
1. Introduction
2. Materials and Methods
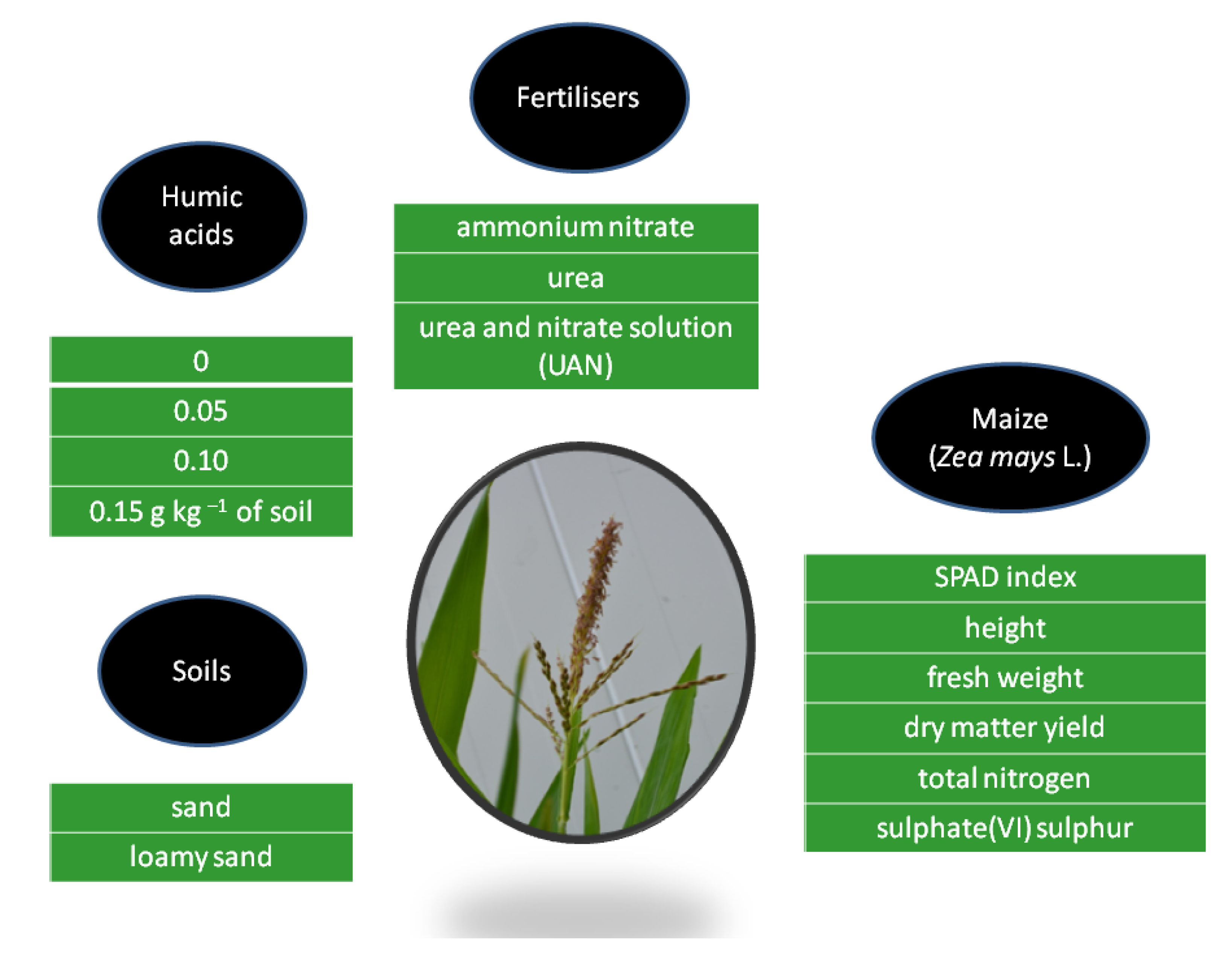
2.1. Methodology of the Plant Growing Experiment
2.2. Methodology of Laboratory and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klavins, M.; Grandovska, S.; Obuka, V.; Ievinsh, G. Comparative study of biostimulant properties of industrially and experimentally produced humic substances. Agronomy 2021, 11, 1250. [Google Scholar] [CrossRef]
- Gümüş, İ.; Şeker, C. Influence of humic acid applications on modulus of rupture, aggregate stability, electrical conductivity, carbon and nitrogen content of a crusting problem soil. Solid Earth 2015, 6, 1231–1236. [Google Scholar] [CrossRef]
- Leite, J.M.; Pitumpe Arachchige, P.S.; Ciampitti, I.A.; Hettiarachchi, G.M.; Maurmann, L.; Trivelin, P.C.O.; Prasad, P.V.V.; Sunoj, S.V.J. Co-addition of humic substances and humic acids with urea enhances foliar nitrogen use efficiency in sugarcane (Saccharum officinarum L.). Heliyon 2020, 6, e05100. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994; pp. 1–512. [Google Scholar]
- Rostami, M.; Shokouhian, A.; Mohebodini, M. Effect of humic acid, nitrogen concentrations and application method on the morphological, yield and biochemical characteristics of strawberry ‘Paros’. Int. J. Fruit Sci. 2022, 22, 203–214. [Google Scholar] [CrossRef]
- Pena-Mendez, M.; Havel, J.; Patocka, J. Humic substances-compounds of still unknown structure: Applications in agriculture, industry, environment, and biomedicine. J. Appl. Biomed. 2005, 3, 13–24. [Google Scholar] [CrossRef]
- Abbas, T.; Ahmad, S.; Ashraf, M.; Adnan Shahid, M.; Yasin, M.; Mukhtar Balal, R.; Pervez, M.A.; Abbas, S. Effect of humic and application at different growth stages of kinnow mandarin (Citrus reticulata blanco) on the basis of physio-biochemical and reproductive responses. Acad. J. Biotechnol. 2013, 1, 014–020. [Google Scholar] [CrossRef]
- Khan, A.; Afridi, M.Z.; Airf, M.; Ali, S.; Muhammad, I. A sustainable approach toward maize production: Effectiveness of farmyad manure and urea nitrogen. Ann. Biol. Sci. 2017, 5, 8–13. [Google Scholar] [CrossRef]
- Hussain, I.; Khan, A.; Akbarm, H. Maize growth in response to beneficial microbes, Humic acid and farmyard manure application. Sarhad J. Agric. 2021, 37, 1426–1435. [Google Scholar] [CrossRef]
- Omonode, R.A.; Halvorson, A.D.; Bernard, G.; Vyn, T.J. Achieving lower nitrogen balance and higher nitrogen recovery efficiency reduces nitrous oxide emissions in North America’s maize cropping systems. Front. Plant Sci. 2017, 8, 1080. [Google Scholar] [CrossRef]
- Ren, B.; Guo, Y.; Liu, P.; Zhao, B.; Zhang, J. Effects of urea-ammonium nitrate solution on yield, N2O emission, and nitrogen efficiency of summer maize under integration of water and fertilizer. Front. Plant Sci. 2021, 12, 700331. [Google Scholar] [CrossRef]
