Pulsatile Controlled Release and Stability Evaluation of Polymeric Particles Containing Piper nigrum Essential Oil and Preservatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil Extraction and Characterization
2.2. Nanoparticle Development and Essential Oil Encapsulation
2.3. Zeta Potential
2.4. Atomic Force Microscopy (AFM)
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Stability Evaluation
- (i)
- Preliminary stability: all systems were subjected to (5 ± 2) °C for the first screening.
- (ii)
- Stability under constant handling at (25 ± 2) °C: Colloidal systems containing preservatives (PNE, PMI, PTI, PED, and NBS) were stored in transparent vials and maintained in a bio-oxygen demand (BOD) incubator at (25 ± 2) °C. All systems were subjected to constant handling (vials were opened and exposed to the laboratory environmental conditions such as air contact, light, and temperature variation) at pre-established time intervals (1–3 days) until reaching an EE% equal to or less than 70%, or presenting alterations (slightly (SA) or intensely Altered (IA)) of their organoleptic properties (color and odor). Physical parameters (pH, electrical conductivity, EE% and turbidity) were measured in all vials on opening. All measurements were performed in triplicate.
- (iii)
- Shelf-life test: The systems selected in the stability test under constant handling at (25 ± 2) °C were submitted to shelf-life tests at (25 ± 2) °C and (35 ± 2) °C. Systems were stored in sealed vials until reaching an EE% equal to or less than 70%, or presenting alterations ((SA) or (IA)) in organoleptic properties (color and odor). Vials were opened every 30 days. Physical parameters (pH, electrical conductivity, EE%, and turbidity) were measured in all vials on opening. All measurements were performed in triplicate.
2.7. Laser Scanning Confocal Microscopy (LSCM) and Fluorescence Measurements
2.8. Attenuated Total Reflectance–Fourier-Transform Infrared Spectroscopy (ATR–FTIR)
2.9. Controlled Release
3. Results and Discussion
3.1. Essential Oil Characterization
3.2. Particle Size Evaluation
3.3. AFM Analysis
3.4. Zeta Potential (ζ)
3.5. Stability of Loaded Particles Containing Preservatives
3.5.1. Preliminary Stability
3.5.2. Stability under Constant Handling
- EE% and Electrical Conductivity
- Turbidity and pH
- Organoleptic Properties
3.5.3. Shelf-Life Evaluation at 25 °C and 35 °C
3.6. Laser Scanning Confocal Microscopy (LSCM) and Fluorescence Measurements
3.7. ATR-FTIR Analysis
3.8. Controlled Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, D.; Das, B.M. Scope of green nanotechnology towards amalgamation of green chemistry for cleaner environment: A review on synthesis and applications of green nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100418. [Google Scholar] [CrossRef]
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2021, 28, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Babaee, M.; Garavand, F.; Rehman, A.; Jafarazadeh, S.; Amini, E.; Cacciotti, I. Biodegradability, physical, mechanical and antimicrobial attributes of starch nanocomposites containing chitosan nanoparticles. Int. J. Biol. Macromol. 2022, 195, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, C.; Qin, Y.; Hu, Y.; Jiao, A.; Jin, Z.; Qiu, C.; Wang, J. Bioactive and functional biodegradable packaging films reinforced with nanoparticles. J. Food Eng. 2022, 312, 110752. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Xiong, J.; Shao, W.; Cui, C.; Sun, N.; Zhang, Y.; Chang, S.; Han, P.; Liu, F.; et al. Biodegradable and high-performance multiscale structured nanofiber membrane as mask filter media via poly(lactic acid) electrospinning. J. Colloid Interface Sci. 2022, 606, 961–970. [Google Scholar] [CrossRef]
- Sharma, R.; Tripathi, A. Green synthesis of nanoparticles and its key applications in various sectors. Mater. Today Proc. 2022, 48, 1626–1632. [Google Scholar] [CrossRef]
- Campelo, P.H.; Junqueira, L.A.; De Resende, J.V.; Zacarias, R.D.; Fernandes, R.V.D.B.; Botrel, D.A.; Borges, S.V. Stability of lime essential oil emulsion prepared using biopolymers and ultrasound treatment. Int. J. Food Prop. 2017, 20, S564–S579. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Wang, B.; Tang, Y.; Oh, Y.; Lamb, N.W.; Xia, S.; Ding, Z.; Chen, B.; Suarez, M.J.; Meng, T.; Kulkarni, V.; et al. Controlled release of dexamethasone sodium phosphate with biodegradable nanoparticles for preventing experimental corneal neovascularization. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 119–123. [Google Scholar] [CrossRef]
- Silva, J.S.M.; Rabelo, M.S.; Lima, S.X.; Rocha, A.N.A.L.F.; Tadei, W.P.; Chaves, F.C.M.; Bezerra, J.D.E.A.; Biondo, M.M.; Campelo, P.H.; Sanches, E.A. Biodegradable nanoparticles loaded with Lippia alba essential oil: A sustainable alternative for Aedes aegypti larvae control. Eur. Acad. Res. 2020, VII, 6237–6258. [Google Scholar]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Khizar, S.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N.; Elaissari, A. Microfluidic-based nanoparticle synthesis and their potential applications. Electrophoresis 2021, 43, 819–838. [Google Scholar] [CrossRef]
- Corrado, I.; Di Girolamo, R.; Regalado-González, C.; Pezzella, C. Polyhydroxyalkanoates-based nanoparticles as essential oil carriers. Polymers 2022, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive carriers of antimicrobial essential oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Bizzo, H.R.; Hovell, A.M.C.; Rezende, C.M. Óleos essenciais no Brasil: Aspectos gerais, desenvolvimento e perspectivas. Quim. Nova 2009, 32, 588–594. [Google Scholar] [CrossRef]
- Sawicka, B.; Skiba, D.; Umachandran, K.; Dickson, A. Alternative and new plants. In Preparation of Phytopharmaceuticals for the Management of Disorders; Egbuna, C., Mishra, A.P., Goyal, M.R., Eds.; Elsevier: Warsaw, Poland, 2021; pp. 491–537. ISBN 978-0-12-820284-5. [Google Scholar]
- Swathy, J.S.; Mishra, P.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Antimicrobial potency of high-energy emulsified black pepper oil nanoemulsion against aquaculture pathogen. Aquaculture 2018, 491, 210–220. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytoscience 2021, 7, 52. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Gulzar, T.; Begum, S.; Afshan, F. Piptigrine, a new insecticidal amide from Piper Nigrum Linn. Nat. Prod. Res. 2004, 18, 473–477. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Jaiswal, G. Evaluation of biological activities of Piper nigrum oil against Tribolium castaneum. Bull. Insectology 2007, 60, 57–61. [Google Scholar]
- Gülçin, İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Perlatti, B.; De Souza Bergo, P.L.; Fernandes, J.B.; Forim, M.R. Polymeric nanoparticle-based insecticides: A controlled release purpose for agrochemicals. In Insecticides—Development of Safer and More Effective Technologies; Trdan, S., Ed.; IntechOpen Limited: London, UK, 2013; pp. 523–550. ISBN 978-953-51-0958-7. [Google Scholar]
- Papanikolaou, N.E.; Kalaitzaki, A.; Karamaouna, F.; Michaelakis, A.; Papadimitriou, V.; Dourtoglou, V.; Papachristos, D.P. Nano-formulation enhances insecticidal activity of natural pyrethrins against Aphis gossypii (Hemiptera: Aphididae) and retains their harmless effect to non-target predators. Environ. Sci. Pollut. Res. 2017, 25, 10243–10249. [Google Scholar] [CrossRef]
- Da Costa, Í.C.; Saraiva Matos, R.S.; De Azevedo, S.G.; Costa, C.A.R.; Sanches, E.A.; Da Fonseca Filho, H. Microscopy-based infrared spectroscopy as a tool to evaluate the influence of essential oil on the surface of loaded bilayered-nanoparticles. Nanotechnology 2021, 32, 345703. [Google Scholar] [CrossRef]
- Mahmoudi Saber, M. Strategies for surface modification of gelatin-based nanoparticles. Colloids Surf. B Biointerfaces 2019, 183, 110407. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Bezerra, J.A.; Campelo, P.H.; Machado, M.B.; Trovati, G.; Santos, L.; Fonseca, D.; et al. Encapsulation of Piper aduncum and Piper hispidinervum essential oils in gelatin nanoparticles: A possible sustainable control tool of Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. J. Sci. Food Agric. 2018, 99, 685–695. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Guia de Estabilidade de Produtos Cosméticos, 1st ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2004; Volume 1, ISBN 8588233150. [Google Scholar]
- Schaffazick, S.R.; Pohlmann, A.R. Caracterização e estudo de estabilidade de suspensões de nanocápsulas e de nanoesferas poliméricas contendo diclofenaco. Acta Farm. Bonaer. 2002, 21, 99–106. [Google Scholar]
- Boyapally, H.; Nukala, R.K.; Bhujbal, P.; Douroumis, D. Controlled release from directly compressible theophylline buccal tablets. Colloids Surf. B Biointerfaces 2010, 77, 227–233. [Google Scholar] [CrossRef]
- Korsmeyer, R.; Peppas, N. Macromolecular and modeling aspects of swelling controlled systems. In Controlled Release Delivery Systems; Springer: Berlin/Heidelberg, 1983; pp. 77–90. [Google Scholar]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Densities of pure substances. In Perry’s Chemical Engineers’ Handbook; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Van Den Dool, H.; Kratz, D.J.A. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Books: Carol Stream, IL, USA, 2009. [Google Scholar]
- McLafferty, F.W.; Stauffer, D.B.; Loh, S.Y. Comparative evaluations of mass spectral data bases. J. Am. Soc. Mass Spectrom. 1991, 2, 438–440. [Google Scholar] [CrossRef] [Green Version]
- NIST. NIST Standard Reference Data Program; National Institute of Standards and Technology: Boulder, CO, USA, 2010. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2014. Available online: http://www.pherobase.net/database/control/control-approach-Mass%20trapping-all.php (accessed on 3 December 2018).