- Pereira, R.V.; Filgueiras, C.C.; Dória, J.; Peñaflor, M.F.G.V.; Willett, D.S. The effects of biostimulants on induced plant defense. Front. Agron. 2021, 3, 630596. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.D.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Ulukan, H. Effect of soil applied humic acid at different sowing times on some yield components of wheat (Triticum spp.) hybrids. Int. J. Bot. 2008, 4, 164–175. [Google Scholar] [CrossRef][Green Version]
- Pukalchik, M.; Kydralieva, K.; Yakimenko, O.; Fedoseeva, E.; Terekhova, V. Outlining the Potential role of humic products in modifying biological properties of the soil—A review. Front. Environ. Sci. 2019, 7, 80. [Google Scholar] [CrossRef]
- Sharif, M.; Khattak, R.A.; Sarir, M.S. Effect of different levels of lignitic coal derived humic acid on growth of maize plants. Commun. Soil Sci. Plant Anal. 2002, 33, 3567–3580. [Google Scholar] [CrossRef]
- Olivares, F.L.; Aguiar, N.O.; Rosa, R.C.C.; Canellas, L.P. Substrate biofortification in combination with foliar sprays of plant growth promoting bacteria and humic substances boosts production of organic tomatoes. Sci. Hortic. 2015, 183, 100–108. [Google Scholar] [CrossRef]
- Schoebitz, M.; López, M.D.; Serrí, H.; Martínez, O.; Zagal, E. Combined application of microbial consortium and humic substances to improve the growth performance of blueberry seedlings. J. Soil Sci. Plant Nutr. 2016, 16, 1010–1023. [Google Scholar] [CrossRef]
- Bharali, A.; Baruah, K.K.; Bhattacharyya, P.; Gorh, D. Integrated nutrient management in wheat grown in a northeast India soil: Impacts on soil organic carbon fractions in relation to grain yield. Soil Tillage Res. 2017, 168, 81–91. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Giovanardi, D.; Dallai, D.; Dondini, L.; Mantovani, V.; Stefani, E. Elicitation of resistance to bacterial canker of stone fruits by humic and fulvic acids (glucohumates): A cDNA-AFLP-dHPLC approach. Sci. Hortic. 2016, 212, 183–192. [Google Scholar] [CrossRef]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Hazmi, M.H. Lignite-derived humic acid effect on growth of wheat plants in different soils. Pedosphere 2011, 2, 124–131. [Google Scholar] [CrossRef]
- Eyheraguibel, B.; Silvestre, J.; Morard, P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour. Technol. 2008, 99, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, L.; McLaughlin, N.B.; Mi, J.; Chen, Q.; Liu, J. Effect of synthetic and natural water absorbing soil amendment soil physical properties under potato production in a semi-arid region. Soil Tillage Res. 2015, 148, 31–39. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Ali, S.; et al. Humic substances: Determining potential molecular regulatory processes in plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Olivares, F.L.; Busato, J.G.; de Paula, A.M.; da Silva Lima, L.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef]