- Ghasemishahrestani, Z.; Mehta, M.; Darne, P.; Yadav, A.; Ranade, S. Tunable synthesis of gelatin nanoparticles employing sophorolipid and plant extract, a promising drug carrier. World J. Pharm. Pharm. Sci. 2015, 4, 1365–1381. [Google Scholar]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.W. (Ed.) Handbook of Lubrication and Tribology; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429137372. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Naidu, B.V.K.; Paulson, A.T. A new method for the preparation of gelatin nanoparticles: Encapsulation and drug release characteristics. J. Appl. Polym. Sci. 2011, 121, 3495–3500. [Google Scholar] [CrossRef]
- Igwe, C.; Yayi, E.; Gbenou, J.; Moudachirou, M. Encapsulation of essential oils of Piper Nigrum and Monodora myristica from South-West Nigeria using gum arabic. Niger. Food J. 2011, 28, 57402. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; Vicente, J.; Dos Santos, C.H.C.; De Carvalho, M.G.; Garcia-Rojas, E.E. Encapsulation of black pepper (Piper nigrum L.) essential oil with gelatin and sodium alginate by complex coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Kapoor, I.P.S.; Singh, B.; Singh, G.; De Heluani, C.S.; De Lampasona, M.P.; Catalan, C.A.N. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum). J. Agric. Food Chem. 2009, 57, 5358–5364. [Google Scholar] [CrossRef]
- Aboutabl, E.A.; Meselhy, K.M.; Elkhreisy, E.M.; Nassar, M.I.; Fawzi, R. Composition and bioactivity of essential oils from leaves and fruits of Myrtus communis and Eugenia supraxillaris (Myrtaceae) grown in Egypt. J. Essent. Oil Bear. Plants 2011, 14, 192–200. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Umadevi, N.P.; Kuttan, R. Antioxidant, anti-inflammatory and antinociceptive properties of black pepper essential oil (Piper nigrum Linn). J. Essent. Oil Bear. Plants 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Morshed, S.; Hossain, M.D.; Ahmad, M.; Junayed, M. Physicochemical characteristics of essential oil of black pepper (Piper nigrum) cultivated in Chittagong, Bangladesh. J. Food Qual. Hazards Control 2017, 4, 66–69. [Google Scholar]
- Costa, J.G.M.; Santos, P.F.; Brito, S.A.; Rodrigues, F.F.G. Composição química e toxicidade de óleos essenciais de espécies de Piper frente a Larvas de Aedes aegypti L. (Diptera: Culicidae). Lat. Am. J. Pharm. 2010, 29, 2383. [Google Scholar] [CrossRef] [Green Version]
- García-Díez, J.; Alheiro, J.; Pinto, A.L.; Soares, L.; Falco, V.; Fraqueza, M.J.; Patarata, L. Behaviour of food-borne pathogens on dry cured sausage manufactured with herbs and spices essential oils and their sensorial acceptability. Food Control 2016, 59, 262–270. [Google Scholar] [CrossRef]
- Tran, T.H.; Ha, L.K.; Nguyen, D.C.; Dao, T.P.; Nhan, L.T.H.; Nguyen, D.H.; Nguyen, T.D.; Vo, D.V.N.; Tran, Q.T.; Bach, L.G. The study on extraction process and analysis of components in essential oils of black pepper (Piper nigrum L.) seeds harvested in Gia Lai province, Vietnam. Processes 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Abdulazeez, M.A.; Sani, I.; James, B.D.; Abdullahi, A.S. Black Pepper (Piper nigrum L.) Oils; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780124166448. [Google Scholar]
- Montanari, R.M.; Barbosa, L.C.A.; Demuner, A.J.; Silva, C.J.; Carvalho, L.S.; Andrade, N.J. Chemical composition and antibacterial activity of essential oils from verbenaceae species: Alternative sources of (E)-Caryophyllene and germacrene-D. Quim. Nova 2011, 34, 1550–1555. [Google Scholar] [CrossRef] [Green Version]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction–gas chromatography, solid-phase microextraction–gas chromatography–mass spectrometry and olfactometry. J. Chromatogr. 2002, 976, 265–275. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, G.; Jawad, M.; Hussein, H. Characterization of bioactive chemical compounds from Aspergillus terreus and evaluation of antibacterial and antifungal activity. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 889–905. [Google Scholar]
- Zhang, C.; Zhao, J.; Famous, E.; Pan, S.; Peng, X.; Tian, J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021, 346, 128845. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.M.; Jensen, H.R.; Philogène, B.J.R.; Arnason, J.T. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem. Rev. 2008, 7, 65–75. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Tropical biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1945–S1953. [Google Scholar] [CrossRef]
- Seibert, J.B.; Viegas, J.S.R.; Almeida, T.C.; Amparo, T.R.; Rodrigues, I.V.; Lanza, J.S.; Frézard, F.J.G.; Soares, R.D.O.A.; Teixeira, L.F.M.; De Souza, G.H.B.; et al. Nanostructured systems improve the antimicrobial potential of the essential oil from Cymbopogon densiflorus leaves. J. Nat. Prod. 2019, 82, 3208–3220. [Google Scholar] [CrossRef] [PubMed]
- Tsuiji, K. Microencapsulation of pesticides and their improved handling safety. J. Microencapsul. 2001, 18, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Costa, Í.C.; Azevedo, S.G.; Sanches, E.A. Caracterização de nanopartículas poliméricas preenchidas com óleo essencial de Piper nigrum por Microscopia de Força Atômica. Matéria 2021, 26, e12981. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Matos, R.S.; Campelo, P.H.; Sanches, E.A.; Da Fonseca Filho, H.D. Evaluation of the nanoscale surface applied to biodegradable nanoparticles containing Allium sativum essential oil. Mater. Lett. 2020, 275, 128111. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; De Lucca Freitas, L.; Pohlmann, A.R. Physicochemical characterization and stability of the polymeric nanoparticle systems for drug administration. Quim. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Campelo, P.H.; Souza, H.J.B.; Figueiredo, J.A.; Fernandes, R.V.B.; Botrel, D.A.; Oliveira, C.R.; Yoshida, M.I.; Borges, S.V. Prebiotic carbohydrates: Effect on reconstitution, storage, release, and antioxidant properties of lime essential oil microparticles. J. Agric. Food Chem. 2016, 65, 445–453. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Da Fonseca Filho, H.D.; Lima, S.X.; Bezerra, J.D.A.; Machado, M.B.; Campelo, P.H.; et al. Alternative biodefensive based on the essential oil from Allium sativum encapsulated in PCL/Gelatin nanoparticles. J. Food Eng. Technol. 2019, 8, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, M.; Domínguez, J.A.; Pascual-Pineda, L.A.; Azuara, E.; Beristain, C.I. Elaboration and characterization of O/W cinnamon (Cinnamomum zeylanicum) and black pepper (Piper nigrum) emulsions. Food Hydrocoll. 2018, 77, 902–910. [Google Scholar] [CrossRef]