- da Silva, M.S.R.A.; dos Santos, B.d.M.S.; da Silva, C.S.R.A.; da Silva, C.S.R.A.; Antunes, L.F.S.; dos Santos, R.M.; Santos, C.H.B.; Rigobelo, E.C. Humic substances in combination with plant growth-promoting bacteria as an alternative for sustainable agriculture. Front. Microbiol. 2021, 12, 719653. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Z.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Effects of humic acid added to controlled-release fertilizer on summer maize yield, nitrogen use efficiency and greenhouse gas emission. Agriculture 2022, 12, 448. [Google Scholar] [CrossRef]
- Susic, M.; Genc, Y.; Lyons, G. Replenishing humic acids in agricultural soils. Agronomy 2016, 6, 45. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S. Content of trace elements in soil fertilized with potassium and nitrogen. Agriculture 2020, 10, 398. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Bujanowicz-Haraś, B. Mineral fertilization and maize cultivation as factors which determine the content of trace elements in soil. Agronomy 2022, 12, 286. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S. Potassium and nitrogen fertilization vs. trace element content of maize (Zea mays L.). Agriculture 2021, 11, 96. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem. Biol. Technol. Agric. 2015, 2, 5. [Google Scholar] [CrossRef]
- Geng, J.; Yang, X.; Huo, X.; Chen, J.; Lei, S.; Li, H.; Lang, Y.; Liu, Q. Effects of controlled-release urea combined with fulvic acid on soil inorganic nitrogen, leaf senescence and yield of cotton. Sci. Rep. 2020, 10, 17135. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; World Soil Resources Report. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources; Reports No. 106; FAO: Rome, Italy, 2015; p. 192. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 18 November 2021).
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties (Agronomy 9); Norman, A.G., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analysis and Evaluation of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- Grzesiuk, W. Nephelometric determination of sulphate sulphur in plants. Rocz. Gleboz. 1968, 1, 167–173. Available online: http://ssa.ptg.sggw.pl/files/artykuly/1968_19/1968_tom_19_nr_1/tom_19_nr_1_167-173.pdf (accessed on 2 June 2022).
- PN-R-04032; Soil and Mineral Materials–Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warsaw, Poland, 1998; pp. 1–12.