- Bravo Cadena, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Schöler, N.; Zimmermann, E.; Katzfey, U.; Hahn, H.; Müller, R.H.; Liesenfeld, O. Preserved solid lipid nanoparticles (SLN) at low concentrations do cause neither direct nor indirect cytotoxic effects in peritoneal macrophages. Int. J. Pharm. 2000, 196, 235–239. [Google Scholar] [CrossRef]
- Guterres, S.S.; Fessi, H.; Barratt, G.; Devissaguet, J.P.; Puisieux, F. Poly (DL-lactide) nanocapsules containing diclofenac: I. Formulation and stability study. Int. J. Pharm. 1995, 113, 57–63. [Google Scholar] [CrossRef]
- Saez, A.; Guzmán, M.; Molpeceres, J.; Aberturas, M.R. Freeze-drying of polycaprolactone and poly (d,l-lactic-glycolic) nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur. J. Pharm. Biopharm. 2000, 50, 379–387. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498726696. [Google Scholar]
- Xing, F.; Cheng, G.; Yi, K.; Ma, L. Nanoencapsulation of capsaicin by complex coacervation of gelatin, acacia, and tannins. J. Appl. Polym. Sci. 2005, 96, 2225–2229. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Quintanar-Guerrero, D.; Del Real-López, A.; Piñon-Segundo, E. Effect of sucrose concentration and pH onto the physical stability of β-carotene nanocapsules. LWT—Food Sci. Technol. 2018, 90, 354–361. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules for cutaneous applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Smittarello, D.; Pinel, V.; Maccaferri, F.; Furst, S.; Rivalta, E.; Cayol, V. Characterizing the physical properties of gelatin, a classic analog for the brittle elastic crust, insight from numerical modeling. Tectonophysics 2021, 812, 228901. [Google Scholar] [CrossRef]
- Lai, P.; Daear, W.; Löbenberg, R.; Prenner, E.J. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly (d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf. B Biointerfaces 2014, 118, 154–163. [Google Scholar] [CrossRef]
- De Oliveira, P.H.R.; Reis, R.R. Ácido benzóico (CAS 65-85-0). Rev. Virtual Química 2017, 9, 2673–2687. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.S. Antimicrobial nanoemulsion formulation based on thyme (Thymus vulgaris) essential oil for UF labneh preservation. J. Mater. Res. Technol. 2020, 10, 1029–1041. [Google Scholar] [CrossRef]
- Daulisio, M.D.C.Z.; Schneider, R.P. Inactivation of Pseudomonas aeruginosa MDC by isothiazolones and biocide stabilizing agents. Int. Biodeterior. Biodegrad. 2020, 155, 105090. [Google Scholar] [CrossRef]
- Puschmann, J.; Herbig, M.E.; Müller-Goymann, C.C. Correlation of antimicrobial effects of phenoxyethanol with its free concentration in the water phase of o/w-emulsion gels. Eur. J. Pharm. Biopharm. 2018, 131, 152–161. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Homs, M.; Calderó, G.; Monge, M.; Morales, D.; Solans, C. Influence of polymer concentration on the properties of nano-emulsions and nanoparticles obtained by a low-energy method. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 204–212. [Google Scholar] [CrossRef]
- Mardones, L.E.; Legnoverde, M.S.; Pereyra, A.M.; Basaldella, E.I. Progress in organic coatings long-lasting isothiazolinone-based biocide obtained by encapsulation in micron-sized mesoporous matrices. Prog. Org. Coatings 2018, 119, 155–163. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Pe, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Pandey, A.K.; Chavez-Gonzalez, M.L.; Silva, A.S.; Singh, P. Essential oils from the genus Thymus as antimicrobial food preservatives: Progress in their use as nanoemulsions—A new paradigm. Trends Food Sci. Technol. 2021, 111, 426–441. [Google Scholar] [CrossRef]
- Liolios, C.C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Asprea, M.; Leto, I.; Bergonzi, M.C.; Bilia, A.R. Thyme essential oil loaded in nanocochleates: Encapsulation efficiency, in vitro release study and antioxidant activity. LWT—Food Sci. Technol. 2017, 77, 497–502. [Google Scholar] [CrossRef]
- Sant’anna, T.B. A interferência do material de embalagem na estabilidade de um medicamento—Estudo de caso (Dietilcarbamazina 50mg). Farmanguinhos/Fiocruz 2013, 1, 77. [Google Scholar]
- Liu, J.; Zhao, Q.; Zhou, L.; Cao, Z.; Shi, C. Influence of environmental temperature during grain filling period on granule size distribution of rice starch and its relation to gelatinization properties. J. Cereal Sci. 2017, 76, 42–55. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Tian, X.-N.; Jiang, Z.-T.; Li, R. Inclusion interactions and molecular microcapsule of Salvia sclarea L. essential oil with β-cyclodextrin derivatives. Eur. Food Res. Technol. 2008, 227, 1001–1007. [Google Scholar] [CrossRef]
- Catauro, M.; Piccolella, S.; Leonelli, C. FT-IR characterization of antimicrobial hybrid materials through sol-gel synthesis. Appl. Sci. 2020, 10, 1180. [Google Scholar] [CrossRef] [Green Version]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of type I collagen and PCL nanofibers using acetic acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.S.; Han, J. Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef]
- Zanetti, M.; Mazon, L.R.; De Meneses, A.C.; Silva, L.L.; De Araújo, P.H.H.; Fiori, M.A.; De Oliveira, D. Encapsulation of geranyl cinnamate in polycaprolactone nanoparticles. Mater. Sci. Eng. C 2019, 97, 198–207. [Google Scholar] [CrossRef]
- Silva, M.D.S.; Cocenza, D.S.; Grillo, R.; De Melo, N.F.S.; Tonello, P.S.; De Oliveira, L.C.; Cassimiro, D.L.; Rosa, A.H.; Fraceto, L.F. Paraquat-loaded alginate/chitosan nanoparticles: Preparation, characterization and soil sorption studies. J. Hazard. Mater. 2011, 190, 366–374. [Google Scholar] [CrossRef]
- Grillo, R.; Dos Santos, N.Z.P.; Maruyama, C.R.; Rosa, A.H.; De Lima, R.; Fraceto, L.F. Poly(ε-caprolactone) nanocapsules as carrier systems for herbicides: Physico-chemical characterization and genotoxicity evaluation. J. Hazard. Mater. 2012, 231, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wu, F. A novel pulsed-release system based on swelling and osmotic pumping mechanism. J. Control. Release 2003, 89, 47–55. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kawabuchi, M.; Sugihara, M.; Sakurai, Y.; Okano, T. Pulsed dextran release from calcium-alginate gel beads. J. Control. Release 1997, 47, 21–29. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.K.; De Souza, A.A.U.; De Oliveira, J.V.; Priamo, W.L.; De Souza, S.M.A.G.U. Modeling of the controlled release of betacarotene into anhydrous ethanol from microcapsules. OpenNano 2016, 1, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Karsa, D.R.; Stephenson, R.A. Encapsulation and Controlled Release; Woodhead Publishing: Swaston, UK, 2005; Volume 53, ISBN 9788578110796. [Google Scholar]