- Tibco. Statistica Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021. [Google Scholar]
- Khan, S.A.; Khan, S.U.; Qayyum, A.; Gurmani, A.R.; Khan, A.; Khan, S.M.; Ahmed, W.; Mehmood, A.; Amin, B.A.Z. Integration of humic acid with nitrogen wields an auxiliary impact on physiological traits, growth and yield of maize (Zea mays L.) varieties. Appl. Ecol. Environ. Res. 2019, 17, 6783–6799. [Google Scholar] [CrossRef]
- Lollato, R.P.; Figueiredo, B.M.; Dhillon, J.S.; Arnall, D.B.; Raun, W.R. Wheat grain yield and grain-nitrogen relationships as affected by N, P, and K fertilization: A synthesis of long-term experiments. Field Crops Res. 2019, 236, 42–57. [Google Scholar] [CrossRef]
- Ichami, S.M.; Shepherd, K.D.; Sila, A.M.; Stoorvogel, J.J.; Hoffland, E. Fertilizer response and nitrogen use efficiency in African smallholder maize farms. Nutr. Cycl. Agroecosyst. 2019, 113, 1–19. [Google Scholar] [CrossRef]
- Ju, X.T.; Gu, B.J. Status-quo, problem and trend of nitrogen fertilization in China. J. Plant Nutr. Fert. 2014, 20, 783–795. [Google Scholar] [CrossRef]
- Zhang, W.F.; Ma, L.; Huang, G.Q.; Wu, L.; Chen, X.P.; Zhang, F.S. The development and contribution of nitrogenous fertilizer in China and challenges faced by the country. Sci. Agric. Sin. 2013, 46, 3161–3171. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Mullen, R.W.; Freeman, K.W.; Thomason, W.E.; Lukina, E.V. Improving nitrogen use efficiency in cereal grain production with optical sensing and variable rate application. Agron. J. 2002, 94, 815–820. [Google Scholar] [CrossRef]
- Gojon, A. Nitrogen nutrition in plants: Rapid progress and new challenges. J. Exp. Bot. 2017, 68, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Bi, Y.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef]
- Goñi, O.; Łangowski, Ł.; Feeney, E.; Quille, P.; O’Connell, S. Reducing nitrogen input in barley crops while maintaining yields using an engineered biostimulant derived from Ascophyllum nodosum to enhance nitrogen use efficiency. Front. Plant Sci. 2021, 12, 664682. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yang, J.S.; Tao, J.Y.; Yao, Y.J. Integrated application of inorganic fertilizer with fulvic acid for improving soil nutrient supply and nutrient use efficiency of winter wheat in a salt-affected soil. Appl. Soil Ecol. 2022, 170, 104255. [Google Scholar] [CrossRef]
- Azeem, K.; Naz, F.; Jalal, A.; Galindo, F.S.; Teixeira Filho, M.C.M.; Khalil, F. Humic acid and nitrogen dose application in corn crop under alkaline soil conditions. Rev. Bras. Eng. Agríc. Ambiental. 2021, 25, 657–663. [Google Scholar] [CrossRef]
- Azeem, K.; Shah, S.; Ahmad, N.; Shah, S.T.; Khan, F.; Arafat, Y.; Naz, F.; Azeem, I.; Ilyas, M. Physiological indices, biomass and economic yield of maize influenced by humic acid and nitrogen levels. Russ. Agric. Sci. 2015, 41, 115–119. [Google Scholar] [CrossRef]
- Baldotto, M.A.; Melo, R.O.; Baldotto, L.B. Field corn yield in response to humic acids application in the absence or presence of liming and mineral fertilization. Semina Ciên. Agrár. 2019, 40, 3299–3304. [Google Scholar] [CrossRef]
- Niaz, A.; Yaseen, M.; Shakar, M.; Sultana, S.; Ehsan, M.; Nazarat, A. Maize production and nitrogen use efficiency in response to nitrogen application with and without humic acid. J. Anim. Plant Sci. 2016, 26, 1641–1651. [Google Scholar]