- Sanchez, A.; Gupta, R.K.; Alonso, M.J.; Siber, G.R.; Langer, R. Pulsed controlled-release system for potential use in vaccine delivery. J. Pharm. Sci. 1996, 85, 547–552. [Google Scholar] [CrossRef]
- Penhasi, A.; Gomberg, M. A specific two-pulse release of rivastigmine using a modified time-controlled delivery system: A proof of concept case study. J. Drug Deliv. Sci. Technol. 2018, 47, 404–410. [Google Scholar] [CrossRef]
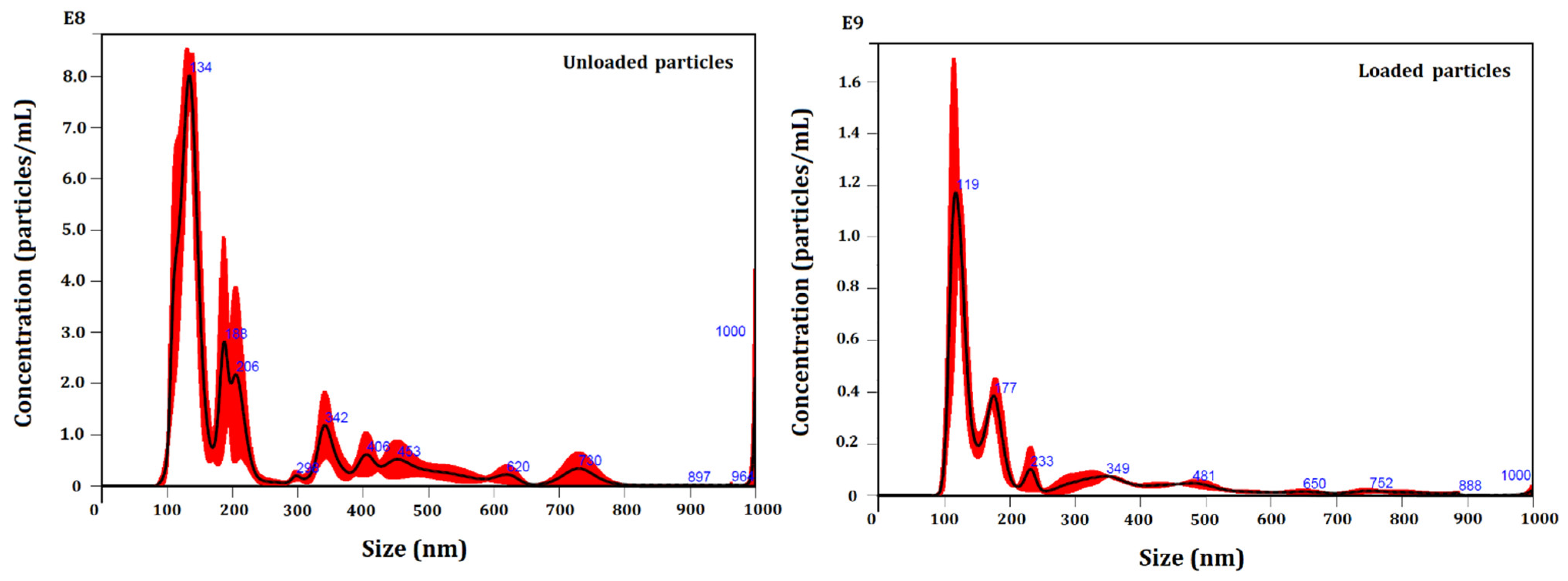

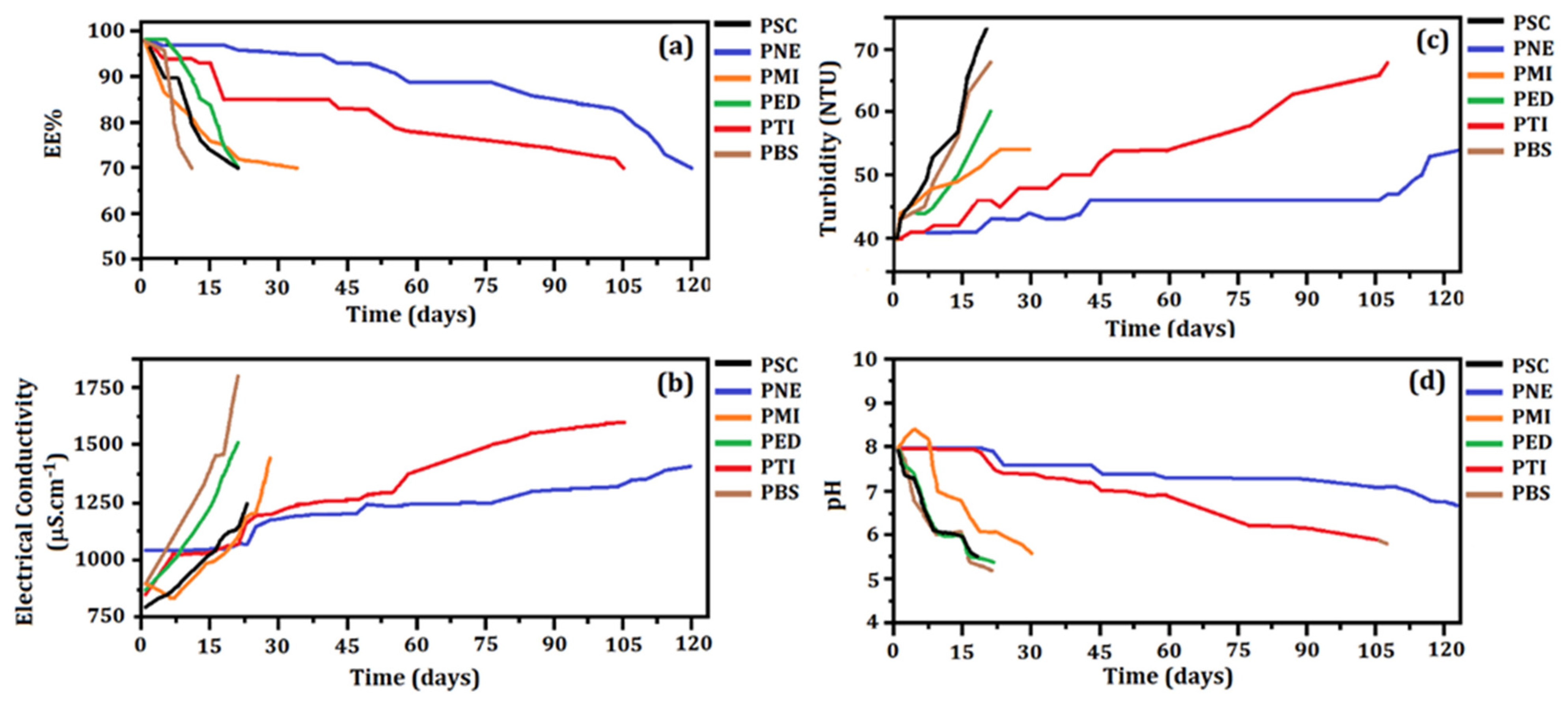
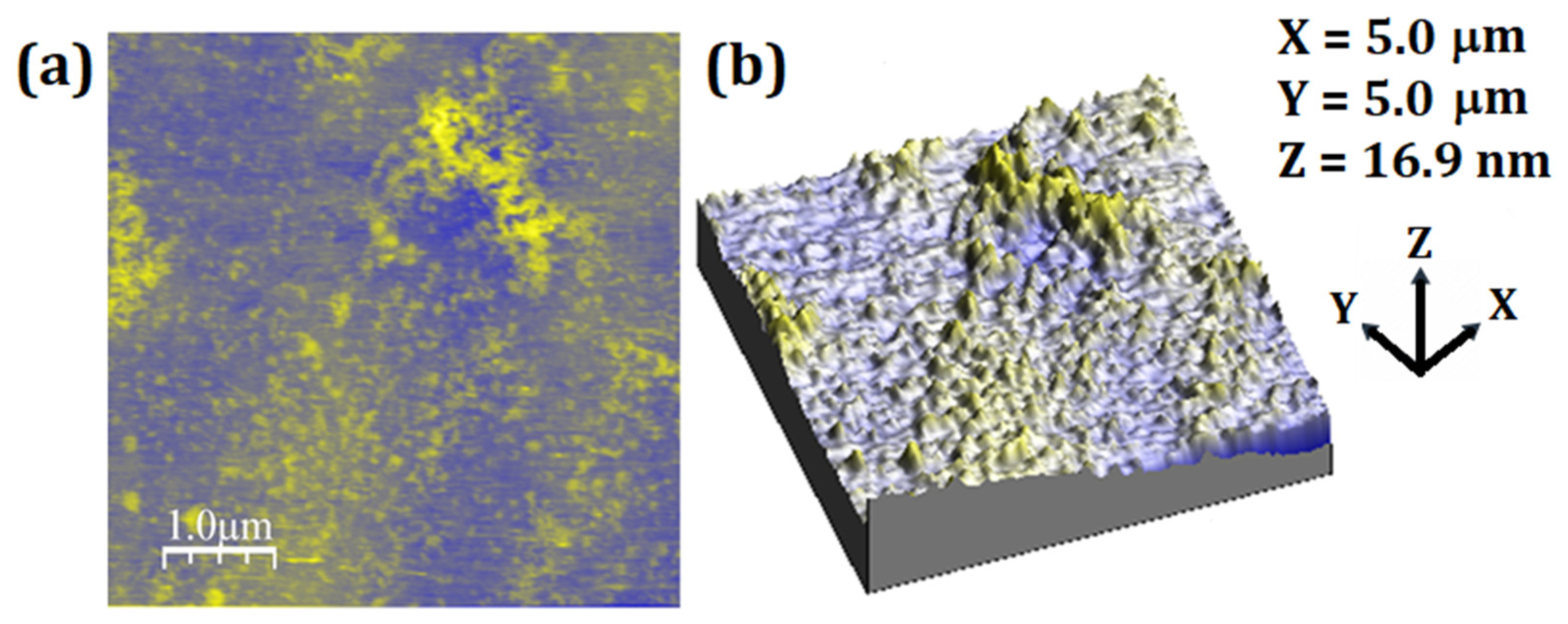



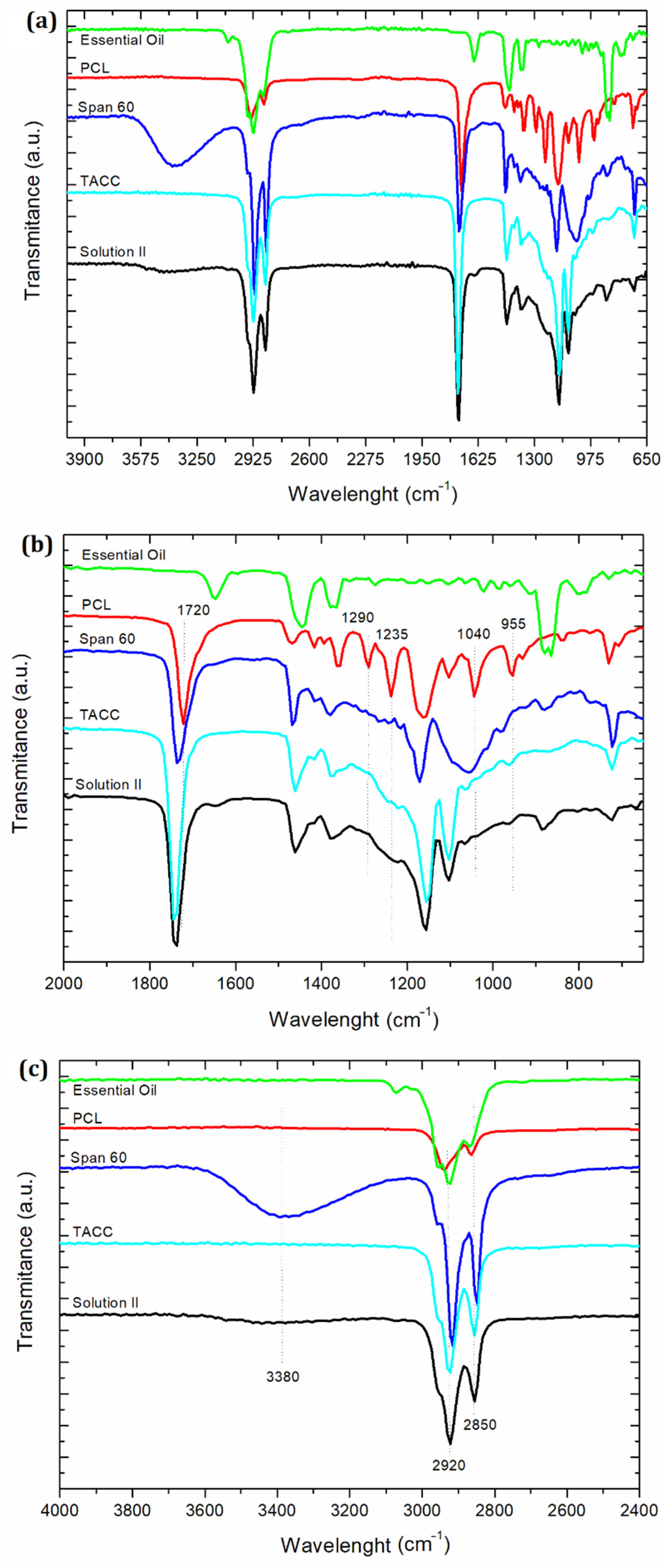


| Parameters | Unloaded Particles | Loaded Particles |
|---|---|---|
| Mean (nm) | 240 ± 8 | 224 ± 9 |
| Mode (nm) | 135 ± 3 | 122 ± 4 |
| SD (nm) | 174 ± 18 | 161 ± 16 |
| D10 (nm) | 118 ± 4 | 114 ± 3 |
| D50 (nm) | 162 ± 2 | 147 ± 4 |
| D90 (nm) | 519 ± 13 | 456 ± 11 |
| Concentration (particles/mL) | (5.0 ± 0.3) × 1010 | (6.0 ± 0.7) × 1010 |
| Parameters | PNE | PMI | PBS | |||
| Time (days) | 0 | 15 | 0 | 15 | 0 | 15 |
| EE (%) | 98 ± 2 | 98 ± 2 | 98 ± 2 | 98 ± 2 | 98 ± 2 | 76 ± 3 |
| Electrical Conductivity (µS·cm−1) | 1043 ± 5 | 1050 ± 5 | 738 ± 5 | 900 ± 5 | 915 ± 5 | 1000 ± 10 |
| Turbidity (NTU) | 40 ± 1 | 45 ± 2 | 40 ± 1 | 40 ± 1 | 40 ± 1 | 60 ± 4 |
| pH | 8.00 ± 0.02 | 7.60 ± 0.03 | 8.00 ± 0.02 | 7.60 ± 0.03 | 8.00 ± 0.02 | 6.70 ± 0.04 |
| Color | NA | NA | NA | NA | NA | SA |
| Odor | NA | NA | NA | NA | NA | SA |
| Parameters | PED | PTI | PSC | |||
| Time (days) | 0 | 15 | 0 | 15 | 0 | 15 |
| EE (%) | 98 ± 2 | 83 ± 3 | 98 ± 2 | 98 ± 2 | 98 ± 2 | 76 ± 3 |
| Electrical Conductivity (µS.cm−1) | 687 ± 5 | 1200 ± 10 | 856 ± 5 | 1100 ± 10 | 628 ± 5 | 5000 ± 10 |
| Turbidity (NTU) | 40 ± 1 | 50 ± 3 | 40 ± 1 | 80 ± 2 | 40 ± 1 | 70 ± 3 |
| pH | 8.00 ± 0.02 | 7.20 ± 0.03 | 8.00 ± 0.02 | 7.00 ± 0.04 | 8.00 ± 0.02 | 6.00 ± 0.03 |
| Cor | NA | SA | NA | SA | NA | SA |
| Odor | NA | SA | NA | SA | NA | SA |
| Systems | Time (Days) | EE (%) | Electrical Conductivity (µS·cm−1) | Turbidity (NTU) | pH | Color | Odor |
|---|---|---|---|---|---|---|---|
| PNE | 0 | (98 ± 2) | (1043 ± 2) | (40 ± 1) | (8.00 ± 0.02) | NA | NA |
| 30 | (98 ± 2) | (1140 ± 3) | (40 ± 2) | (8.00 ± 0.03) | NA | NA | |
| 60 | (97 ± 1) | (1204 ± 4) | (42 ± 3) | (7.90 ± 0.04) | NA | NA | |
| 90 | (95 ± 2) | (1301 ± 3) | (45 ± 2) | (7.80 ± 0.06) | NA | NA | |
| 120 | (90 ± 2) | (1400 ± 4) | (45 ± 2) | (7.70 ± 0.06) | NA | NA | |
| 150 | (89 ± 2) | (1450 ± 3) | (46 ± 3) | (7.60 ± 0.07) | NA | NA | |
| 180 | (85 ± 2) | (1500 ± 3) | (48 ± 2) | (7.50 ± 0.08) | NA | NA | |
| 210 | (84 ± 1) | (1532 ± 4) | (50 ± 2) | (7.40 ± 0.09) | NA | NA | |
| 240 | (80 ± 1) | (1580 ± 6) | (53 ± 2) | (7.3 ± 0.1) | SA | SA | |
| 270 | (70 ± 1) | (1630 ± 7) | (55 ± 2) | (7.1 ± 0.1) | SA | SA | |
| PTI | 0 | (98 ± 3) | (856 ± 2) | (40 ± 3) | (8.00 ± 0.02) | NA | NA |
| 30 | (95 ± 2) | (1000 ± 4) | (41 ± 1) | (7.70 ± 0.02) | NA | NA | |
| 60 | (90 ± 4) | (1121 ± 4) | (44 ± 2) | (7.50 ± 0.04) | NA | NA | |
| 90 | (85 ± 4) | (1203 ± 5) | (47 ± 2) | (7.20 ± 0.04) | NA | NA | |
| 120 | (80 ± 2) | (1342 ± 5) | (48 ± 1) | (7.00 ± 0.02) | NA | NA | |
| 150 | (75 ± 2) | (1389 ± 6) | (50 ± 3) | (6.80 ± 0.03) | SA | SA | |
| 180 | (72 ± 2) | (1409 ± 7) | (55 ± 4) | (6.50 ± 0.03) | SA | SA | |
| 210 | (70 ± 2) | (1500 ± 10) | (60 ± 5) | (5.90 ± 0.03) | SA | SA | |
| PSC | 0 | (98 ± 2) | (800 ± 2) | (40 ± 3) | (8.00 ± 0.03) | NA | NA |
| 30 | (90 ± 2) | (1100 ± 2) | (50 ± 7) | (7.00 ± 0.03) | SA | SA | |
| 60 | (70 ± 2) | (1500 ± 9) | (60 ± 5) | (5.40 ± 0.03) | SA | SA |
| Systems | Time (Days) | EE (%) | Electrical Conductivity (µS·cm−1) | Turbidity (NTU) | pH | Color | Odor |
|---|---|---|---|---|---|---|---|
| PNE | 0 | (98 ± 2) | (1043 ± 5) | (40 ± 1) | (8.00 ± 0.02) | NA | NA |
| 30 | (90 ± 2) | (1043 ± 4) | (42 ± 3) | (7.90 ± 0.03) | NA | NA | |
| 60 | (78 ± 2) | (1060 ± 4) | (45 ± 3) | (7.70 ± 0.06) | NA | NA | |
| 90 | (73 ± 2) | (1070 ± 3) | (49 ± 2) | (7.00 ± 0.08) | NA | NA | |
| 120 | (70 ± 2) | (1080 ± 5) | (50 ± 3) | (6.50 ± 0.05) | SA | SA | |
| PTI | 0 | (98 ± 2) | (856 ± 2) | (40 ± 3) | (8.00 ± 0.02) | NA | NA |
| 30 | (70 ± 4) | (1621 ± 4) | (70 ± 2) | (5.00 ± 0.04) | IA | IA | |
| PSC | 0 | (98 ± 2) | (800 ± 2) | (40 ± 3) | (8.00 ± 0.03) | NA | NA |
| 30 | (50 ± 2) | (1550 ± 4) | (70 ± 7) | (5.00 ± 0.03) | IA | IA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, S.G.; Rocha, A.L.F.; de Aguiar Nunes, R.Z.; da Costa Pinto, C.; Ţălu, Ş.; da Fonseca Filho, H.D.; de Araújo Bezerra, J.; Lima, A.R.; Guimarães, F.E.G.; Campelo, P.H.; et al. Pulsatile Controlled Release and Stability Evaluation of Polymeric Particles Containing Piper nigrum Essential Oil and Preservatives. Materials 2022, 15, 5415. https://doi.org/10.3390/ma15155415
Azevedo SG, Rocha ALF, de Aguiar Nunes RZ, da Costa Pinto C, Ţălu Ş, da Fonseca Filho HD, de Araújo Bezerra J, Lima AR, Guimarães FEG, Campelo PH, et al. Pulsatile Controlled Release and Stability Evaluation of Polymeric Particles Containing Piper nigrum Essential Oil and Preservatives. Materials. 2022; 15(15):5415. https://doi.org/10.3390/ma15155415
Chicago/Turabian StyleAzevedo, Sidney Gomes, Ana Luisa Farias Rocha, Ronald Zico de Aguiar Nunes, Camila da Costa Pinto, Ştefan Ţălu, Henrique Duarte da Fonseca Filho, Jaqueline de Araújo Bezerra, Alessandra Ramos Lima, Francisco Eduardo Gontijo Guimarães, Pedro Henrique Campelo, and et al. 2022. "Pulsatile Controlled Release and Stability Evaluation of Polymeric Particles Containing Piper nigrum Essential Oil and Preservatives" Materials 15, no. 15: 5415. https://doi.org/10.3390/ma15155415
APA StyleAzevedo, S. G., Rocha, A. L. F., de Aguiar Nunes, R. Z., da Costa Pinto, C., Ţălu, Ş., da Fonseca Filho, H. D., de Araújo Bezerra, J., Lima, A. R., Guimarães, F. E. G., Campelo, P. H., Bagnato, V. S., Inada, N. M., & Sanches, E. A. (2022). Pulsatile Controlled Release and Stability Evaluation of Polymeric Particles Containing Piper nigrum Essential Oil and Preservatives. Materials, 15(15), 5415. https://doi.org/10.3390/ma15155415