- Suntari, R.; Retnowati, R.; Soemarno, S.; Munir, M. Determination of urea-humic acid dosage of vertisols on the growth and production of rice. AGRIVITA J. Agric. Sci. 2015, 37, 185–192. [Google Scholar] [CrossRef]
- Chen, X.G.; Kou, M.; Tang, Z.H.; Zhang, A.J.; Li, H.M. The use of humic acid urea fertilizer for increasing yield and utilization of nitrogen in sweet potato. Plant Soil Environ. 2017, 63, 201–206. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Yuan, L.; Li, W.; Lin, Z.A.; Li, Y.T.; Hu, S.W.; Zhao, B.Q. Effects of urea enhanced with different weathered coal-derived humic acid components on maize yield and fate of fertilizer nitrogen. J. Intergrative Agric. 2019, 18, 656–666. [Google Scholar] [CrossRef]
- Pei, R.J.; Yuan, T.Y.; Wang, J.Z.; Hu, N.; Li, Y.N. Effects of application of humic acid on yield, nitrogen use efficiency of summer maize. Sci. Agric. Sin. 2017, 50, 2189–2198. [Google Scholar] [CrossRef]
- Gao, F.; Li, Z.; Du, Y.; Duan, J.; Zhang, T.; Wei, Z.; Guo, L.; Gong, W.; Liu, Z.; Zhang, M. The combined application of urea and fulvic acid solution improved maize carbon and nitrogen metabolism. Agronomy 2022, 12, 1400. [Google Scholar] [CrossRef]
- Canellas, L.P.; Balmori, D.M.; Médici, L.O.; Aguiar, N.O.; Campostrini, E.; Rosa, R.C.; Façanha, A.R.; Olivares, F.L. A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant Soil 2013, 366, 119–132. [Google Scholar] [CrossRef]
- El-Bassioung, H.S.M.; Bakry, B.A.; El-Monem Attia, A.A.; Abd Allah, M.M. Physiological role of humic acid and nicotinamide on improving plant growth, yield, and mineral nutrient of wheat (Triticum durum) grown under newly reclaimed sandy soil. Agric. Sci. 2014, 5, 687–700. [Google Scholar] [CrossRef]
- Kazemi, M. Effect of foliar application of humic acid and potassium nitrate on cucumber growth. Bull. Environ. Pharmacol. Life Sci. 2013, 11, 3–6. [Google Scholar]
- Mahmoudi, M.; Samavat, S.; Mostafavi, M.; Khalighi, A.; Cherati, A. The effects of proline and humic acid on quantitative properties of kiwifruit. Int. Res. J. Appl. Basic Sci. 2013, 6, 1117–1119. [Google Scholar]
- Denre, M.; Bandopadhyay, P.K.; Chakravarty, A.; Pal, S.; Bhattacharya, A. Effect of foliar application of humic acid, zinc and boron on biochemical changes related to productivity of pungent pepper (Capsicum annuum L.). Afr. J. Plant Sci. 2014, 8, 320–335. [Google Scholar] [CrossRef]
- Reeza, A.A.; Ahmed, O.H.; Majid, N.M.N.A.; Jalloh, M.B. Reducing ammonia loss from urea by mixing with humic and fulvicacids isolated from coal. Am. J. Environ. Sci. 2009, 5, 420–426. [Google Scholar] [CrossRef]




| Humic Acid Dose g kg−1 of Soil | Sand | Loamy Sand | ||||||
|---|---|---|---|---|---|---|---|---|
| Ammonium Nitrate | Urea | UAN | Average | Ammonium Nitrate | Urea | UAN | Average | |
| Height (cm) | ||||||||
| 0 | 192.9 ab | 209.7 ab | 204.7 ab | 202.4 A | 195.1 ab | 206.7 ab | 193.7 ab | 198.5 A |
| 0.05 | 204.0 ab | 212.1 ab | 184.9 ab | 200.3 A | 202.6 ab | 210.9 ab | 206.0 ab | 206.5 A |
| 0.10 | 205.8 ab | 213.4 b | 180.1 ab | 199.8 A | 208.3 ab | 203.3 ab | 209.9 ab | 207.2 A |
| 0.15 | 206.1 ab | 207.6 ab | 163.3 a | 192.3 A | 205.8 ab | 191.1 ab | 201.0 ab | 199.3 A |
| Average | 202.2 AB | 210.7 A | 183.3 B | 198.7 A | 203.0 AB | 203.0 A | 202.7 AB | 202.9 A |
| r | 0.853 | −0.251 | −0.978 | −0.904 | 0.852 | −0.824 | 0.476 | 0.086 |
| Aerial parts fresh weight yield (g pot−1) | ||||||||
| 0 | 738.9 cd | 753.0 de | 816.5 de | 769.5 ACD | 745.6 cd | 799.0 de | 805.6 de | 783.4 AD |
| 0.05 | 741.0 cd | 786.4 de | 641.8 bc | 723.1 B–D | 758.7 cd | 810.7 de | 838.6 de | 802.7 A |
| 0.10 | 754.6 cd | 783.0 de | 581.1 ab | 706.2 BC | 774.8 c–e | 828.9 de | 900.2 e | 834.6 A |
| 0.15 | 783.7 de | 782.8 de | 489.4 a | 685.3 B | 762.8 c–e | 829.6 de | 876.6 de | 823.0 A |
| Average | 754.6 A | 776.3 AB | 632.2 D | 721.0 A | 760.5 A | 817.1 BC | 855.3 C | 810.9 B |
| r | 0.926 | 0.711 | −0.975 | −0.971 | 0.726 | 0.955 | 0.850 | 0.861 |
| Aerial parts dry matter yield (g pot−1) | ||||||||
| 0.05 | 127.0 d–f | 152.7 gh | 100.0 bc | 126.6 AC | 131.8 d–h | 140.3 d-h | 148.1 gh | 140.1 B |
| 0.10 | 129.5 d–f | 141.6 d–h | 84.7 ab | 118.6 CD | 134.8 d–h | 149.4 gh | 148.9 f–h | 144.4 A |
| 0.15 | 128.0 d–f | 140.7 d–h | 62.9 a | 110.5 D | 132.3 d–h | 133.6 d-h | 144.6 e–h | 136.8 AB |
| Average | 126.4 B | 140.3 AC | 95.4 D | 120.7 A | 130.9 BC | 138.7 A | 146.8 A | 138.8 B |
| r | 0.814 | 0.389 | −0.987 | −0.951 | 0.769 | 0.251 | −0.100 | 0.384 |
| Humic Acid Dose g kg−1 of Soil | Sand | Loamy Sand | ||||||
|---|---|---|---|---|---|---|---|---|
| Ammonium Nitrate | Urea | UAN | Average | Ammonium Nitrate | Urea | UAN | Average | |
| Total-N content (g kg−1 DM) | ||||||||
| 0 | 8.59 ab | 10.17 b–g | 11.85 h–j | 10.20 AB | 9.33 a–e | 8.87 a–c | 11.57 g–j | 9.92 A |
| 0.05 | 8.87 a-c | 10.55 d–h | 12.97 i–k | 10.80 B | 9.89 b–f | 9.05 a–d | 13.16 jk | 10.70 AB |
| 0.10 | 10.55 d-h | 10.92 e–h | 18.01 l | 13.16 C | 11.11 f–h | 10.64 d–h | 14.00 k | 11.92 D |
| 0.15 | 8.12 a | 10.36 c–h | 21.28 m | 13.25 C | 11.48 f-i | 10.08 b–g | 9.33 a-e | 10.30 AB |
| Average | 9.03 A | 10.50 B | 16.03 D | 11.85 B | 10.45 B | 9.66 A | 12.02 C | 10.71 A |
| r | 0.033 | 0.379 | 0.976 | 0.940 | 0.980 | 0.799 | −0.370 | 0.349 |
| Sulphate(VI) sulphur content (g S-SO4 kg−1 DM) | ||||||||
| 0 | 0.118 a | 0.147 a | 0.099 a | 0.121 A | 0.120 a | 0.128 a | 0.093 a | 0.114 A |
| 0.05 | 0.127 a | 0.092 a | 0.122 a | 0.114 A | 0.146 a | 0.111 a | 0.102 a | 0.120 A |
| 0.10 | 0.134 a | 0.099 a | 0.131 a | 0.121 A | 0.120 a | 0.098 a | 0.112 a | 0.110 A |
| 0.15 | 0.108 a | 0.103 a | 0.148 a | 0.120 A | 0.112 a | 0.116 a | 0.086 a | 0.105 A |
| Average | 0.122 A | 0.110 A | 0.125 A | 0.119 A | 0.125 A | 0.113 A | 0.098 A | 0.112 A |
| r | −0.264 | −0.648 | 0.987 | 0.095 | −0.436 | −0.509 | −0.126 | −0.751 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of Organic Materials to Limit the Potential Negative Effect of Nitrogen on Maize in Different Soils. Materials 2022, 15, 5755. https://doi.org/10.3390/ma15165755
Brodowska MS, Wyszkowski M, Kordala N. Use of Organic Materials to Limit the Potential Negative Effect of Nitrogen on Maize in Different Soils. Materials. 2022; 15(16):5755. https://doi.org/10.3390/ma15165755
Chicago/Turabian StyleBrodowska, Marzena S., Mirosław Wyszkowski, and Natalia Kordala. 2022. "Use of Organic Materials to Limit the Potential Negative Effect of Nitrogen on Maize in Different Soils" Materials 15, no. 16: 5755. https://doi.org/10.3390/ma15165755
APA StyleBrodowska, M. S., Wyszkowski, M., & Kordala, N. (2022). Use of Organic Materials to Limit the Potential Negative Effect of Nitrogen on Maize in Different Soils. Materials, 15(16), 5755. https://doi.org/10.3390/ma15165755






